Abstract
Camelina (Camelina sativa (L.) Crantz) is an alternative oilseed crop that is garnering increasing popularity due to its multiple applications and greater tolerance to adverse environmental conditions than oilseed rape. The study analyzed selected traits of 10 Canadian and Polish spring camelina genotypes grown in a field experiment in north-eastern Poland in 2015–2018. The greatest differences were observed in seed yield where the effect of weather and environmental conditions explained 72.7% of variance, the effect of genotype explained 5.9% of variance, and the effect of the genotype-by-environment interaction explained 5.7% of total variance. In contrast, 1000-seed weight was not affected by environmental conditions, and it was differentiated only by genotype which explained 73.3% of variance. Genotype was responsible for 4.5%–25.3% of the variance in the remaining traits. The genotype-by-environment interaction explained 2.0%–18.8% of variance in the examined traits. The additive main effects and multiplicative interaction model (AMMI) revealed that genotype 13CS0787-15 was potentially most suited for cultivation in the temperate climate of north-eastern Poland, Central Europe. This genotype was characterized by the highest seed yields and straw yields, as well as the greatest yield stability.
1. Introduction
Camelina (Camelina sativa (L.) Crantz) is one of the oldest crops of the family Brassicaceae. Its exact origin is unknown, but it is considered to be a native species of south-eastern Europe and south-western Asia [1]. In Europe, camelina was farmed already in the Bronze Age, and on the territory of modern Poland (Central Europe), it was grown during the period of the Lusatian culture (1350/1300 BCE–500/400 BCE). Camelina was probably introduced to North America as impurities in hemp seeds and other crop seeds [1,2]. It was imported to Canada in 1863, where it was initially farmed on a small scale and became more widely produced only in the late 1990s. A camelina breeding program was initiated in 2005 by the Saskatoon Research and Development Center of Agriculture and Agri-Food Canada (AAFC). In 2011, a similar program was undertaken in joint effort by the AAFC and Linnaeus Plant Sciences Inc., and the first commercial variety of camelina, Midas, was developed by Dr. Kevin Falk, senior plant breeder at the AAFC Research Station. Certified planting seeds of Midas have been available to producers since 2013. In Poland, the registered varieties of winter and spring camelina that are adapted to local environmental conditions have been developed by the Department of Genetics and Plant Breeding of the Poznań University of Life Sciences. Winter cultivars Maczuga and Luna were entered into the register of varieties protected by National Plant Breeders’ rights in 2012, and spring cultivar Omega was registered in 2013 [3].
New camelina cultivars are bred selectively for various traits, including improved seed yield, oil content, and seed size. Camelina seeds are very small, and they are difficult to sow, in particular under dry conditions [4]. Some breeding programs aim to increase camelina’s tolerance to diseases, including downy mildew [5,6] and aster yellows [7]. Both diseases can significantly compromise camelina production.
Camelina is an alternative oilseed crop which is garnering increasing popularity due to its multiple applications [8]. Camelina seeds are processed into edible oil with a high content of omega 3 fatty acids, as well as into high-protein feed for cattle, fish, poultry, and pigs. Camelina has also numerous industrial applications, including in the production of biofuels and bioproducts such as bioplastics for packaging [9]. The analyzed crop is more resistant to adverse soil than oilseed rape [10] and more environmental adaptability [11]. Therefore, camelina can be grown on low-quality soils that are not suitable for other crops, and the acquisition of camelina oil does not compromise food production. Both spring and winter cultivars of camelina are grown. Winter cultivars are characterized by higher yields than spring cultivars [12]. Camelina increases the biological diversity of arable land. Further research is thus required to improve the agronomic treatments and genetic traits of camelina.
Despite camelina’s low soil and agricultural requirements, its yield potential is determined by genetic factors and responses to environmental conditions and agronomic practices. These factors affect yields by exerting a direct influence on yield components. The response of quantitative yield components to varied environmental conditions is determined by the additive main effects of genotype (G) and environmental conditions (E), as well as by the non-additive effects of genotype-by-environment (GE) interactions [13]. These interactions are well described by the additive main effects and multiplicative interaction model (AMMI) [14,15]. In the AMMI model, the effects of GE interactions are represented by multiplicative parameters for genotypes and environments (see Model 1 in the statistical analysis). The AMMI model combines the analysis of variance (ANOVA) with additive parameters and the principal component analysis (PCA) with multiplicative parameters in a single analysis. These models are most useful when multiplicative GE terms have agricultural interpretability [16,17]. AMMI models are widely used in plant breeding. In recent years (2017–2019), they have been applied to evaluate various plant species, from the most popular crops such as wheat [18,19,20,21], oilseed rape [17,22], maize [23,24,25], rice [26,27,28,29], and sugar beet [30] to cassava [31], peanuts [32], and non-edible crops such as cotton [33] and willow [16].
The aim of this study was to analyze variation in selected parameters of 10 Canadian and Polish cultivars and breeding lines of spring camelina under different environmental conditions during a four-year field experiment. An attempt was made to verify the research hypothesis postulating that groups of genotypes characterized by differences in yield stability under varied environmental conditions, potentially high seed yields or higher biomass yields can be identified among the studied cultivars and breeding lines. The results of the study can be used to improve the selection of cultivars/breeding lines that are optimally adapted to local environmental conditions, thus increasing productivity.
2. Materials and Methods
2.1. Field Experiment
A field experiment was conducted in 2015–2018 in the Research Station in Łężany (N 53°57′, E 21°08′) operated by the University of Warmia and Mazury in Olsztyn, Poland. The experiment was established on Eutric Cambisol composed of medium-quality sandy loam with a well-developed humic horizon and favorable soil-water relationships. Winter wheat was the preceding crop. After wheat harvest, the stubble was broken with a cultivator, and the field was plowed before winter. Secondary tillage involved a seedbed cultivator and a harrow with a string roller. Mineral fertilizer was applied at up to 120 kg ha−1 N, and the fertilizer rate was calculated including soil-available nitrogen. Ten cultivars/breeding lines of spring camelina (Table 1) were sown in a stand of 500 germinating seeds per 1 m2 with 15 cm row spacing.

Table 1.
Spring camelina genotypes tested in the experiment in 2015–2018.
The experiment had a randomized complete block design (RCBD) with four replications in each year of the study. Plot size was 9 m2 each. The plots with the tested cultivars/lines were separated by buffer plots sown with camelina cv. Midas which were not taken into account in the analysis. Camelina seeds were sown with a plot seeder to a depth of 1.5–2.0 cm on 14 April 2015, 7 April 2016, 11 April 2017, and 16 April 2018. Before harvest, final plant density was determined per 1 m2 of plot area, and 10 plants were sampled for measurements of plant height, number of branches, plant mass, and seed mass per plant. Camelina plants were harvested with the Wintersteiger Classic plot harvester. Each year, the analyzed cultivars were harvested on the same date. Straw yield per plot was weighed at harvest, and straw samples were collected to determine moisture content on a dry matter basis. Seeds were cleaned in a seed cleaning machine and weighed. Thousand-seed weight was determined. The dry matter yield of straw and seeds was determined per hectare (Mg ha−1).
2.2. Statistical Analysis
In the first stage of the statistical analysis, the variation in the selected parameters of 10 spring camelina genotypes were determined by mixed-model ANOVA for RCBD at the α = 0.05. To detect structure in the relationships between selected traits of spring camelina factor analysis (FA) with varimax rotation was conducted. In the second stage, the additive main effects and multiplicative interaction (AMMI) model was incorporated into the RCBD model [14,15,16]:
where yGEr is the response of genotype G in environmental conditions E to replicate r, µ is the grand mean, αG is the genotype deviation from the grand mean (genotype mean minus grand mean), βE is the deviation of environmental conditions, λn is the singular value of the nth IPCA axis, γn is the eigenvector value of genotype G on the IPCA axis n, δEn is the eigenvector value of environmental conditions E on the IPCA axis n, ρGE is the residual, κr(E) is the block effect for replication r within the environment E, and εGEr is the experimental error.
yGEr = µ + αG + βE + Σn λn γGn δEn + ρGE + κr(E) + εGEr
If the primary component of the IPCA1 for the effect of the genotype-by-environment (GE) interaction was significant in the AMMI model, the results were presented graphically in the AMMI-1 biplot where the additive main effects of genotype-by-environment interactions were plotted on the x-axis in units of yield, and IPCA1 scores were plotted on the y-axis as the square root of yield [14]. The vertical dashed line represents the grand mean of the yield. All objects on the right side of the vertical line are characterized by average yields above the grand mean, and the objects on the left side of the line are characterized by average yields below the grand mean. The objects’ position relative to the horizontal IPCA1 axis indicates their contribution to the additive main effect. The line intersects the zero point of IPCA1. The points distributed more distantly around the line denote greater contribution to the interaction. Genotypes located close to the horizontal line are characterized by stable yields regardless of environmental conditions. Genotypes and environments with same-sign IPCA1 scores are bound by positive interactions, and scores with opposite signs denote negative interactions [16].
AMMI stability value (ASV) was calculated as follows:
where SSIPCA1 is the sum of squares for IPCA1, SSIPCA2 is the sum of squares for IPCA2. Lower ASV value indicate a more stable genotype across environments.
Yield stability index (YSI) was calculated for each genotype as:
where RYi is rank mean yield for ith genotype, RASVi is rank for the ASV value for ith genotype [34]. In addition, Kang non-parametric rank-sum stability statistic for crop traits was also estimated. This parameter uses both yield and Shukla stability variance. The genotype with the lowest rank-sum is the most desirable one [35].
YSI = RYi + RASVi
The mixed model ANOVA for RBCD, factor analysis, and AMMI analysis were performed in STATISTICA v. 13 [36]. All yield stability statistics were performed in the R environment for statistical computing v. 3.6.2 [37] with the package ‘stability: Stability Analysis of Genotype by Environment Interaction (GEI)’ v. 0.5.0.
3. Results
3.1. Weather Conditions during the Experiment
The experimental period (2015–2018) was characterized by varied weather conditions in different stages of spring camelina genotypes development. The last year of this study 2018 was a hot and very dry year, whereas 2016 was a very wet year (Figure 1). Weather conditions in 2015 and 2017 differed mainly during the growing season. The growing season of 2017 was characterized by moderate precipitation and temperatures close to the long-term average, whereas the growing season of 2015 was characterized by very low precipitation (181 mm) between April and August and the lowest temperatures between May and July.
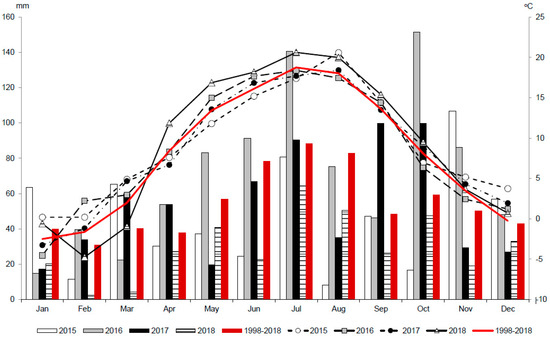
Figure 1.
Weather conditions during the experimental period of 2015–2018 and the referenced long-term average (1998–2018). Bars represent precipitation and curves represent air temperatures.
3.2. Camelina Genotypes and Traits
In the mixed model statistical analysis, the values of final density, number branches, and seed mass per plant validated the null hypothesis (H0) postulating the absence of significant differences between the mean values of the analyzed genotypes. The grand means of the investigated traits were determined at: Final density—235 plants m−2, number branches—7.21, seed mass per plant—1.27 g. Plant height, plant mass, straw yield, 1000-seed weight, and seed yield differed significantly between genotypes. The analyzed genotypes differed significantly in plant height (71.3–84.1 cm), and significantly in plant mass (3.68–5.27 g), and 1000-seed weight (1.18–1.69 g); seed yield ranged from 1.70 to 2.21 Mg ha−1 DM (grand mean of 2.02 Mg ha−1 DM) and straw yield ranged from 1.77 to 2.81 Mg ha−1 DM (grand mean of 2.38 Mg ha−1 DM).
Three factor components were identified in the factor analysis (Table 2). The first component F1 was plant mass, seed mass per plant, number of branches, and final density. The final density score had an opposite sign to the remaining scores, which implies that it was negatively correlated with other traits. The lower the final density, the higher the number of branches, the higher the seed mass and plant mass. The first component explained 32.9% of total variance. The second component F2 explained 23.1% of total variance, and it was composed of positively correlated plant height, seed yield, and straw yield. The third component F3 was 1000-seed weight, and it explained 14.8% of total variance. When combined, F1, F2, and F3 explained 70.8% of total variance in the analyzed traits of spring camelina genotypes.

Table 2.
Factor loads in factor analysis with varimax rotation.
The biplot as a graphical presentation of the relationship between all traits and genotypes revealed that genotype 14CS0887 differed most considerably from the group, whereas the remaining genotypes formed small clusters (Figure 2). Genotypes 13CS0787-05 and 14CS0886 were highly similar in terms of a high number of branches, seed mass, and plant mass. Genotypes 13CS0787-08 and 13CS0787-15 were characterized by the highest seed yield, relatively tall plants, and average values of the remaining traits.
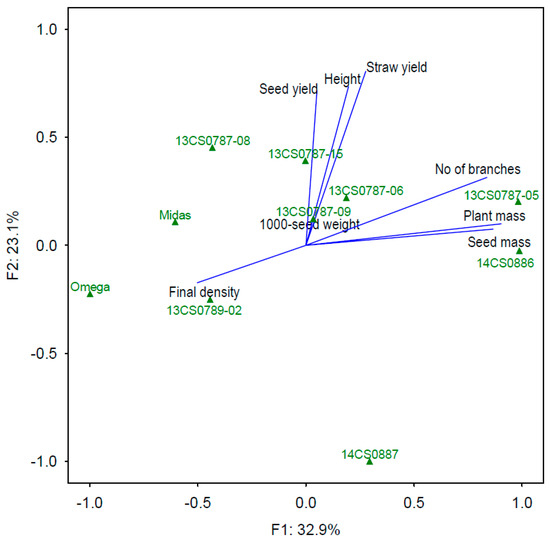
Figure 2.
The biplot of the relationship between all traits and 10 spring camelina genotypes (triangles) across four experimental years (2015–2018).
3.3. Genotype-by-Environment (GE) Interaction
The results of the AMMI analysis of selected traits in the studied spring camelina genotypes are presented in Table 3. The table presents the effects of the environment (weather and habitat conditions during the four-year experiment), genotype, block effect within environment (years), and GE interactions represented by three IPCA components. The number of IPCA components was equal to the number of the environmental degrees of freedom. The effect of environmental conditions was significant for observed traits, and in most cases, it was more effective in explaining the variance in the analyzed traits than the effect of genotype. Environmental conditions explained 72.7% of variance in seed yield and 71.5% of variance in plant height. The only exception was 1000-seed weight whose variance was determined by the effect of genotype in 73.3%. The remaining traits were influenced by genotype in 4.5% to 25.3%.

Table 3.
The additive main effects and multiplicative interaction (AMMI) analysis of variance in the traits of 10 camelina genotypes across four growing seasons (environments).
The effect of the GE interaction plays a very important role in selective breeding. In this study, GE interaction exerted the greatest effect on straw yield, where the values of both IPCA1 and IPCA2 were significant. In regards to the number of branches, 1000-seed weight and seed yield, the significance of the GE interaction was determined only by the IPCA1 score. The GE interaction explained 2.0% to 18.8% of variance in camelina traits.
Due to different commercial uses of camelina plants, the GE interaction was analyzed in greater detail based on seed yield (oil) and straw yield (biomass). On average, seed yields were highest in genotypes 13CS0787-15 and 13CS0787-08, and lowest in genotype 14CS0887 (Figure 3). Average seed yields were highest in the hot and dry year 2018 and lowest in the relatively warm and wet year 2016. The effect of the GE interaction was manifested in 2015 and 2017. In the relatively cool and dry year 2015 the greatest increase in seed yield was noted in genotype 13CS0789-02. In 2017 with moderate precipitation levels and temperatures similar to the long-term average, seed yields increased in genotypes 13CS0787-15, 13CS0787-05, Midas and 13CS0787-06, and the highest seed yield was noted in genotype 13CS0787-08. It should be stressed that IPCA1 scores with opposite signs denote negative responses of genotypes. Thus, environmental conditions in 2015 did not support the development of genotypes located above the horizontal IPCA1 axis with positive IPCA1 scores, whereas environmental conditions in 2017 did not promote the development of genotypes located below the IPCA1 axis with negative scores (Figure 3). In the group of highest-yielding genotypes, genotype 13CS0787-15 was characterized by the highest yield stability, i.e., average seed yields were high and stable regardless of environmental conditions. In the AMMI-1 biplot, the above is represented by the point closest to the horizontal line on the right side of the diagram. Genotype 14CS0887 is equally close to the horizontal IPCA1 axis, but it is located on the left side of the diagram, which indicates that its seed yields were lowest and stable regardless of environmental conditions (Figure 3). The same was confirmed by the yield stability index (YSI) with AMMI stability values and Kang’s sums of ranks with Shukla’s stability variances (Table 4). Lower ASV and Shukla’s value indicated a more stable genotype across environments, and the genotype with the lowest rank-sum was the most desirable one.
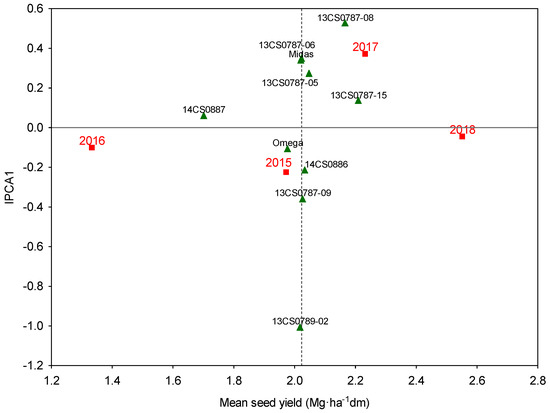
Figure 3.
AMMI-1 biplot of the seed yield of 10 camelina genotypes (triangles) across four experimental years (2015–2018) with varied weather conditions (squares).

Table 4.
The genotype means and stability indices for seed yield and straw yield. The number in square brackets denote rang value.
The average straw yield was highest in 2018 and lowest in 2017. The genotype 13CS0787-15 (ASV = 0.41; Shukla = 0.055) was characterized by the highest average straw yield, and genotype 14CS0887 (ASV = 0.57; Shukla = 0.050)—by the significantly lowest straw yield (Figure 4). The scatter of points in the AMMI-1 diagram indicates that many genotypes contributed significantly to the effect of the GE interaction. The greatest contribution was made by genotypes 14CS0886, 13CS0787-06, 13CS0787-08, and cv. Midas.
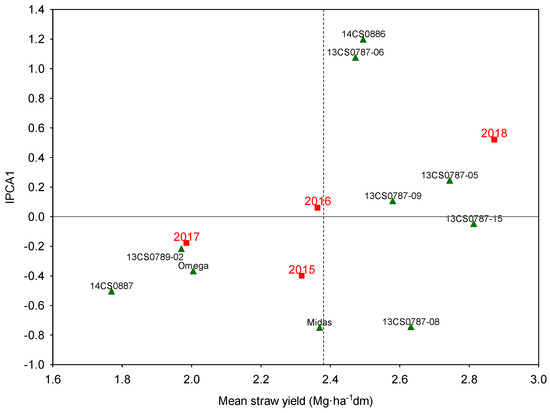
Figure 4.
AMMI-1 biplot of the straw yield of 10 camelina genotypes (triangles) across four experimental years (2015–2018) with varied weather conditions (squares).
4. Discussion and Conclusions
The variation in selected traits of 10 spring camelina genotypes grown in the temperate climate of north-eastern Poland (Central Europe) was analyzed. Studies of the type usually involve a series of varietal experiments in several locations or, less frequently, varietal experiments conducted in a single location over a period of several years [13,38]. The developed AMMI models demonstrated that the analyzed traits of spring camelina plants were influenced mainly by environmental conditions and were significantly less affected by genotype. The effect of environmental conditions explained 72.7% of variance in seed yield. The above could explain the highest average seed yields in the dry year and the lowest average seed yields in the wet year in all genotypes. According to Gesch and Cermak [39] and Berti et al. [9], camelina is relatively tolerant to drought, but highly susceptible to waterlogging in the field. Drought tolerance could be partially attributed to camelina’s ability to extract water from deep soil horizons. However, camelina has a short life cycle, which probably plays an important role in its low water use and tolerance to severe drought stress [40]. The effect of the GE interaction was significant, but it explained less than 6% of variance, and was manifested only in years when weather conditions were stable and approximated the long-term average. Camelina seed yields were high (grand mean of 2.02 Mg ha−1 DM) relative to the grand means reported in other countries [9]: Austria (1.91 Mg ha−1 DM), Canada (1.05 Mg ha−1 DM), Chile (1.41 Mg ha−1 DM), Denmark (1.82 Mg ha−1 DM), Germany (1.88 Mg ha−1 DM), Italy (2.25 Mg ha−1 DM), Romania (2.33 Mg ha−1 DM), Slovenia (0.60 Mg ha−1 DM), Turkey (0.78 Mg ha−1 DM), and the USA (1.30 Mg ha−1 DM). However, the present experiment was established on medium-quality soil which is suitable for the cultivation of wheat and exceeds the soil requirements for camelina. Regardless of the above, the AMMI analysis identified genotype 13CS0787-15 as most suitable for cultivation in the temperate climate of north-eastern Poland. This genotype was characterized by the highest seed and straw yields, and, most importantly, the highest yield stability (seed yield: ASV = 0.23 and Shukla = 0.003; straw yield: ASV = 0.41 and Shukla = 0.055). The genotype 13CS0787-08 was also characterized by above-average seed yields, but considerably lower seed yield stability (ASV = 0.70 and Shukla = 0.025). The high average yields of genotype 13CS0787-08 can be attributed to the strong effect of the GE interaction in the supportive years of 2017 and 2018, whereas low yields were noted under less favorable weather conditions in 2016. Low yield stability is a considerable defect in locations characterized by varied weather and environmental conditions [13,18,38]. The genotype 14CS0887 is definitely not suitable for cultivation in north-eastern Poland.
Camelina seed yields are often analyzed jointly with the oil content, protein content, and fatty acid profile of seeds. The theoretical oil and protein yields per unit area can be calculated by multiplying “seed yield × oil content of seeds” and “seed yield × protein content of seeds”, respectively, but it is generally more influenced by the quantitative trait [11]. In genotypes with similar seed yield, oil yields are more likely to be determined by qualitative trait. In addition to genetic factors, seed oil content is also affected by environmental conditions [41,42]. Zanetti et al. [11] found a strong negative correlation between oil content and the protein content of seeds. Krzyżaniak et al. [42] analyzed the same 10 spring camelina genotypes under identical environmental conditions similar to our experiment. In their study, genotype 13CS0787-15, which was selected as most suitable for cultivation in our study, was characterized by a high content of oil with high concentrations of polyunsaturated fatty acids (PUFAs), and low concentrations of monounsaturated fatty acids (MUFAs) and saturated fatty acids (SFAs). As a result, genotype 13CS0787-15 was characterized by the highest potential oil yield per hectare [42]. When this trait is combined with high yield stability, 13CS0787-15 emerges as a genotype that delivers stable yields of high-quality oil regardless of environmental conditions.
The seed yield, oil content, protein content, and the fatty acid profile of camelina seeds have been widely researched in the literature, but the effect of the GE interaction on the above parameters remains insufficiently investigated. In the USA, such studies have been carried out by Guy et al. [43], George et al. [44], and Obour et al. [45] to select the optimal camelina genotypes as an alternative crop for cultivation in very dry regions. Zanetti et al. [11] investigated the effect of the GE interaction on spring camelina grown in various countries (Canada, Greece, Italy, Poland). These authors analyzed that locations differed in weather and environmental conditions, but mixed model two-way ANOVA did not reveal a significant effect of the GE interaction on seed yield or the content of oil, protein, SFAs, MUFAs, or PUFAs in camelina seeds [11].
In the present study, straw yields produced nearly exemplary data for describing the effect of the GE interaction. Straw yields were equally influenced by environment and genotype (25% each). Due to the significant effect of the GE interaction (IPCA1 and PICA2 scores), the variation in straw yield could be accurately investigated in 10 genotypes under exposure to varied weather conditions over a period of four years. The results were used to describe the optimal conditions for each genotype and to maximize biomass yields. The analysis also revealed which weather and environmental factors contributed to a negative effect of the GE interaction and a decrease in the straw yields of spring camelina.
In our study, spring camelina plants were able to compensate for low final density through high values of the remaining yield components. In the factor analysis the first component (F1) grouped plant mass, seed mass per plant, number of branches, and final density, where the sign of final density was opposite to the sign of the remaining variables. Therefore, lower final density was compensated by a higher number of branches, higher plant mass, and higher seed mass per plant. These correlated traits were significantly differentiated by environmental conditions, but not by genotype. Similar observations were made by Urbaniak et al. [46] or Berti et al. [47] who also incorporated the number of siliques per plant and the number of seeds per plant in their analyses. These correlations should be associated with seed yield and straw yield which were grouped in the second component (F2). The first two components of FA are most important because they explain most of the variance in the analyzed data (Table 2, Figure 2).
In contrast, the 1000-seed weight of spring camelina plants was affected by genotype, but not by environmental conditions. The proportion in 1000-seed weight across the analyzed genotypes (i.e., the proportion between seeds sown and seed yield) was similar under different environmental conditions. According to Jankowski and Budzyński [48], 1000-seed weight plays a key role in the seed yield of spring camelina, whereas the remaining yield components are strongly correlated, but do not significantly influence seed yield per unit area. These observations were not confirmed in the present study where 1000-seed weight was identified as the third, separate factor component (F3) that explained less than 15% of total variance. In contrast, yield components and yields were the first two factor components (F1 + F2) that explained 56% of total variance. The above implies that 1000-seed weight was not directly correlated with seed yield.
Since breeders intend to increase camelina seed size [4], it should be noted that in our experiment, the 1000-seed weight determined based on the produced camelina yield and the seeds supplied by breeders was higher in all tested breeding lines than in the previously developed cv. Midas. According to Berti et al. [9], larger seeded forms may exhibit better seedling emergence and crop establishment under dry seeding conditions. However, Vollmann et al. [41,49] observed that larger camelina seeds (heavier than 1.5 g) may be of low agronomic value due to concomitant reductions of both seed yield and oil content.
In conclusion, climate change can profoundly influence agricultural crops, which spurs the search for plant species that can be cultivated under less favorable conditions. Camelina could be one of such species due to its wide range of applications and high tolerance to adverse environmental conditions. The breeding success of new spring camelina varieties will be determined by yield stability and adaptability to varied climatic and environmental conditions.
Author Contributions
Conceptualization, M.K., J.T., M.J.S., D.Z., and J.K.; Methodology, M.K., J.T., M.J.S., D.Z., and J.K.; Validation, D.Z., M.K., J.T., M.J.S., and J.K.; Formal analysis, D.Z.; Investigation, M.K. and J.T.; Resources, M.K. and J.T.; Data curation, M.K. and D.Z.; Writing—original draft preparation, D.Z. and J.T.; Writing—review and editing, D.Z. and J.T.; Visualization, D.Z.; Supervision, M.K. and J.T.; Project administration, M.K. and J.T. All authors have read and agreed to the published version of the manuscript.
Funding
Project financially supported by MINISTER OF SCIENCE AND HIGHER EDUCATION in the range of the program entitled “Regional Initiative of Excellence” for the years 2019–2022, Project No. 010/RID/2018/19, amount of funding 12.000.000 PLN.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Francis, A.; Warwick, S.I. The biology of Canadian weeds. 142. Camelina alyssum (Mill.) Thell.; C. microcarpa Andrz. ex DC.; C. sativa (L.) Crantz. Can. J. Plant Sci. 2009, 89, 791–810. [Google Scholar] [CrossRef]
- Putnam, D.H.; Budin, J.T.; Field, L.A.; Breene, W.M. Camelina: A promising low-input oilseed. In New Crops; Janick, J., Simon, J.E., Eds.; Wiley: New York, NY, USA, 1993; pp. 314–322. [Google Scholar]
- Research Centre for Cultivar Testing Diariusz. Polish Gaz. Plant Breeders’ Rights Natl. List 2018, 3, 16.
- Eynck, C.; Falk, K. Camelina (Camelina sativa). In BiofuelCrops: Production, Physiology and Genetics; Singh, B., Ed.; CABI: Fort Valley, GA, USA, 2013; p. 369. [Google Scholar]
- Putnam, M.L.; Serdani, M.; Ehrensing, D.; Curtis, M. Camelina Infected by Downy Mildew (Hyaloperonospora camelinae ) in the Western United States: A First Report. Plant Heal. Prog. 2009, 10, 40. [Google Scholar] [CrossRef]
- Gesch, R.W. Influence of genotype and sowing date on camelina growth and yield in the north central U.S. Ind. Crops Prod. 2014, 54, 209–215. [Google Scholar] [CrossRef]
- Soroka, J.; Olivier, C.; Grenkow, L.; Séguin-Swartz, G. Interactions between Camelina sativa (Brassicaceae) and insect pests of canola. Can. Entomol. 2015, 147, 193–214. [Google Scholar] [CrossRef]
- Masella, P.; Martinelli, T.; Galasso, I. Agronomic evaluation and phenotypic plasticity of Camelina sativa growing in Lombardia, Italy. Crop Pasture Sci. 2014, 65, 453. [Google Scholar] [CrossRef]
- Berti, M.; Gesch, R.; Eynck, C.; Anderson, J.; Cermak, S. Camelina uses, genetics, genomics, production, and management. Ind. Crops Prod. 2016, 94, 690–710. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Krzyżaniak, M.; Tworkowski, J.; Załuski, D.; Kwiatkowski, J.; Szczukowski, S. Camelina and crambe production – Energy efficiency indices depending on nitrogen fertilizer application. Ind. Crops Prod. 2019, 137, 386–395. [Google Scholar] [CrossRef]
- Zanetti, F.; Eynck, C.; Christou, M.; Krzyżaniak, M.; Righini, D.; Alexopoulou, E.; Stolarski, M.J.; Van Loo, E.N.; Puttick, D.; Monti, A. Agronomic performance and seed quality attributes of Camelina (Camelina sativa L. crantz) in multi-environment trials across Europe and Canada. Ind. Crops Prod. 2017, 107, 602–608. [Google Scholar] [CrossRef]
- Kurasiak-Popowska, D.; Tomkowiak, A.; Człopińska, M.; Bocianowski, J.; Weigt, D.; Nawracała, J. Analysis of yield and genetic similarity of Polish and Ukrainian Camelina sativa genotypes. Ind. Crops Prod. 2018, 123, 667–675. [Google Scholar] [CrossRef]
- Madry, W.; Gacek, E.S.; Paderewski, J.; Gozdowski, D.; Drzazga, T. Adaptive yield response of winter wheat cultivars across environments in Poland using combined AMMI and cluster analyses. Int. J. Plant Prod. 2011, 5, 299–309. [Google Scholar]
- Gauch, H.G. Statistical Analysis of Regional Yield Trials: AMMI Analysis of Factorial Designs; Elsevier: Ithaca, NY, USA, 1992; ISBN 9780444892409. [Google Scholar]
- Gauch, H.G. A Simple Protocol for AMMI Analysis of Yield Trials. Crop Sci. 2013, 53, 1860–1869. [Google Scholar] [CrossRef]
- Fabio, E.S.; Volk, T.A.; Miller, R.O.; Serapiglia, M.J.; Gauch, H.G.; Van Rees, K.C.J.; Hangs, R.D.; Amichev, B.Y.; Kuzovkina, Y.A.; Labrecque, M.; et al. Genotype × environment interaction analysis of North American shrub willow yield trials confirms superior performance of triploid hybrids. GCB Bioenergy 2017, 9, 445–459. [Google Scholar] [CrossRef]
- Bocianowski, J.; Niemann, J.; Nowosad, K. Genotype-by-environment interaction for seed quality traits in interspecific cross-derived Brassica lines using additive main effects and multiplicative interaction model. Euphytica 2019, 215, 7. [Google Scholar] [CrossRef]
- Paderewski, J.; Rodrigues, P.C. Constrained AMMI Model: Application to Polish Winter Wheat Post-Registration Data. Crop Sci. 2018, 58, 1458. [Google Scholar] [CrossRef]
- Mohammadi, R.; Armion, M.; Zadhasan, E.; Ahmadi, M.M.; Amri, A. The use of AMMi model for interpreting genotype × environment interaction in durum wheat. Exp. Agric. 2018, 54, 670–683. [Google Scholar] [CrossRef]
- Krishnappa, G.; Ahlawat, A.K.; Shukla, R.B.; Singh, S.K.; Singh, S.K.; Singh, A.M.; Singh, G.P. Multi-environment analysis of grain quality traits in recombinant inbred lines of a biparental cross in bread wheat (Triticum aestivum L.). Cereal Res. Commun. 2019, 47, 334–344. [Google Scholar] [CrossRef]
- Sardouei-Nasab, S.; Mohammadi-Nejad, G.; Nakhoda, B. Yield stability in bread wheat germplasm across drought stress and non-stress conditions. Agron. J. 2019, 111, 175–181. [Google Scholar] [CrossRef]
- Nowosad, K.; Liersh, A.; Popławska, W.; Bocianowski, J. Genotype by environment interaction for oil content in winter oilseed rape (Brassica napus L.) using additive main effects and multiplicative interaction model. Indian J. Genet. Plant Breed. 2017, 77, 293–297. [Google Scholar] [CrossRef]
- Brankovic-Radojcic, D.; Babic, V.; Girek, Z.; Zivanovic, T.; Radojĉic, A.; Filipovic, M.; Srdic, J. Evaluation of maize grain yield and yield stability by AMMI analysis. Genetika 2018, 50, 1067–1080. [Google Scholar] [CrossRef]
- Bernardo Júnior, L.A.Y.; da Silva, C.P.; de Oliveira, L.A.; Nuvunga, J.J.; Pires, L.P.M.; Von Pinho, R.G.; Balestre, M. AMMI Bayesian Models to Study Stability and Adaptability in Maize. Agron. J. 2018, 110, 1765. [Google Scholar] [CrossRef]
- Das, A.K.; Muthusamy, V.; Zunjare, R.U.; Chauhan, H.S.; Sharma, P.K.; Bhat, J.S.; Guleria, S.K.; Saha, S.; Hossain, F. Genetic variability-, genotype × environment interactions- and combining ability-analyses of kernel tocopherols among maize genotypes possessing novel allele of γ-tocopherol methyl transferase (ZmVTE4). J. Cereal Sci. 2019, 86, 1–8. [Google Scholar] [CrossRef]
- Jain, B.T.; Sarial, A.K.; Saharan, R.P.; HariKesh; Anuragi, H. AMMI biplot analysis for stability in basmati rice (Oryza sativa L.) in different production systems. Electron. J. Plant Breed. 2018, 9, 502–510. [Google Scholar] [CrossRef]
- Ponnuswamy, R.; Rathore, A.; Vemula, A.; Das, R.R.; Singh, A.K.; Balakrishnan, D.; Arremsetty, H.S.; Vemuri, R.B.; Ram, T. Analysis of multi-location data of hybrid rice trials reveals complex genotype by environment interaction. Cereal Res. Commun. 2018, 46, 146–157. [Google Scholar] [CrossRef]
- Inabangan-Asilo, M.A.; Mallikarjuna Swamy, B.P.; Amparado, A.F.; Descalsota-Empleo, G.I.L.; Arocena, E.C.; Reinke, R. Stability and G × E analysis of zinc-biofortified rice genotypes evaluated in diverse environments. Euphytica 2019, 215, 61. [Google Scholar] [CrossRef]
- Torres, R.O. Yield stability of selected rice breeding lines and donors across conditions of mild to moderately severe drought stress. F. Crop. Res. 2018, 220, 37–45. [Google Scholar] [CrossRef]
- Hassani, M.; Heidari, B.; Dadkhodaie, A.; Stevanato, P. Genotype by environment interaction components underlying variations in root, sugar and white sugar yield in sugar beet (Beta vulgaris L.). Euphytica 2018, 214, 79. [Google Scholar] [CrossRef]
- Fotso, A.K.; Hanna, R.; Kulakow, P.; Parkes, E.; Iluebbey, P.; Ngome, F.A.; Suh, C.; Massussi, J.; Choutnji, I.; Wirnkar, V.L. AMMI analysis of cassava response to contrasting environments: Case study of genotype by environment effect on pests and diseases, root yield, and carotenoids content in Cameroon. Euphytica 2018, 214, 155. [Google Scholar] [CrossRef]
- Patil, A.S.; Hedvat, I.; Levy, Y.; Galili, S.; Hovav, R. Genotype-by-environment effects on the performance of recombinant inbred lines of Virginia-type peanut. Euphytica 2018, 214, 83. [Google Scholar] [CrossRef]
- Riaz, M.; Farooq, J.; Ahmed, S.; Amin, M.; Chattha, W.S.; Ayoub, M.; Kainth, R.A. Stability analysis of different cotton genotypes under normal and water-deficit conditions. J. Integr. Agric. 2019, 18, 1257–1265. [Google Scholar] [CrossRef]
- Bocianowski, J.; Księżak, J.; Nowosad, K. Genotype by environment interaction for seeds yield in pea (Pisum sativum L.) using additive main effects and multiplicative interaction model. Euphytica 2019, 215, 191. [Google Scholar] [CrossRef]
- Kang, M.S. A rank-sum method for selecting high-yielding, stable corn genotypes. Cereal Res. Commun. 1988, 16, 113–115. [Google Scholar]
- TIBCO Software Inc. Statistica (Data Analysis Software System), Version 13. Available online: http://statistica.io (accessed on 27 November 2019).
- R Core Team. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-project.org (accessed on 27 November 2019).
- Gauch, H.G.; Piepho, H.P.; Annicchiarico, P. Statistical analysis of yield trials by AMMI and GGE: Further considerations. Crop Sci. 2008, 48, 866–889. [Google Scholar] [CrossRef]
- Gesch, R.W.; Cermak, S.C. Sowing Date and Tillage Effects on Fall-Seeded Camelina in the Northern Corn Belt. Agron. J. 2011, 103, 980. [Google Scholar] [CrossRef]
- Hunsaker, D.J.; French, A.N.; Clarke, T.R.; El-Shikha, D.M. Water use, crop coefficients, and irrigation management criteria for camelina production in arid regions. Irrig. Sci. 2011, 29, 27–43. [Google Scholar] [CrossRef]
- Vollmann, J.; Moritz, T.; Kargl, C.; Baumgartner, S.; Wagentristl, H. Agronomic evaluation of camelina genotypes selected for seed quality characteristics. Ind. Crops Prod. 2007, 26, 270–277. [Google Scholar] [CrossRef]
- Krzyżaniak, M.; Stolarski, M.J.; Tworkowski, J.; Puttick, D.; Eynck, C.; Załuski, D.; Kwiatkowski, J. Yield and seed composition of 10 spring camelina genotypes cultivated in the temperate climate of Central Europe. Ind. Crops Prod. 2019, 138, 111443. [Google Scholar] [CrossRef]
- Guy, S.O.; Wysocki, D.J.; Schillinger, W.F.; Chastain, T.G.; Karow, R.S.; Garland-Campbell, K.; Burke, I.C. Camelina: Adaptation and performance of genotypes. F. Crop. Res. 2014, 155, 224–232. [Google Scholar] [CrossRef]
- George, N.; Hollingsworth, J.; Yang, W.R.; Kaffka, S. Canola and camelina as new crop options for cool-season production in California. Crop Sci. 2017, 57, 693–712. [Google Scholar] [CrossRef]
- Obour, A.K.; Obeng, E.; Mohammed, Y.A.; Ciampitti, I.A.; Durrett, T.P.; Aznar-Moreno, J.A.; Chen, C. Camelina seed yield and fatty acids as influenced by genotype and environment. Agron. J. 2017, 109, 947–956. [Google Scholar] [CrossRef]
- Urbaniak, S.D.; Caldwell, C.D.; Zheljazkov, V.D.; Lada, R.; Luan, L. The effect of seeding rate, seeding date and seeder type on the performance of Camelina sativa L. in the Maritime Provinces of Canada. Can. J. Plant Sci. 2008, 88, 501–508. [Google Scholar] [CrossRef]
- Berti, M.; Wilckens, R.; Fischer, S.; Solis, A.; Johnson, B. Seeding date influence on camelina seed yield, yield components, and oil content in Chile. Ind. Crops Prod. 2011, 34, 1358–1365. [Google Scholar] [CrossRef]
- Jankowski, K.J.; Budzyński, W.S. The role of yield components in the management of yielding of some spring oilseed crops. Rośliny Oleiste Oilseed Crop. 2003, 23, 443–454. [Google Scholar]
- Vollmann, J.; Damboeck, A.; Eckl, A.; Schrems, H.; Ruckenbauer, P. Improvement of Camelina sativa, an underexploited oilseed. In Progress in New Crops; Janick, J., Ed.; ASHS Press: Alexandria, VA, USA, 1996; pp. 357–362. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).