Response of U.S. Rice Cultivars Grown under Non-Flooded Irrigation Management
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Experimental Design, and Cultural Management
2.2. Trait Measurements
2.3. Statistical Analysis
3. Results
3.1. Analysis of Variance (ANOVA)
3.2. Variety and Trait Response to Soil Moisture Levels
3.3. Correlations Between Plant Traits
3.4. Identification of Traits Related to Crop Resiliency to Non-Flooded Irrigation Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khush, G.S. Green revolution: The way forward. Nat. Rev. Genet. 2001, 2, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Espe, M.B.; Hill, J.E.; Leinfelder-Miles, M.; Espino, L.A.; Mutters, R.; Mackill, D.; van Kessel, C.; Linquist, B.A. Rice yield improvements through plant breeding are offset by inherent yield declines over time. Field Crop. Res. 2018, 222, 59–65. [Google Scholar] [CrossRef]
- Grassini, P.; Eskridge, K.M.; Cassman, K.G. Distinguishing between yield advances and yield plateaus in historical crop production trends. Nat. Commun. 2013, 4, 2918. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.; Deryng, D.; Müller, C.; Frieler, K.; Konzmann, M.; Gerten, D.; Glotter, M.; Flörke, M.; Wada, Y.; Best, N.; et al. Constraints and potentials of future irrigation water availability on agricultural production under climate change. Proc. Natl. Acad. Sci. USA 2014, 111, 3239–3244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gheewala, S.; Silalertruksa, T.; Nilsalab, P.; Mungkung, R.; Perret, S.; Chaiyawannakarn, N. Water footprint and impact of water consumption for food, feed, fuel crops production in Thailand. Water 2014, 6, 1698–1718. [Google Scholar] [CrossRef] [Green Version]
- Ringler, C.; Zhu, T. Water resources and food security. Agron. J. 2015, 107, 1533–1538. [Google Scholar] [CrossRef] [Green Version]
- Childs, N.; Skorbiansky, S. Rice Outlook. USDA Economic Research Service. RCS-181. Available online: https://www.ers.usda.gov/webdocs/publications/90130/rcs-18i.pdf?v=2685.6 (accessed on 14 September 2018).
- Reba, M.L.; Daniels, M.; Chen, Y.; Sharpley, A.; Bouldin, J.; Teague, T.G.; Daniel, P.; Henry, C.G. A statewide network for monitoring agricultural water quality and water quantity in Arkansas. J. Soil Water Conserv. 2013, 68, 45A–49A. [Google Scholar] [CrossRef] [Green Version]
- Czarnecki, J.B.; Hays, P.D.; Mckee, P.W. The Mississippi River Valley Alluvial Aquifer in Arkansas: A Sustainable Water Resource; Fact Sheet FS-041-0; USGS: Reston, VI, USA, 2002; 4p. [CrossRef]
- Arkansas Natural Resources Commission. Arkansas Groundwater Protection and Management Report for 2011; Arkansas Natural Resources Commission: Little Rock, AR, USA, 2012.
- Clark, B.R.; Hart, R.M.; Gurdak, J.J. Groundwater Availability of the Mississippi Embayment; Professional Paper 1785; U.S. Geological Survey: Reston, VA, USA, 2011; 62p. Available online: https://pubs.usgs.gov/pp/1785/ (accessed on 14 September 2018).
- Upholt, B. An Interstate Battle for Groundwater. The Atlantic Daily. Available online: https://www.theatlantic.com/science/archive/2015/12/mississippi-memphis-tennesee-groundwater-aquifer/418809/ (accessed on 14 September 2018).
- FAO. Water Resource Issues and Agriculture. Available online: http://www.fao.org/docrep/003/t0800e/t0800e0a.htm (accessed on 15 July 2019).
- Korres, N.E.; Norsworthy, J.K.; Burgos, N.R.; Oosterhuis, D.M. Temperature and drought impacts on rice production: An agronomic perspective regarding short- and long-term adaptation measures. Water Resour. Rural Dev. 2017, 9, 12–27. [Google Scholar] [CrossRef]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [Green Version]
- Davies, W.J.; Zhang, J.; Yang, J.; Dodd, I.C. Novel crop science to improve yield and resource use efficiency in water-limited agriculture. J. Agric. Sci. 2010, 149, 123–131. [Google Scholar] [CrossRef]
- Blum, A. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crop. Res. 2009, 112, 119–123. [Google Scholar] [CrossRef]
- De Avila, L.A.; Martini, L.F.D.; Mezzomo, R.F.; Refatti, J.P.; Campos, R.; Cezimbra, D.M.; Machado, S.L.O.; Massey, J.H.; Carlesso, R.; Marchesan, E. Rice water use efficiency and yield under continuous and intermittent irrigation. Agron. J. 2015, 107, 442–448. [Google Scholar] [CrossRef]
- Hameed, F.; Xu, J.; Rahim, S.F.; Wei, Q.; Rehman Khalil, A.u.; Liao, Q. Optimizing nitrogen options for improving nitrogen use efficiency of rice under different water regimes. Agronomy 2019, 9, 39. [Google Scholar] [CrossRef] [Green Version]
- Massey, J.H.; Mark Stiles, C.; Epting, J.W.; Shane Powers, R.; Kelly, D.B.; Bowling, T.H.; Leighton Janes, C.; Pennington, D.A. Long-term measurements of agronomic crop irrigation made in the Mississippi delta portion of the lower Mississippi River Valley. Irrig. Sci. 2017, 35, 297–313. [Google Scholar] [CrossRef]
- Massey, J.H.; Walker, T.W.; Anders, M.M.; Smith, M.C.; Avila, L.A. Farmer adaptation of intermittent flooding using multiple-inlet rice irrigation in Mississippi. Agric. Water Manag. 2014, 146, 297–304. [Google Scholar] [CrossRef]
- Oo, A.Z.; Sudo, S.; Inubushi, K.; Chellappan, U.; Yamamoto, A.; Ono, K.; Mano, M.; Hayashida, S.; Koothan, V.; Osawa, T.; et al. Mitigation potential and yield-scaled global warming potential of early-season drainage from a rice paddy in Tamil Nadu, India. Agronomy 2018, 8, 202. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.C.; Massey, J.H.; Branson, J.; Epting, J.W.; Pennington, D.; Tacker, P.L.; Thomas, J.; Vories, E.D.; Wilson, C. Water use estimates for various rice production systems in Mississippi and Arkansas. Irrig. Sci. 2006, 25, 141–147. [Google Scholar] [CrossRef]
- Linquist, B.A.; Anders, M.M.; Adviento-Borbe, M.A.A.; Chaney, R.L.; Nalley, L.L.; da Rosa, E.F.F.; Kessel, C. Reducing greenhouse gas emissions, water use, and grain arsenic levels in rice systems. Glob. Chang. Biol. 2015, 21, 407–417. [Google Scholar] [CrossRef]
- Takahashi, Y.; Minamikawa, R.; Hattori, K.H.; Kurishima, K.; Kihou, N.; Yuita, K. Arsenic behavior in paddy fields during the cycle of flooded and non-flooded periods. Environ. Sci. Technol. 2004, 38, 1038–1044. [Google Scholar] [CrossRef]
- Barnaby, J.Y.; Rohila, J.S.; Henry, C.G.; Sicher, R.C.; Reddy, V.R.; McClung, A.M. Physiological and metabolic responses of rice to reduced soil moisture: Relationship of water stress tolerance and grain production. Int. J. Mol. Sci. 2019, 20, 1846. [Google Scholar] [CrossRef] [Green Version]
- Gealy, D.R.; Rohila, J.S.; Boykin, D.L. Genetic potential of rice under alternate-wetting-and-drying irrigation management for barnyardgrass (Echinochloa crus-galli) suppression and grain yield production. Weed Sci. 2019, 67, 453–462. [Google Scholar] [CrossRef]
- Sandhu, N.; Kumar, A. Bridging the rice yield gaps under drought: QTLs, genes, and their use in breeding programs. Agronomy 2017, 7, 27. [Google Scholar] [CrossRef]
- Torres, R.O.; McNally, K.L.; Cruz, C.V.; Serraj, R.; Henry, A. Screening of rice genebank germplasm for yield and selection of new drought tolerance donors. Field Crop. Res. 2013, 147, 12–22. [Google Scholar] [CrossRef]
- Lone, A.A.; Jumaa, S.H.; Wijewardana, C.; Taduri, S.; Redona, E.D.; Reddy, K.R. Drought stress tolerance screening of elite American breeding rice genotypes using low-cost pre-fabricated mini-hoop modules. Agronomy 2019, 9, 199. [Google Scholar] [CrossRef] [Green Version]
- Verma, H.; Borah, J.L.; Sarma, R.N. Variability assessment for root and drought tolerance traits and genetic diversity analysis of rice germplasm using SSR markers. Sci. Rep. 2019, 9, 16513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espe, M.B.; Cassman, K.G.; Yang, H.; Guilpart, N.; Grassini, P.; Van Wart, J.; Anders, M.; Beighley, D.; Harrell, D.; Linscombe, S.; et al. Yield gap analysis of US rice production systems shows opportunities for improvement. Field Crop. Res. 2016, 196, 276–283. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-Y.; Wang, J.; Zeigler, R.S. The 3,000 rice genomes project: New opportunities and challenges for future rice research. GigaScience 2014, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, T.; International Rice Genome Sequencing Project. The map-based sequence of the rice genome. Nature 2005, 436, 793–800. [Google Scholar] [CrossRef]
- Lu, H.; Redus, M.A.; Coburn, J.R.; Rutger, J.N.; McCouch, S.R.; Tai, T.H. Population structure and breeding patterns of 145 U.S. rice cultivars based on SSR marker analysis. Crop Sci. 2005, 45, 66–76. [Google Scholar] [CrossRef] [Green Version]
- Khush, G.S. Origin, dispersal, cultivation and variation of rice. Plant Mol. Biol. 1997, 35, 25–34. [Google Scholar] [CrossRef]
- Monkham, T.; Jongdee, B.; Pantuwan, G.; Sanitchon, J.; Mitchell, J.H.; Fukai, S. Genotypic variation in grain yield and flowering pattern in terminal and intermittent drought screening methods in rainfed lowland rice. Field Crop. Res. 2015, 175, 26–36. [Google Scholar] [CrossRef]
- Torres, R.O.; Henry, A. Yield stability of selected rice breeding lines and donors across conditions of mild to moderately severe drought stress. Field Crop. Res. 2016, 220, 37–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xangsayasane, P.; Jongdee, B.; Pantuwan, G.; Fukai, S.; Mitchell, J.H.; Inthapanya, P.; Jothiyangkoon, D. Genotypic performance under intermittent and terminal drought screening in rainfed lowland rice. Field Crop. Res. 2014, 156, 281–292. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [Green Version]
- Dixit, S.; Singh, A.; Kumar, A. Rice breeding for high grain yield under drought: A strategic solution to a complex problem. Int. J. Agron. 2014, 2014, 1–15. [Google Scholar] [CrossRef]
- Vikram, P.; Swamy, B.P.M.; Dixit, S.; Singh, R.; Singh, B.P.; Miro, B.; Kohli, A.; Henry, A.; Singh, N.K.; Kumar, A. Drought susceptibility of modern rice varieties: An effect of linkage of drought tolerance with undesirable traits. Sci. Rep. 2015, 5, 14799. [Google Scholar] [CrossRef] [Green Version]
- Tardieu, F.; Simonneau, T.; Muller, B. The physiological basis of drought tolerance in crop plants: A scenario-dependent probabilistic approach. Annu. Rev. Plant Biol. 2018, 69, 733–759. [Google Scholar] [CrossRef] [Green Version]
- Faralli, M.; Matthews, J.; Lawson, T. Exploiting natural variation and genetic manipulation of stomatal conductance for crop improvement. Curr. Opin. Plant Biol. 2019, 49, 1–7. [Google Scholar] [CrossRef]
- Das, A.; Rushton, P.J.; Rohila, J.S. Metabolomic profiling of soybeans (Glycine max L.) reveals the importance of sugar and nitrogen metabolism under drought and heat stress. Plants 2017, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Palanog, A.D.; Swamy, B.P.M.; Shamsudin, N.A.A.; Dixit, S.; Hernandez, J.E.; Boromeo, T.H.; Cruz, P.C.S.; Kumar, A. Grain yield QTLs with consistent-effect under reproductive-stage drought stress in rice. Field Crop. Res. 2014, 161, 46–54. [Google Scholar] [CrossRef]
- Zou, G.H.; Mei, H.W.; Liu, H.Y.; Liu, G.L.; Hu, S.P.; Yu, X.Q.; Li, M.S.; Wu, J.H.; Luo, L.J. Grain yield responses to moisture regimes in a rice population: Association among traits and genetic markers. Theor. Appl. Genet. 2005, 112, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Kamoshita, A.; Yamagishi, J. Preflowering abortion reduces spikelet number in upland rice (Oryza sativa L.) under water stress. Crop Sci. 2008, 48, 2389–2395. [Google Scholar] [CrossRef]
- Liu, G.; Mei, H.; Liu, H.; Yu, X.; Zou, G.; Luo, L. Sensitivities of rice grain yield and other panicle characters to late-stage drought stress revealed by phenotypic correlation and QTL analysis. Mol. Breed. 2010, 25, 603–613. [Google Scholar] [CrossRef]
- Saxton, K.E.; Rawls, W.J. Soil water characteristic estimates by texture and organic matter for hydrologic solutions. Soil Sci. Soc. Am. J. 2006, 70, 1569–1578. [Google Scholar] [CrossRef] [Green Version]
- Counce, P.A.; Keisling, T.C.; Mitchell, A.J. A uniform, objective, and adaptive system for expressing rice development. Crop Sci. 2000, 40, 436–443. [Google Scholar] [CrossRef] [Green Version]
- Counce, P.A.; Watkins, K.B.; Brye, K.R.; Siebenmorgen, T.J. A model to predict safe stages of development for rice field draining and field tests of the model predictions in the Arkansas grand prairie. Agron. J. 2009, 101, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Nelson, J.C.; McClung, A.M.; Fjellstrom, R.G.; Moldenhauer, K.A.; Boza, E.; Jodari, F.; Oard, J.H.; Linscombe, S.; Scheffler, B.E.; Yeater, K.M. Mapping QTL main and interaction influences on milling quality in elite US rice germplasm. Theor. Appl. Genet. 2011, 122, 291–309. [Google Scholar] [CrossRef]
- Blum, A. PlantStress.com. Canopy Temperature and it Measurement by the Hand-Held Infrared Thermometer in the Field. Available online: http://www.plantstress.com/methods/IRT_protocol.htm (accessed on 14 September 2018).
- Haroldsen, V.M.; Chi-Ham, C.L.; Kulkarni, S.; Lorence, A.; Bennett, A.B. Constitutively expressed DHAR and MDHAR influence fruit, but not foliar ascorbate levels in tomato. Plant Physiol. Biochem. 2011, 49, 1244–1249. [Google Scholar] [CrossRef] [Green Version]
- McCouch, S.R.; Wright, M.H.; Tung, C.W.; Maron, L.G.; McNally, K.L.; Fitzgerald, M.; Singh, N.; DeClerck, G.; Agosto-Perez, F.; Korniliev, P.; et al. Open access resources for genome-wide association mapping in rice. Nat. Commun. 2016, 7, 10532. [Google Scholar] [CrossRef] [Green Version]
- Pinson, S.R.M.; Liu, G.; Jia, M.H.; Jia, Y.; Fjellstrom, R.G.; Sharma, A.; Wang, Y.; Tabien, R.E.; Li, Z. Registration of a rice gene-mapping population consisting of ‘TeQing’-into-‘Lemont’ backcross introgression lines. J. Plant Regist. 2012, 6, 128–135. [Google Scholar] [CrossRef] [Green Version]
- Sandhu, N.; Dixit, S.; Swamy, B.P.M.; Vikram, P.; Venkateshwarlu, C.; Catolos, M.; Kumar, A. Positive interactions of major-effect QTLs with genetic background that enhances rice yield under drought. Sci. Rep. 2018, 8, 1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeJonge, K.C.; Taghvaeian, S.; Trout, T.J.; Comas, L.H. Comparison of canopy temperature-based water stress indices for maize. Agric. Water Manag. 2015, 156, 51–62. [Google Scholar] [CrossRef]
- Kaler, A.S.; Ray, J.D.; Schapaugh, W.T.; Asebedo, A.R.; King, C.A.; Gbur, E.E.; Purcell, L.C. Association mapping identifies loci for canopy temperature under drought in diverse soybean genotypes. Euphytica 2018, 214, 135. [Google Scholar] [CrossRef]
- Sathishraj, R.; Bheemanahalli, R.; Ramachandran, M.; Dingkuhn, M.; Muthurajan, R.; Krishna, J.S.V. Capturing heat stress induced variability in spikelet sterility using panicle, leaf and air temperature under field conditions. Field Crop. Res. 2016, 190, 10–17. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; Yin, X.; Stomph, T.-J.; Struik, P.C. Can exploiting natural genetic variation in leaf photosynthesis contribute to increasing rice productivity? A simulation analysis. Plant Cell Environ. 2014, 37, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yu, J.; Huang, B. Elevated CO2-mitigation of high temperature stress associated with maintenance of positive carbon balance and carbohydrate accumulation in kentucky bluegrass. PLoS ONE 2014, 9, e89725. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, A.; Kondo, K.; Ikka, T.; Takai, T.; Tanabata, T.; Yamamoto, T. A novel QTL associated with rice canopy temperature difference affects stomatal conductance and leaf photosynthesis. Breed. Sci. 2018, 68, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Basu, S.; Roychoudhury, A.; Saha, P.P.; Sengupta, D.N. Differential antioxidative responses of indica rice cultivars to drought stress. Plant Growth Regul. 2010, 60, 51. [Google Scholar] [CrossRef]
- Guo, Z.; Ou, W.; Lu, S.; Zhong, Q. Differential responses of antioxidative system to chilling and drought in four rice cultivars differing in sensitivity. Plant Physiol. Biochem. 2006, 44, 828–836. [Google Scholar] [CrossRef]
- Roy, S.; Arora, A.; Chinnusamy, V.; Singh, V.P. Endogenous reduced ascorbate: An indicator of plant water deficit stress in wheat. Indian J. Plant Physiol. 2017, 22, 365–368. [Google Scholar] [CrossRef]
- Seminario, A.; Song, L.; Zulet, A.; Nguyen, H.T.; González, E.M.; Larrainzar, E. Drought stress causes a reduction in the biosynthesis of ascorbic acid in soybean plants. Front. Plant Sci. 2017, 8, 1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variety | Sub-Population 1 | Year of Release for Production in U.S.A. | Commercialized Acreage in Southern U.S.A. | Grain Shape | Parents of Mapping Population | Bi-parental Mapping Population |
|---|---|---|---|---|---|---|
| Katy | TRJ | 1990 | Major | Long | A1 | A |
| PI 312777 | Indica | . | None | Short | A2 | |
| Cybonnet | TRJ | 2006 | Minor | Long | B1 | B |
| Saber | TRJ | 2004 | Minor | Long | B2 | |
| Francis | TRJ | 2007 | Major | Long | C1 | C |
| Rondo | Indica | 2010 | Minor | Long | C2 | |
| Kaybonnet | TRJ | 1994 | Major | Long | D1 | D |
| Zhe 733 | Indica | . | None | Long | D2 | |
| Lemont | TRJ | 1985 | Major | Long | E1 | E |
| Teqing | Indica | . | None | Medium | E2 | |
| Roy J | TRJ | 2013 | Major | Long | . | . |
| Lagrue | TRJ | 1995 | Major | Long | . | . |
| Mars | TRJ | 1979 | Major | Medium | . | . |
| CL 151 | TRJ | 2011 | Major | Long | . | . |
| Jupiter | TRJ | 2006 | Major | Long | . | . |
| Trait | Total Sums of Squares | Year Effect | Variety Effect | Irrigation Effect | Variety × Irrigation Effect | % of Total Sums of Squares Explained by Significant Interaction | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year Sums of Squares | p Value > F | Variety Sums of Squares | p Value > F | Irrigation Sums of Squares | p Value > F | Variety × Irrigation Sums of Squares | p Value > F | |||
| Days to heading | 56,372 | 27,257 | <0.0001 | 13,472 | <0.0001 | 2161 | <0.022 | 1056 | ns | - |
| Days to maturity | 47,553 | 17,453 | <0.0001 | 12,790 | <0.0001 | 295 | ns | 166 | ns | - |
| Plant height | 87,473 | 22,272 | <0.0001 | 24,948 | <0.0001 | 11,583 | <0.006 | 3908 | <0.0003 | 4.5 |
| GY | 115,592 | 573 | ns | 31,832 | <0.0001 | 12,046 | <0.05 | 220 | <0.005 | 0.2 |
| EGP | 295 | 21 | <0.0001 | 86 | <0.0001 | 3 | ns | 15 | ns | - |
| Avg soil moisture | 25,072 | 4869 | <0.0001 | 933 | <0.0001 | 11,716 | <0.0001 | 783 | ns | - |
| Panicle stress | 1372 | 69 | <0.0001 | 135 | <0.0001 | 378 | <0.002 | 63 | ns | - |
| Leaf stress | 215 | 0.05 | ns | 24 | <0.0001 | 11 | ns | 20 | ns | - |
| Grain length | 132 | 0.12 | <0.05 | 116 | <0.0001 | 1 | <0.001 | 1.16 | ns | - |
| Grain width | 23 | 0.05 | <0.0013 | 19 | <0.0001 | 0.39 | <0.012 | 0.25 | <0.03 | 1.1 |
| Length:Width | 99 | 0.06 | <0.006 | 90 | <0.0001 | 0.09 | ns | 0.23 | ns | - |
| Chalk | 15,401 | 553 | <0.0001 | 10,422 | <0.0001 | 49 | ns | 619 | <0.02 | 4.0 |
| TKW | 1661 | 142 | <0.0001 | 761 | <0.0001 | 161 | <0.0001 | 56 | ns | - |
| Grain thickness | 3.55 | 0.04 | <0.0001 | 2.57 | <0.0001 | 0.015 | ns | 0.13 | <0.02 | 3.6 |
| Grainfill days | 14,892 | 3055 | <0.0001 | 3146 | <0.0001 | 166 | ns | 1580 | ns | - |
| Canopy temp ^ | 4251 | 111 | <0.0003 | 222 | <0.02 | 1322 | <0.012 | 310 | ns | - |
| GDD1 ^ | 16,679,867 | 12,509,660 | <0.0001 | 157,448 | <0.0001 | 40,353 | ns | 195,696 | ns | - |
| GDD2 ^ | 3,280,359 | 420,046 | <0.0001 | 1,043,113 | <0.0001 | 104,726 | ns | 308,436 | ns | - |
| Irrigation Treatment | Avg Soil Moisture (%VWC) | Days to Heading | Plant Height (cm) | GY (g plant−1) | Panicle Stress Score | Grain Length (mm) | Grain Width (mm) | TKW (g) | Canopy Temperature (°C) ^ |
|---|---|---|---|---|---|---|---|---|---|
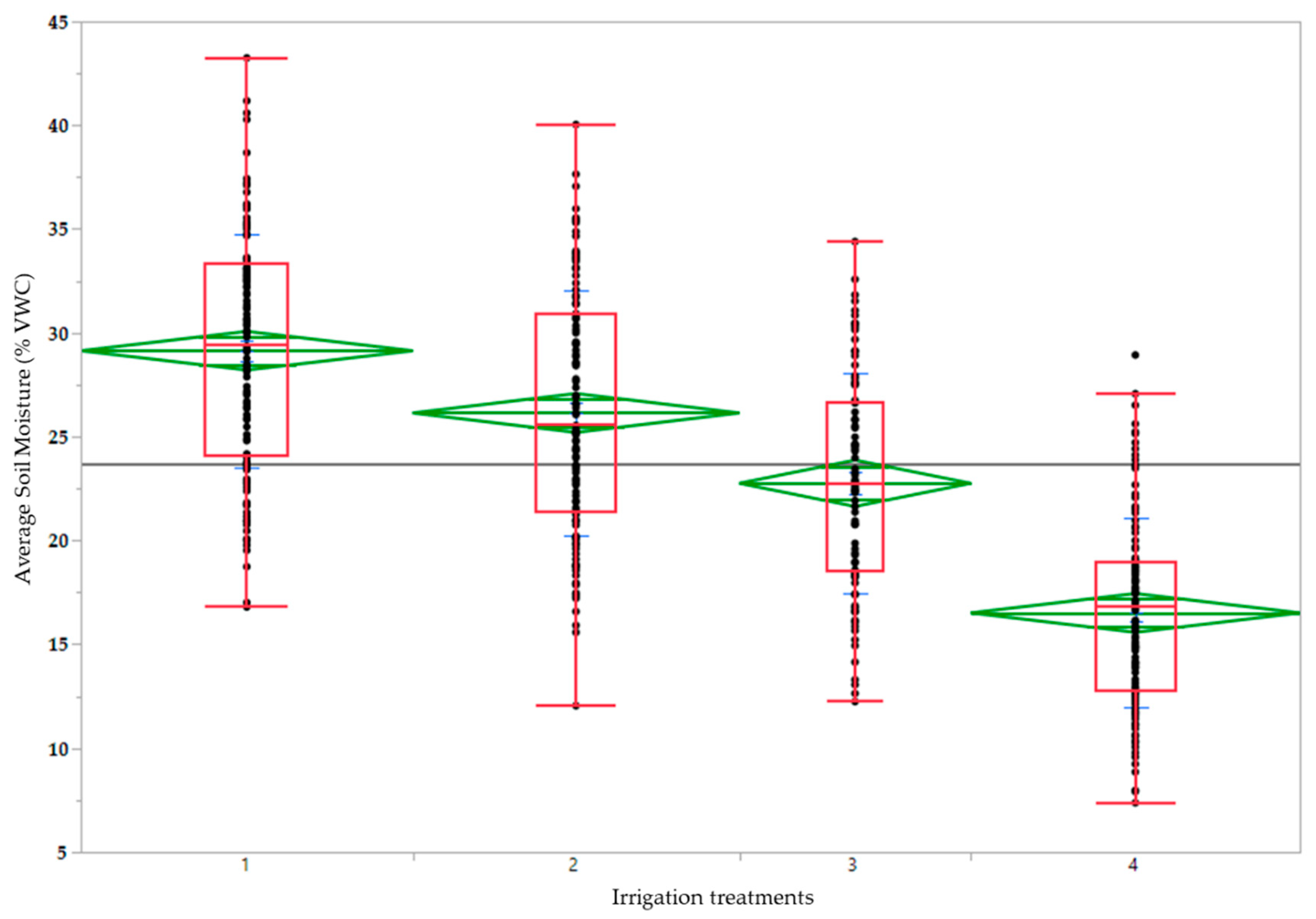
| 1 | 29.1 a | 86.6 c | 91.3 a | 26.2 a | 2.4 d | 6.27 a | 2.27 a | 17.9 a | 31.8 c |
| 2 | 26.1 b | 89.5 b | 89.6 a | 26.3 a | 3.2 c | 6.25 a | 2.23 b | 17.8 a | 32.2 c |
| 3 | 22.8 c | 90.4 b | 84.5 b | 23.2 a | 3.9 b | 6.18 b | 2.21 b | 17.1 b | 33.6 b |
| 4 | 16.5 d | 93.8 a | 79.4 c | 14.4 b | 5.3 a | 6.13 b | 2.18 c | 16.3 c | 35.1 a |
| Variety | Canopy Temperature (°C) | Days to Heading | Plant Height (cm) | Grain Yield (g) | Leaf Stress Score | Panicle Stress Score | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slope | p Value | Intercept | Slope | p Value | Intercept | Slope | p Value | Intercept | Slope | p Value | Intercept | Slope | p Value | Intercept | Slope | p Value | Intercept | |
| Katy | −0.261 | 0.012 | 39.710 | −0.610 | 0.012 | 108.934 | 1.322 | <0.0001 | 59.779 | 0.758 | 0.001 | −8.622 | −0.037 | 0.154 | 4.086 | −0.262 | 0.000 | 11.599 |
| PI 312777 | −0.180 | 0.046 | 37.106 | −0.708 | <0.0001 | 112.015 | 1.201 | <0.0001 | 51.639 | 1.461 | <0.0001 | −2.746 | −0.004 | 0.790 | 3.444 | −0.130 | 0.031 | 6.840 |
| Cybonnet | −0.139 | 0.004 | 36.851 | −0.389 | 0.039 | 95.517 | 0.520 | 0.003 | 70.348 | 0.620 | 0.017 | 1.323 | −0.019 | 0.352 | 3.554 | −0.166 | <0.0001 | 7.747 |
| Saber | −0.144 | 0.026 | 35.740 | −0.616 | 0.001 | 100.968 | 0.717 | 0.002 | 70.587 | 0.478 | 0.089 | 13.648 | 0.030 | 0.375 | 2.229 | −0.145 | 0.001 | 6.463 |
| Francis | −0.143 | 0.010 | 36.223 | −0.539 | 0.001 | 95.810 | 0.909 | <0.0001 | 72.617 | 1.205 | 0.001 | 5.592 | 0.053 | 0.041 | 1.775 | −0.156 | 0.000 | 6.734 |
| Rondo | −0.172 | 0.034 | 37.438 | −0.394 | 0.003 | 102.252 | 0.816 | <0.0001 | 59.837 | 0.494 | 0.163 | 20.171 | −0.010 | 0.468 | 3.775 | −0.127 | 0.005 | 5.768 |
| Kaybonnet | −0.166 | 0.039 | 37.183 | −0.290 | 0.057 | 96.007 | 0.641 | 0.000 | 81.809 | 0.575 | 0.019 | −1.320 | 0.011 | 0.680 | 2.777 | −0.232 | <0.0001 | 9.290 |
| Zhe733 | −0.243 | 0.000 | 39.525 | −0.322 | 0.012 | 78.234 | 0.800 | 0.000 | 57.996 | 0.985 | 0.000 | 1.686 | −0.032 | 0.057 | 4.574 | −0.201 | <0.0001 | 8.518 |
| Lemont | −0.199 | 0.009 | 38.341 | −0.506 | 0.001 | 105.133 | 0.677 | 0.004 | 58.616 | 0.668 | 0.002 | −3.289 | −0.031 | 0.321 | 3.860 | −0.135 | 0.017 | 8.354 |
| Teqing | −0.186 | 0.052 | 36.513 | −0.663 | 0.002 | 111.764 | 1.634 | <0.0001 | 49.407 | 1.964 | 0.002 | −1.897 | −0.028 | 0.190 | 3.546 | −0.272 | 0.000 | 9.406 |
| CL151 | −0.217 | 0.004 | 37.987 | −0.634 | 0.002 | 97.723 | 0.491 | 0.006 | 76.299 | 0.848 | 0.043 | 3.685 | 0.018 | 0.497 | 2.758 | −0.105 | 0.037 | 6.531 |
| Jupiter | −0.217 | 0.001 | 38.411 | −0.262 | 0.051 | 91.575 | 0.563 | 0.002 | 68.532 | 0.576 | 0.098 | 8.544 | −0.025 | 0.232 | 4.051 | −0.114 | 0.004 | 6.456 |
| Lagrue | −0.115 | 0.091 | 35.421 | −0.841 | 0.000 | 110.081 | 1.012 | 0.001 | 67.501 | 0.578 | 0.021 | 3.717 | −0.042 | 0.036 | 4.185 | −0.118 | 0.052 | 7.627 |
| Mars | −0.134 | 0.093 | 37.810 | −0.604 | 0.008 | 100.445 | 0.714 | 0.003 | 74.084 | 0.784 | 0.002 | −6.670 | −0.003 | 0.895 | 3.377 | −0.151 | 0.012 | 8.136 |
| RoyJ | −0.064 | 0.158 | 33.686 | −0.483 | 0.001 | 107.706 | 0.534 | 0.006 | 85.231 | 0.898 | 0.000 | 4.065 | −0.049 | 0.008 | 3.996 | −0.193 | <0.0001 | 8.877 |
| Variety | Chalk (%) | Grain Length (mm) | Grain Width (mm) | Grain Thickness (mm) | 1000 Kernel Weight (g) | Length:Width Ratio | No. of Traits with Significant Slope * | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slope | p Value | Intercept | Slope | p Value | Intercept | Slope | p Value | Intercept | Slope | p Value | Intercept | Slope | p Value | Intercept | Slope | p Value | Intercept | ||
| Katy | −0.017 | 0.534 | 2.528 | 0.018 | 0.051 | 6.179 | 0.005 | 0.043 | 1.852 | 0.003 | 0.073 | 1.475 | 0.137 | 0.027 | 12.057 | 0.000 | 0.916 | 3.352 | 9 |
| PI 312777 | −0.405 | <0.0001 | 13.234 | 0.009 | 0.000 | 4.926 | 0.007 | 0.003 | 2.387 | 0.001 | 0.080 | 1.692 | 0.133 | 0.000 | 13.744 | −0.002 | 0.083 | 2.077 | 12 |
| Cybonnet | −0.059 | 0.012 | 3.906 | 0.001 | 0.909 | 6.593 | −0.001 | 0.755 | 2.123 | 0.001 | 0.311 | 1.584 | 0.040 | 0.325 | 15.903 | 0.002 | 0.653 | 3.082 | 6 |
| Saber | −0.026 | 0.163 | 3.022 | 0.016 | <0.0001 | 5.759 | 0.007 | <.0001 | 1.803 | 0.001 | 0.407 | 1.559 | 0.112 | 0.000 | 11.996 | −0.003 | 0.094 | 3.197 | 9 |
| Francis | −0.079 | 0.035 | 4.481 | 0.020 | <0.0001 | 5.978 | 0.002 | 0.114 | 2.080 | −0.001 | 0.199 | 1.662 | 0.075 | 0.012 | 15.680 | 0.008 | 0.001 | 2.852 | 10 |
| Rondo | −0.001 | 0.959 | 1.727 | 0.016 | 0.000 | 6.072 | 0.004 | 0.001 | 2.087 | 0.001 | 0.094 | 1.579 | 0.126 | <0.0001 | 14.598 | 0.001 | 0.352 | 2.919 | 8 |
| Kaybonnet | 0.001 | 0.951 | 1.539 | 0.008 | 0.050 | 6.384 | 0.003 | 0.074 | 1.893 | 0.002 | 0.078 | 1.502 | 0.082 | 0.020 | 13.397 | −0.002 | 0.571 | 3.383 | 9 |
| Zhe733 | 0.502 | 0.021 | 9.007 | 0.011 | 0.000 | 5.811 | 0.003 | 0.020 | 2.377 | −0.003 | 0.005 | 1.849 | 0.069 | 0.056 | 18.512 | 0.001 | 0.722 | 2.460 | 11 |
| Lemont | −0.014 | 0.669 | 2.941 | 0.006 | 0.149 | 6.553 | 0.003 | 0.049 | 2.122 | −0.001 | 0.439 | 1.682 | 0.143 | 0.039 | 14.744 | −0.001 | 0.618 | 3.076 | 7 |
| Teqing | −0.162 | 0.139 | 11.884 | 0.021 | <0.0001 | 4.876 | 0.012 | <0.0001 | 2.347 | 0.006 | <0.0001 | 1.654 | 0.237 | <0.0001 | 12.748 | −0.002 | 0.178 | 2.082 | 9 |
| CL151 | −0.116 | 0.054 | 6.422 | 0.005 | 0.055 | 6.312 | 0.003 | 0.053 | 2.112 | 0.000 | 0.724 | 1.642 | 0.069 | 0.007 | 15.918 | −0.003 | 0.258 | 2.998 | 9 |
| Jupiter | −0.006 | 0.770 | 1.911 | 0.010 | <0.0001 | 5.202 | 0.007 | 0.000 | 2.421 | 0.002 | 0.006 | 1.736 | 0.122 | <0.0001 | 15.473 | −0.001 | 0.357 | 2.123 | 9 |
| Lagrue | −0.007 | 0.775 | 2.649 | 0.009 | 0.105 | 6.478 | 0.001 | 0.269 | 2.030 | 0.000 | 0.639 | 1.620 | 0.038 | 0.212 | 17.181 | 0.003 | 0.163 | 3.167 | 6 |
| Mars | 0.022 | 0.266 | 1.990 | 0.007 | 0.047 | 5.544 | 0.008 | 0.049 | 2.187 | 0.003 | 0.052 | 1.609 | 0.107 | 0.037 | 14.455 | −0.006 | 0.183 | 2.556 | 10 |
| RoyJ | −0.032 | 0.121 | 2.463 | 0.005 | 0.067 | 6.761 | 0.001 | 0.486 | 2.013 | 0.000 | 0.894 | 1.667 | 0.052 | 0.095 | 17.150 | 0.001 | 0.339 | 3.354 | 7 |
| Parent of Mapping Populations | Variety | Sub-Population * | No. of Responsive Traits for Each Mapping Parent 1 | No. of Responsive Traits in Common between Mapping Parents 2 | No. of Stable Traits in Common between Mapping Parents 3 | No. of Complimentary Traits between Mapping Parents 4 |
|---|---|---|---|---|---|---|
| A1 | Katy | TRJ | 9 | 9 | 1 | 3 |
| A2 | PI 312777 | Indica | 12 | |||
| B1 | Cybonnet | TRJ | 6 | 5 | 3 | 5 |
| B2 | Saber | TRJ | 9 | |||
| C1 | Francis | TRJ | 10 | 6 | 1 | 6 |
| C2 | Rondo | Indica | 8 | |||
| D1 | Kaybonnet | TRJ | 9 | 8 | 2 | 3 |
| D2 | Zhe 733 | Indica | 11 | |||
| E1 | Lemont | TRJ | 7 | 7 | 4 | 2 |
| E2 | Teqing | Indica | 9 |
| Parent of Mapping Populations | Chalk (%) | Canopy Temp (°C) ^ | Grain Length (mm) | Grain Width (mm) | Length: Width Ratio | Grain Thickness (mm) | TKW (g) | Days to Heading | Days to Maturity | Plant Height (cm) | GY (g plant−1) | Leaf Stress Score | Panicle Stress Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | 1.94 b | 33.2 a | 6.6 a | 1.98 a | 3.34 a | 1.53 a | 15.4 a | 97 a | 126 b | 93 a | 10 a | 3.1 a | 4.6 a |
| A2 | 5.18 a | 33.3 a | 5.13 a | 2.53 a | 2.03 b | 1.74 a | 16.69 a | 97 a | 134 a | 76 a | 27 a | 3.4 a | 3.9 a |
| Mean of A | 3.56 | 33.3 | 5.87 | 2.26 | 2.69 | 1.64 | 16.05 | 97 | 130 | 85 | 19 | 3.3 | 4.3 |
| B1 | 2.09 b | 33.3 a | 6.57 a | 2.09 a | 3.14 a | 1.61 a | 16.73 a | 87 a | 120 a | 83 a | 18 a | 3.0 a | 3.3 a |
| B2 | 2.34 a | 32.6 a | 6.12 b | 1.97 b | 3.13 b | 1.59 a | 14.46 b | 90 a | 121 a | 86 a | 25 a | 2.9 a | 3.0 a |
| Mean of B | 2.22 | 32.9 | 6.35 | 2.03 | 3.14 | 1.60 | 15.60 | 89 | 121 | 85 | 22 | 3.0 | 3.2 |
| C1 | 2.67 a | 33.2 a | 6.42 a | 2.12 b | 3.02 a | 1.63 a | 17.23 a | 85 a | 122 a | 92 a | 32 a | 3.0 b | 3.0 a |
| C2 | 1.61 b | 33.4 a | 6.47 a | 2.19 a | 2.95 b | 1.61 b | 17.66 a | 93 a | 127 a | 79 a | 33 a | 3.6 a | 2.7 a |
| Mean of C | 2.14 | 33.3 | 6.45 | 2.16 | 2.99 | 1.62 | 17.45 | 89 | 125 | 86 | 33 | 3.3 | 2.9 |
| D1 | 1.26 b | 33.2 a | 6.58 a | 1.97 a | 3.35 a | 1.55 a | 15.29 a | 90 a | 120 a | 97 a | 13 a | 3.1 b | 3.3 a |
| D2 | 21.9 a | 33.6 a | 6.08 a | 2.45 a | 2.48 b | 1.78 a | 20.15 a | 71 a | 116 a | 75 a | 10 a | 3.8 a | 3.5 a |
| Mean of D | 11.58 | 33.4 | 6.33 | 2.21 | 2.92 | 1.67 | 17.72 | 81 | 118 | 86 | 12 | 3.5 | 3.4 |
| E1 | 2.57 a | 33.1 a | 6.71 a | 2.2 a | 3.05 a | 1.68 b | 18.22 a | 94 a | 126 a | 75 a | 13 a | 3.0 a | 4.6 a |
| E2 | 9.86 a | 32.6 a | 5.33 b | 2.6 a | 2.05 a | 1.79 a | 17.81 a | 98 a | 135 a | 84 a | 41 a | 2.9 a | 3.2 a |
| Mean of E | 6.22 | 32.8 | 6.02 | 2.40 | 2.55 | 1.74 | 18.02 | 96 | 131 | 80 | 27 | 3.0 | 3.9 |
| Trait | * p Value > F | % of Total Sum of Squares Explained by Significant Interaction | ||
|---|---|---|---|---|
| Variety | Irrigation | Variety × Irrigation | ||
| Days to heading | 0.0001 | ns | 0.04 | 6 |
| Days to maturity | 0.0001 | ns | 0.02 | 16 |
| Grainfill days | 0.0001 | ns | ns | . |
| GDD2 | 0.0001 | ns | ns | . |
| GDD1 | 0.0001 | ns | 0.02 | 16 |
| Plant height | 0.0001 | ns | 0.02 | 14 |
| GY | 0.0001 | ns | 0.02 | 13 |
| EGP | 0.0001 | ns | ns | . |
| Avg soil moisture | 0.03 | 0.0001 | ns | . |
| Panicle stress score | 0.0001 | 0.0002 | ns | . |
| Leaf stress score | 0.0001 | 0.04 | 0.02 | 11 |
| Grain length | 0.0001 | 0.01 | ns | . |
| Grain width | 0.0001 | 0.005 | ns | . |
| Length:Width ratio | 0.0001 | ns | ns | . |
| Chalk | 0.0001 | ns | 0.0001 | 11 |
| TKW | 0.0001 | 0.0001 | ns | . |
| Grain thickness | 0.0001 | 0.02 | ns | . |
| Canopy temp | 0.0005 | 0.04 | ns | . |
| Vegetative height rate | 0.001 | ns | ns | . |
| Reproductive height rate | 0.0001 | ns | ns | . |
| Leaf ascorbic acid at V8 | Ns | 0.02 | ns | . |
| SPAD | 0.0001 | 0.002 | ns | . |
| Trait 1 | Trait 2 | * Correlation Coefficient |
|---|---|---|
| Reproductive height rate | Vegetative height rate | −0.72 |
| GDD1 | Days to heading | 0.70 |
| GDD1 | Days to maturity | 0.99 |
| GDD1 | Reproductive height rate | −0.44 |
| Chalk | Reproductive height rate | 0.42 |
| GDD2 | Grainfill days | 0.98 |
| Grain thickness | GDD2 | 0.44 |
| TKW | Avg soil moisture | 0.39 |
| Plant height | Avg soil moisture | 0.39 |
| Leaf ascorbic acid at V8 | Avg soil moisture | 0.39 |
| Canopy temp | Avg soil moisture | −0.57 |
| Canopy temp | Leaf stress score | 0.49 |
| Canopy temp | SPAD | 0.41 |
| Avg soil moisture | SPAD | −0.57 |
| Days to maturity | SPAD | 0.39 |
| Panicle stress score | SPAD | 0.43 |
| Panicle stress score | Leaf stress score | 0.42 |
| (a) All three years combined | ||||||
| Step | Variable Entered | Partial R2 | Model R2 | C(p) | F Value | p Value > F |
| 1 | Panicle stress score | 0.11 | 0.11 | 52.90 | 18.81 | <0.0001 |
| 2 | Canopy temp | 0.11 | 0.22 | 29.84 | 21.32 | <0.0001 |
| 3 | GDD1 | 0.07 | 0.28 | 16.58 | 14.10 | 0.0002 |
| 4 | Plant height | 0.01 | 0.30 | 15.75 | 2.64 | 0.1063 |
| 5 | Chalk | 0.02 | 0.32 | 12.10 | 5.43 | 0.0211 |
| 6 | Days to maturity | 0.03 | 0.35 | 6.35 | 7.79 | 0.0059 |
| (b) 2016 alone | ||||||
| Step | Variable Entered | Partial R2 | Model R2 | C(p) | F Value | p Value > F |
| 1 | Panicle stress score | 0.11 | 0.11 | 57.55 | 18.81 | <0.0001 |
| 2 | Canopy temp | 0.11 | 0.22 | 33.92 | 21.32 | <0.0001 |
| 3 | GDD1 | 0.07 | 0.28 | 20.31 | 14.10 | 0.0002 |
| 4 | Vegetative height rate | 0.03 | 0.31 | 16.14 | 5.75 | 0.0178 |
| 5 | Chalk | 0.01 | 0.33 | 14.65 | 3.30 | 0.0711 |
| 6 | Days to maturity | 0.04 | 0.36 | 7.82 | 8.78 | 0.0035 |
| 7 | Plant height | 0.02 | 0.38 | 5.51 | 4.38 | 0.0381 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McClung, A.M.; Rohila, J.S.; Henry, C.G.; Lorence, A. Response of U.S. Rice Cultivars Grown under Non-Flooded Irrigation Management. Agronomy 2020, 10, 55. https://doi.org/10.3390/agronomy10010055
McClung AM, Rohila JS, Henry CG, Lorence A. Response of U.S. Rice Cultivars Grown under Non-Flooded Irrigation Management. Agronomy. 2020; 10(1):55. https://doi.org/10.3390/agronomy10010055
Chicago/Turabian StyleMcClung, Anna M., Jai S. Rohila, Christopher G. Henry, and Argelia Lorence. 2020. "Response of U.S. Rice Cultivars Grown under Non-Flooded Irrigation Management" Agronomy 10, no. 1: 55. https://doi.org/10.3390/agronomy10010055
APA StyleMcClung, A. M., Rohila, J. S., Henry, C. G., & Lorence, A. (2020). Response of U.S. Rice Cultivars Grown under Non-Flooded Irrigation Management. Agronomy, 10(1), 55. https://doi.org/10.3390/agronomy10010055






