Production and Implication of Bio-Activated Organic Fertilizer Enriched with Zinc-Solubilizing Bacteria to Boost up Maize (Zea mays L.) Production and Biofortification under Two Cropping Seasons
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Bacterial Strain and Production of Zn Solubilizing Bacteria Enriched Bio-Organic Fertilizers
2.2. Soil Characterization
2.3. Field Experimental Layout
2.4. Crop Harvesting and Analyses
2.4.1. Physiological Parameters
2.4.2. Growth and Yield Parameters
2.4.3. Chemical Analysis of Zinc
2.4.4. Quality Parameters
2.5. Statistical Analysis
3. Results
3.1. Field Soil Characterization
3.2. Effects of Zn Sources on Maize Growth and Yield
3.3. Effect of Zn Sources on Maize Physiology
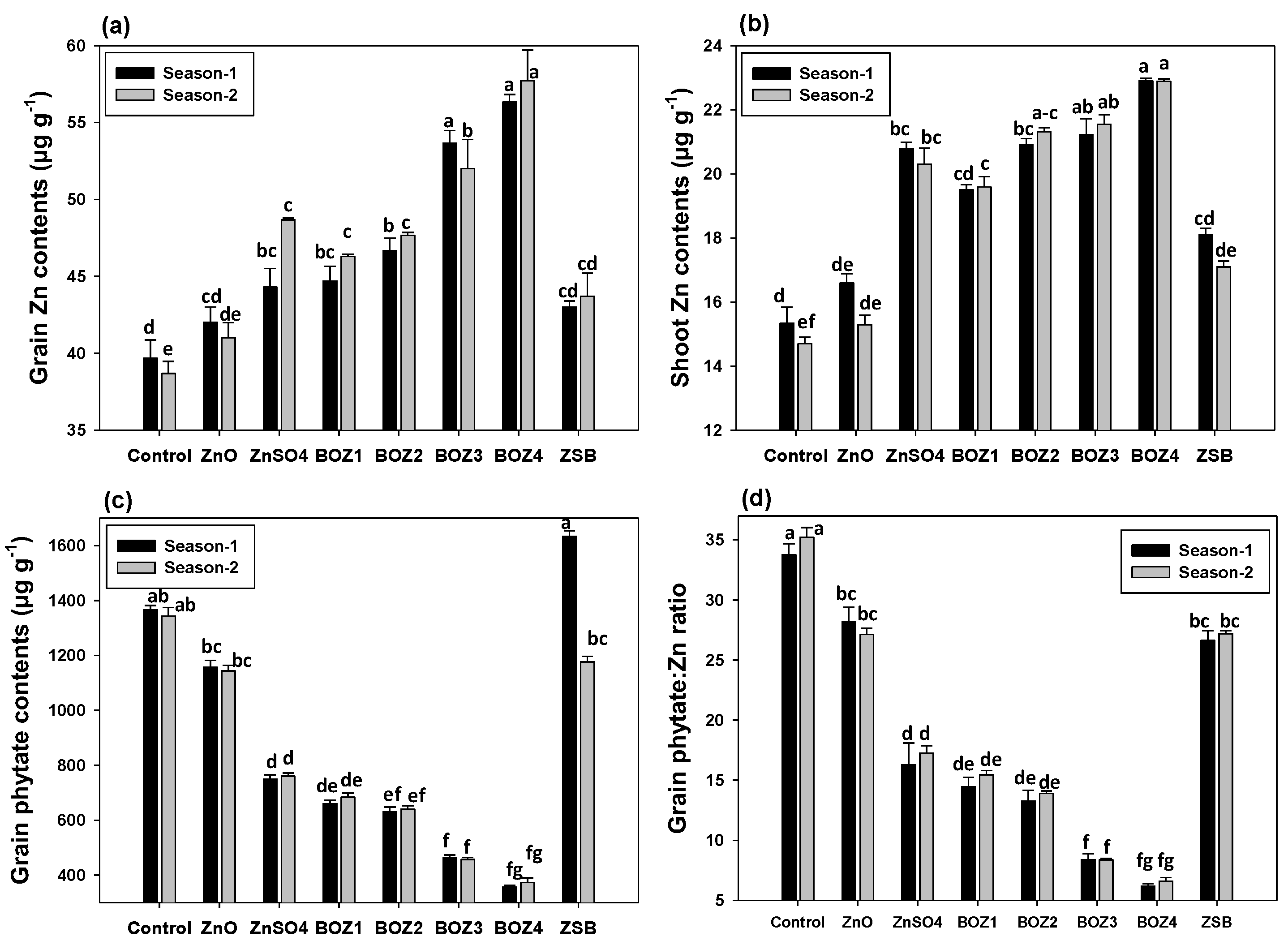
3.4. Effect of Zn Sources on Zn and Phytate Contents of Maize
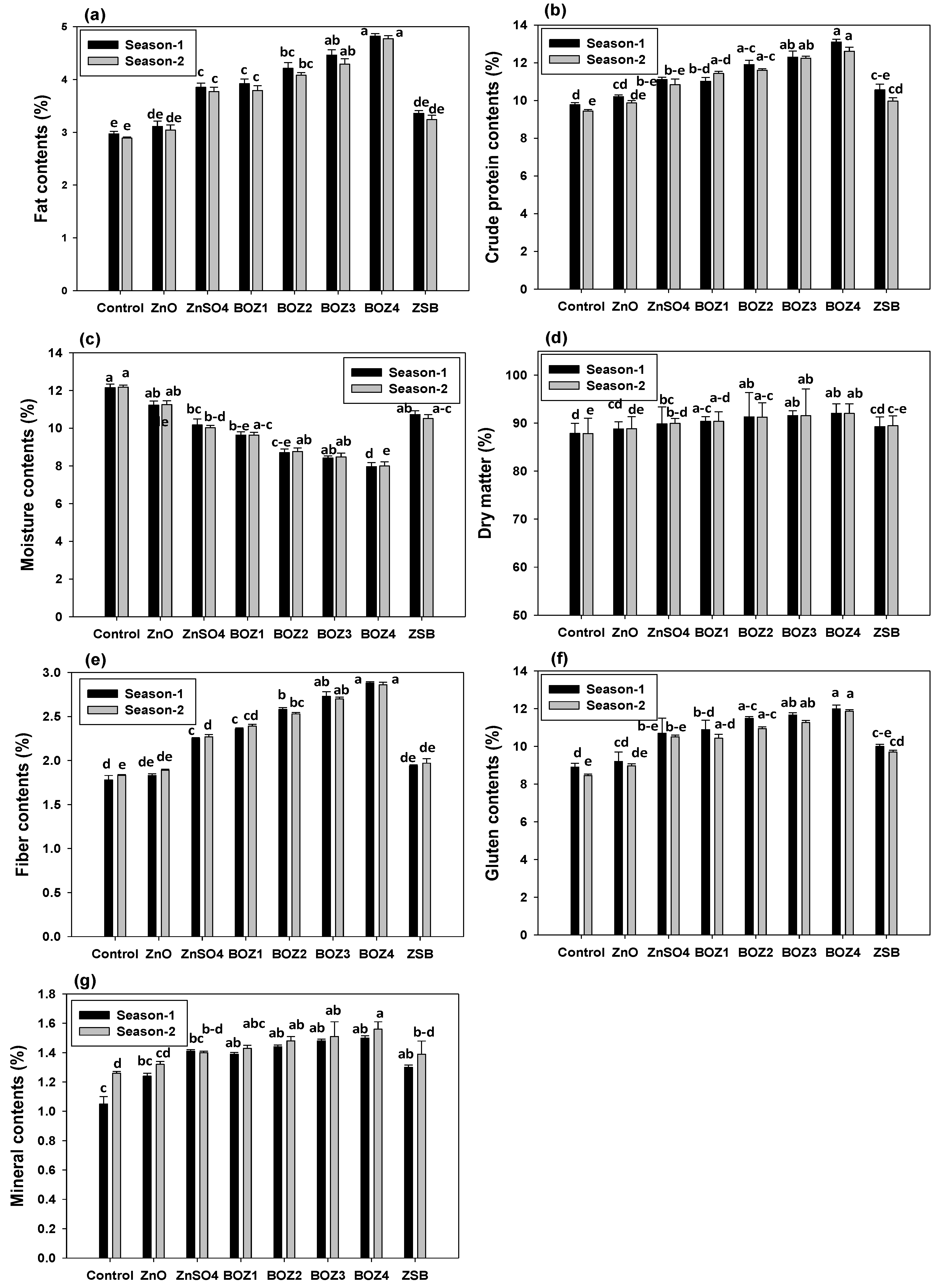
3.5. Effect of Zn Sources on Maize Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zastrow, M.L.; Pecoraro, V.L. Designing Hydrolytic Zinc Metalloenzymes. Biochemistry 2014, 53, 957–978. [Google Scholar] [CrossRef] [PubMed]
- Alloway, B. Zinc in SoilS and Crop Nutrition, 2nd ed.; International Zinc Association and International Fertilizer Industry Association: Paris, France, 2008. [Google Scholar]
- Cakmak, I. Tansley Review No. 111: Possible Roles of Zinc in Protecting Plant Cells from Damage by Reactive Oxygen Species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef]
- Palmer, C.; Guerinotm, M.L. Facing the Challenges of Cu, Fe and Zn Homeostasis in Plants. Nat. Chem. Biol. 2009, 5, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, B.; Khanif, Y.; Saleem, M. Role of Zinc in Plant Nutrition-A Review. Am. J. Exp. Agric. 2013, 3, 374–391. [Google Scholar] [CrossRef]
- Alloway, B. Soil Factors Associated with Zinc Deficiency in Crops and Humans. Environ. Geochem. Health 2009, 31, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Asghar, H.; Akhtar, M.J.; Arshad, M. Impact of Phosphate Solubilizing Bacteria on Growth and Yield of Maize. Soil Environ. 2013, 32, 71–78. [Google Scholar]
- Fageria, N.; Baligar, V.; Clark, R. Micronutrients in Crop Production. Adv. Agron. 2002, 77, 185–268. [Google Scholar]
- Bell, R.; Dell, B. Micronutrients for Sustainable Food, Feed, Fibre and Bioenergy Production; International Fertilizer Industry Association (IFA): Paris, France, 2008. [Google Scholar]
- Shivay, Y.; Prasad, R.; Kaur, R.; Pal, M. Relative Efficiency of Zinc-Coated Urea and Soil and Foliar Application of Zinc Sulphate on Yield, Nitrogen, Phosphorus, Potassium, Zinc and Iron Biofortification grains and uptake by basmati rice. J. Agric. Sci. 2015, 7, 161–173. [Google Scholar] [CrossRef]
- World Health Organization. The World Health Report 2002: Reducing Risks, Promoting Healthy Life; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Salim, H.; Zhao, K.; Liao, L.; Xu, J. Adsorption-Desorption Kinetics of Zn in Soils: Influence of Phosphate. In Molecular Environmental Soil Science at the Interfaces in the Earth’s Critical Zone; Xu, J., Huang, P.M., Eds.; Springer: Berlin, Germany, 2010; pp. 88–90. [Google Scholar]
- Kausar, M.; Chaudhry, F.M.; Rashid, A.; Latif, A.; Alam, S. Micronutrient Availability to Cereals from Calcareous Soils. Plant Soil 1977, 47, 297–302. [Google Scholar] [CrossRef]
- Fageria, N.K. Dry Matter Yield and Nutrient Uptake by Lowland Rice at Different Growth Stages. J. Plant Nutr. 2004, 27, 947–958. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of Fertilizers with Zinc: An Excellent Investment for Humanity and Crop Production in India. J. Trace Elem. Med. Biol. 2009, 23, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Beebout, S.; Angeles, O.; Alberto, M.; Buresh, R. Simultaneous Minimization of Nitrous Oxide and Methane Emission from Rice Paddy Soils Is Improbable Due to Redox Potential Changes with Depth in a Greenhouse. Geoderma 2009, 149, 45–53. [Google Scholar] [CrossRef]
- Hefferon, K. Biotechnological Approaches for Generating Zinc-Enriched Crops to Combat Malnutrition. Nutrients 2019, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Shivay, Y.S.; Kumar, D.; Prasad, R. Effect of Zinc-Enriched Urea on Productivity, Zinc Uptake and Efficiency of an Aromatic Rice-Wheat Cropping System. Nutr. Cycl. Agroecosyst. 2008, 81, 229–243. [Google Scholar] [CrossRef]
- Fasim, F.; Ahmed, N.; Parsons, R.; Gadd, G.M. Solubilization of Zinc Salts by a Bacterium Isolated from the Air Environment of a Tannery. FEMS Microbiol. Lett. 2002, 213. [Google Scholar] [CrossRef]
- Ahmad, M.; Pataczek, L.; Hilger, T.H.; Zahir, Z.A.; Hussain, A.; Rasche, F.; Schafleitner, R.; Solberg, S.O. Perspectives of Microbial Inoculation for Sustainable Development and Environmental Management. Front. Microbiol. 2018, 9, 2992. [Google Scholar] [CrossRef]
- Wu, S.; Cheung, K.; Luo, Y.; Wong, H. Effects of Inoculation of Plant Growth-Promoting Rhizobacteria on Metal Uptake by Brassica Juncea. Environ. Pollut. 2006, 40, 124–135. [Google Scholar] [CrossRef]
- Havlin, J.; Tisdale, S.; Nelson, W.; Beaton, J. Soil Fertility and Fertilizers: An Introduction to Nutrient Management, 7th ed.; Pearson Education, Inc.: Upper Saddle River, NJ, USA, 2005. [Google Scholar]
- Saravanan, V.; Subramoniam, S.; Raj, S. Assessing in Vitro Solubilization Potential of Different Zinc Solubilizing Bacterial (ZSB) Isolates. Braz. J. Microbiol. 2004, 34, 121–125. [Google Scholar] [CrossRef]
- Tariq, M.; Hameed, S.; Malik, K.; Hafeez, F. Plant Root Associated Bacteria for Zinc Mobilization in Rice Biofortification View Project Research Project on Antimicrobial Activity and Bacteriocin Isolation from Probiotic Bacteria. Characterization of Indigenous Probiotics from Pakistan View Project. Pak. J. Bot. 2007, 39, 245–253. [Google Scholar]
- Hussain, A. Efficacy of Bio-Activated Zn for Improving Yield and Quality of Maize, Institute of Soil & Environmental Sciences. Ph.D. Thesis, University of Agriculture, Faisalabad, Pakistan, 2015. [Google Scholar]
- Khalid, A.; Arshad, M.; Shaharoona, B.; Mahmood, T. Plant Growth Promoting Rhizobacteria and Sustainable Agriculture. In Microbial Strategies for Crop Improvement; Springer: Berlin/Heidelberg, Germany, 2009; pp. 133–160. [Google Scholar]
- Nadeem, S.; Haq, R.; Khan, Z. Numerical Study of MHD Boundary Layer Flow of a Maxwell Fluid Past a Stretching Sheet in the Presence of Nanoparticles. J. Taiwan Inst. Chem. Eng. 2014, 45, 121–126. [Google Scholar] [CrossRef]
- Hussain, A.; Arshad, M.; Zahir, Z.A.; Asghar, M. Prospects of Zinc Solubilizing Bacteria for Enhancing Growth of Maize Development of Novel Rhizobial Inoculants for Inducing Drought Tolerance in Cereals View Project m.Phil View Project. Pak. J. Agric. Sci. 2015, 52, 915–922. [Google Scholar]
- Ahmad, M.; Akhtar, M.; Jamil, M.; Latif, M. Pesticide Tolerant Plant Growth Promoting Rhizobacteria Isolated from Rhizosphere of Okra. Soil Environ. 2015, 34, 111–118. [Google Scholar]
- Subramanian, K.; Tenshia, V.; Jayalakshmi, K.; Ramachandran, V. Role of Arbuscular Mycorrhizal Fungus (Glomus Intraradices) (Fungus Aided) in Zinc Nutrition of Maize. J. Agric. Biotechnol. Sustain. Dev. 2009, 1, 29–38. [Google Scholar]
- Liang, Y.; Chen, Q.; Liu, Q.; Zhang, W.; Ding, R. Exogenous Silicon (Si) Increases Antioxidant Enzyme Activity and Reduces Lipid Peroxidation in Roots of Salt-Stressed Barley (Hordeum vulgare L.). J. Plant Physiol. 2003, 160, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Arshad, M.; Khalid, A.; Kanwal, S. Perspectives of Rhizosphere Microflora for Improving Zn Bioavailability and Acquisition by Higher Plants. Int. J. Agric. Biol. 2014, 16, 653–662. [Google Scholar]
- Hussain, A.; Zahir, Z.A.; Asghar, H.N.; Imran, M.; Ahmad, M.; Hussain, S. Integrating the Potential of Bacillus SP. AZ6 and Organic Waste for Zinc Oxide Bio-Activation to Improve Growth, Yield and Zinc Content of Maize Grains. Pak. J. Agric. Sci. 2020, 57, 123–130. [Google Scholar]
- Gee, G.; Bauder, J. Particle-Size Analysis. In Methods of Soil Analysis, Part 1. Physical and Mineralogical Methods, 2nd ed.; Klute, A., Ed.; Agronomy Monograph No. 9; American Society of Agronomy/Soil Science Society of America: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- US Salinity Lab. Staff. Diagnosis and Improvement of Saline and Alkali Soils. In Agriculture Handbook 60; United States Department of Agriculture: Washington, DC, USA, 1954; pp. 83–100. [Google Scholar]
- Moodie, C.; Smith, H.; McCreery, R. Laboratory Manual for Soil Fertility; Department of Agronomy, State College of Washington Pullman: Washington, DC, USA, 1959. [Google Scholar]
- Jackson, M. Soil Chemical Analysis; Prentice Hall, Inc.: Englwood Cliff, NY, USA, 1958. [Google Scholar]
- Watanabe, F.; Olsen, S. Test of an Ascorbic Acid Method for Determining Phosphorus in Water and NaHCO3 Extracts from Soil. Soil Sci. Soc. Am. 1965, 29, 677–678. [Google Scholar] [CrossRef]
- Simard, R. Ammonium Acetate Extractable Elements. In Soil Sampling and Methods of Analysis; Carter, M., Ed.; CRC Press: Washington, DC, USA, 1993; pp. 39–42. [Google Scholar]
- Soltanpour, P.; Schwab, A. A New Soil Test for Simultaneous Extraction of Macro and Micro-Nutrients in Alkaline Soils. Commun. Soil Sci. Plant Anal. 1977, 8, 195–207. [Google Scholar] [CrossRef]
- Dwivedi, R.; Randhawa, N. Evaluation of a Rapid Test for the Hidden Hunger of Zinc in Plants. Plant Soil 1974, 40, 445–451. [Google Scholar] [CrossRef]
- Lutts, S.; Kinet, J.; Bouharmont, J. Changes in Plant Response to NaCl during Development of Rice (Oryza sativa L.) Varieties Differing in Salinity Resistance. J. Exp. Bot. 1995, 46, 1843–1852. [Google Scholar] [CrossRef]
- De Carvalho, L.M.J.; Gomes, P.B.; de Oliveira Godoy, R.L.; Pacheco, S.; do Monte, P.H.F.; de Carvalho, J.L.V.; Nutti, M.R.; Neves, A.C.L.; Vieira, A.C.R.A.; Ramos, S.R.R. Total Carotenoid Content, α-Carotene and β-Carotene, of Landrace Pumpkins (Cucurbita moschata Duch): A Preliminary Study. Food Res. Int. 2012, 47, 337–340. [Google Scholar] [CrossRef]
- Jones, J.; Case, V. Sampling, Handling and Analyzing Plant Tissue Samples. In Soil Testing and Plant Analysis; Westerman, R.L., Ed.; Book Series; Soil Science Society of America: Madison, WI, USA, 1990; pp. 389–447. [Google Scholar]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic Peroxidase-like Activity of Ferromagnetic Nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- AACC. Approved Methods of the American Association of Cereal Chemists, 10th ed.; AACC: St. Paul, MN, USA, 2000. [Google Scholar]
- Steel, R.; Torrie, J.; Dicky, D. Principles and Procedures of Statistics, A Biometrical Approach, 3rd ed.; McGraw Hill, Inc. Book Co.: New York, NY, USA, 1997. [Google Scholar]
- Mumtaz, M.Z.; Ahmad, M.; Jamil, M.; Hussain, T. Zinc Solubilizing Bacillus spp. Potential Candidates for Biofortification in Maize. Microbiol. Res. 2017, 202, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Joshi, M.; Prasanna, R.; Shivay, Y. Biofortification of Wheat through Inoculation of Plant Growth Promoting Rhizobacteria and Cyanobacteria. Eur. J. Soil Biol. 2012, 50, 118–126. [Google Scholar] [CrossRef]
- Mehta, P.; Walia, A.; Kulshrestha, S.; Chauhan, A.; Shirkot, C.K. Efficiency of Plant Growth-Promoting P-Solubilizing Bacillus Circulans CB7 for Enhancement of Tomato Growth under Net House Conditions. J. Basic Microbiol. 2015, 55, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Ditta, A.; Arshad, M.; Zahir, Z.A.; Jamil, A. Comparative Efficacy of Rock Phosphate Enriched Organic Fertilizer vs. Mineral Phosphatic Fertilizer for Nodulation, Growth and Yield of Lentil Theoretical Studies of Silaaromatics View Project Bioremediation of Emerging Pollutants View Project. Int. J. Agric. Biol. 2015, 17, 589–595. [Google Scholar] [CrossRef]
- Mumtaz, M.Z.; Ahmad, M.; Jamil, M.; Asad, S.A.; Hafeez, F. Bacillus Strains as Potential Alternate for Zinc Biofortification of Maize Grains. Int. J. Agric. Biol. 2018, 20, 1779–1786. [Google Scholar]
- Ahmad, M.; Adil, Z.; Hussain, A.; Mumtaz, M.Z.; Nafees, M.; Ahmad, I.; Jamil, M. Potential of Phosphate Solubilizing Bacillus Strains for Improving Growth and Nutrient Uptake in Mungbean and Maize Crops. Pak. J. Agric. Sci. 2019, 56, 283–289. [Google Scholar]
- Fatima, I.; Jamil, M.; Hussain, A.; Mumtaz, M.Z.; Luqman, M.; Hussain, S.; Kashif, S.R.; Ahmad, M. Zinc Solubilizing Bacillus sp. ZM20 and Bacillus Aryabhattai ZM31 Promoted the Productivity in Okra (Abelmoschus esculentus L.). Biologia 2018, 64, 179–185. [Google Scholar]
- Kamilova, F.; Kravchenko, L.V.; Shaposhnikov, A.I.; Makarova, N.; Lugtenberg, B. Effects of the Tomato Pathogen Fusarium Oxysporum f. sp. Radicis-Lycopersici and of the Biocontrol Bacterium Pseudomonas Fluorescens WCS365 on the Composition of Organic Acids and Sugars in Tomato Root Exudate. Mol. Plant Microbe Interact. 2006, 19, 1121–1126. [Google Scholar] [CrossRef]
- Ditta, A.; Muhammad, J.; Imtiaz, M.; Mehmood, S.; Qian, Z.; Tu, S. Application of Rock Phosphate Enriched Composts Increases Nodulation, Growth and Yield of Chickpea. Int. J. Recycl. Org. Waste Agric. 2018, 7, 33–40. [Google Scholar] [CrossRef]
- Ditta, A.; Imtiaz, M.; Mehmood, S.; Rizwan, M.S.; Mubeen, F.; Aziz, O.; Qian, Z.; Ijaz, R.; Tu, S. Rock Phosphate-Enriched Organic Fertilizer with Phosphate-Solubilizing Microorganisms Improves Nodulation, Growth, and Yield of Legumes. Commun. Soil Sci. Plant Anal. 2018, 49, 2715–2725. [Google Scholar] [CrossRef]
- Ditta, A.; Khalid, A. Bio-Organo-Phos: A Sustainable Approach for Managing Phosphorus Deficiency in Agricultural Soils. In Organic Fertilizers: From Basic Concepts to Applied Outcomes; Larramendy, M.L., Soloneski, S., Eds.; InTech: Rijeka, Kroatia, 2016; pp. 109–136. [Google Scholar]
- Hussain, A.; Ahmad, M.; Mumtaz, M.Z.; Nazli, F.; Farooqi, M.A.; Khalid, I.; Iqbal, Z.; Arshad, H. Impact of Integrated Use of Enriched Compost, Biochar, Humic Acid and Alcaligenes sp. AZ9 on Maize Productivity and Soil Biological Attributes in Natural Field Conditions. Ital. J. Agron. 2019, 14, 101–107. [Google Scholar] [CrossRef]
- Sabah, N.; Sarwar, G.; Tahir, M. Depicting the Role of Organic Amendments for Bio Available Phosphorus Release from Different Sources of Rock Phosphate and Uptake by Maize Crop. Pak. J. Bot. 2018, 50, 117–122. [Google Scholar]
- Arif, M.; Shahzad, S.; Riaz, M.; Yasmeen, T.; Shahzad, T.; Akhtar, M.J.; Bragazza, L.; Buttler, A. Nitrogen-enriched Compost Application Combined with Plant Growth-promoting Rhizobacteria (PGPR) Improves Seed Quality and Nutrient Use Efficiency of Sunflower. J. Plant Nutr. Soil Sci. 2017, 180, 464–473. [Google Scholar] [CrossRef]
- Vimal, S. Effect of Plant Growth Promoting Rhizobacteria (PGPR) and Farmyard Manure (FYM) Amendment on Growth Parameters and Antioxidant Level in Paddy (Oryza sativa L.) Crop under Soil Salinity, Department of Environmental Microbiology, School for Environmental Sciences. Ph.D. Thesis, Babasaheb Bhimrao Ambedkar University, Lucknow, India, 2018. [Google Scholar]
- Chang, H.; Lin, C.; Huang, H.J. Zinc-Induced Cell Death in Rice (Oryza sativa L.) Roots. Plant Growth Regul. 2005, 46, 261–266. [Google Scholar] [CrossRef]
- Cakmak, I. Role of Mineral Nutrients in Tolerance of Crop Plants to Environmental Stress Factors. In Fertigation: Optimizing the Utilization of Water and Nutrients; International Symposium on Fertigation: Beijing, China, 2008; pp. 35–48. [Google Scholar]
- Escudero-Almanza, D.; Ojeda-Barrios, D.; Hernández-Rodríguez, O.; Chávez, E.; Ruíz-Anchondo, T.; Sida-Arreola, J. Carbonic Anhydrase and Zinc in Plant Physiology. Chil. J. Agric. Res. 2012, 72, 140–146. [Google Scholar] [CrossRef]
- Edwards, G.; Mohamed, A. Reduction in Carbonic Anhydrase Activity in Zinc Deficient Leaves of Phaseolus vulgaris L. 1. Crop Sci. 1973, 13, 351–354. [Google Scholar] [CrossRef]
- Randall, P.; Bouma, D. Zinc Deficiency, Carbonic Anhydrase, and Photosynthesis in Leaves of Spinach. Plant Physiol. 1973, 52, 229–232. [Google Scholar] [CrossRef]
- Mumtaz, M.; Barry, K.; Baker, A.; Nichols, D.; Ahmad, M.; Zahir, Z.; Britz, M. Production of Lactic and Acetic Acids by Bacillus sp. ZM20 and Bacillus Cereus Following Exposure to Zinc Oxide: A Possible Mechanism for Zn Solubilization. Rhizosphere 2019, 12, 100170. [Google Scholar] [CrossRef]
- Whiting, S.N.; De Souza, M.P.; Terry, N. Rhizosphere Bacteria Mobilize Zn for Hyperaccumulation by Thlaspi Caerulescens. Environ. Sci. Technol. 2001, 35, 3144–3150. [Google Scholar] [CrossRef] [PubMed]
- Tejada, M.; Hernandez, M. Application of Two Organic Amendments on Soil Restoration: Effects on the Soil Biological Properties. J. Environ. Qual. 2006, 35, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Alves, P.D.D.; de Siqueira, F.F.; Facchin, S.; Horta, C.C.R.; Victória, J.M.N.; Kalapothakis, E. Survey of Microbial Enzymes in Soil, Water, and Plant Microenvironments. Open Microbiol. J. 2014, 8, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Kucukural, A.; Zhang, Y. I-TASSER: A Unified Platform for Automated Protein Structure and Function Prediction. Nat. Protoc. 2010, 5, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Watts, D.B.; Kloepper, J.W.; Torbert, H.A. Influence of Plant Growth-Promoting Rhizobacteria on Corn Growth Under Different Fertility Sources. Commun. Soil Sci. Plant Anal. 2018, 49, 1239–1255. [Google Scholar] [CrossRef]
- Eleiwa, M.E.; Hamed, E.R.; Shehata, H.S. The Role of Biofertilizers and/or Some Micronutrients on Wheat Plant (Triticum aestivum L.) Growth in Newly Reclaimed Soil. Afr. J. Ecol. 2012, 50, 464–475. [Google Scholar] [CrossRef]
- Nain, L.; Yadav, R.; Saxena, J. Characterization of Multifaceted Bacillus sp. RM-2 for Its Use as Plant Growth Promoting Bioinoculant for Crops Grown in Semi Arid Deserts. Appl. Soil Ecol. 2012, 59, 124–135. [Google Scholar]
- Bona, E.; Lingua, G.; Todeschini, V. Effect of Bioinoculants on the Quality of Crops. In Bioformulations: For Sustainable Agriculture; Arora, N., Mehnaz, S., Balestrini, R., Eds.; Springer International Publishing: New Delhi, India, 2016; pp. 93–124. [Google Scholar]
- Bationo, A.; Kimetu, J.; Ikerra, S.; Kimani, S.; Mugendi, D.; Odendo, M.; Silver, M.; Swift, M.; Sanginga, N. The African Network for Soil Biology and Fertility: New Challenges and Opportunities. In Managing Nutrient Cycles to Sustain Soil Fertility in Sub-Saharan Africa; Bationo, A., Ed.; Academy Science Publishers (ASP): Nairobi, Kenya, 2003; pp. 1–24. [Google Scholar]
- El-Shafey, A.; El-Hawary, M.M. Integrated Effect of Bio-Organic and/or Nitrogen Fertilizer on Growth and Yield of Maize (Zea maize L.). Zagazig J. F. Crop Sci. 2016, 43, 1105–1119. [Google Scholar]
| Characteristics | Units | Season-I | Season-II |
|---|---|---|---|
| Sand | % | 51.2 | 53.1 |
| Silt | % | 29.6 | 27.5 |
| Clay | % | 19.2 | 19.4 |
| Textural class | -- | Sandy clay loam | Sandy clay loam |
| Saturation percentage | % | 33.0 | 33.0 |
| pH | -- | 7.9 | 7.7 |
| Electrical Conductivity | dS m−1 | 1.41 | 1.43 |
| Organic Matter | % | 0.68 | 0.71 |
| Total nitrogen | % | 0.06 | 0.05 |
| Available phosphorous | mg kg−1 | 6.79 | 5.30 |
| Extractable potassium | mg kg−1 | 84 | 89 |
| Available zinc | mg kg−1 | 0.61 | 0.58 |
| Treatments | Plant Height (cm) | Fresh Shoots Biomass (t ha−1) | Dry Shoots Biomass (t ha−1) | |||
|---|---|---|---|---|---|---|
| Season-I | Season-II | Season-I | Season-II | Season-I | Season-II | |
| Control | 146.78 ± 1.836 d * | 140.77 ± 3.938 d | 51.23 ± 1465.53 c | 52.20 ± 1429.45 c | 18.62 ± 756.78 d | 17.62 ± 583.20 d |
| ZnO | 150.63 ± 1.833 d | 143.97 ± 2.614 d | 54.66 ± 683.94 bc | 54.43 ± 674.13 bc | 19.55 ± 266.67 c | 18.85 ± 223.88 cd |
| ZnSO4 | 183.26 ± 5.045 bc | 185.03 ± 4.884 c | 61.96 ± 1562.41 ab | 61.63 ± 1599.39 ab | 22.29 ± 526.25 bc | 21.95 ± 338.78 bc |
| BOZ1 | 179.23 ± 2.082 c | 180.70 ± 2.515 c | 61.90 ± 2858.32 ab | 62.16 ± 1231.27 ab | 21.45 ± 480.76 bc | 20.78 ± 688.29 cd |
| BOZ2 | 198.95 ± 5.194 ab | 201.14 ± 5.262 b | 62.53 ± 995.55 ab | 62.50 ± 133.33 ab | 24.29 ± 605.64 ab | 23.95 ± 472.58 bc |
| BOZ3 | 210.99 ± 2.962 a | 210.99 ± 2.962 ab | 64.66 ± 4102.98 a | 65.50 ± 119.29 a | 25.21 ± 532.28 ab | 24.54 ± 768.97 b |
| BOZ4 | 219.53 ± 1.161 a | 219.53 ± 2.890 a | 67.01 ± 2951.27 a | 66.86 ± 1885.32 a | 26.70 ± 701.43 a | 26.36 ± 827.54 a |
| ZSB | 162.74 ± 4.289 cd | 173.74 ± 5.138 c | 60.56 ± 5773.89 ab | 57.10 ± 2318.04 bc | 19.85 ± 541.39 c | 19.19 ± 713.53 cd |
| Treatments | Grain Yield (g cob−1) | Grain Yield (kg ha−1) | Stover Yield (g cob−1) | Stover Yield (kg ha−1) | 1000-Grain Weight (g) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Season-I | Season-II | Season-I | Season-II | Season-I | Season-II | Season-I | Season-II | Season-I | Season-II | |
| Control | 247.7 ± 10.493 c * | 231.7 ± 10.713 c | 9366.7 ± 371.18 d | 9113.3 ± 154.96 e | 46.8 ± 0.601 d | 46.8 ± 1.073 d | 3050.0 ± 65.06 c | 3050.0 ± 28.87 c | 268.13 ± 1.108 d | 270.72 ± 3.782 c |
| ZnO | 256.0 ± 11.468 bc | 253.6 ± 13.872 bc | 9806.7 ± 148.47 c | 9590.3 ± 261.09 de | 48.2 ± 1.156 d | 48.5 ± 2.285 cd | 3070.0 ± 94.52 c | 3126.7 ± 46.31 bc | 285.14 ± 3.528 c | 284.29 ± 4.373 bc |
| ZnSO4 | 280.0 ± 9.019 ab | 280.7 ± 9.871 ab | 10566.7 ± 352.77 a–c | 10673.3 ± 421.16 b-d | 59.2 ± 3.812 bc | 59.8 ± 1.175 ab | 3266.7 ± 84.53 bc | 3680.0 ± 126.21 a | 295.47 ± 3.752 bc | 302.05 ± 3.885 ab |
| BOZ1 | 279.3 ± 4.702 ab | 279.3 ± 4.177 ab | 10760.0 ± 502.93 ab | 10636.7 ± 220.63 b-d | 60.8 ± 1.946 bc | 60.5 ± 1.685 ab | 3283.3 ± 92.08 bc | 3526.7 ± 52.07 ab | 290.33 ± 3.269 c | 301.08 ± 4.832 ab |
| BOZ2 | 283.7 ± 10.333 ab | 285.0 ± 5.033 ab | 10900.0 ± 611.01 bc | 10866.7 ± 284.80 bc | 61.7 ± 1.453 bc | 58.8 ± 1.271 ab | 3463.3 ± 73.11 a-c | 3683.3 ± 81.10 a | 296.25 ± 4.006 bc | 307.56 ± 5.236 ab |
| BOZ3 | 287.0 ± 11.150 a | 289.3 ± 5.365 ab | 11033.3 ± 240.37 ab | 11500.0 ± 416.33 ab | 64.3 ± 3.245 ab | 63.5 ± 3.952 a | 3543.3 ± 173.82 ab | 3753.3 ± 115.66 a | 301.64 ± 2.403 b | 310.77 ± 4.505 a |
| BOZ4 | 295.3 ± 2.028 a | 294.0 ± 3.001 a | 11646.6 ± 202.10 a | 12013.3 ± 252.01 a | 68.7 ± 1.167 a | 65.2 ± 2.645 a | 3736.7 ± 169.59 a | 3873.3 ± 140.71 a | 314.03 ± 1.024 a | 314.69 ± 3.934 a |
| ZSB | 271.7 ± 8.413 ab | 275.7 ± 2.028 ab | 10510.0 ± 409.19 bc | 10266.7 ± 581.19 cd | 57.7 ± 2.848 c | 55.5 ± 3.621 bc | 3116.7 ± 116.67 bc | 3540.0 ± 133.02 ab | 292.80 ± 4.362 bc | 291.32 ± 4.391 a–c |
| Treatment | Photosynthetic Rate | Transpiration Rate | Stomatal Conductance | Chlorophyll Contents | Electrolyte Leakage | Carotenoids Contents | Carbonic Anhydrase | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (µmol m−2 s−1) | (mmol m−2 s−1) | (mmol m−2 s−1) | (SPAD Value) | (%) | μmol (CO2)kg−1 s−1 | |||||||||
| Season-I | Season-II | Season-I | Season-II | Season-I | Season-II | Season-I | Season-II | Season-I | Season-II | Season-I | Season-II | Season-I | Season-II | |
| Control | 28.0 ± 1.227 d* | 19.5 ± 0.784 e | 9.71 ± 0.835 c | 3.82 ± 0.549 f | 395.7 ± 25.740 c | 183.7 ± 3.215 e | 28.97 ± 1.065 e | 28.30 ± 0.462 d | 59.3 ± 0.882 a | 55.7 ± 0.667 a | 0.063 ± 0.0026 c | 0.062 ± 0.0021 d | 256.0 ± 7.371 f | 253.0 ± 4.042 e |
| ZnO | 29.3 ± 0.970 cd | 22.6 ± 0.723 de | 10.82 ± 0.340 bc | 4.56 ± 0.291 ef | 416.3 ± 15.857 bc | 193.0 ± 15.271 de | 31.60 ± 0.379 de | 29.53 ± 0.754 d | 57.0 ± 1.155 a | 57.7 ± 0.760 a | 0.065 ± 0.0019 bc | 0.064 ± 0.0015 cd | 278.7 ± 5.175 ef | 270.3 ± 7.753 de |
| ZnSO4 | 35.6 ± 1.886 a-c | 24.0 ± 0.491 cd | 11.82 ± 0.762 a-c | 4.82 ± 0.375 c-e | 498.0 ± 38.974 a-c | 234.3 ± 9.262 cd | 36.33 ± 1.004 bc | 37.28 ± 1.344 bc | 52.0 ± 1.045 b | 47.7 ± 1.848 b | 0.073 ± 0.0025 ac | 0.073 ± 0.0017 a-d | 308.3 ± 2.028 c-e | 310.3 ± 1.202 b-d |
| BOZ1 | 33.8 ± 1.486 a-d | 24.0 ± 0.731 c | 11.55 ± 0.823 a-c | 4.74 ± 0.239 cd | 491.0 ± 32.083 a-c | 229.7 ± 4.807 cd | 36.13 ± 1.443 b-d | 36.87 ± 1.797 bc | 47.0 ± 0.577 c | 47.0 ± 0.547 b | 0.074 ± 0.0021 ac | 0.072 ± 0.0013 a-d | 319.0 ± 3.055 b-d | 317.7 ± 7.513 bc |
| BOZ2 | 36.6 ± 1.713 ab | 24.3 ± 0.949 bc | 12.06 ± 0.968 ab | 4.93 ± 0.118 bc | 541.7 ± 9.387 ab | 241.6 ± 17.755 bc | 38.23 ± 0.953 bc | 41.90 ± 1.685 ab | 38.0 ± 0.754 d | 32.7 ± 1.028 c | 0.078 ± 0.0015 ab | 0.078 ± 0.0024 a-c | 338.7 ± 4.485 a-c | 341.0 ± 11.136 ab |
| BOZ3 | 38.4 ± 1.893 a | 26.3 ± 1.557 b | 12.14 ± 0.309 ab | 5.06 ± 0.237 ab | 551.0 ± 10.583 ab | 253.3 ± 19.462 ab | 39.77 ± 1.549 ab | 43.63 ± 1.178 a | 33.0 ± 1.156 e | 33.0 ± 0.882 cd | 0.082 ± 0.0008 a | 0.081 ± 0.0012 ab | 350.3 ± 9.319 ab | 348.3 ± 13.346 ab |
| BOZ4 | 40.2 ± 1.596 a | 29.5 ± 1.815 a | 13.33 ± 0.763 a | 5.87 ± 0.164 a | 580.3 ± 24.037 a | 261.3 ± 19.238 a | 43.73 ± 0.504 a | 46.47 ± 0.974 a | 30.0 ± 0.585 e | 30.0 ± 1.202 d | 0.085 ± 0.0023 a | 0.086 ± 0.0021 a | 365.0 ± 7.768 a | 366.0 ± 4.726 a |
| ZSB | 31.5 ± 1.285 b-d | 23.4 ± 1.408 de | 11.29 ± 0.965 a-c | 4.61 ± 0.202 d-f | 446.0 ± 16.828 a-c | 201.7 ± 4.3716 de | 34.10 ± 0.985 cd | 32.27 ± 1.213 cd | 59.0 ± 0.882 a | 61.0 ± 1.404 a | 0.068 ± 0.0019 bc | 0.070 ± 0.0012 b-d | 295.0 ± 5.033 de | 286.7 ± 9.956 c-e |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, A.; Zahir, Z.A.; Ditta, A.; Tahir, M.U.; Ahmad, M.; Mumtaz, M.Z.; Hayat, K.; Hussain, S. Production and Implication of Bio-Activated Organic Fertilizer Enriched with Zinc-Solubilizing Bacteria to Boost up Maize (Zea mays L.) Production and Biofortification under Two Cropping Seasons. Agronomy 2020, 10, 39. https://doi.org/10.3390/agronomy10010039
Hussain A, Zahir ZA, Ditta A, Tahir MU, Ahmad M, Mumtaz MZ, Hayat K, Hussain S. Production and Implication of Bio-Activated Organic Fertilizer Enriched with Zinc-Solubilizing Bacteria to Boost up Maize (Zea mays L.) Production and Biofortification under Two Cropping Seasons. Agronomy. 2020; 10(1):39. https://doi.org/10.3390/agronomy10010039
Chicago/Turabian StyleHussain, Azhar, Zahir Ahmad Zahir, Allah Ditta, Muhammad Usman Tahir, Maqshoof Ahmad, Muhammad Zahid Mumtaz, Khizar Hayat, and Shahzad Hussain. 2020. "Production and Implication of Bio-Activated Organic Fertilizer Enriched with Zinc-Solubilizing Bacteria to Boost up Maize (Zea mays L.) Production and Biofortification under Two Cropping Seasons" Agronomy 10, no. 1: 39. https://doi.org/10.3390/agronomy10010039
APA StyleHussain, A., Zahir, Z. A., Ditta, A., Tahir, M. U., Ahmad, M., Mumtaz, M. Z., Hayat, K., & Hussain, S. (2020). Production and Implication of Bio-Activated Organic Fertilizer Enriched with Zinc-Solubilizing Bacteria to Boost up Maize (Zea mays L.) Production and Biofortification under Two Cropping Seasons. Agronomy, 10(1), 39. https://doi.org/10.3390/agronomy10010039








