Flaming, Glyphosate, Hot Foam and Nonanoic Acid for Weed Control: A Comparison
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Set Up, Design and Treatment
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Weed Control
3.2. Weed Regrowth
3.3. Weed Dry Biomass
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duke, S.O.; Heap, I. Evolution of weed resistance to herbicides: What have we learned after seventy years. In Biology, Physiology and Molecular Biology of Weeds; Jugulam, M., Ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Heap, I. Global perspective of herbicide-resistant weeds. Pest Manag. Sci. 2014, 70, 1306–1315. [Google Scholar] [CrossRef]
- Wei, D.; Liping, C.; Zhijun, M.; Guangwei, W.; Ruirui, Z. Review of non-chemical weed management for green agriculture. Int. J. Agric. Biol. Eng. 2010, 3, 52–60. [Google Scholar]
- Craig, I.P.; Hewitt, A.; Terry, H. Rotary atomiser design requirements for optimum pesticide application efficiency. Crop Prot. 2014, 66, 34–39. [Google Scholar] [CrossRef]
- Matthews, G.A. Pesticide Application Methods; Longman (Group UK): Harlow, Essex, UK, 1992. [Google Scholar]
- Woods, N.; Craig, I.; Dorr, G.; Young, B. Spray drift of pesticides arising from aerial application in cotton. J. Environ. Qual. 2001, 30, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Doĝan, M.N.; Öğüt, D.; Mülleder, N.; Boz, Ö.; Brants, I.; Voegler, W. Effect of Water Volume and Water Quality on the Efficacy of Glyphosate on some Important Weed Species in Turkey. In Proceedings of the 25th German Conference on Weed Biology and Weed Control, Braunschweig, Germany, 13–15 March 2012; pp. 229–234. [Google Scholar]
- Webber, C.L., III; Taylor, M.J.; Shrefler, J.W. Weed control in yellow squash using sequential postdirected applications of pelargonic acid. HortTechnology 2014, 24, 25–29. [Google Scholar] [CrossRef]
- Melander, B.; Liebman, M.; Davis, A.S.; Gallandt, E.R.; Bàrberi, P.; Moonen, A.C.; Rasmussen, J.; van der Weide, R.; Vidotto, F. Non-chemical weed management. Thermal weed control. In Weed Research: Expanding Horizons; Hatcher, P.E., Froud Williams, R.J., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 259–264. [Google Scholar]
- Senseman, S.A. Herbicide Handbook, 9th ed.; Weed Science Society of America: Lawrence, KS, USA, 2007; pp. 379–381. [Google Scholar]
- Ulloa, S.M.; Datta, A.; Knezevic, S.Z. Tolerance of selected weed species to broadcast flaming at different growth stages. Crop Prot. 2010, 29, 1381–1388. [Google Scholar] [CrossRef]
- Cederlund, H.; Börjesson, E. Hot foam for weed control—Do alkyl polyglucoside surfactants used as foaming agents affect the mobility of organic contaminants in soil? J. Hazard. Mater. 2016, 314, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Raffaelli, M.; Martelloni, L.; Frasconi, C.; Fontanelli, M.; Peruzzi, A. Development of machines for flaming weed control on hard surfaces. Appl. Eng. Agric. 2013, 29, 663–673. [Google Scholar]
- Martelloni, L.; Fontanelli, M.; Frasconi, C.; Raffaelli, M.; Peruzzi, A. Cross-flaming application for intra-row weed control in maize. Appl. Eng. Agric. 2016, 32, 569–578. [Google Scholar]
- Martelloni, L.; Raffaelli, M.; Frasconi, C.; Fontanelli, M.; Peruzzi, A.; D’Onofrio, C. Using flaming as an alternative method to vine suckering. Agronomy 2019, 9, 147. [Google Scholar] [CrossRef]
- Ascard, J. Effects of flame weeding on weed species at different developmental stages. Weed Res. 1995, 35, 397–411. [Google Scholar] [CrossRef]
- Rajamannan, A.H.J.; Washington, D.C. U.S. Patent and Trademark Office. Method of Using Hot Air Foam to Kill Vegetation and Pests. U.S. Patent No. 5,575,111, 19 November 1996. [Google Scholar]
- Martelloni, L.; Frasconi, C.; Sportelli, M.; Fontanelli, M.; Raffaelli, M.; Peruzzi, A. The use of different hot foam doses for weed Control. Agronomy 2019, 9, 490. [Google Scholar] [CrossRef]
- Peerzada, A.M.; Chauhan, B.S. Chapter 2. Thermal Weed Control: History, Mechanisms, and Impacts. In Non-Chemical Weed Control; Jabran, K., Chauhan, B.S., Eds.; Elsevier: London, UK, 2018; pp. 9–31. [Google Scholar]
- Kup, F.; Saglam, R. Weed destruction in cotton fields using hot foam method and its comparison to certain other methods. ARPN J. Agric. Biol. Sci. 2014, 9, 301–307. [Google Scholar]
- De Cauwer, B.; Bogaert, S.; Claerhout, S.; Bulcke, R.; Reheul, D. Efficacy and reduced fuel use for hot water weed control on pavements. Weed Res. 2014, 55, 195–205. [Google Scholar] [CrossRef]
- Boonen, E.; Beeldens, A.; Fagot, M.; De Cauwer, B.; Reheulc, D.; Bulcke, R. Preventive Weed Control on Pavements: Reducing the Environmental Impact of Herbicides Part 1: A Field Survey Study. In Proceedings of the 10th International Conference on Concrete Block Paving, Shanghai, China, 24–26 November 2012; pp. 1–14. [Google Scholar]
- Melander, B.; Holst, N.; Grundy, A.C.; Kempenaar, C.; Riemens, M.M.; Verschwele, A.; Hansson, D. Weed occurrence on pavements in five North European towns. Weed Res. 2009, 49, 516–525. [Google Scholar] [CrossRef]
- Peruzzi, A.; Martelloni, L.; Frasconi, C.; Fontanelli, M.; Pirchio, M.; Raffaelli, M. Machines for nonchemical intra-row weed control in narrow and wide-row crops: A. review. J. Agric. Eng. 2017, 48, 57–70. [Google Scholar] [CrossRef]
- Martelloni, L.; Fontanelli, M.; Frasconi, C.; Raffaelli, M.; Pirchio, M.; Peruzzi, A. A combined flamer-cultivator for weed control during the harvesting season of asparagus green spears. Span. J. Agric. Res. 2017, 15, e0203. [Google Scholar] [CrossRef]
- Hess, M.; Barralis, G.; Bleiholder, H.; Buhr, L.; Eggers, T.H.; Hack, H.; Stauss, R. Use of the extended BBCH scale—General for the description of the growth stages of mono- and dicotyledonous weed species. Weed Res. 1997, 37, 433–441. [Google Scholar] [CrossRef]
- Mankar. ULV Herbicide Spraying System. Available online: http://agri-flex.com/wp-content/uploads/2017/03/0005.pdf (accessed on 24 October 2019).
- Weedingtech. Foamstream M1200. Available online: https://www.weedingtech.com/product/foamstream-m1200/ (accessed on 6 November 2019).
- Weedingtech. Our Technology. Available online: https://www.weedingtech.com/why-foamstream/our-technology/ (accessed on 6 November 2019).
- Nesbit, M.; Fergusson, M.; Colsa, A.; Ohlendorf, J.; Hayes, C.; Paquel, K.; Schweitzer, J.P. Comparative Study on the Differences between the EU and US Legislation on Emissions in the Automotive Sector; European Parliament: Brussel, Belgium, 2016; Available online: http://www.europarl.europa.eu/RegData/etudes/STUD/2016/587331/IPOL_STU(2016)587331_EN.pdf (accessed on 6 November 2019).
- IMAGING Crop Response Analyser. Ver. 0.4.. 2018. Available online: http://imaging-crops.dk (accessed on 17 July 2019).
- Rasmussen, J.; Norremark, M.; Bibby, B.M. Assessment of leaf cover and crop soil cover in weed harrowing research using digital images. Weed Res. 2007, 47, 299–310. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://www.R-project.org/ (accessed on 17 July 2019).
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Soft. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 3rd ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Webber, C.L., III; Shrefler, J.W.; Brandenberger, L.P.; Davis, A.R. AXXE® (Pelargonic Acid) and Racer® (Ammonium Nonanoate): Weed Control Comparisons; Vegetable Weed Control Studies; Oklahoma State University, Division of Agricultural Sciences and Natural Resources, Department of Horticulture & Landscape Architecture: Stillwater, OK, USA, 2012; pp. 1–4. [Google Scholar]
- Rowley, M.A.; Ransom, C.V.; Reeve, J.R.; Black, B.L. Mulch and organic herbicide combinations for in-row orchard weed suppression. Int. J. Fruit Sci. 2011, 11, 316–331. [Google Scholar] [CrossRef]
- Ward, J.S.; Todd, L.M. Nonchemical and herbicide treatments for management of Japanese stiltgrass (Microstegium vimineum). Invasive Plant Sci. Manag. 2012, 5, 9–19. [Google Scholar] [CrossRef]

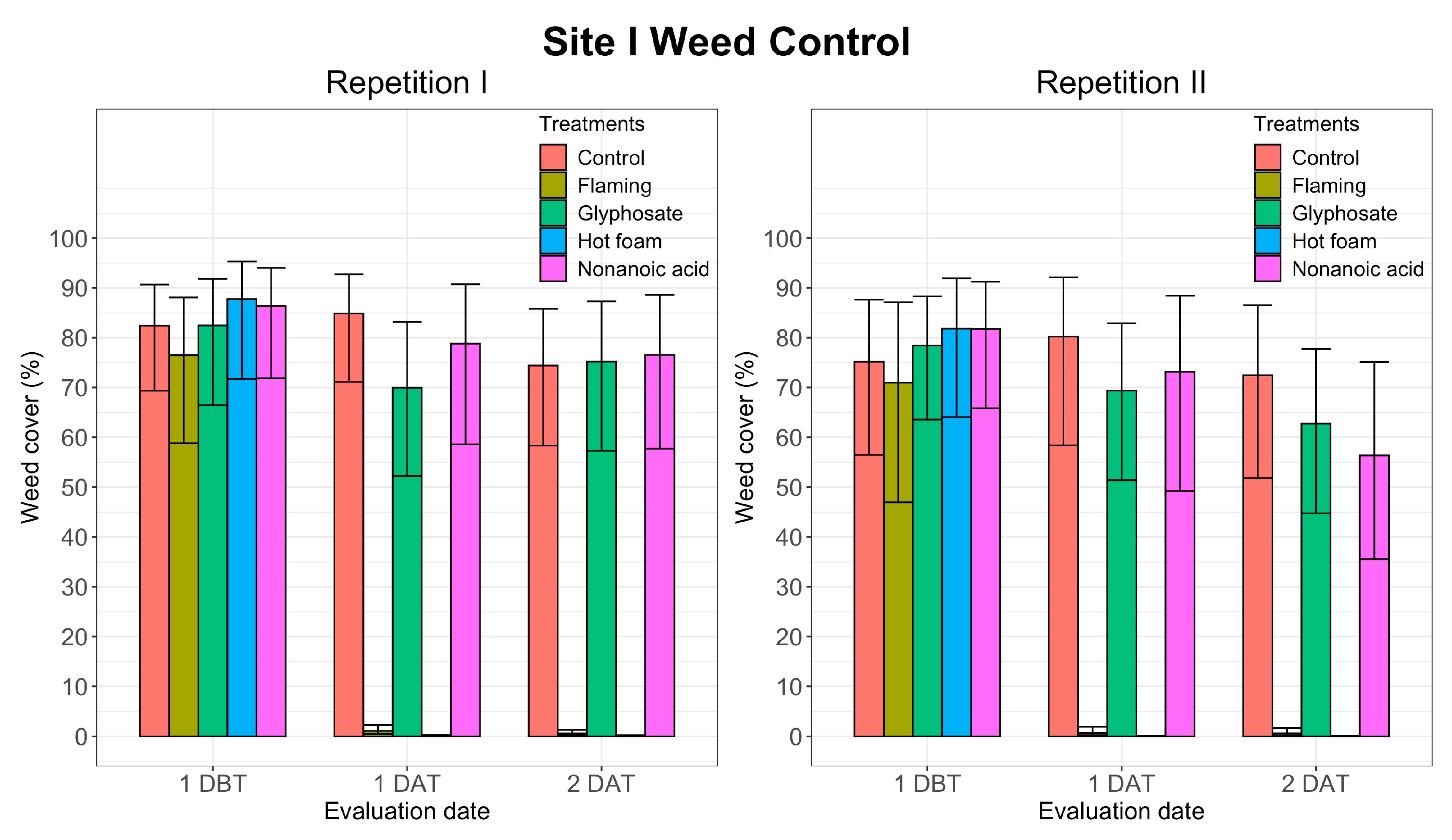
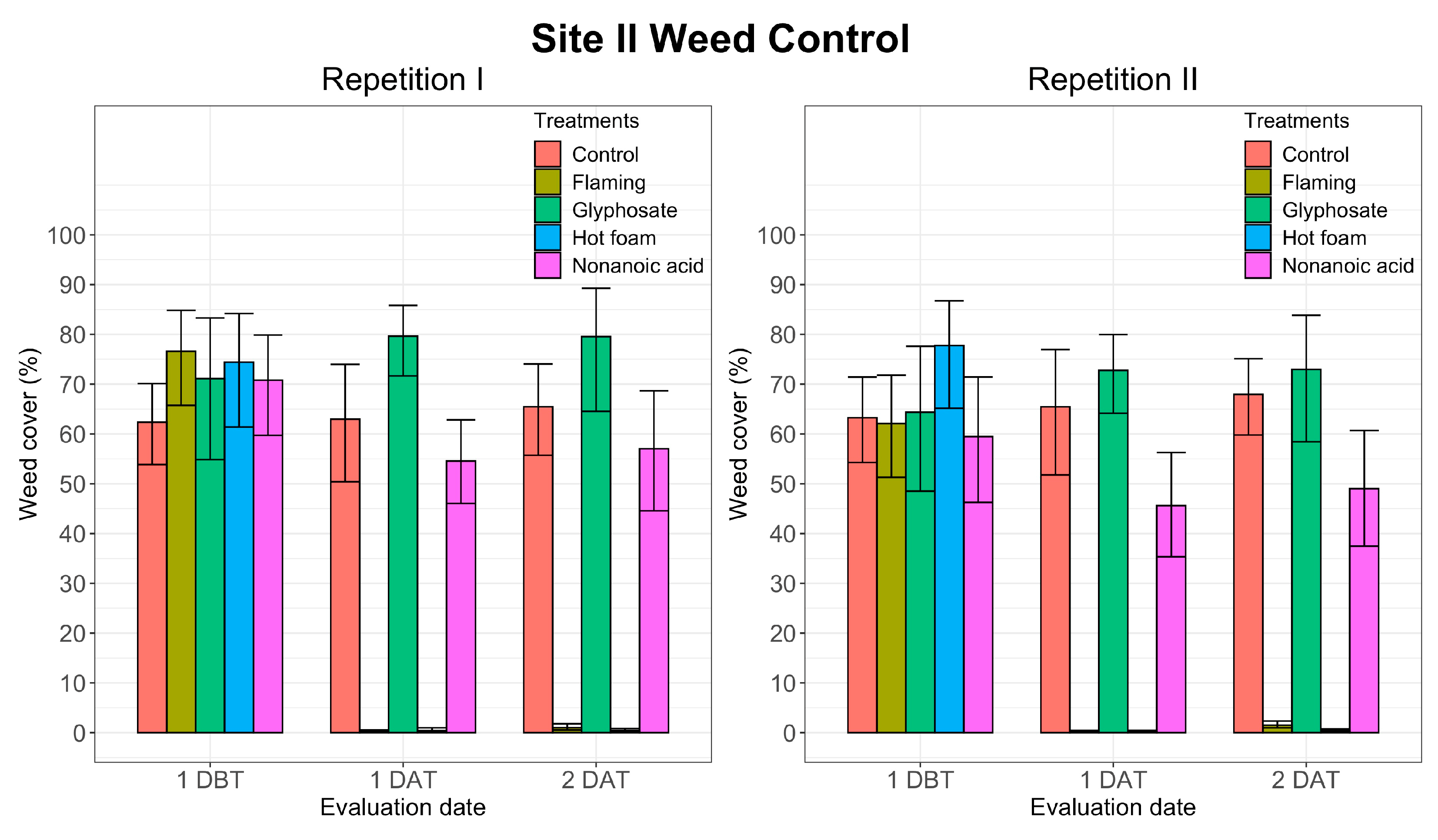
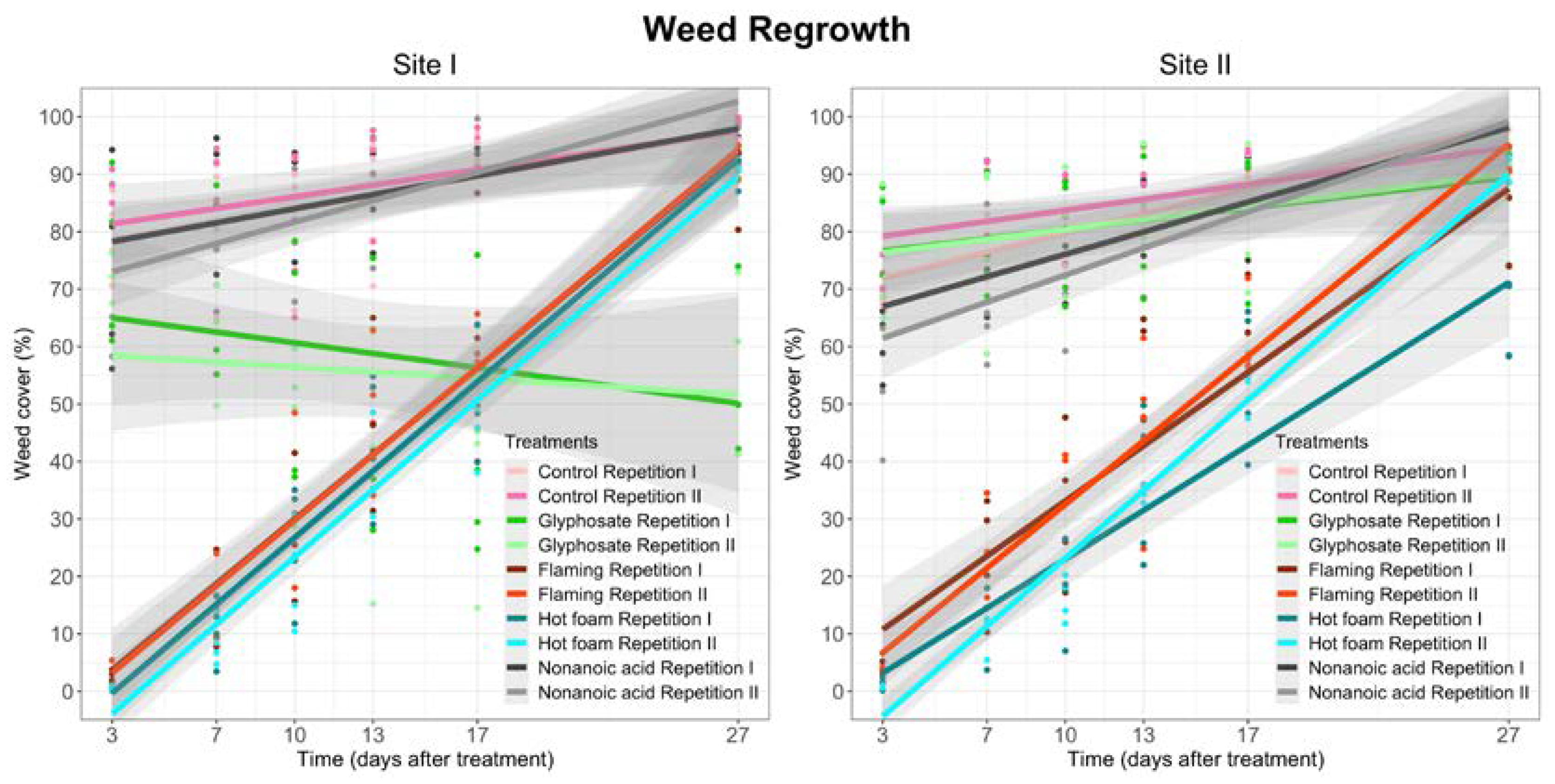
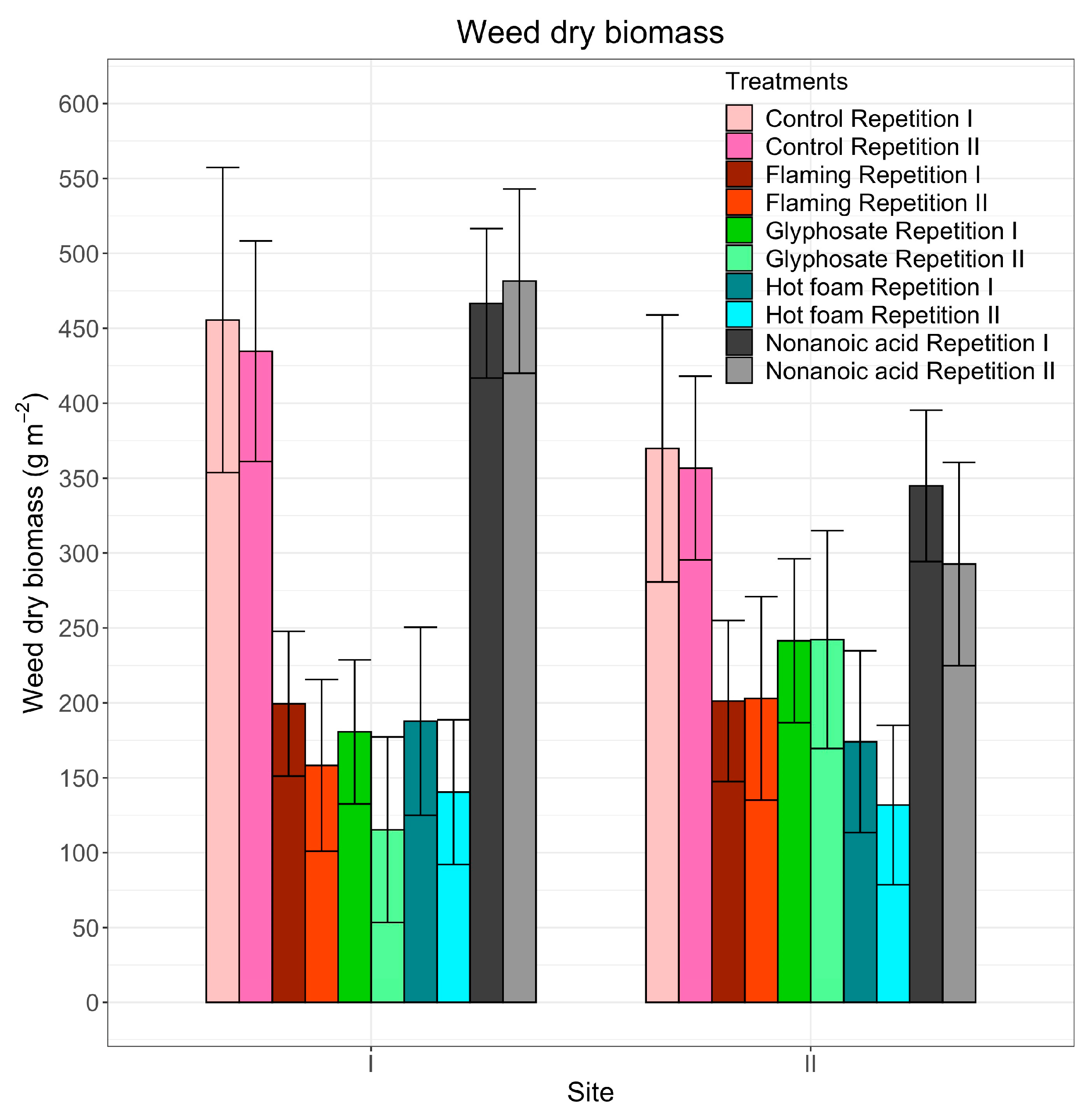
| Site I | Site II | |
|---|---|---|
| p-Value | p-Value | |
| Treatment | <0.001 | <0.001 |
| Date | <0.001 | <0.001 |
| Repetition | 0.161 | 0.134 |
| Treatment: Date | <0.001 | <0.001 |
| Treatment: repetition | 0.588 | 0.112 |
| Date: repetition | 0.896 | 0.160 |
| Treatment: date: repetition | 0.865 | 0.037 |
| Treatment | Logit [Weed Cover (%)] (±SE) | ||
|---|---|---|---|
| Repetition I | |||
| 1 DBT | 1 DAT | 2 DAT | |
| Control | 1.55 (0.365) | 1.72 (0.399) | 1.07 (0.365) |
| Flaming | 1.18 (0.400) | −4.53 (0.381) | −5.16 (0.399) |
| Glyphosate | 1.55 (0.415) | 0.85 (0.375) | 1.11 (0.397) |
| Hot foam | 1.97 (0.476) | −6.98 (0.507) | −7.07 (0.466) |
| Nonanoic acid | 1.84 (0.430) | 1.31 (0.451) | 1.18 (0.417) |
| Repetition II | |||
| Control | 1.11 (0.409) | 1.40 (0.483) | 0.97 (0.426) |
| Flaming | 0.89 (0.468) | −5.04 (0.494) | −5.15 (0.482) |
| Glyphosate | 1.29 (0.367) | 0.82 (0.378) | 0.52 (0.366) |
| Hot foam | 1.50 (0.437) | −8.34 (0.511) | −7.73 (0.442) |
| Nonanoic acid | 1.50 (0.406) | 1.00 (0.473) | 0.26 (0.410) |
| Treatment | logit [Weed Cover (%)] (±SE) | ||
|---|---|---|---|
| Repetition I | |||
| 1 DBT | 1 DAT | 2 DAT | |
| Control | 0.50 (0.175) | 0.53 (0.234) | 0.64 (0.197) |
| Flaming | 1.19 (0.240) | −5.70 (0.218) | −4.59 (0.262) |
| Glyphosate | 0.90 (0.298) | 1.36 (0.207) | 1.36 (0.316) |
| Hot foam | 1.07 (0.264) | −5.48 (0.351) | −5.37 (0.270) |
| Nonanoic acid | 0.89 (0.226) | 0.18 (0.172) | 0.28 (0.229) |
| Repetition II | |||
| Control | 0.54 (0.183) | 0.64 (0.252) | 0.75 (0.177) |
| Flaming | 0.49 (0.208) | −5.82 (0.198) | −4.17 (0.209) |
| Glyphosate | 0.59 (0.280) | 0.98 (0.194) | 0.99 (0.280) |
| Hot foam | 1.25 (0.271) | −6.30 (0.364) | −5.41 (0.257) |
| Nonanoic acid | 0.38 (0.240) | −0.18 (0.204) | −0.04 (0.220) |
| p-Values | ||
|---|---|---|
| Site I | Site II | |
| Treatment | <0.001 | <0.001 |
| Date | <0.001 | <0.001 |
| Repetition | 0.447 | 0.492 |
| Treatment: Date | <0.001 | <0.001 |
| Treatment: repetition | 0.834 | 0.476 |
| Date: repetition | 0.494 | 0.056 |
| Treatment: date: repetition | 0.914 | 0.019 |
| Regression Coefficient (±SE) | ||
|---|---|---|
| Site I | Site II | |
| Intercept | 0.760 (0.049) | 0.686 (0.035) |
| Glyphosate | −0.091 (0.069) | 0.064 (0.050) |
| Flaming | −0.835 (0.069) | −0.675 (0.050) |
| Hot foam | −0.879 (0.069) | −0.739 (0.050) |
| Nonanoic acid | −0.002 (0.069) | −0.055 (0.050) |
| Evaluation date | 0.008 (0.003) | 0.011 (0.002) |
| Repetition II | 0.033 (0.069) | 0.087 (0.050) |
| Glyphosate: evaluation date | −0.014 (0.005) | −0.006 (0.003) |
| Flaming: evaluation date | 0.030 (0.005) | 0.021 (0.003) |
| Hot foam: evaluation date | 0.031 (0.005) | 0.017 (0.003) |
| Nonanoic acid: evaluation date | 0.000 (0.005) | 0.002 (0.003) |
| Glyphosate: repetition II | −0.109 (0.098) | −0.091 (0.071) |
| Flaming: repetition II | −0.040 (0.098) | −0.142 (0.071) |
| Hot foam: repetition II | −0.070 (0.098) | −0.195 (0.071) |
| Nonanoic acid: repetition II | −0.098 (0.098) | −0.150 (0.071) |
| Evaluation date: repetition II | −0.001 (0.005) | −0.005 (0.003) |
| Glyphosate: evaluation date: repetition II | 0.005 (0.007) | 0.005 (0.005) |
| Flaming: evaluation date: repetition II | 0.002 (0.007) | 0.010 (0.005) |
| Hot foam: evaluation date: repetition II | 0.002 (0.007) | 0.016 (0.005) |
| Nonanoic acid: evaluation date: repetition II | 0.005 (0.007) | 0.007 (0.005) |
| Treatment | Estimated Time (Days) (±SE) for Weed Cover Percentage Regrowth | |||||
|---|---|---|---|---|---|---|
| Repetition I | Repetition II | |||||
| Site I | ||||||
| ET10 (±SE) | ET50 (±SE) | ET90 (±SE) | ET10 (±SE) | ET50 (±SE) | ET90 (±SE) | |
| Control | NA | NA | 17.6 (±3.69) | NA | NA | 15.8 (±4.00) |
| Flaming | 4.7 (±0.97) | 15.3 (±0.70) | 26.0 (±1.32) | 4.8 (±0.96) | 15.3 (±0.70) | 25.9 (±1.30) |
| Glyphosate | NA | NA | NA | NA | NA | NA |
| Hot foam | 5.7 (±0.89) | 16.0 (±0.70) | 26.4 (±1.31) | 6.6 (±0.83) | 16.8 (0.73) | 27.1 (±1.35) |
| Nonanoic acid | NA | NA | 17.3 (±3.55) | NA | NA | 16.7 (±2.28) |
| Site II | ||||||
| ET10 (±SE) | ET50 (±SE) | ET90 (±SE) | ET10 (±SE) | ET50 (±SE) | ET90 (±SE) | |
| Control | NA | NA | 19.2 (±2.12) | NA | NA | 19.8 (±3.79) |
| Flaming | 2.8 (±0.93) | 15.3 (±0.60) | 27.8 (±1.24) | 3.9 (±0.75) | 14.7 (±0.51) | 25.6 (±0.95) |
| Glyphosate | NA | NA | 28.0 (±7.48) | NA | NA | 26.8 (±6.52) |
| Hot foam | 5.4 (±0.89) | 19.3 (±1.85) | 33.7 (±1.85) | 6.7 (±0.59) | 16.8 (±0.52) | 27.0 (±0.97) |
| Nonanoic acid | NA | NA | 20.7 (±2.01) | NA | NA | 21.2 (±1.71) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martelloni, L.; Frasconi, C.; Sportelli, M.; Fontanelli, M.; Raffaelli, M.; Peruzzi, A. Flaming, Glyphosate, Hot Foam and Nonanoic Acid for Weed Control: A Comparison. Agronomy 2020, 10, 129. https://doi.org/10.3390/agronomy10010129
Martelloni L, Frasconi C, Sportelli M, Fontanelli M, Raffaelli M, Peruzzi A. Flaming, Glyphosate, Hot Foam and Nonanoic Acid for Weed Control: A Comparison. Agronomy. 2020; 10(1):129. https://doi.org/10.3390/agronomy10010129
Chicago/Turabian StyleMartelloni, Luisa, Christian Frasconi, Mino Sportelli, Marco Fontanelli, Michele Raffaelli, and Andrea Peruzzi. 2020. "Flaming, Glyphosate, Hot Foam and Nonanoic Acid for Weed Control: A Comparison" Agronomy 10, no. 1: 129. https://doi.org/10.3390/agronomy10010129
APA StyleMartelloni, L., Frasconi, C., Sportelli, M., Fontanelli, M., Raffaelli, M., & Peruzzi, A. (2020). Flaming, Glyphosate, Hot Foam and Nonanoic Acid for Weed Control: A Comparison. Agronomy, 10(1), 129. https://doi.org/10.3390/agronomy10010129










