Assessing the Performance of Maize (Zea mays L.) as Trap Crops for the Management of Sunflower Broomrape (Orobanche cumana Wallr.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment 1. Maize-Broomrape Coculture Experiment
2.1.1. Plant Materials and Chemicals
2.1.2. Experiment Design
2.2. Experiment 2. Maize Rotation Experiment
2.2.1. Plant and Soil Materials
2.2.2. Experiment Design
2.2.3. Statistical Analyses
3. Results
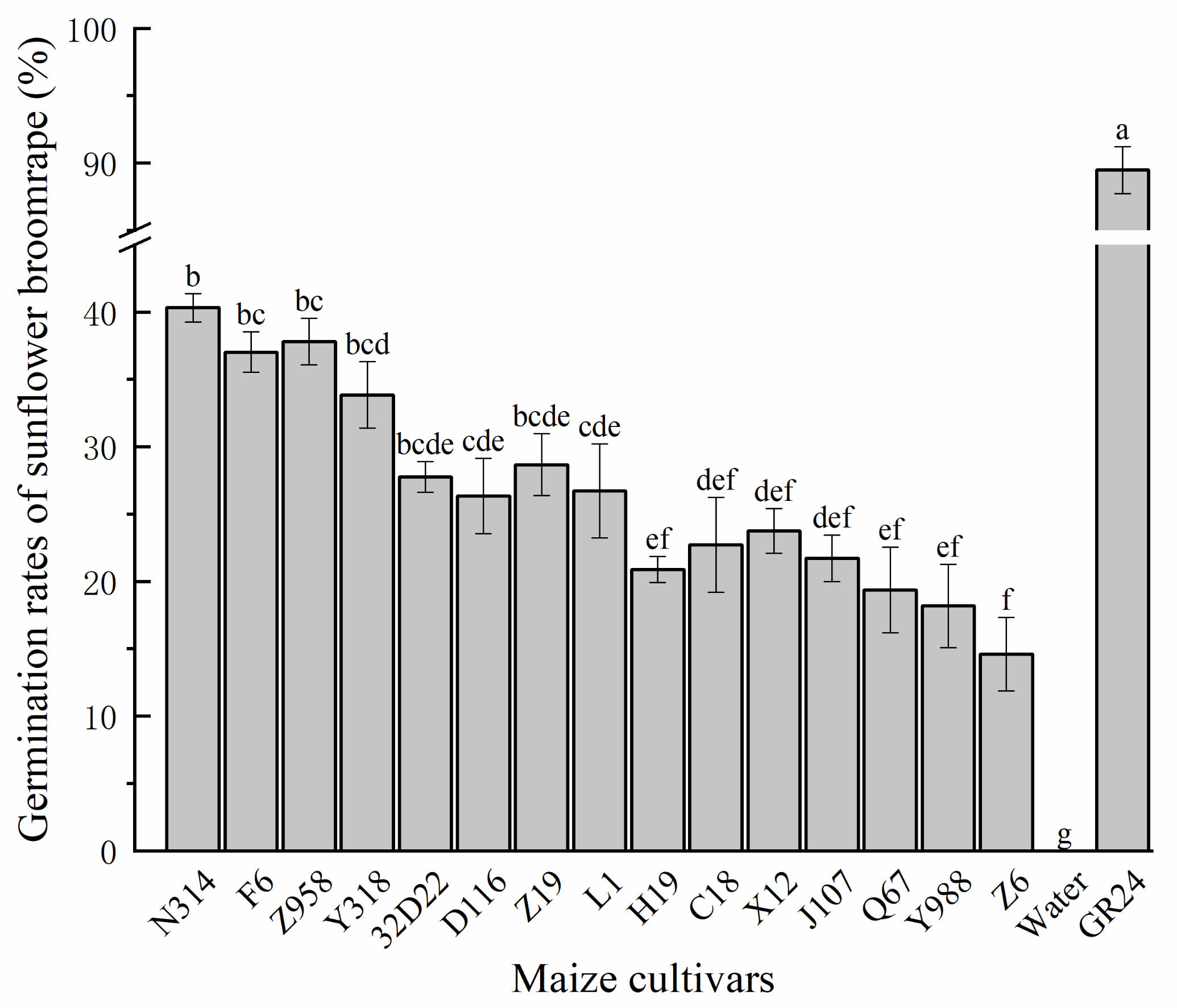
3.1. Germination Responses of Sunflower Broomrape to Various Maize Cultivars
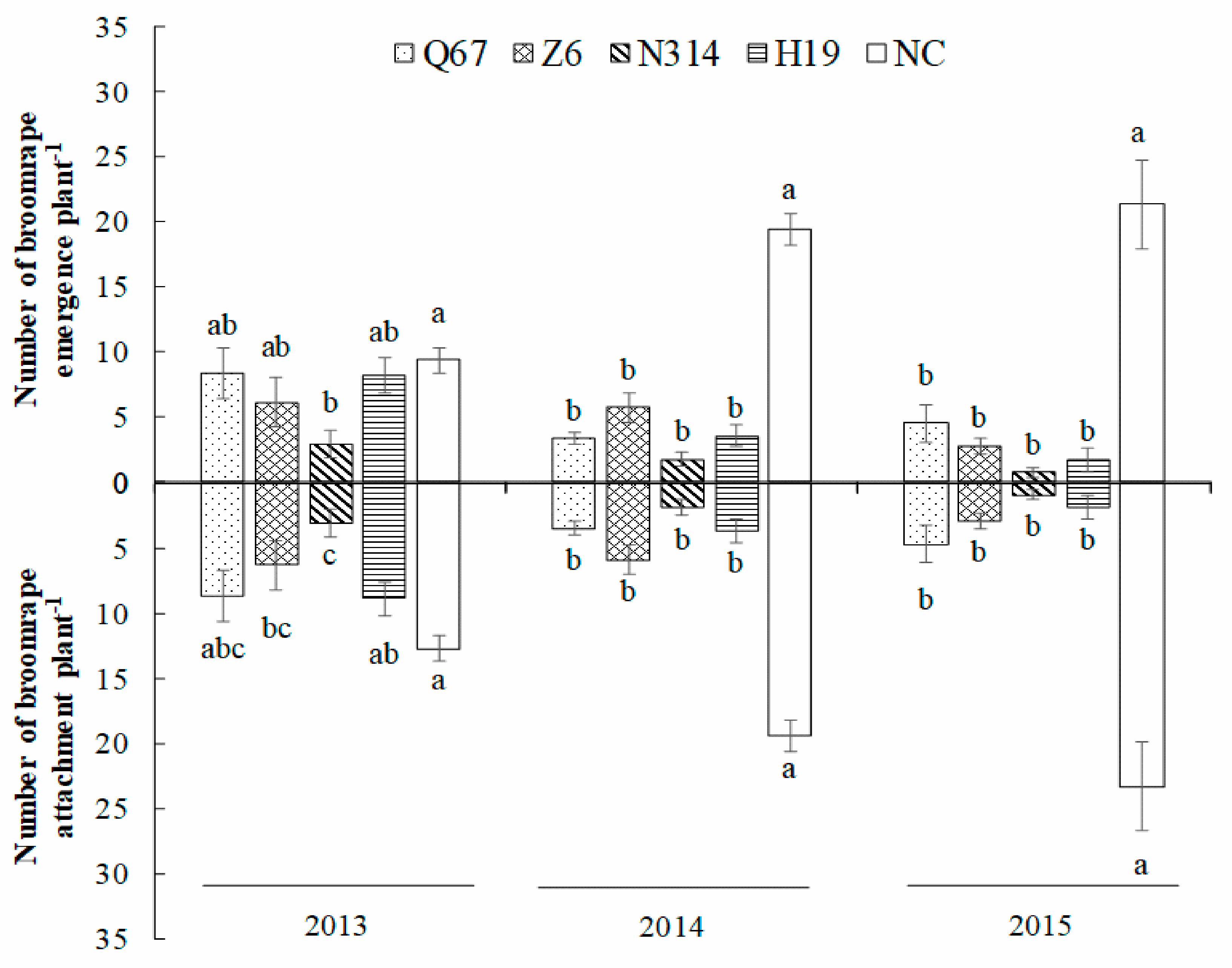
3.2. Broomrape Infection Levels after Rotation with Maize
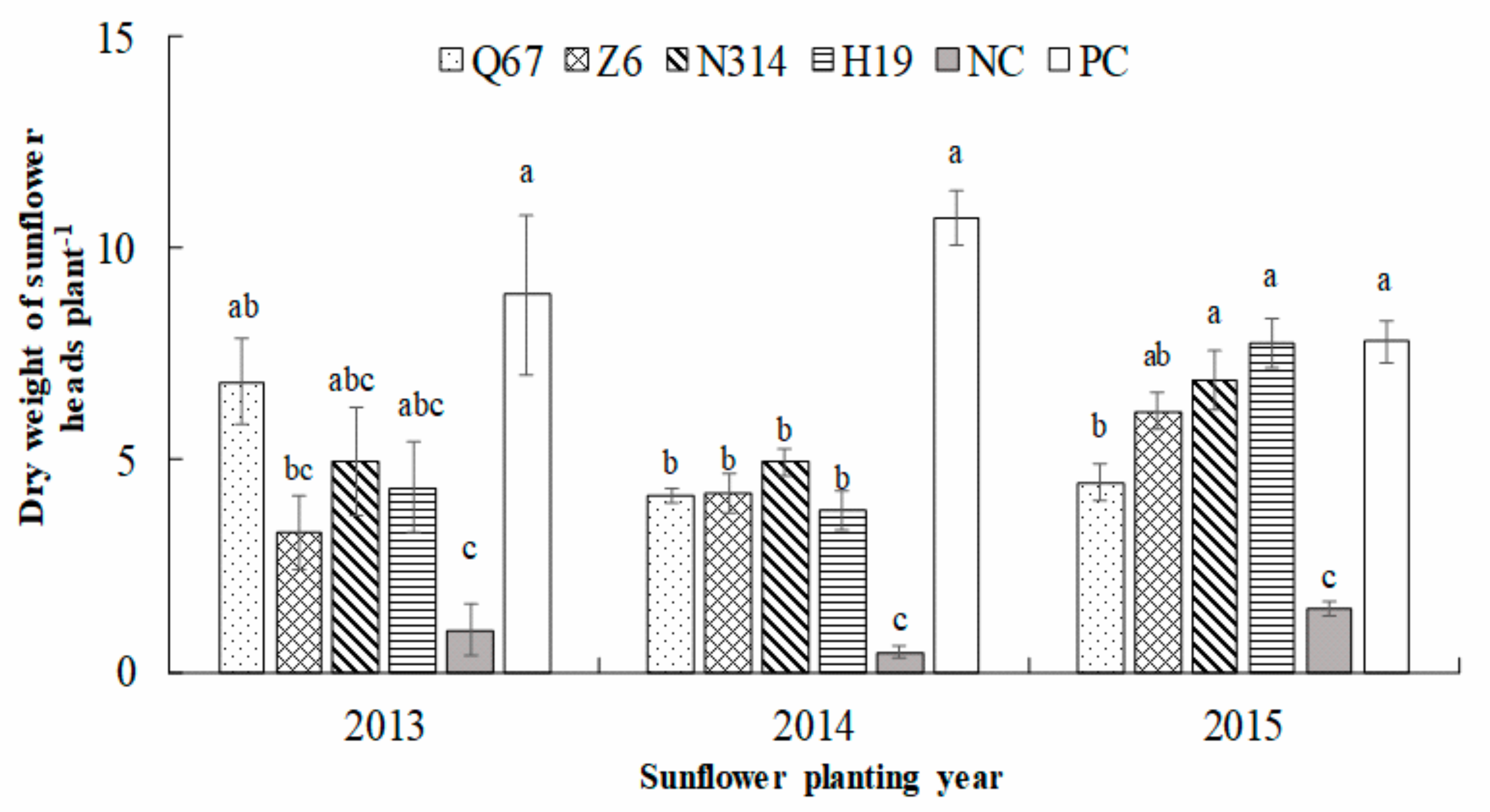
3.3. Sunflower Performance after Rotation with Maize
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chris, P. The Parasitic Weeds of the Orobanchaceae. In Parasitic Orobanchaceae: Parasitic Mechanisms and Control Strategies; Joel, D.M., Gressel, J., Musselman, L.J., Eds.; Springer: Berlin, Germany, 2013; pp. 313–344. [Google Scholar]
- Shi, B.; Chen, G.; Zhang, Z.; Hao, J.; Jing, L.; Zhou, H.; Zhao, J. First Report of Race Composition and Distribution of Sunflower Broomrape, Orobanche cumana, in China. Plant Dis. 2015, 99, 291. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Chao, C.; Yazhen, L.; Na, D.; Haiyan, W. present situation and prospect of the parasitic weed sunflower broomrape research. Jiangsu Agric. Sci. 2015, 43, 144–147, (In Chinese with English abstract). [Google Scholar]
- Parker, C.; Riches, C.R. Parasitic Weeds of the World: Biology and Control; CAB International: Wallingford, UK, 1993; pp. 111–163. [Google Scholar]
- Cook, C.; Whichard, L.P.; Wall, M.; Egley, G.H.; Coggon, P.; Luhan, P.A.; McPhail, A. Germination stimulants. II. Structure of strigol, a potent seed germination stimulant for witchweed (Striga lutea). J. Am. Chem. Soc. 1972, 94, 6198–6199. [Google Scholar] [CrossRef]
- Butler, L.G. Chemical communication between the parasitic weed Striga and its crop host. A new dimension in allelochemistry. In Insights into Allelopathy, ACS Symposium Series; Inderjit, K., Dakshini, K.M.M., Einhellig, F.A., Eds.; ACS Books: Washington, DC, USA, 1995; pp. 158–168. [Google Scholar]
- Molinero-Ruiz, L.; Delavault, P.; Pérez-Vich, B.; Pacureanu-Joita, M.; Bulos, M.; Altieri, E.; Domínguez, J. History of the race structure of Orobanche cumana and the breeding of sunflower for resistance to this parasitic weed: A review. Span. J. Agric. Res. 2015, 13, e10R01. [Google Scholar] [CrossRef]
- Rubiales, D.; FernÁNdez-Aparicio, M.; Wegmann, K.; Joel, D.M. Revisiting strategies for reducing the seedbank of Orobanche and Phelipanche spp. Weed Res. 2009, 49, 23–33. [Google Scholar] [CrossRef]
- Akiyama, K.; Hayashi, H. Strigolactones: Chemical signals for fungal symbionts and parasitic weeds in plant roots. Ann. Bot. 2006, 97, 925–931. [Google Scholar] [CrossRef]
- Samejima, H.; Sugimoto, Y. Recent research progress in combatting root parasitic weeds. Biotechnol. Biotechnol. Equip. 2018, 32, 221–240. [Google Scholar] [CrossRef]
- Antonova, T.; Alonso, L.; Strel’nikov, E.; Araslanova, N. Stimulating effect of the root exudates of sorghum, millet, and Sudan grass on the seed germination of broomrape (Orobanche cumana Wallr.) infesting sunflowers in Russia. Russ. Agric. Sci. 2015, 41, 347–351. [Google Scholar] [CrossRef]
- Lins, R.D.; Colquhoun, J.B.; Mallory-Smith, C.A. Investigation of wheat as a trap crop for control of Orobanche minor. Weed Res. 2006, 46, 313–318. [Google Scholar] [CrossRef]
- Ye, X.; Chen, J.; McErlean, C.S.P.; Zhang, M.; Yu, R.; Ma, Y. The potential of foxtail millet as a trap crop for sunflower broomrape. Acta Physiol. Plant. 2016, 39, 1. [Google Scholar] [CrossRef]
- Fernandez-Aparicio, M.; Reboud, X.; Gibot-Leclerc, S. Broomrape weeds. underground mechanisms of parasitism and associated strategies for their control: A Review. Front. Plant Sci. 2016, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Al-thahabi, S.A. Small Broomrape (Orobanche minor) Management Using Wheat (Triticum aestivum) As a False Host. Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 2006. [Google Scholar]
- Ma, Y.Q.; Jia, J.N.; An, Y.; Wang, Z.; Mao, J.C. Potential of some hybrid maize lines to induce germination of sunflower broomrape. Crop Sci. 2013, 53, 260–270. [Google Scholar] [CrossRef]
- Sunderland, N. The production of the Striga and Orobanche germination stimulants by maize roots II. Conditions of synthesis in the root. J. Exp. Bot. 1960, 11, 356–366. [Google Scholar] [CrossRef]
- Zehhar, N.; Labrousse, P.; Arnaud, M.-C.; Boulet, C.; Bouya, D.; Fer, A. Study of resistance to Orobanche ramosa in host (oilseed rape and carrot) and non-host (maize) plants. Eur. J. Plant Pathol. 2003, 109, 75–82. [Google Scholar] [CrossRef]
- Awad, A.A.; Sato, D.; Kusumoto, D.; Kamioka, H.; Takeuchi, Y.; Yoneyama, K. Characterization of strigolactones, germination stimulants for the root parasitic plants Striga and Orobanche, produced by maize, millet and sorghum. Plant Growth Regul. 2006, 48, 221–227. [Google Scholar]
- Charnikhova, T.V.; Gaus, K.; Lumbroso, A.; Sanders, M.; Vincken, J.-P.; De Mesmaeker, A.; Ruyter-Spira, C.P.; Screpanti, C.; Bouwmeester, H.J. Zeapyranolactone—A novel strigolactone from maize. Phytochem. Lett. 2018, 24, 172–178. [Google Scholar] [CrossRef]
- Charnikhova, T.V.; Gaus, K.; Lumbroso, A.; Sanders, M.; Vincken, J.P.; De Mesmaeker, A.; Ruyter-Spira, C.P.; Screpanti, C.; Bouwmeester, H.J. Zealactones. Novel natural strigolactones from maize. Phytochemistry 2017, 137, 123–131. [Google Scholar] [CrossRef]
- Siame, B.A.; Weerasuriya, Y.; Wood, K.; Ejeta, G.; Butler, L.G. Isolation of strigol, a germination stimulant for Striga asiatica, from host plants. J. Agric. Food Chem. 1993, 41, 1486–1491. [Google Scholar] [CrossRef]
- Xing, N.; Zhang, Y.; Wang, L. The Study on Dryland Agriculture in North China, Chinese Version; Chinese Agriculture Press: Beijing, China, 2001. [Google Scholar]
- Parker, C.; Hitchcock, A.; Ramaiah, K. The germination of Striga species by crop root exudates; Techniques for selecting resistant crop cultivars. In Proceedings of the Asian-Pacific Weed Science Society 6th Conference, Djakarta, Indonesia, 11–17 July 1977. [Google Scholar]
- Bartlett, M. Some examples of statistical methods of research in agriculture and applied biology. Suppl. J. R. Stat. Soc. 1937, 4, 137–183. [Google Scholar] [CrossRef]
- Owen, M.D.K.; Beckie, H.J.; Leeson, J.Y.; Norsworthy, J.K.; Steckel, L.E. Integrated pest management and weed management in the United States and Canada. Pest Manag. Sci. 2015, 71, 357–376. [Google Scholar] [CrossRef]
- Kebreab, E.; Murdoch, A.J. Simulation of integrated control strategies for Orobanche spp. based on a life cycle model. Exp. Agric. 2001, 37, 37–51. [Google Scholar] [CrossRef]
- Abu-Shall, A.M.H.; Ragheb, E.I.M. Management of Orobanche crenata using trap crops and phytomyza orobanchia Kalt. in Broad Bean (Vicia faba) field in Egypt. Egypt. J. Biol. Pest Control 2014, 24, 217–223. [Google Scholar]
- Kleifeld, Y.; Goldwasser, Y.; Herzlinger, G.; Joel, D.; Golan, S.; Kahana, D. The effects of flax (Linum usitatissimum L.) and other crops as trap and catch crops for control of Egyptian broomrape (Orobanche aegyptiaca Pers.). Weed Res. 1994, 34, 37–44. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Yoneyama, K.; Rubiales, D. The role of strigolactones in host specificity of Orobanche and Phelipanche seed germination. Seed Sci. Res. 2011, 21, 55–61. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, Y.Q.; Wang, Z.; Ye, X.X.; Shui, J.F. Some soybean cultivars have ability to induce germination of sunflower broomrape. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Babaei, S.; Alizadeh, H.; Jahansouz, M.R.; Mashhadi, H.R.; Moeini, M.M. Management of Phelipanche aegyptiaca Pomel. using trap crops in rotation with tomato (Solanum lycopersicom L.). Aust. J. Crop Sci. 2010, 4, 437–442. [Google Scholar]
- Qasem, J. Parasitic weeds and allelopathy: From the hypothesis to the proofs. In Allelopathy: A Physiological Process with Ecological Implications; Reigosa, M., Pedrol, N., González, L., Eds.; Springer: Berlin, Germany, 2006; pp. 565–637. [Google Scholar]
- Kato-Noguchi, H.; Sakata, Y.; Takenokuchi, K.; Kosemura, S.; Yamamura, S. Allelopathy in Maize I.: Isolation and identification of allelochemicals in maize seedlings. Plant Prod. Sci. 2000, 3, 43–46. [Google Scholar] [CrossRef]
- Emechebe, A.M.; Ahonsi, M.O. Ability of excised root and stem pieces of maize, cowpea and soybean to cause germination of Striga hermonthica seeds. Crop Prot. 2003, 22, 347–353. [Google Scholar] [CrossRef]
- Fernandez-Aparicio, M.; Flores, F.; Rubiales, D. Recognition of root exudates by seeds of broomrape (Orobanche and Phelipanche) species. Ann. Bot. 2009, 103, 423–431. [Google Scholar] [CrossRef]
- Dong, S.; Ma, Y.; Wu, H.; Shui, J.; Hao, Z. Stimulatory effects of wheat (Triticum aestivum L.) on seed germination of Orobanche minor Sm. Allelopath. J. 2012, 30, 247–258. [Google Scholar]
- Berner, D.; Winslow, M.; Awad, A.; Cardwell, K.; Raj, D.M.; Kim, S. Striga Research Methods; Manual; The Pan-African Striga Control Network (PASCON) and the International Institute of Tropical Agriculture: Idban, Nigeria, 1997; pp. 13–20. [Google Scholar]
- Song, W.; Zhou, W.; Jin, Z.; Cao, D.; Joel, D.; Takeuchi, Y.; Yoneyama, K. Germination response of Orobanche seeds subjected to conditioning temperature, water potential and growth regulator treatments. Weed Res. 2005, 45, 467–476. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, M.; Dong, S.; Ma, Y. Conditioning duration and agents involved in broomrape seeds responding to germination stimulants. Plant Growth Regul. 2017, 81, 221–230. [Google Scholar] [CrossRef]
- Ye, X. Effectiveness of Broomrape Management Using Maize and Foxtail Millet As Trap Crops. Ph.D. Thesis, Northwest A&F Univ., Shaanxi, China, 2017. (In Chinese with English abstract). [Google Scholar]
- Fernandez-Aparicio, M.; Westwood, J.H.; Rubiales, D. Agronomic, breeding, and biotechnological approaches to parasitic plant management through manipulation of germination stimulant levels in agricultural soils. Botany 2011, 89, 813–826. [Google Scholar] [CrossRef]
- Abebe, G.; Sahile, G.; Al-Tawaha, A.-R.M. Evaluation of potential trap crops on Orobanche soil seed bank and tomato yield in the Central Rift Valley of Ethiopia. World J. Agric. Sci. 2005, 1, 148–151. [Google Scholar]
- Lins, R.D.; Colquhoun, J.B.; Mallory-Smith, C.A. Effect of small broomrape (Orobanche minor) on red clover growth and dry matter partitioning. Weed Sci. 2007, 55, 517–520. [Google Scholar] [CrossRef]
- Van Ast, A.; Bastiaans, L.; Kropff, M. A comparative study on Striga hermonthica interaction with a sensitive and a tolerant sorghum cultivar. Weed Res. 2000, 40, 479–493. [Google Scholar] [CrossRef]
- Taylor, A.; Martin, J.; Seel, W. Physiology of the parasitic association between maize and witchweed (Striga hermonthica): Is ABA involved? J. Exp. Bot. 1996, 47, 1057–1065. [Google Scholar] [CrossRef]
- Shen, H.; Ye, W.; Hong, L.; Cao, H.; Wang, Z. Influence of the obligate parasite Cuscuta campestris on growth and biomass allocation of its host Mikania micrantha. J. Exp. Bot. 2005, 56, 1277–1284. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Flores, F.; Rubiales, D. The effect of Orobanche crenata infection severity in faba bean, field pea, and grass pea productivity. Front. Plant Sci. 2016, 7, 1409. [Google Scholar] [CrossRef]
- Stewart, G.R.; Press, M.C. The physiology and biochemistry of parasitic angiosperms. Annu. Rev. Plant Biol. 1990, 41, 127–151. [Google Scholar] [CrossRef]
- Alcántara, E.; Morales-García, M.; Díaz-Sánchez, J. Effects of broomrape parasitism on sunflower plants: Growth, development, and mineral nutrition. J. Plant Nutr. 2006, 29, 1199–1206. [Google Scholar] [CrossRef]
- Pacureanu-Joita, M.; Raranciuc, S.; Sava, E.; Stanciu, D.; Nastase, D. Virulence and aggressiveness of sunflower broomrape (Orobanche cumana Wallr.) populations in Romania. Helia 2009, 32, 119–126. [Google Scholar]
- Ortiz-Bustos, C.M.; Pérez-Bueno, M.L.; Barón, M.; Molinero-Ruiz, L. Fluorescence imaging in the red and far-red region during growth of sunflower plantlets. Diagnosis of the early infection by the parasite Orobanche cumana. Front. Plant Sci. 2016, 7, 884. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Bustos, C.M.; Pérez-Bueno, M.L.; Barón, M.; Molinero-Ruiz, L. Use of blue-green fluorescence and thermal imaging in the early detection of sunflower infection by the root parasitic weed Orobanche cumana Wallr. Front. Plant Sci. 2017, 8, 833. [Google Scholar] [CrossRef]
| Year | Artificially Infested with Broomrape Seeds | Sowing Date | Harvest Date | |||||
|---|---|---|---|---|---|---|---|---|
| 2012 | No crop | No crop | No crop | Maize | Maize | Maize | 5 May | 14 September |
| 2013 | Sunflower | No crop | No crop | Sunflower | Maize | Maize | 24 April | 29 September |
| 2014 | Sunflower | No crop | Sunflower | Maize | 10 May | 26 September | ||
| 2015 | Sunflower | Sunflower | 1 May | 2 October | ||||
| Without Broomrape Seeds | ||||||||
| 2012 | No crop | No crop | No crop | 5 May | 14 September | |||
| 2013 | Sunflower | No crop | No crop | 24 April | 29 September | |||
| 2014 | Sunflower | No crop | 10 May | 26 September | ||||
| 2015 | Sunflower | 1 May | 2 October | |||||
| Source | df | F-Value | df | F-Value | ||||
|---|---|---|---|---|---|---|---|---|
| No. of Emerged Sunflower Broomrape | No. of Attached Sunflower Broomrape | Seedling Height | Shoot Dry Weight | Root Dry Weight | Sunflower Head Dry Weight | |||
| Previous crop | 4 | 49.52 ** | 13.11 ** | 5 | 1.35 | 21.18 ** | 2.77 * | 10.46 ** |
| Planting years | 2 | 0.37 | 6.76 ** | 2 | 4.19 * | 2.95 | 1.73 | 5.07 ** |
| Interaction effect | 8 | 7.79 ** | 4.01 ** | 10 | 1.04 | 0.66 | 0.59 | 1.58 |
| Maize Cultivars | Seedling Height (cm) | Shoot Dry Weight (g plant−1) | Root Dry Weight (g plant−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2013 | 2014 | 2015 | 2013 | 2014 | 2015 | |
| Q67 | 80.2 ± 4.9 a | 78.6 ± 1.0 a | 104.8 ± 12.1 ab | 21.2 ± 3.1 a | 20.6 ± 1.6 ab | 19.5 ± 0.9 b | 2.8 ± 0.4 a | 3.0 ± 0.4 a | 3.5 ± 0.2 a |
| Z6 | 78.8 ± 2.3 a | 76.9 ± 5.2 a | 98.2 ± 11.3 ab | 20.4 ± 5.0 a | 20.1 ± 2.6 ab | 20.3 ± 1.2 b | 2.7 ± 0.3 a | 2.3 ± 0.4 a | 2.9 ± 0.1 ab |
| N314 | 86.7 ± 2.4 a | 78.9 ± 5.3 a | 112.8 ± 28.3 ab | 24.0 ± 3.1 a | 21.4 ± 1.3 ab | 28.7 ± 1.2 a | 3.0 ± 0.6 a | 2.8 ± 0.4 a | 3.6 ± 0.2 a |
| H19 | 75.2 ± 7.6 a | 64.0 ± 4.2 a | 128.8 ± 47.3 a | 21.6 ± 1.2 a | 17.4 ± 2.1 b | 27.3 ± 0.5 a | 2.9 ± 0.3 a | 2.6 ± 0.3 a | 3.0 ± 0.5 ab |
| NC | 77.4 ± 11.5 a | 61.8 ± 6.4 a | 56.6 ± 5.5 b | 5.3 ± 0.6 b | 5.1 ± 0.2 c | 9.9 ± 0.5 c | 2.4 ± 0.2 a | 2.5 ± 0.1 a | 2.0 ± 0.2 b |
| PC | 81.2 ± 5.8 a | 81.6 ± 7.9 a | 81.6 ± 6.1 ab | 29.3 ± 6.5 a | 31.0 ± 2.7 a | 29.5 ± 1.6 a | 2.9 ± 0.4 a | 3.0 ± 0.3 a | 3.4 ± 0.4 ab |
| Pearson Correlation | Seedling Height | Shoot Dry Mass | Root Dry Mass | Head Dry Mass | No. of Emergent Broomrape |
|---|---|---|---|---|---|
| Shoot dry mass | 0.197 | ||||
| Root dry mass | 0.293 ** | 0.215 * | |||
| Head dry mass | 0.232 * | 0.795 ** | 0.219 * | ||
| No. of Emergent broomrape | −0.271 ** | −0.616 ** | −0.374 ** | −0.620 ** | |
| No. of Attached broomrape | −0.266 * | −0.638 ** | −0.377 ** | −0.633 ** | 0.990 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, X.; Zhang, M.; Zhang, M.; Ma, Y. Assessing the Performance of Maize (Zea mays L.) as Trap Crops for the Management of Sunflower Broomrape (Orobanche cumana Wallr.). Agronomy 2020, 10, 100. https://doi.org/10.3390/agronomy10010100
Ye X, Zhang M, Zhang M, Ma Y. Assessing the Performance of Maize (Zea mays L.) as Trap Crops for the Management of Sunflower Broomrape (Orobanche cumana Wallr.). Agronomy. 2020; 10(1):100. https://doi.org/10.3390/agronomy10010100
Chicago/Turabian StyleYe, Xiaoxin, Meng Zhang, Manyun Zhang, and Yongqing Ma. 2020. "Assessing the Performance of Maize (Zea mays L.) as Trap Crops for the Management of Sunflower Broomrape (Orobanche cumana Wallr.)" Agronomy 10, no. 1: 100. https://doi.org/10.3390/agronomy10010100
APA StyleYe, X., Zhang, M., Zhang, M., & Ma, Y. (2020). Assessing the Performance of Maize (Zea mays L.) as Trap Crops for the Management of Sunflower Broomrape (Orobanche cumana Wallr.). Agronomy, 10(1), 100. https://doi.org/10.3390/agronomy10010100





