SelectedAspects of Iodate and Iodosalicylate Metabolism in Lettuce Including the Activity of Vanadium Dependent Haloperoxidases as Affected by Exogenous Vanadium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatments
2.2. Activity of Vanadium-Dependent Haloperoxidases (vHPO)
2.3. Analysis of Dry Samples of Roots and Leaves
2.4. Statistical Analysis
3. Results
3.1. Plant Biomass
3.2. Iodine Uptake and Accumulation by Lettuce Plants
3.3. Vanadium Uptake and Accumulation by Lettuce.
3.4. Effect of Iodine and Vanadium on vHPO Activity in Lettuce Plants
3.5. The Content of BeA, SA, Iodosalicylates, Iodobenzoates, and T3 in Lettuce Plants
4. Discussion
4.1. Biomass Production and Vanadium Accumulation in Lettuce Plants
4.2. Iodine Accumulation vs. Vanadium Application and vHPO Activity
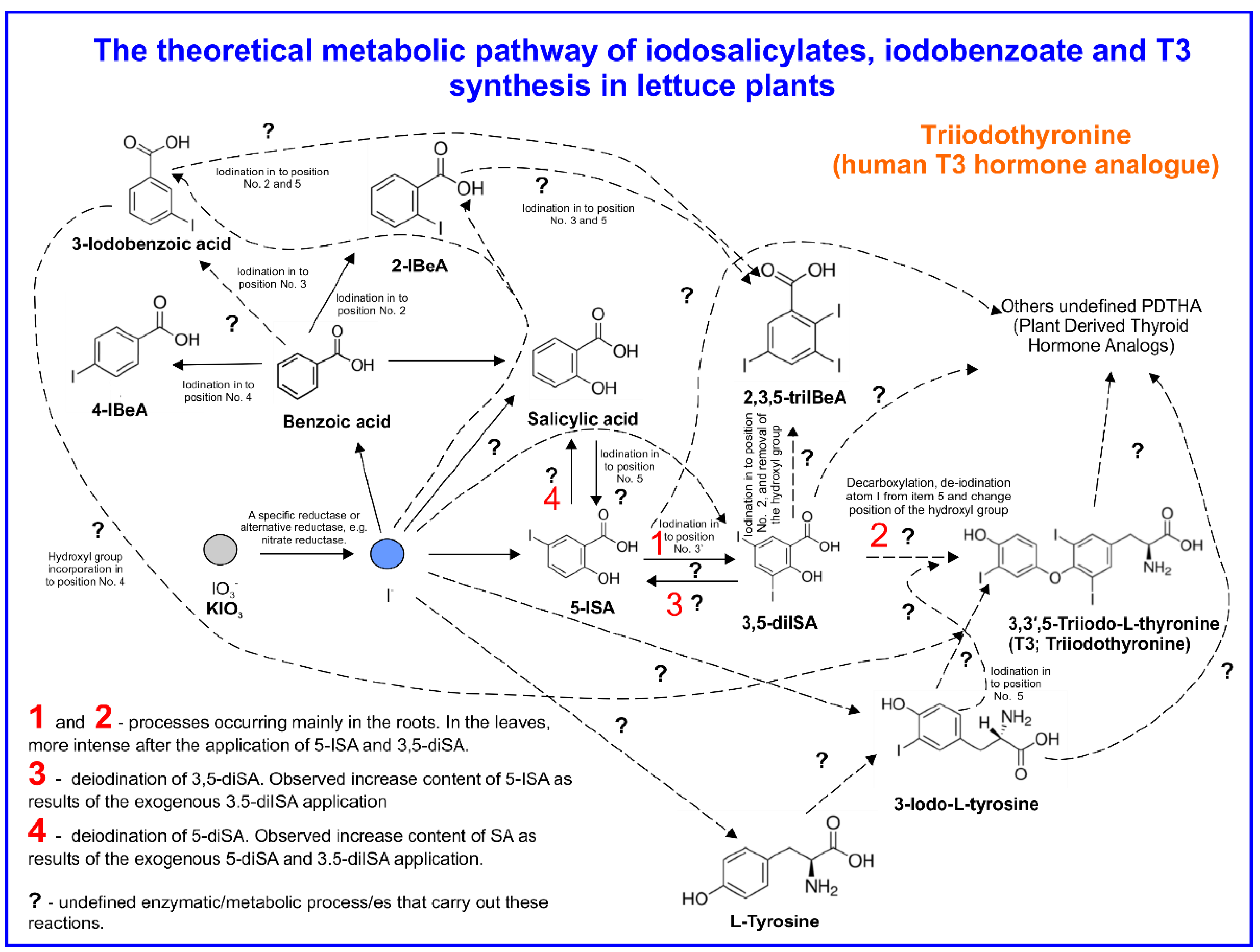
4.3. SA and Iodosalicylate Metabolism
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anke, M.; Illing-Günther, H.; Gürtler, H.; Holzinger, S.; Jaritz, M.; Anke, S.; Schäfer, U. Vanadium—An essential element for animals and humans. In Trace Elements in Man and Animals 10; Roussel, A.M., Anderson, R.A., Favrier, A.E., Eds.; Springer: Boston, MA, USA, 2002; pp. 221–225. [Google Scholar] [CrossRef]
- Mukherjee, B.; Patra, B.; Mahapatra, S.; Banerjee, P.; Tiwari, A.; Chatterjee, M. Vanadium—An element of atypical biological significance. Toxicol. Lett. 2004, 150, 135–143. [Google Scholar] [CrossRef]
- Gruzewska, K.; Michno, A.; Pawelczyk, T.; Bielarczyk, H. Essentiality and toxicity of vanadium supplements in health and pathology. J. Physiol. Pharmacol. 2014, 65, 603–611. [Google Scholar]
- Medrano-Macías, J.; Leija-Martínez, P.; González-Morales, S.; Juárez-Maldonado, A.; Benavides-Mendoza, A. Use of iodine to biofortify and promote growth and stress tolerance in crops. Front. Plant Sci. 2016, 7, 1146. [Google Scholar] [CrossRef] [Green Version]
- Antonyak, H.L.; Panas, N.E.; Pershyn, O.I.; Polishchuk, A.I.; Hoyvanovych, N.K. Iodine in abiotic and biotic environments. Studia Biol. 2018, 12, 117–134. [Google Scholar] [CrossRef]
- WHO. Vanadium. In Trace Elements in Human Nutrition and Health; World Health Organization: Geneva, Switzerland, 1996; pp. 180–184. [Google Scholar]
- WHO. Vanadium Pentoxide and Other Inorganic Vanadium Compounds; World Health Organization: Geneva, Switzerland, 2001; pp. 1–54. [Google Scholar]
- Afkhami, A.M.; Karimi, M.; Nourian, F.; Soheylikhah, S. Comparison of the effects of sodium metavanadate and zinc sulfate supplementation on lipid and glucose in patients with type 2 diabetes. Iran. J. Diabetes Obes. 2009, 1, 22–29. [Google Scholar]
- Harland, B.F.; Harden-Williams, B.A. Is vanadium of human nutritional importance yet? J. Acad. Nutr. Diet. 1994, 94, 891–894. [Google Scholar] [CrossRef]
- Bressman, R.B.; Miles, R.D.; Comer, C.W.; Wilson, H.R.; Butcher, G.D. Effect of dietary supplementation of vanadium commercial egg-type laying hens. J. Appl. Poultry Res. 2002, 11, 46–53. [Google Scholar] [CrossRef]
- Roberts, G.K.; Stout, M.D.; Sayers, B.; Fallacara, D.M.; Hejtmancik, M.R.; Waidyanatha, S.; Hooth, M.J. 14-day toxicity studies of tetravalent and pentavalent vanadium compounds in Harlan Sprague Dawley rats and B6C3F1/N mice via drinking water exposure. Toxicol. Rep. 2016, 3, 531–538. [Google Scholar] [CrossRef] [Green Version]
- Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary reference intakes: Vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Acad. Nutr. Diet. 2001, 101, 294–301. [Google Scholar]
- Pennington, J.A.T.; Jones, J.W. Molybdenum, nickel, cobalt, vanadium, and strontium in total diets. J. Am. Diet. Assoc. 1987, 87, 1644–1650. [Google Scholar] [PubMed]
- Andersson, M.; de Benoist, B.; Darnton-Hill, I.; Delange, F. Iodine Deficiency in Europe: A Continuing Public Health Problem; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Charlton, K.E.; Phil, M.; Gemming, L.; Yeatman, H.; Ma, G. Suboptimal iodine status of Australian pregnant women reflects poor knowledge and practices related to iodine nutrition. Nutrition 2010, 26, 963–968. [Google Scholar] [CrossRef]
- Melse-Boonstra, A.; Jaiswal, N. Iodine deficiency in pregnancy, infancy and childhood and its consequences for brain development. Best Pract. Res. 2010, 24, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Welch, R.M.; Huffman, E.W. Vanadium and plant nutrition: The growth of lettuce (Lactuca sativa L.) and tomato (Lycopersicon esculentum Mill.) plants in nutrient solutions low in vanadium. Plant Physiol. 1973, 52, 183–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilbeam, D.J.; Drihem, K. Vanadium. In Handbook of Plant Nutrition, 1st ed.; Barker, A.V., Pilbeam, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 585–596. [Google Scholar]
- Kaplan, D.I.; Adriano, D.C.; Carlson, C.L.; Sajwan, K.S. Vanadium—toxicity and accumulation by beans. Water Air Soil Pollut. 1990, 49, 81–91. [Google Scholar] [CrossRef]
- Sentíes-Herrera, H.E.; Trejo-Téllez, L.I.; Volke-Haller, V.H.; Cadena-Íñiguez, J.; Sánchez-García, P.; Gómez-Merino, F.C. Iodine, silicon, and vanadium differentially affect growth, flowering, and quality components of stalks in sugarcane. Sugar Tech 2018, 20, 518–533. [Google Scholar] [CrossRef]
- Basiouny, F.M. Distribution of vanadium and its influence on chlorophyll formation and iron metabolism in tomato plants. J. Plant Nutr. 1984, 7, 1059–1073. [Google Scholar] [CrossRef]
- Blasco, B.; Rios, J.J.; Cervilla, L.M.; Sánchez-Rodrigez, E.; Ruiz, J.M.; Romero, L. Iodine biofortification and antioxidant capacity of lettuce: Potential benefits for cultivation and human health. Ann. Appl. Biol. 2008, 152, 289–299. [Google Scholar] [CrossRef]
- Umaly, R.C.; Poel, L.W. Effects of iodine in various formulations on the growth of barley and pea plants in nutrient solution culture. Ann. Bot. 1971, 35, 127–131. [Google Scholar] [CrossRef]
- Weng, H.X.; Yan, A.L.; Hong, C.L.; Xie, L.L.; Qin, Y.C.; Cheng, C.Q. Uptake of different species of iodine by water spinach and its effect to growth. Biol. Trace Elem. Res. 2008, 124, 184–194. [Google Scholar] [CrossRef]
- Smoleń, S.; Ledwożyw-Smoleń, I.; Halka, M.; Sady, W.; Kováčik, P. The absorption of iodine from 5-iodosalicylic acid by hydroponically grown lettuce. Sci. Hortic. 2017, 225, 716–725. [Google Scholar] [CrossRef]
- Halka, M.; Klimek-Chodacka, M.; Smoleń, S.; Barański, R.; Ledwożyw-Smoleń, I.; Sady, W. Organic iodine supply affects tomato plants differently than inorganic iodine. Physiol. Plant. 2018, 164, 290–306. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, C.; Colin, C.; Cosse, A.; Delage, L.; La Barre, S.; Morin, P.; Fiévet, B.; Voiseux, C.; Ambroise, Y.; Verhaeghe, E.; et al. Iodine transfers in the coastal marine environment: The key role of brown algae and of their vanadium-dependent haloperoxidases. Biochemie 2006, 88, 1773–1785. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.G.; Humanes, M.; Melo, R.; Silva, J.A.; Fraústo da Silva, J.J.R.; Wever, R. Purification and characterisation of vanadium haloperoxidases from the brown alga Pelvetia canaliculata. Phytochemistry 2000, 54, 5–11. [Google Scholar] [CrossRef]
- Kongkiattikajorn, J.; Pongdam, S. Vanadium haloperoxidase from the Red Alga Gracilaria fisheri. Sci. Asia 2006, 32, 25–30. [Google Scholar] [CrossRef]
- Colin, C.; Leblanc, C.; Wagner, E.; Delage, L.; Leize-Wagner, E.; Van Dorsselaer, A.; Potin, P. The brown algal kelp Laminaria digitata features distinct bromoperoxidase and iodoperoxidase activities. J. Biol. Chem. 2003, 278, 23545–23552. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.B., Jr. Hydroponics: A Practical Guide for the Soilless Grower, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 1–440. [Google Scholar]
- Stępowska, A.; Rogowska, M. Lettuce Cultivation in the Field and Under the Cover; Plant Press: Kraków, Poland, 2004; pp. 1–144. [Google Scholar]
- Verhaeghe, E.; Buisson, D.; Zekri, E.; Leblanc, C.; Potin, P.; Ambroise, Y. A colorimetric assay for steady-state analyses of iodo-and bromoperoxidase activities. Anal. Biochem. 2008, 379, 60–65. [Google Scholar] [CrossRef]
- Fournier, J.B.; Rebuffet, E.; Delage, L.; Grijol, R.; Meslet-Cladière, L.; Rzonca, J.; Leblanc, C. The vanadium iodoperoxidase from the marine Flavobacteriaceae species Zobellia galactanivorans reveals novel molecular and evolutionary features of halide specificity in the vanadium haloperoxidase enzyme family. Appl. Environ. Microbiol. 2014, 80, 7561–7573. [Google Scholar] [CrossRef] [Green Version]
- Waterborg, J.H. The Lowry method for protein quantitation. In The Protein Protocols Handbook, 3rd ed.; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2002; pp. 7–10. [Google Scholar]
- Smoleń, S.; Kowalska, I.; Kováčik, P.; Halka, M.; Sady, W. Biofortification of six varieties of lettuce (Lactuca sativa L.) with iodine and selenium in combination with the application of salicylic acid. Front. Plant Sci. 2019, 10, 143. [Google Scholar] [CrossRef] [Green Version]
- PN-EN 15111—008. Foodstuffs—Determination of Trace Elements—Determination of Iodine by ICP-MS (Inductively Coupled Plasma Mass Spectrometry); Polish Committee of Standardization: Warsaw, Poland, 2007. (In Polish)
- Pasławski, P.; Migaszewski, Z.M. The quality of element determinations in plant materials by instrumental methods. Pol. J. Environ. Stud. 2006, 15, 154–164. [Google Scholar]
- Halka, M.; Smoleń, S.; Czernicka, M.; Klimek-Chodacka, M.; Pitala, J.; Tutaj, K. Iodine biofortification through expression of HMT, SAMT and S3H genes in Solanum lycopersicum L. Plant Physiol. Biochem. 2019, 144, 35–48. [Google Scholar] [CrossRef]
- Vachirapatama, N.; Jirakiattiku, Y.; Dicinoski, G.W.; Townsend, A.T.; Haddad, P.R. Effect of vanadium on plant growth and its accumulation in plant tissues. Songklanakarin J. Sci. Technol. 2011, 33, 255–261. [Google Scholar]
- Akoumianaki-Ioannidou, A.; Barouchas, P.E.; Ilia, E.; Kyramariou, A.; Moustakas, N.K. Effect of vanadium on dry matter and nutrient concentration in sweet basil (Ocimum basilicum L.). Aust. J. Crop Sci. 2016, 10, 199–206. [Google Scholar]
- Chongkid, B.; Vachirapattama, N.; Jirakiattikul, Y. Effects of vanadium on rice growth and vanadium accumulation in rice tissues kasetsart. J. Natl. Agric. 2007, 41, 28–33. [Google Scholar]
- Jásik, J.; Lux, A.; Hudák, J.; Mikuš, M. Plastid degeneration induced by vanadium. Biol. Plant. 1987, 29, 73–75. [Google Scholar] [CrossRef]
- Gil, J.; Álvarez, C.E.; Martinez, M.C.; Pérez, N. Effect of vanadium on lettuce growth, cationic nutrition, and yield. J. Environ. Sci. Health Part A 1995, 30, 73–87. [Google Scholar] [CrossRef] [Green Version]
- Nagatoshi, Y.; Nakamura, T. Characterization of three halide methyltransferases in Arabidopsis thaliana. Plant Biotechnol. 2007, 24, 503–506. [Google Scholar] [CrossRef] [Green Version]
- Redeker, K.R.; Wang, N.; Low, J.C.; McMillan, A.; Tyler, S.C.; Cicerone, R.J. Emissions of methyl halides and methane from rice paddies. Science 2000, 290, 966–969. [Google Scholar] [CrossRef] [Green Version]
- Gonzali, S.; Kiferle, C.; Perata, P. Iodine biofortification of crops: Agronomic biofortification, metabolic engineering and iodine bioavailability. Curr. Opin. Biotechnol. 2017, 44, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Wever, R.; Hemrika, W. Vanadium Haloperoxidases. In Handbook of Metalloproteins; John Wiley Sons, Ltd.: Chichester, UK, 2006. [Google Scholar] [CrossRef]
- Colin, C.; Leblanc, C.; Michel, G.; Wagner, E.; Leize-Wagner, E.; Van Dorsselaer, A.; Potin, P. Vanadium-dependent iodoperoxidases in Laminaria digitata, a novel biochemical function diverging from brown algal bromoperoxidases. JBIC J. Biol. Inorg. Chem. 2005, 10, 156–166. [Google Scholar] [CrossRef]
- Kato, S.; Wachi, T.; Yoshihira, K.; Nakagawa, T.; Ishikawa, A.; Takagi, D.; Tezuka, A.; Yoshida, H.; Yoshida, S.; Sekimoto, H.; et al. Rice (Oryza sativa L.) roots have iodate reduction activity in response to iodine. Front. Plant Sci. 2013, 4, 227. [Google Scholar] [CrossRef] [Green Version]
- Gust, A.A.; Nürnberger, T. Plant immunology: A life or death switch. Nature 2012, 486, 198–199. [Google Scholar] [CrossRef] [PubMed]
- Saniewski, M.; Góraj, J.; Węgrzynowicz-Lesiak, E.; Miyamoto, K.; Ueda, J. Differential of auxin polar transport inhibitors on rooting in some Crassulaceae species. Acta Agrobot. 2014, 67, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Van de Wouwer, D.; Vanholme, R.; Decou, R.; Goeminne, G.; Audenaert, D.; Nguyen, L.; Höfer, R.; Pesquet, E.; Vanholme, B.; Boerjan, W. Chemical genetics uncovers novel inhibitors of lignification, including p-oodobenzoic acid targeting cinnamate-4-hydroxylase. Plant Physiol. 2016, 172, 198–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crisan, M. Iodo substitutient position effect on Cucumber germination seeds of benzoic acid’s monoethanolamine salts. Ann. West Univ. Timis. Chem. 2008, 17, 23–26. [Google Scholar]
- Lima, S.T.C.; Merrigan, T.L.; Rodrigues, E.D. Synthetic and plant derived thyroid hormone analogs. In Thyroid and Parathyroid Diseases—New Insights into Some Old and Some New Issues; Ward, L.S., Ed.; In Tech: Rijeka, Croatia, 2012; pp. 221–237. [Google Scholar] [CrossRef] [Green Version]
- Fowden, L. Radioactive iodine incorporation into organic compounds of various angiosperms. Physiol. Plant. 1959, 12, 657–664. [Google Scholar] [CrossRef]
- Fenical, W. Halogenation in Rhodophyta 1, 2. A review. J. Phycol. 1975, 11, 245–259. [Google Scholar] [CrossRef]

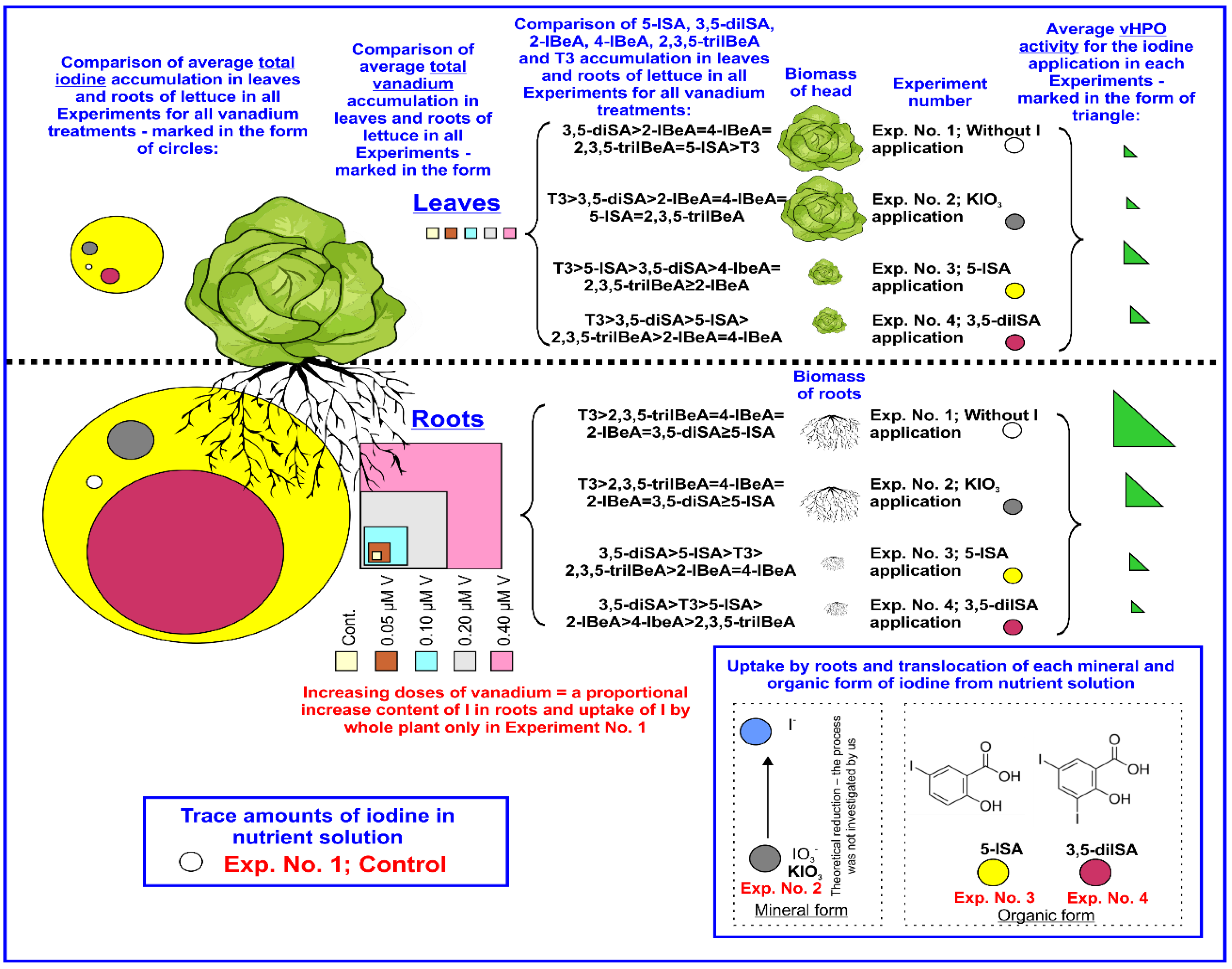
| Experimental Factor | Experiment No. 1 | Experiment No. 2 | Experiment No. 3 | Experiment No. 4 |
|---|---|---|---|---|
| Dose of vanadium as ammonium metavanadate (µM V nutrient solution). | 0 (control *) | 0 (control *) | 0 (control *) | 0 (control *) |
| 0.05 | 0.05 | 0.05 | 0.05 | |
| 0.1 | 0.1 | 0.1 | 0.1 | |
| 0.2 | 0.2 | 0.2 | 0.2 | |
| 0.4 | 0.4 | 0.4 | 0.4 | |
| Concentration and chemical form of iodine in nutrient solution (the same for each treatments in separateexperiment) ** | Control * (0.0204 µM I) | KIO3 | 5-ISA | 3,5-diISA |
| (potassium iodate) | (5-iodosalicylic acid) | (3,5-diiodo-salicylic acid) | ||
| 10 µM | 10 µM | 10 µM | ||
| (10 µM I) | (10 µM I) | (20 µM I) | ||
| Growing season | Autumn | Autumn | Autumn-winter | Autumn-winter |
| Seed sowing | 28 August 2018 | 28 August 2018 | 22 October 2018 | 22 October 2018 |
| Planting seedlings to NFT gutter | 20 September 2018 | 20 September 2018 | 16 November 2018 | 16 November 2018 |
| Iodine and vanadium application in rosette stage of plants. | 05 October 2018 | 05 October 2018 | 07 December 2018 | 07 December 2018 |
| Harvest of the lettuce heads (number of days after sowing/after planting seedlings to NFT gutter) | 05 November 2018 (69/46 days) | 05 November 2018 (69/46 days) | 08 January 2019 (78/53 days) | 08 January 2019 (78/53 days) |
| Parameters | ||||
| Temperature for heating strategy (day/night) | 16 °C/10 °C | 16 °C/10 °C | 16 °C/10 °C | 16 °C/10 °C |
| Temperature for ventilation strategy (day/night) | 22 °C/15 °C | 22 °C/15 °C | 22 °C/15 °C | 22 °C/15 °C |
| Hours of natural light supplementation with 600-W high-pressure sodium lamps | 5.00–8.00 and 16.00–19.00 | 5.00–8.00 and 16.00–19.00 | 5.00–8.00 and 16.00–19.00 | 5.00–8.00 and 16.00–19.00 |
| Exp. No. | Treatments | Leaf Biomass (Lettuce Head, g) | Root Biomass from One Plant (g) | Whole Plant Biomass (Roots + Leaves, g) |
|---|---|---|---|---|
| 1 | Control | 238.5 ± 6.06a | 22.6 ± 0.72a | 261.1 ± 6.60a |
| 0.05 µM V | 253.1 ± 12.78a | 25.1 ± 1.60a | 278.3 ± 13.94a | |
| 0.10 µM V | 267.6 ± 5.21a | 27.1 ± 1.14a | 294.8 ± 4.62a | |
| 0.20 µM V | 269.7 ± 3.51a | 27.4 ± 1.05a | 297.0 ± 3.04a | |
| 0.40 µM V | 247.4 ± 18.76a | 24.4 ± 1.59a | 271.8 ± 19.68a | |
| 2 | Cont. + KIO3 | 254.4 ± 3.37a | 23.8 ± 0.63a | 278.1 ± 3.43a |
| 0.05 µM V + KIO3 | 233.8 ± 3.74a | 23.6 ± 0.86a | 257.3 ± 3.71a | |
| 0.10 µM V + KIO3 | 246.7 ± 14.74a | 25.5 ± 1.26a | 272.2 ± 15.03a | |
| 0.20 µM V + KIO3 | 252.5 ± 8.37a | 24.8 ± 1.58a | 277.3 ± 9.59a | |
| 0.40 µM V + KIO3 | 245.6 ± 13.26a | 21.4 ± 1.79a | 267.0 ± 14.94a | |
| 3 | Cont. + 5-ISA | 29.50 ± 1.14a | 4.50 ± 0.46a | 34.0 ± 1.58a |
| 0.05 µM V + 5-ISA | 29.25 ± 1.90a | 4.88 ± 0.47a | 34.1 ± 2.37a | |
| 0.10 µM V + 5-ISA | 28.50 ± 1.85a | 4.38 ± 0.55a | 32.9 ± 2.11a | |
| 0.20 µM V + 5-ISA | 27.33 ± 1.85a | 4.25 ± 0.60a | 31.6 ± 2.16a | |
| 0.40 µM V + 5-ISA | 32.38 ± 0.80a | 4.13 ± 0.24a | 36.5 ± 0.79a | |
| 4 | Cont. + 3,5-diISA | 40.94 ± 3.36ab | 3.59 ± 0.30ab | 44.5 ± 3.64ab |
| 0.05 µM V + 3,5-diISA | 39.38 ± 1.14ab | 4.69 ± 0.18bc | 44.1 ± 1.16ab | |
| 0.10 µM V + 3,5-diISA | 43.13 ± 4.29ab | 5.63 ± 0.26c | 48.8 ± 4.51b | |
| 0.20 µM V + 3,5-diISA | 49.22 ± 2.98b | 5.16 ± 0.47c | 54.4 ± 3.20b | |
| 0.40 µM V + 3,5-diISA | 29.84 ± 2.82a | 3.13 ± 0.26a | 33.0 ± 3.05a |
| Exp. No. | Treatments | Content of Iodine (mg I∙kg−1 D.W.) | |
|---|---|---|---|
| in Leaves | in Roots | ||
| 1 | Control | 0.21 ± 0.006a | 2.91 ± 0.440a |
| 0.05 µM V | 0.51 ± 0.009d | 10.46 ± 0.149b | |
| 0.10 µM V | 0.55 ± 0.005e | 12.89 ± 0.252c | |
| 0.20 µM V | 0.29 ± 0.003b | 15.21 ± 0.156d | |
| 0.40 µM V | 0.32 ± 0.002c | 17.43 ± 0.345e | |
| 2 | Cont. + KIO3 | 10.56 ± 0.184d | 33.51 ± 0.565a |
| 0.05 µM V + KIO3 | 8.01 ± 0.044b | 45.77 ± 1.256c | |
| 0.10 µM V + KIO3 | 9.14 ± 0.140c | 52.88 ± 1.117d | |
| 0.20 µM V + KIO3 | 8.65 ± 0.285bc | 35.41 ± 0.163b | |
| 0.40 µM V + KIO3 | 6.99 ± 0.146a | 46.80 ± 1.019c | |
| 3 | Cont. + 5-ISA | 286.77 ± 3.462b | 1 111.30 ± 20.512a |
| 0.05 µM V + 5-ISA | 243.50 ± 0.583a | 1 029.87 ± 4.949b | |
| 0.10 µM V + 5-ISA | 247.68 ± 7.197a | 941.42 ± 11.650a | |
| 0.20 µM V + 5-ISA | 233.31 ± 1.982a | 919.30 ± 18.200a | |
| 0.40 µM V + 5-ISA | 238.82 ± 1.480a | 943.14 ± 7.584a | |
| 4 | Cont. + 3,5-diISA | 9.68 ± 0.084b | 646.79 ± 54.627bc |
| 0.05 µM V + 3,5-diISA | 8.82 ± 0.036a | 678.97 ± 34.856bc | |
| 0.10 µM V + 3,5-diISA | 12.11 ± 0.127d | 764.66 ± 18.047c | |
| 0.20 µM V + 3,5-diISA | 9.50 ± 0.329ab | 603.05 ± 19.232ab | |
| 0.40 µM V + 3,5-diISA | 11.23 ± 0.135c | 466.95 ± 36.763a | |
| Exp. No. | Treatments | Iodine Uptake | Vanadium Uptake | ||||
|---|---|---|---|---|---|---|---|
| by Single Head (μg I∙head−1) | by Roots (μg I roots∙plant −1) | by Whole Plant (Head + Roots) (μg I plant −1) | by Single Head (μg V∙head−1) | by Roots (μg V roots∙plant −1) | by Whole Plant (Head + Roots) (μg V plant −1) | ||
| 1 | Control | 1.85 ± 0.05a | 1.92 ± 0.1a | 3.76 ± 0.1a | 8.54 ± 0.2a | 1.95 ± 0.1a | 10.49 ± 0.2a |
| 0.05 µM V | 4.99 ± 0.09c | 8.28 ± 0.1b | 13.27 ± 0.1b | 9.44 ± 0.1bc | 4.26 ± 0.1b | 13.70 ± 0.1b | |
| 0.10 µM V | 5.60 ± 0.04d | 12.61 ± 0.2c | 18.20 ± 0.2d | 10.09 ± 0.2c | 9.34 ± 0.1c | 19.43 ± 0.1c | |
| 0.20 µM V | 3.03 ± 0.03b | 13.95 ± 0.1d | 16.98 ± 0.1c | 9.93 ± 0.2c | 10.98 ± 0.7d | 20.91 ± 0.6c | |
| 0.40 µM V | 3.15 ± 0.02b | 14.37 ± 0.3d | 17.52 ± 0.2cd | 8.76 ± 0.2ab | 19.10 ± 0.1e | 27.86 ± 0.1d | |
| 2 | Cont. + KIO3 | 106.0 ± 1.8c | 24.5 ± 0.4a | 130.5 ± 2.1d | 9.51 ± 0.1bc | 1.19 ± 0. 1a | 10.7 ± 0.1a |
| 0.05 µM V + KIO3 | 75.4 ± 0.4a | 33.7 ± 0.9b | 109.2 ± 0.8ab | 8.43 ± 0.1a | 3.86 ± 0.1b | 12.3 ± 0.1a | |
| 0.10 µM V + KIO3 | 84.5 ± 1.3b | 36.9 ± 0.8c | 121.4 ± 1.9c | 8.57 ± 0.2a | 6.71 ± 0.1c | 15.3 ± 0.3b | |
| 0.20 µM V + KIO3 | 85.5 ± 2.8b | 31.3 ± 0.1b | 116.8 ± 2.7bc | 8.81 ± 0.1ab | 10.36 ± 0.1d | 19.2 ± 0.2c | |
| 0.40 µM V + KIO3 | 70.0 ± 1.5a | 31.8 ± 0.7b | 101.7 ± 1.3a | 9.59 ± 0.1c | 16.14 ± 1.2e | 25.7 ± 1.2d | |
| 3 | Cont. + 5-ISA | 502.8 ± 6.0b | 218.0 ± 4.0b | 720.9 ± 8.8ab | 2.73 ± 0.1a | 0.11 ± 0.1a | 2.83 ± 0.1a |
| 0.05 µM V + 5-ISA | 469.4 ± 1.1a | 256.3 ± 1.2c | 725.7 ± 2.2b | 3.03 ± 0.1ab | 0.48 ± 0.1b | 3.51 ± 0.1a | |
| 0.10 µM V + 5-ISA | 511.6 ± 14.8b | 221.7 ± 2.7b | 733.4 ± 14.5b | 3.58 ± 0.1b | 1.20 ± 0.1c | 4.78 ± 0.1b | |
| 0.20 µM V + 5-ISA | 457.8 ± 3.8a | 229.0 ± 4.5b | 686.8 ± 2.5a | 3.44 ± 0.1b | 4.03 ± 0.1d | 7.47 ± 0.1c | |
| 0.40 µM V + 5-ISA | 531.8 ± 3.3b | 171.5 ± 1.5a | 703.3 ± 2.7a | 3.67 ± 0.1b | 6.19 ± 0.1e | 9.86 ± 0.3d | |
| 4 | Cont. + 3,5-diISA | 28.1 ± 0.2b | 164.8 ± 13.9b | 192.8 ± 13.8b | 4.68 ± 0.1bc | 0.33 ± 0.1a | 5.01 ± 0.1a |
| 0.05 µM V + 3,5-diISA | 23.9 ± 0.1a | 226.2 ± 11.6c | 250.1 ± 11.7c | 4.13 ± 0.2b | 1.63 ± 0.1b | 5.76 ± 0.2a | |
| 0.10 µM V + 3,5-diISA | 37.1 ± 0.4d | 322.9 ± 7.6d | 360.3 ± 8.0d | 4.83 ± 0.2c | 3.17 ± 0.1c | 8.00 ± 0.2b | |
| 0.20 µM V + 3,5-diISA | 31.7 ± 1.1c | 223.6 ± 7.1c | 256.8 ± 6.9c | 6.06 ± 0.1d | 6.76 ± 0.1d | 12.82 ± 0.1c | |
| 0.40 µM V + 3,5-diISA | 22.0 ± 0.2a | 107.4 ± 8.5a | 127.4 ± 8.3a | 3.40 ± 0.1a | 8.63 ± 0.1e | 12.03 ± 0.2c | |
| Exp. No. | Treatments | Content of Vanadium (mg V∙kg−1 D.W.) | |
|---|---|---|---|
| in Leaves | in Roots | ||
| 1 | Control | 0.95 ± 0.024ab | 2.96 ± 0.027a |
| 0.05 µM V | 0.96 ± 0.011ab | 5.38 ± 0.035b | |
| 0.10 µM V | 0.99 ± 0.014b | 9.55 ± 0.146c | |
| 0.20 µM V | 0.95 ± 0.018ab | 11.98 ± 0.802d | |
| 0.40 µM V | 0.90 ± 0.021a | 23.18 ± 0.111e | |
| 2 | Cont. + KIO3 | 0.95 ± 0.015a | 1.64 ± 0.014a |
| 0.05 µM V + KIO3 | 0.89 ± 0.019a | 5.24 ± 0.115b | |
| 0.10 µM V + KIO3 | 0.93 ± 0.024a | 9.45 ± 0.169c | |
| 0.20 µM V + KIO3 | 0.89 ± 0.018a | 13.51 ± 0.115d | |
| 0.40 µM V + KIO3 | 0.96 ± 0.009a | 23.76 ± 1.846e | |
| 3 | Cont. + 5-ISA | 1.55 ± 0.018a | 0.56 ± 0.009a |
| 0.05 µM V + 5-ISA | 1.57 ± 0.077a | 1.94 ± 0.033b | |
| 0.10 µM V + 5-ISA | 1.73 ± 0.029a | 5.11 ± 0.072c | |
| 0.20 µM V + 5-ISA | 1.75 ± 0.039a | 16.19 ± 0.169d | |
| 0.40 µM V + 5-ISA | 1.25 ± 0.132a | 34.05 ± 0.581e | |
| 4 | Cont. + 3,5-diISA | 1.62 ± 0.027 a | 1.29 ± 0.081a |
| 0.05 µM V + 3,5-diISA | 1.53 ± 0.079 a | 4.90 ± 0.051b | |
| 0.10 µM V + 3,5-diISA | 1.58 ± 0.083 a | 7.50 ± 0.059c | |
| 0.20 µM V + 3,5-diISA | 1.82 ± 0.022 a | 18.23 ± 0.053d | |
| 0.40 µM V + 3,5-diISA | 1.73 ± 0.045 a | 37.50 ± 0.582e | |
| Exp. No. | Treatments | vHPO Activity (U∙µg−1 protein) | |
|---|---|---|---|
| in Leaves | in Roots | ||
| 1 | Control | 0.298 ± 0.021a | 3.110 ± 0.036a |
| 0.05 µM V | 0.505 ± 0.040ab | 5.163 ± 0.012a | |
| 0.10 µM V | 0.728 ± 0.010bc | 5.413 ± 0.007a | |
| 0.20 µM V | 1.243 ± 0.011d | 3.690 ± 0.006a | |
| 0.40 µM V | 0.903 ± 0.040c | 3.548 ± 0.014a | |
| 2 | Cont. + KIO3 | 1.213 ± 0.030bc | 3.000 ± 0.043ab |
| 0.05 µM V + KIO3 | 0.310 ± 0.010a | 2.840 ± 0.027ab | |
| 0.10 µM V + KIO3 | 0.465 ± 0.022ab | 1.645 ± 0.009a | |
| 0.20 µM V + KIO3 | 0.238 ± 0.020a | 2.883 ± 0.030ab | |
| 0.40 µM V + KIO3 | 1.908 ± 0.010c | 5.105 ± 0.010b | |
| 3 | Cont. + 5-ISA | 1.585 ± 0.040b | 0.750 ± 0.041a |
| 0.05 µM V + 5-ISA | 0.910 ± 0.020ab | 0.484 ± 0.078a | |
| 0.10 µM V + 5-ISA | 1.385 ± 0.052b | 0.593 ± 0.051a | |
| 0.20 µM V + 5-ISA | 1.088 ± 0.01b | 0.590 ± 0.015a | |
| 0.40 µM V + 5-ISA | 0.568 ± 0.020a | 0.530 ± 0.052a | |
| 4 | Cont. + 3,5-diISA | 1.066 ± 0.027b | 0.397 ± 0.061a |
| 0.05 µM V + 3,5-diISA | 0.901 ± 0.008b | 0.313 ± 0.060a | |
| 0.10 µM V + 3,5-diISA | 0.587 ± 0.005ab | 0.342 ± 0.063a | |
| 0.20 µM V + 3,5-diISA | 0.499 ± 0.014ab | 0.347 ± 0.059a | |
| 0.40 µM V + 3,5-diISA | 0.263 ± 0.005a | 0.421 ± 0.080a | |
| Exp. No. | Part of Plants | Treatments | (mg∙kg−1 D.W.) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BeA | SA | 5-ISA | 3,5-diISA | 2-IBeA | 4-IBeA | 2,3,5-TIBA | T3 | |||
| 1 | Leaves | Control | 2.18 ± 0.83a | 2.86 ± 0.07b | 2.92 ± 0.01b | 4.78 ± 0.28b | 2.12 ± 0.05b | 2.04 ± 0.06b | 2.13 ± 0.08b | 1.34 ± 0.10a |
| 0.05 µM V | 3.49 ± 0.64a | 2.79 ± 0.01b | 2.96 ± 0.04b | 5.01 ± 0.09b | 2.06 ± 0.03b | 1.98 ± 0.03b | 2.13 ± 0.01b | 1.01 ± 0.08a | ||
| 0.10 µM V | 2.38 ± 0.43a | 2.82 ± 0.04b | 3.11 ± 0.06b | 5.26 ± 0.13b | 2.14 ± 0.03b | 2.12 ± 0.05b | 2.21 ± 0.05b | 1.14 ± 0.07a | ||
| 0.20 µM V | 4.19 ± 0.45a | 3.33 ± 0.03c | 4.09 ± 0.03c | 6.91 ± 0.05c | 2.75 ± 0.03c | 2.92 ± 0.06c | 2.96 ± 0.07c | 1.45 ± 0.07a | ||
| 0.40 µM V | 2.45 ± 0.15a | 1.83 ± 0.05a | 1.93 ± 0.06a | 3.32 ± 0.09a | 1.36 ± 0.03a | 1.73 ± 0.04a | 1.49 ± 0.03a | 1.26 ± 0.39a | ||
| Roots | Control | 4.79 ± 1.60a | 1.51 ± 0.02e | 0.02 ± 0.001b | 0.03 ± 0.002a | 0.033 ± 0.003a | 0.039 ± 0.001bc | 0.022 ± 0.001a | 33.02 ± 0.56d | |
| 0.05 µM V | 2.67 ± 0.95a | 1.21 ± 0.01d | 0.02 ± 0.002ab | 0.02 ± 0.003a | 0.026 ± 0.005a | 0.042 ± 0.002c | 0.025 ± 0.003a | 24.38 ± 0.85bc | ||
| 0.10 µM V | 3.32 ± 0.51a | 1.04 ± 0.01c | 0.02 ± 0.001ab | 0.04 ± 0.006a | 0.033 ± 0.002a | 0.048 ± 0.002c | 0.137 ± 0.107a | 26.14 ± 0.49c | ||
| 0.20 µM V | 4.35 ± 1.29a | 0.78 ± 0.01a | 0.02 ± 0.001a | 0.03 ± 0.001a | 0.028 ± 0.001a | 0.029 ± 0.004b | 0.026 ± 0.001a | 20.19 ± 1.23a | ||
| 0.40 µM V | 3.91 ± 0.55a | 0.87 ± 0.02b | 0.02 ± 0.001a | 0.02 ± 0.001a | 0.035 ± 0.002a | 0.014 ± 0.002a | 0.023 ± 0.001a | 21.59 ± 0.61ab | ||
| 2 | Leaves | Cont. + KIO3 | 3.52 ± 0.33ab | 1.04 ± 0.01c | 0.45 ± 0.005a | 0.80 ± 0.016b | 0.38 ± 0.019a | 0.46 ± 0.010a | 0.39 ± 0.005a | 1.35 ± 0.08a |
| 0.05 µM V + KIO3 | 2.49 ± 0.39a | 0.93 ± 0.01b | 0.46 ± 0.008a | 0.81 ± 0.011b | 0.37 ± 0.006a | 0.49 ± 0.037a | 0.38 ± 0.006a | 1.26 ± 0.20a | ||
| 0.10 µM V + KIO3 | 2.25 ± 0.49a | 0.92 ± 0.01b | 0.45 ± 0.007a | 0.81 ± 0.014b | 0.43 ± 0.058a | 0.46 ± 0.004a | 0.40 ± 0.019a | 1.57 ± 0.20a | ||
| 0.20 µM V + KIO3 | 3.87 ± 1.33ab | 1.15 ± 0.01d | 0.46 ± 0.004a | 0.72 ± 0.027a | 0.39 ± 0.007a | 0.51 ± 0.012a | 0.39 ± 0.007a | 1.44 ± 0.05a | ||
| 0.40 µM V + KIO3 | 5.77 ± 0.46b | 0.52 ± 0.01a | 0.45 ± 0.005a | 0.81 ± 0.005b | 0.40 ± 0.012a | 0.49 ± 0.008a | 0.41 ± 0.005a | 1.82 ± 0.30a | ||
| Roots | Cont. + KIO3 | 2.45 ± 0.64a | 0.73 ± 0.01a | 0.025 ± 0.001c | 0.13 ± 0.005b | 0.028 ± 0.002a | 0.023 ± 0.002a | 0.021 ± 0.001a | 24.43 ± 0.94a | |
| 0.05 µM V + KIO3 | 2.18 ± 0.22a | 0.83 ± 0.02b | 0.013 ± 0.001a | 0.03 ± 0.002a | 0.034 ± 0.002a | 0.018 ± 0.003a | 0.022 ± 0.001a | 22.35 ± 1.15a | ||
| 0.10 µM V + KIO3 | 1.94 ± 0.72a | 0.76 ± 0.02ab | 0.016 ± 0.001ab | 0.03 ± 0.002a | 0.031 ± 0.003a | 0.020 ± 0.004a | 0.021 ± 0.002a | 26.33 ± 1.38a | ||
| 0.20 µM V + KIO3 | 1.37 ± 0.33a | 1.37 ± 0.02c | 0.019 ± 0.001b | 0.03 ± 0.002a | 0.027 ± 0.004a | 0.027 ± 0.005a | 0.022 ± 0.003a | 26.30 ± 1.73a | ||
| 0.40 µM V + KIO3 | 2.33 ± 0.38a | 0.73 ± 0.01a | 0.031 ± 0.001d | 0.41 ± 0.004c | 0.075 ± 0.011a | 0.028 ± 0.002a | 0.039 ± 0.000a | 23.75 ± 0.89a | ||
| 3 | Leaves | Cont. + 5-ISA | 0.81 ± 0.17a | 1.14 ± 0.33a | 2.38 ± 0.07c | 0.20 ± 0.009a | 0.09 ± 0.002a | 0.12 ± 0.005a | 0.10 ± 0.003a | 7.50 ± 0.49a |
| 0.05 µM V + 5-ISA | 0.94 ± 0.26a | 1.16 ± 0.01a | 1.95 ± 0.06b | 0.26 ± 0.012b | 0.09 ± 0.005a | 0.12 ± 0.006a | 0.10 ± 0.005a | 8.30 ± 0.53a | ||
| 0.10 µM V + 5-ISA | 0.72 ± 0.25a | 1.24 ± 0.04a | 1.90 ± 0.04b | 0.23 ± 0.021ab | 0.09 ± 0.003a | 0.10 ± 0.001a | 0.10 ± 0.001a | 8.38 ± 0.15a | ||
| 0.20 µM V + 5-ISA | 1.20 ± 0.36a | 1.18 ± 0.05a | 1.24 ± 0.02a | 0.18 ± 0.009a | 0.09 ± 0.009a | 0.10 ± 0.010a | 0.10 ± 0.002a | 8.07 ± 0.46a | ||
| 0.40 µM V + 5-ISA | 0.99 ± 0.26a | 1.40 ± 0.05a | 1.92 ± 0.06b | 0.23 ± 0012ab | 0.09 ± 0.003a | 0.10 ± 0.003a | 0.10 ± 0.001a | 7.13 ± 0.36a | ||
| Roots | Cont. + 5-ISA | 0.86 ± 0.13a | 16.47 ± 0.61c | 14.24 ± 0.24a | 30.3 ± 0.26a | 0.07 ± 0.005b | 0.03 ± 0.004ab | 0.03 ± 0.004a | 9.70 ± 0.18b | |
| 0.05 µM V + 5-ISA | 2.54 ± 0.55b | 8.80 ± 0.13a | 28.18 ± 0.33e | 31.0 ± 0.60a | 0.06 ± 0.001a | 0.03 ± 0.003ab | 0.02 ± 0.002a | 6.47 ± 0.07a | ||
| 0.10 µM V + 5-ISA | 2.30 ± 0.36ab | 10.37 ± 0.40b | 16.34 ± 0.73b | 42.8 ± 2.08c | 0.07 ± 0.002ab | 0.05 ± 0.003b | 0.02 ± 0.001a | 6.48 ± 0.73a | ||
| 0.20 µM V + 5-ISA | 1.84 ± 0.09ab | 11.12 ± 0.15b | 21.34 ± 0.27d | 36.1 ± 0.18b | 0.07 ± 0.001ab | 0.03 ± 0.002a | 0.03 ± 0.007a | 7.72 ± 1.08ab | ||
| 0.40 µM V + 5-ISA | 1.52 ± 0.45ab | 8.11 ± 0.14a | 18.79 ± 0.30c | 35.6 ± 0.21b | 0.07 ± 0.003ab | 0.03 ± 0.005a | 0.03 ± 0.002a | 6.10 ± 0.46a | ||
| 4 | Leaves | Cont. + 3,5-diISA | 1.22 ± 0.37a | 0.64 ± 0.03c | 0.24 ± 0.004a | 10.46 ± 0.41c | 0.08 ± 0.007a | 0.09 ± 0.004a | 0.09 ± 0.003a | 10.52 ± 0.24a |
| 0.05 µM V + 3,5-diISA | 0.83 ± 0.19a | 0.69 ± 0.01c | 0.26 ± 0.002ab | 6.28 ± 0.06a | 0.09 ± 0.007a | 0.08 ± 0.005a | 0.10 ± 0.005ab | 11.12 ± 0.27ab | ||
| 0.10 µM V + 3,5-diISA | 0.98 ± 0.15a | 0.53 ± 0.01b | 0.31 ± 0.005b | 7.62 ± 0.26b | 0.09 ± 0.004a | 0.09 ± 0.008a | 0.11 ± 0.005ab | 13.36 ± 0.73c | ||
| 0.20 µM V + 3,5-diISA | 1.80 ± 0.63a | 0.41 ± 0.02a | 0.27 ± 0.004c | 8.07 ± 0.04b | 0.10 ± 0.003a | 0.09 ± 0.002a | 0.11 ± 0.005ab | 14.44 ± 0.37c | ||
| 0.40 µM V + 3,5-diISA | 1.64 ± 0.36a | 0.55 ± 0.01b | 0.35 ± 0.011d | 11.38 ± 0.28c | 0.10 ± 0.010a | 0.11 ± 0.011a | 0.12 ± 0.007b | 12.96 ± 0.52bc | ||
| Roots | Cont. + 3,5-diISA | 1.33 ± 0.43a | 5.91 ± 0.08c | 3.34 ± 0.04a | 701.8 ± 2.31a | 0.03 ± 0.001b | 0.013 ± 0.002b | 0.003 ± 0.0006ab | 8.20 ± 0.93a | |
| 0.05 µM V + 3,5-diISA | 2.54 ± 0.41a | 6.68 ± 0.03d | 4.99 ± 0.02c | 712.4 ± 4.58a | 0.02 ± 0.001a | 0.011 ± 0.001ab | 0.005 ± 0.0001b | 9.41 ± 0.89a | ||
| 0.10 µM V + 3,5-diISA | 1.54 ± 0.41a | 6.79 ± 0.06d | 3.07 ± 0.04a | 694.4 ± 11.33a | 0.01 ± 0.001a | 0.009 ± 0.001ab | 0.003 ± 0.0004ab | 10.61 ± 0.25a | ||
| 0.20 µM V + 3,5-diISA | 1.88 ± 0.40a | 5.54 ± 0.02b | 3.20 ± 0.02a | 716.7 ± 5.02a | 0.01 ± 0.001a | 0.007 ± 0.0008ab | 0.001 ± 0.0003a | 9.69 ± 0.79a | ||
| 0.40 µM V + 3,5-diISA | 1.80 ± 0.77a | 5.24 ± 0.06a | 3.72 ± 0.13b | 681.9 ± 14.15a | 0.04 ± 0.001c | 0.005 ± 0.0006a | 0.002 ± 0.0005ab | 8.89 ± 0.38a | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smoleń, S.; Kowalska, I.; Halka, M.; Ledwożyw-Smoleń, I.; Grzanka, M.; Skoczylas, Ł.; Czernicka, M.; Pitala, J. SelectedAspects of Iodate and Iodosalicylate Metabolism in Lettuce Including the Activity of Vanadium Dependent Haloperoxidases as Affected by Exogenous Vanadium. Agronomy 2020, 10, 1. https://doi.org/10.3390/agronomy10010001
Smoleń S, Kowalska I, Halka M, Ledwożyw-Smoleń I, Grzanka M, Skoczylas Ł, Czernicka M, Pitala J. SelectedAspects of Iodate and Iodosalicylate Metabolism in Lettuce Including the Activity of Vanadium Dependent Haloperoxidases as Affected by Exogenous Vanadium. Agronomy. 2020; 10(1):1. https://doi.org/10.3390/agronomy10010001
Chicago/Turabian StyleSmoleń, Sylwester, Iwona Kowalska, Mariya Halka, Iwona Ledwożyw-Smoleń, Marlena Grzanka, Łukasz Skoczylas, Małgorzata Czernicka, and Joanna Pitala. 2020. "SelectedAspects of Iodate and Iodosalicylate Metabolism in Lettuce Including the Activity of Vanadium Dependent Haloperoxidases as Affected by Exogenous Vanadium" Agronomy 10, no. 1: 1. https://doi.org/10.3390/agronomy10010001
APA StyleSmoleń, S., Kowalska, I., Halka, M., Ledwożyw-Smoleń, I., Grzanka, M., Skoczylas, Ł., Czernicka, M., & Pitala, J. (2020). SelectedAspects of Iodate and Iodosalicylate Metabolism in Lettuce Including the Activity of Vanadium Dependent Haloperoxidases as Affected by Exogenous Vanadium. Agronomy, 10(1), 1. https://doi.org/10.3390/agronomy10010001






