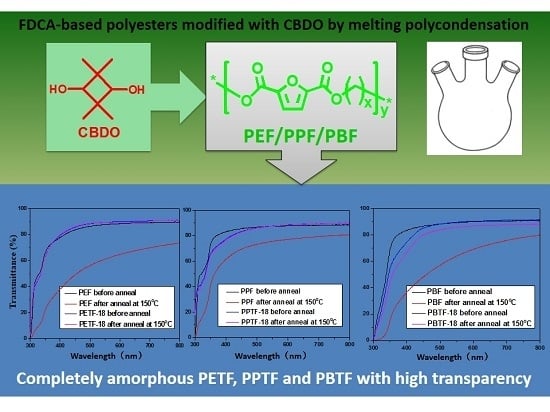
Copolyesters Based on 2,5-Furandicarboxylic Acid (FDCA): Effect of 2,2,4,4-Tetramethyl-1,3-Cyclobutanediol Units on Their Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Dimethyl Furan-2,5-dicarboxylate (DMFD)
2.3. Synthesis of PEF/PPF/PBF and Their Copolyesters
2.4. Measurements
3. Results and Discussion
3.1. Synthesis of FDCA-Based Copolyesters
3.2. Chemical Structures and Composition of the Synthesized Copolyesters
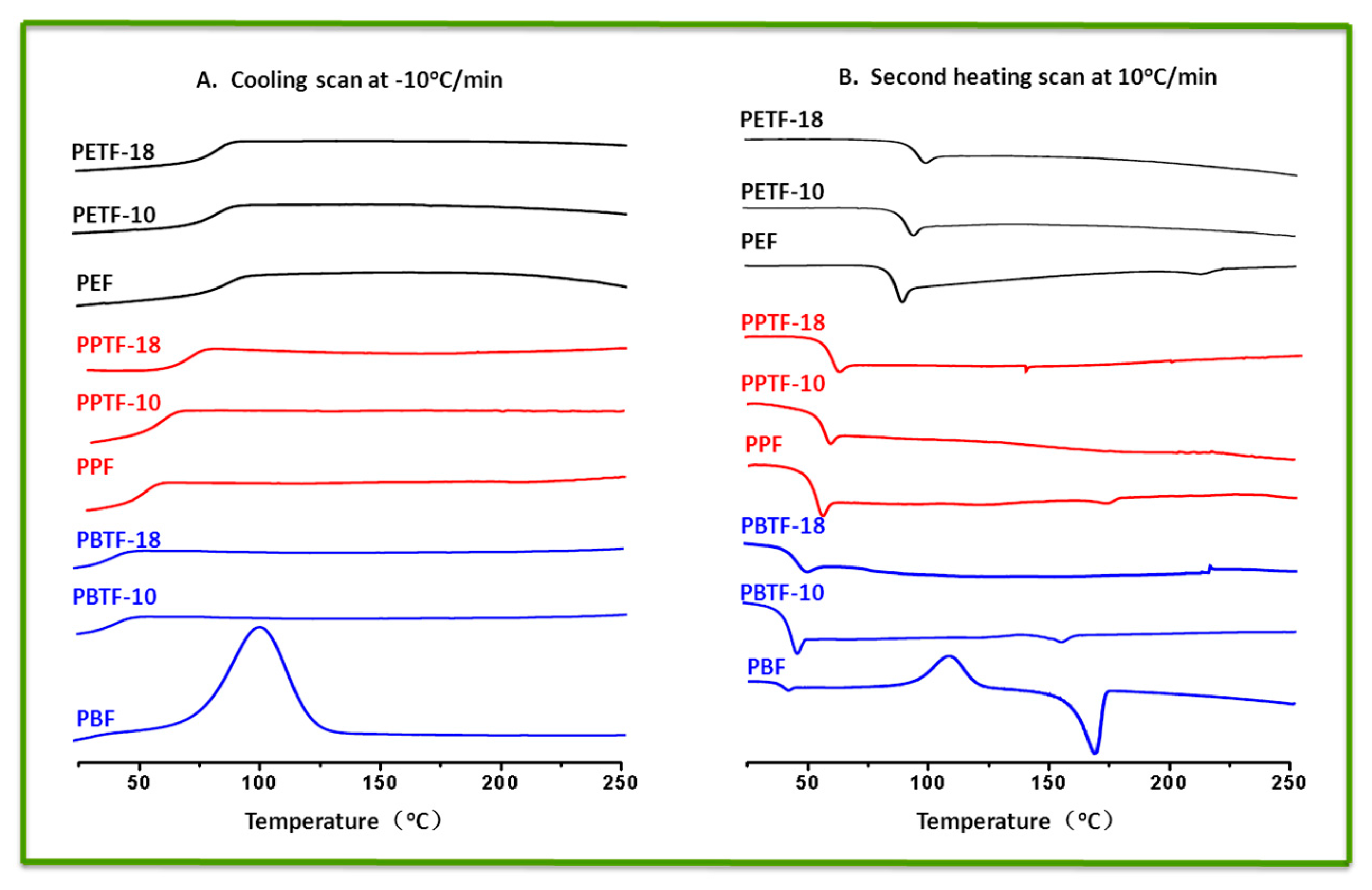
3.3. Thermal Properties Investigation
3.4. Mechanical Properties
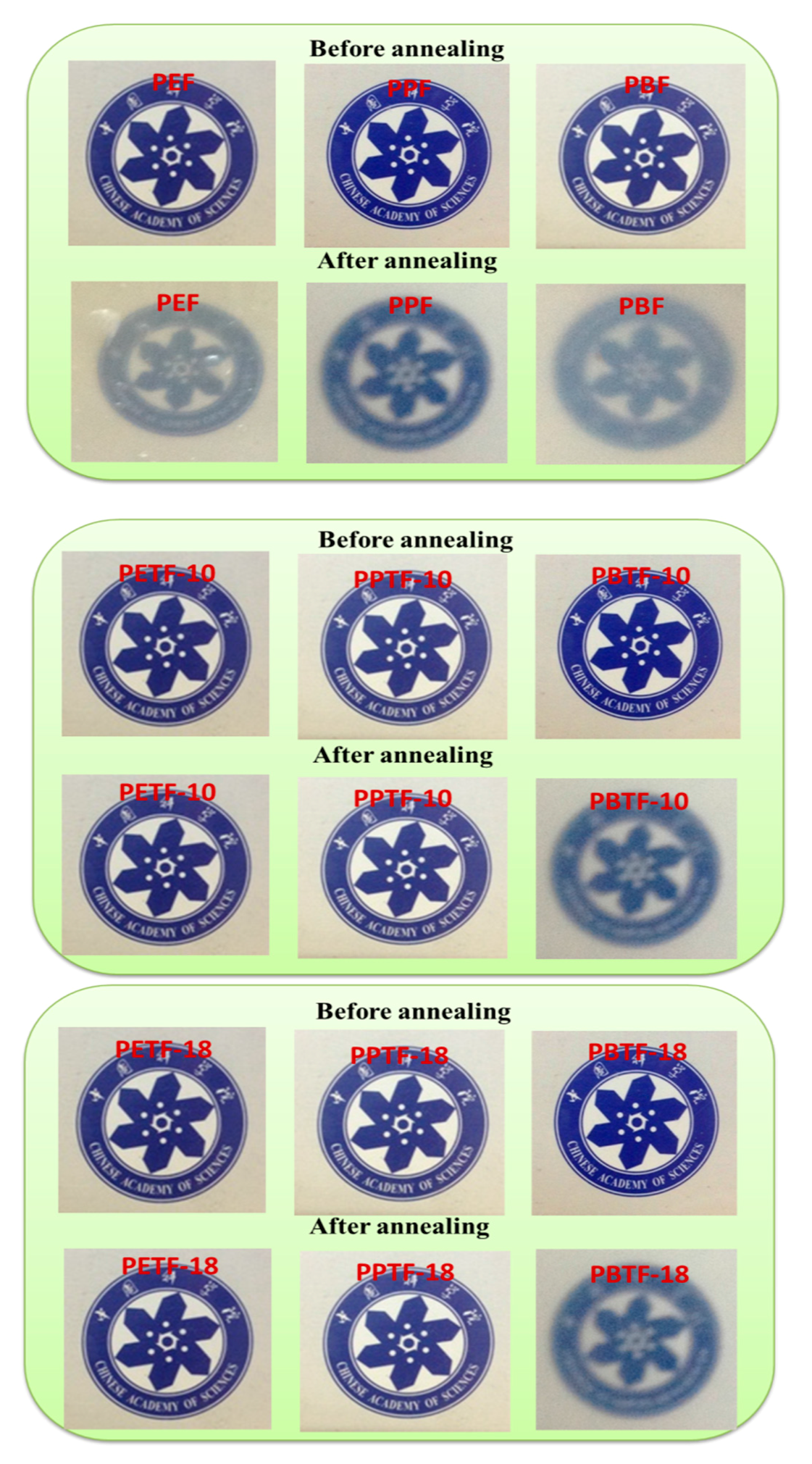
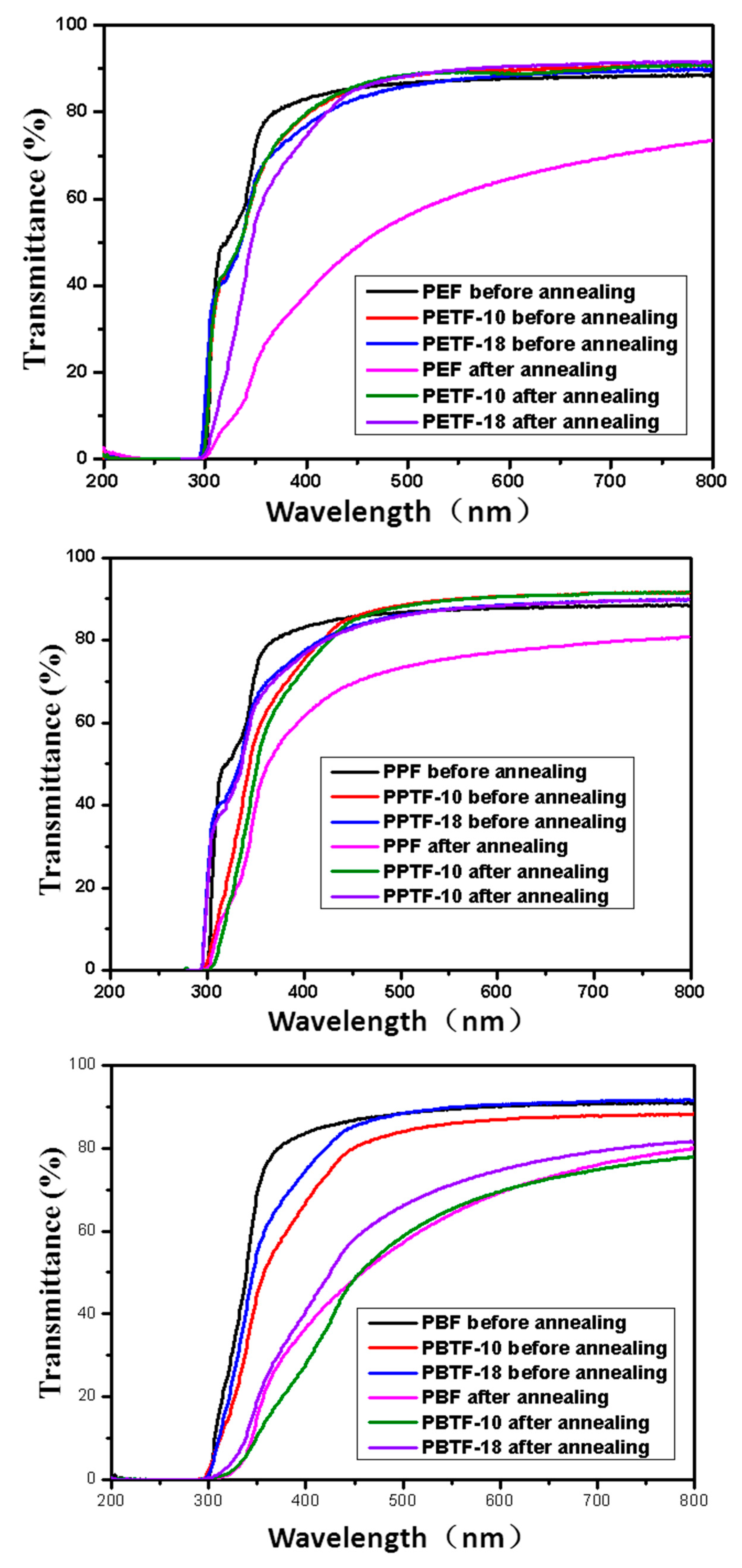
3.5. Transparency Investigation
3.6. Barrier Properties
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, B.; Shen, C.; Chen, S. Ductile PLA Modified with methacryloyloxyalkyl isocyanate improves mechanical properties. Polymer 2010, 51, 4667–4672. [Google Scholar] [CrossRef]
- Reddy, C.S.K.; Ghai, R.; Kalia, R. Polyhydroxyalkanoates: An overview. Bioresour. Technol. 2003, 87, 137–146. [Google Scholar] [CrossRef]
- Ye, H.M.; Wang, R.; Liu, J.; Xu, J.; Guo, B. Isomorphism in poly(butylene succinate-co-butylene fumarate) and its application as polymeric nucleating agent for poly(butylene succinate). Macromolecules 2012, 45, 5667–5675. [Google Scholar] [CrossRef]
- Putten, R.J.; Waal, J.C.; Jong, E.D.; Rasrendra, C.B.; Heeres, H.J.; Vries, J.G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Liu, Q.; Zhang, Q.; Ye, C.; Zhou, G. A series of furan-aromatic Polyesters synthesized via direct esterification method based on renewable resources. J. Polym. Sci. A 2012, 50, 1026–1036. [Google Scholar] [CrossRef]
- Gubbels, E.; Jasinska-Walc, L.; Koning, C.E. Synthesis and characterization on novel renewable polyesters based on 2,5-furandicarboxylic acid and 2,3-butanediol. J. Polym. Sci. A 2013, 51, 890–898. [Google Scholar] [CrossRef]
- Storbeck, R.; Ballauff, M. Synthesis and properties of polyesters based on 2,5-Furandicarboxylic Acid and 1,4:3,6-dianhydrohexitols. Polymer 1993, 34, 5003–5006. [Google Scholar] [CrossRef]
- Gandini, A.; Silvestre, A.J.D.; Neto, C.P.; Sousa, A.F.; Gomes, M. The furan counterpart of polyethylene terephthalate: An alternative material based on renewable resources. J. Polym. Sci. A 2009, 47, 295–298. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Papageorgiou, D.G.; Terzopoulou, Z.; Bikiaris, D.N. Production of bio-based 2,5-furan dicarboxylate polyester: Recent grogress and critical aspects in their synthesis and thermal properties. Eur. Polym. J. 2016, 83, 202–229. [Google Scholar] [CrossRef]
- Mitiakoudis, A.; Gandini, A. Synthesis and characterization of furanic polyamides. Macromolecules 1991, 24, 649–653. [Google Scholar] [CrossRef]
- Gandini, A.; Belgacem, M.N. Furans in polymer chemistry. Prog. Polym. Sci. 1997, 22, 1203–1379. [Google Scholar] [CrossRef]
- Moreau, C.; Belgacem, M.N.; Gandini, A. Recent Catalytic Advances in the chemistry of substituted furans from carbohydrates and in the ensuing polymers. Top. Catal. 2004, 35, 11–30. [Google Scholar] [CrossRef]
- Boufi, S.; Belgacem, M.N.; Quillerou, J.; Gandini, A. Urethanes and uolyurethanes bearing furan moieties. 4, synthesis, kinetics and characterization of linear polymers. Macromolecules 1993, 26, 6706–6717. [Google Scholar] [CrossRef]
- Azzam, R.A.; Mohamed, S.K.; Tol, R.; Everaert, V.; Reynaers, H.; Goderis, B. Synthesis and thermo-mechanical characterization of high performance polyurethane elastomers based on heterocyclic and aromatic diamine chain extenders. Polym. Degrad. Stab. 2007, 92, 1316–1325. [Google Scholar] [CrossRef]
- Deng, J.; Liu, X.; Li, C.; Jiang, Y.; Zhu, J. Synthesis and properties of a bio-based epoxy resin from 2,5-furandicarboxylic acid (FDCA). RSC Adv. 2015, 5, 15930–15939. [Google Scholar] [CrossRef]
- Jong, E.; Dam, M.A.; Sipos, L.; Gruter, G.J. Furandicarboxylic acid (FDCA), a versatile building block for a very interesting class of polyesters. ACS Symp. 2012, 1105, 1–13. [Google Scholar]
- Vannini, M.; Marchese, P.; Celli, A.; Lorenzetti, C. Fully biobased poly(propylene 2,5-furan dicarboxylate) for packaging applications: excellent barrier properties as a function of crystallinity. Green Chem. 2015, 17, 4162–4166. [Google Scholar] [CrossRef]
- Wu, B.S.; Xu, Y.T.; Bu, Z.Y.; Wu, L.B.; Li, B.G.; Dubois, P. Biobased poly(butylene 2,5-furandicarboxylate) and poly(butylene adipate-co-butylene 2,5-furandicarboxylate)s: From synthesis using highly purified 2,5-furandicarboxylic acid to thermo-mechanical properties. Polymer 2014, 55, 3648–3655. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Tsanaktsis, V.; Papageorgiou, D.G.; Exarhopoulos, S.; Papageorgiou, M.; Bikiaris, D.N. Evaluation of polyesters from renewable resources as alternatives to the current fossil-based polymers, phase transitions of poly(butylene 2,5-furan-dicarboxylate). Polymer 2014, 55, 3846–3858. [Google Scholar] [CrossRef]
- Wu, L.; Mincheva, R.; Xu, Y.; Raquez, M.J.; Dubois, P. High molecular weight poly(butylene succinate-co-butylene furandicarboxylate) copolyesters: From catalyzed polycondensation reaction to thermomechanical properties. Biomacromolecules 2012, 13, 2973–2981. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yu, X.; Xu, J.; Pang, Y. Synthesis and crystallinity of poly(butylene 2,5-furandicarboxylate). Polymer 2012, 53, 4145–4151. [Google Scholar] [CrossRef]
- Burgess, S.K.; Kriege, R.M.; Koros, W.J. Carbon dioxide sorption and transport in amorphous poly(ethylene furanoate). Macromolecules 2015, 48, 2184–2193. [Google Scholar] [CrossRef]
- Burgess, S.K.; Mikkilineni, D.; Yu, D.B.; Kim, D.J.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Water sorption in poly(ethylene furanoate) compared to poly(ethylene terephthalate), part 2: Kinetic sorption. Polymer 2014, 55, 4748–4756. [Google Scholar] [CrossRef]
- Knoop, R.J.; Vogelzang, W.; Haveren, J.V.; Es, D.S. High molecular weight poly(ethylene-2,5-furanoate); critical aspects in synthesis and mechanical property determination. J. Polym. Sci. A 2013, 21, 4191–4199. [Google Scholar] [CrossRef]
- Berkel, J.G.; Guigo, N.; Kolstad, J.J.; Sipos, L.; Wang, B.; Dam, M.A.; Sbirrazzuoli, N. Isothermal Crystallization Kinetics Of Poly (Ethylene 2,5-Furandicarboxylate). Macromol. Mater. Eng. 2015, 300, 466–474. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Papageorgiou, D.G.; Tsanaktsis, V.; Bikiaris, D.N. Synthesis of the bio-based polyester poly(propylene 2,5-furan dicarboxylate). Comparison of thermal behaviour and solid state structure with its terephthalate and naphthalate homologues. Polymer 2015, 62, 28–38. [Google Scholar] [CrossRef]
- Gomes, M.; Gandini, A.; Silvestre, A.J.D.; Reis, B. Synthesis and Characterization of poly(2,5-furan dicarboxylate)s based on a variety of diols. J. Polym. Sci. A 2011, 49, 3759–3768. [Google Scholar] [CrossRef]
- Wang, J.G.; Liu, X.Q.; Zhang, Y.J.; Liu, F.; Zhu, J. Modification of poly(ethylene 2,5-furandicarboxylate) with 1,4-cyclohexanedimethylene: Influence of composition on mechanical and barrier properties. Polymer 2016, 103, 1–8. [Google Scholar] [CrossRef]
- Andreia, F.S.; Marina, M.; Carmen, S.R.F.; Armando, J.D.S.; Jorge, F.J.C. New copolyesters derived from terephthalic and 2,5-furandicarboxylic acids: A step forward in the development of biobased polyesters. Polymer 2013, 54, 513–519. [Google Scholar]
- Papageorgiou, D.G.; Guigo, N.; Tsanaktsis, V.; Exarhopoulos, S.; Bikiaris, D.N.; Sbirrazzuoli, N.; Papageorgiou, G.Z. Fast crystallization and melting behaviour of a long spaced aliphatic furandicarboxylate biobased polyester, poly(dodecylene 2,5-furanoate). Ind. Eng. Chem. Res. 2016, 55, 5315–5326. [Google Scholar] [CrossRef]
- Beal, G.W.; Powell, C.E.; Hancock, J.; Kindinger, M.; Mckenzie, H.; Bray, A.V.; Booth, C. Physical properties of CBDO based co-polyterephthalate nanocomposites. J. Appl. Clay Sci. 2007, 37, 295–306. [Google Scholar] [CrossRef]
- Zhang, M.; Moore, M.B.; Long, T.E. Melt transesterification and characterization of segmented block copolyesters containing 2,2,4,4-tetramethyl-1,3-cyclobutanediol. J. Polym. Sci. A 2012, 50, 3710–3718. [Google Scholar] [CrossRef]
- Hasek, R.H.; Elam, E.U.; Martin, J.C.; Nations, R.G. Chemistry of dimethylketene dimer. I. catalytic hydrogenation and ring cleavage by alcohols. J. Org. Chem. 1961, 26, 700–704. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Papageorgiou, D.G.; Exarhopoulos, S.; Bikiaris, D.N.; Papageorgiou, G.Z. On the crystallization and polymorphism of poly(ethylene furanoate). Cryst. Growth Des. 2015, 15, 5505–5512. [Google Scholar] [CrossRef]
- Lee, J.S.; Leisen, J.; Choudhury, R.P.; Kriegel, R.M.; Beckham, H.W.; Koros, W.J. Antiplasticization-based enhancement of poly(ethylene terephthalate) barrier properties. Polymer 2012, 53, 213–222. [Google Scholar] [CrossRef]
- Light, R.R.; Seymour, R.W. Effect of Sub-Tg Relaxations on the gas transport properties of polyesters. Polym. Eng. Sci. 1982, 22, 857–864. [Google Scholar] [CrossRef]

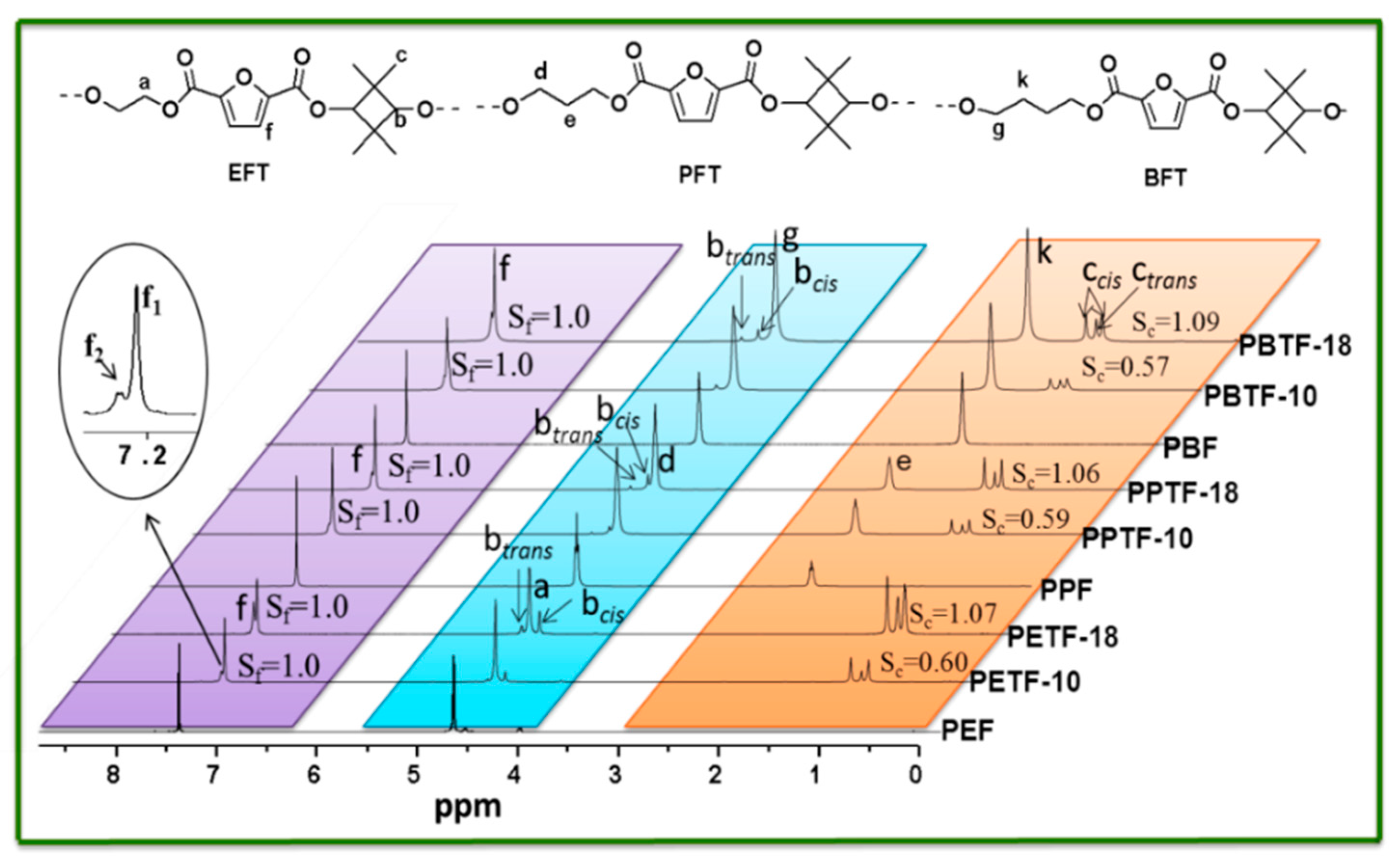
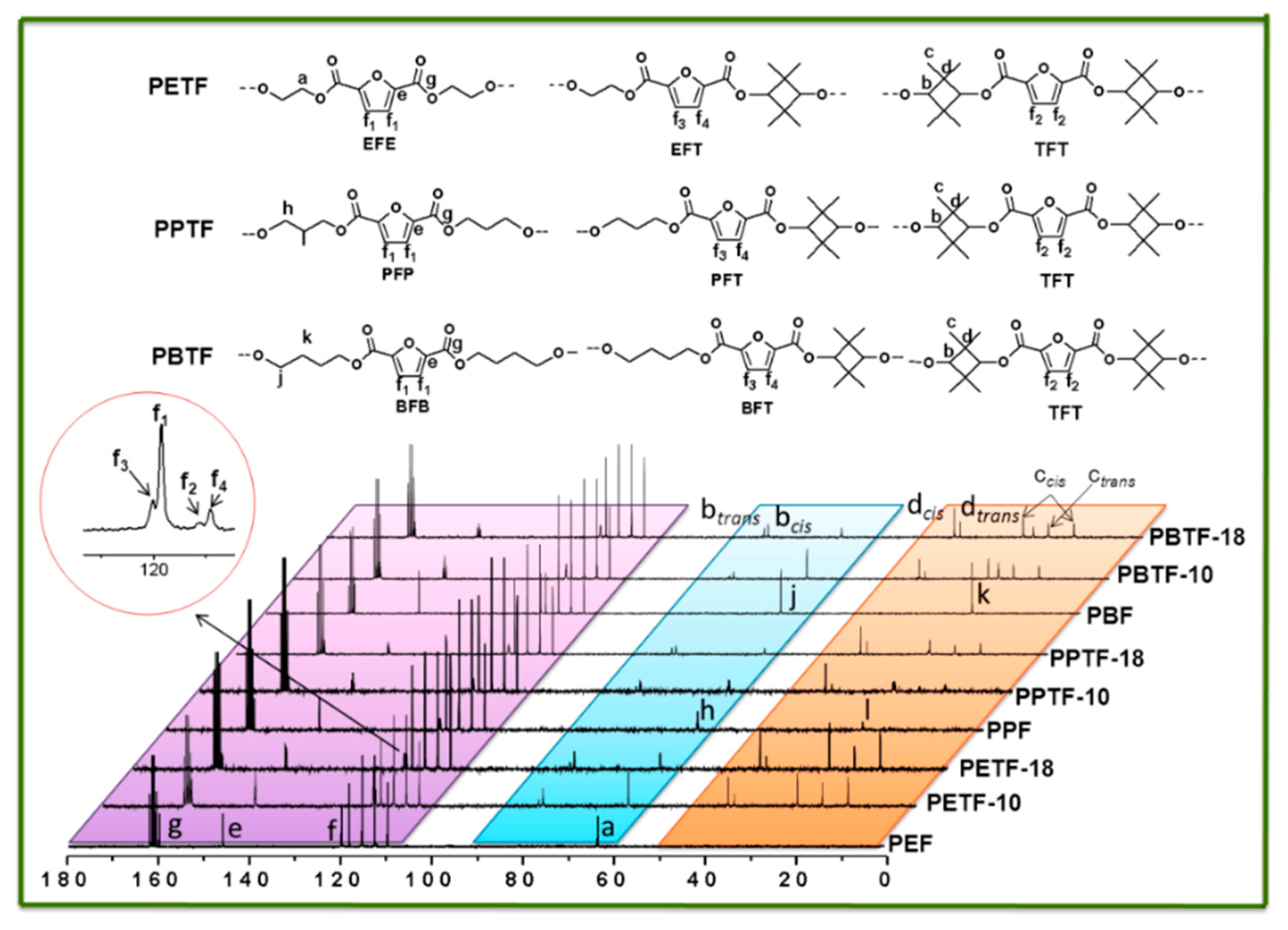

| Sample | In Feed | In Copolyesters a | Triads Component b mol % | Average Sequence Length | Degree of Random | Intrinsic Viscosity | Molecule Weight | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DMFD (mol) | CBDO (mol) | Diol (mol) | CBDO/Diol (mol) | NDFD | NDFT+TFD | NTFT | NDF | NTF | R | [η] c | Mv d | |
| PEF | 0.50 | 0 | 0.8 | 0:100 | 100 | - | - | - | - | - | 0.92 | 6.9 × 104 |
| PETF-10 | 0.50 | 0.075 | 0.725 | 10.3:89.7 | 78.1 | 20.1 | 1.8 | 8.7 | 1.1 | 0.97 | 0.79 | 5.6 × 104 |
| PETF-18 | 0.50 | 0.12 | 0.68 | 18.2:81.8 | 69.0 | 27.4 | 3.6 | 5.0 | 1.3 | 0.99 | 0.74 | 5.1 × 104 |
| PPF | 0.50 | 0 | 0.80 | 0:100 | 100 | - | - | - | - | - | 0.88 | 6.5 × 104 |
| PPTF-10 | 0.50 | 0.075 | 0.725 | 9.8:90.2 | 79.4 | 18.7 | 1.9 | 9.5 | 1.2 | 0.94 | 0.93 | 7.1 × 104 |
| PPTF-18 | 0.50 | 0.12 | 0.68 | 17.8:82.2 | 67.2 | 27.9 | 4.9 | 5.8 | 1.3 | 0.94 | 0.76 | 5.3 × 104 |
| PBF | 0.50 | 0 | 0.80 | 0:100 | 100 | - | - | - | - | - | 0.98 | 7.6 × 104 |
| PBTF-10 | 0.50 | 0.075 | 0.725 | 9.6:90.4 | 80.2 | 17.9 | 1.9 | 9.9 | 1.2 | 0.93 | 0.96 | 7.4 × 104 |
| PBTF-18 | 0.50 | 0.12 | 0.68 | 17.9:82.1 | 69.3 | 26.1 | 4.6 | 5.9 | 1.3 | 0.94 | 0.92 | 6.9 × 104 |
| Sample | DSC | TGA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Second Heating Scan at 10 °C/min | Scan after Annealing at 150 °C for 30 min | |||||||||
| Tg (°C) | Tcc (°C) | ΔHcc (J/g) | Tm (°C) | ΔHm (J/g) | Tm (°C) | ΔHm (J/g) | Td,5% (°C) | Td,max (°C) | R650 (wt %) | |
| PEF | 87.0 | nd | nd | 211.9 | 0.9 | 208.2 | 29.6 | 365 | 401 | 7.9 |
| PETF-10 | 90.9 | nd | nd | nd | nd | nd | nd | 368 | 403 | 7.4 |
| PETF-18 | 91.1 | nd | nd | nd | nd | nd | nd | 369 | 400 | 7.6 |
| PPF | 55.5 | nd | nd | 173.6 | 0.5 | 173.6 | 1.7 | 367 | 405 | 6.1 |
| PPTF-10 | 61.1 | nd | nd | nd | nd | nd | nd | 370 | 403 | 6.5 |
| PPTF-18 | 63.5 | nd | nd | nd | nd | nd | nd | 361 | 397 | 5.5 |
| PBF | 39.0 | 109.4 | 30.4 | 168.8 | 31.1 | 168.6 | 32.6 | 367 | 398 | 5.8 |
| PBTF-10 | 42.5 | nd | nd | 154.4 | 0.5 | 153.4 | 0.8 | 368 | 405 | 4.6 |
| PBTF-18 | 43.5 | nd | nd | nd | nd | nd | nd | 365 | 404 | 4.8 |
| Sample | Tensile Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|
| PEF | 2800 ± 120 | 85 ± 9 | 5 ± 1 |
| PETF-10 | 3100 ± 100 | 97 ± 4 | 9 ± 5 |
| PETF-18 | 3300 ± 100 | 98 ± 2 | 4 ± 1 |
| PPF | 2700 ± 30 | 53 ± 2 | 50 ± 7 |
| PPTF-10 | 2750 ± 20 | 63 ± 3 | 56 ± 11 |
| PPTF-18 | 2800 ± 40 | 78 ± 11 | 30 ± 10 |
| PBF | 2000 ± 30 | 62 ± 3 | 290 ± 6 |
| PBTF-10 | 2100 ± 80 | 72 ± 2 | 274 ± 10 |
| PBTF-18 | 2200 ± 60 | 80 ± 2 | 220 ± 18 |
| Sample | Amorphous | 150 °C for 30 min | ||
|---|---|---|---|---|
| Transmittance % (450 nm) | Transmittance % (700 nm) | Transmittance % 450 nm) | Transmittance % (700 nm) | |
| PEF | 85.7 | 88.4 | 48.9 | 71.9 |
| PETF-10 | 85.1 | 90.8 | 86.0 | 90.4 |
| PETF-18 | 83.9 | 90.2 | 84.3 | 89.8 |
| PPF | 85.7 | 88.4 | 69.5 | 80.0 |
| PPTF-10 | 85.5 | 91.1 | 84.6 | 90.2 |
| PPTF-18 | 84.4 | 89.7 | 83.7 | 89.7 |
| PBF | 85.7 | 90.8 | 48.2 | 75.9 |
| PBTF-10 | 84.1 | 89.8 | 48.4 | 74.8 |
| PBTF-18 | 85.3 | 91.3 | 60.1 | 79.0 |
| Sample a | Temperature (°C) | CO2 Permeability Coefficient (Barrer b) | BIFP | Reference |
|---|---|---|---|---|
| PET | 30 | 0.13 | 1 | This work |
| PEF | 30 | 0.010 | 13.0 | This work |
| PETF-10 | 30 | 0.019 | 6.8 | This work |
| PETF-18 | 30 | 0.059 | 2.2 | This work |
| PPF | 30 | 0.016 | 8.1 | This work |
| PPTF-10 | 30 | 0.018 | 7.2 | This work |
| PPTF-18 | 30 | 0.020 | 6.5 | This work |
| PBF | 30 | 0.018 | 7.2 | This work |
| PBTF-10 | 30 | 0.027 | 4.9 | This work |
| PBTF-18 | 30 | 0.055 | 2.4 | This work |
| PET | 30 | 0.32 | 1 | [35] |
| PEN | 30 | 0.11 | 2.9 | [35] |
| Sample a | Temperature (°C) | O2 Permeability Coefficient (Barrier b) | BIFP | Reference |
|---|---|---|---|---|
| PET | 30 | 0.060 | 1 | This work |
| PEF | 30 | 0.011 | 5.5 | This work |
| PETF-10 | 30 | 0.013 | 4.6 | This work |
| PETF-18 | 30 | 0.028 | 2.1 | This work |
| PPF | 30 | 0.09 | 6.7 | This work |
| PPTF-10 | 30 | 0.010 | 6.0 | This work |
| PPTF-18 | 30 | 0.036 | 1.7 | This work |
| PBF | 30 | 0.018 | 3.3 | This work |
| PBTF-10 | 30 | 0.025 | 2.4 | This work |
| PBTF-18 | 30 | 0.042 | 1.4 | This work |
| PET | 30 | 0.054 | 1 | [34] |
| PEN | 30 | 0.019 | 2.9 | [34] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Liu, X.; Zhu, J.; Jiang, Y. Copolyesters Based on 2,5-Furandicarboxylic Acid (FDCA): Effect of 2,2,4,4-Tetramethyl-1,3-Cyclobutanediol Units on Their Properties. Polymers 2017, 9, 305. https://doi.org/10.3390/polym9090305
Wang J, Liu X, Zhu J, Jiang Y. Copolyesters Based on 2,5-Furandicarboxylic Acid (FDCA): Effect of 2,2,4,4-Tetramethyl-1,3-Cyclobutanediol Units on Their Properties. Polymers. 2017; 9(9):305. https://doi.org/10.3390/polym9090305
Chicago/Turabian StyleWang, Jinggang, Xiaoqing Liu, Jin Zhu, and Yanhua Jiang. 2017. "Copolyesters Based on 2,5-Furandicarboxylic Acid (FDCA): Effect of 2,2,4,4-Tetramethyl-1,3-Cyclobutanediol Units on Their Properties" Polymers 9, no. 9: 305. https://doi.org/10.3390/polym9090305
APA StyleWang, J., Liu, X., Zhu, J., & Jiang, Y. (2017). Copolyesters Based on 2,5-Furandicarboxylic Acid (FDCA): Effect of 2,2,4,4-Tetramethyl-1,3-Cyclobutanediol Units on Their Properties. Polymers, 9(9), 305. https://doi.org/10.3390/polym9090305