Restoration of Impaired Metabolic Energy Balance (ATP Pool) and Tube Formation Potential of Endothelial Cells under “high glucose”, Diabetic Conditions by the Bioinorganic Polymer Polyphosphate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Endothelial Cell Tube Formation Assay
2.3. Cultivation of HUVEC Cells
2.4. MTT Viability Assay
2.5. TACS Assay
2.6. Scanning Electron Microscopy
2.7. Determination of the Intracellular ATP Pool
2.8. Statistical Analysis
3. Results
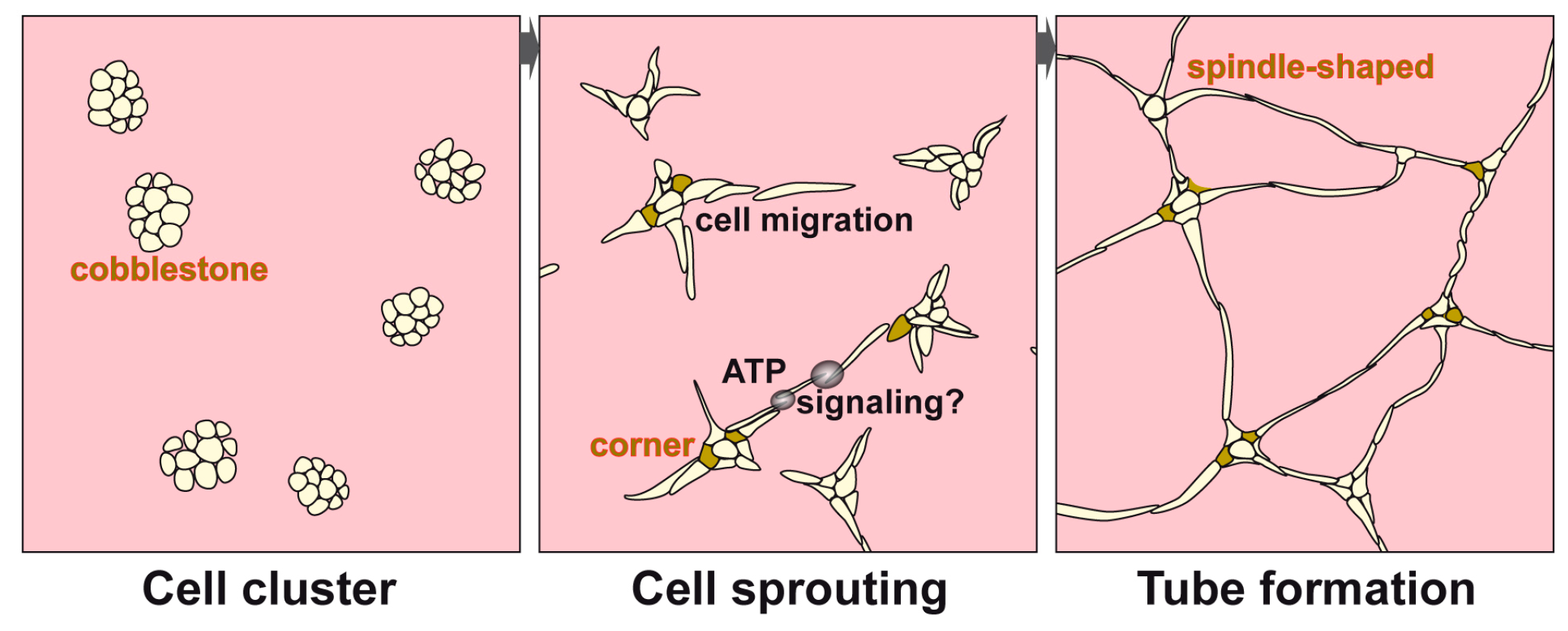
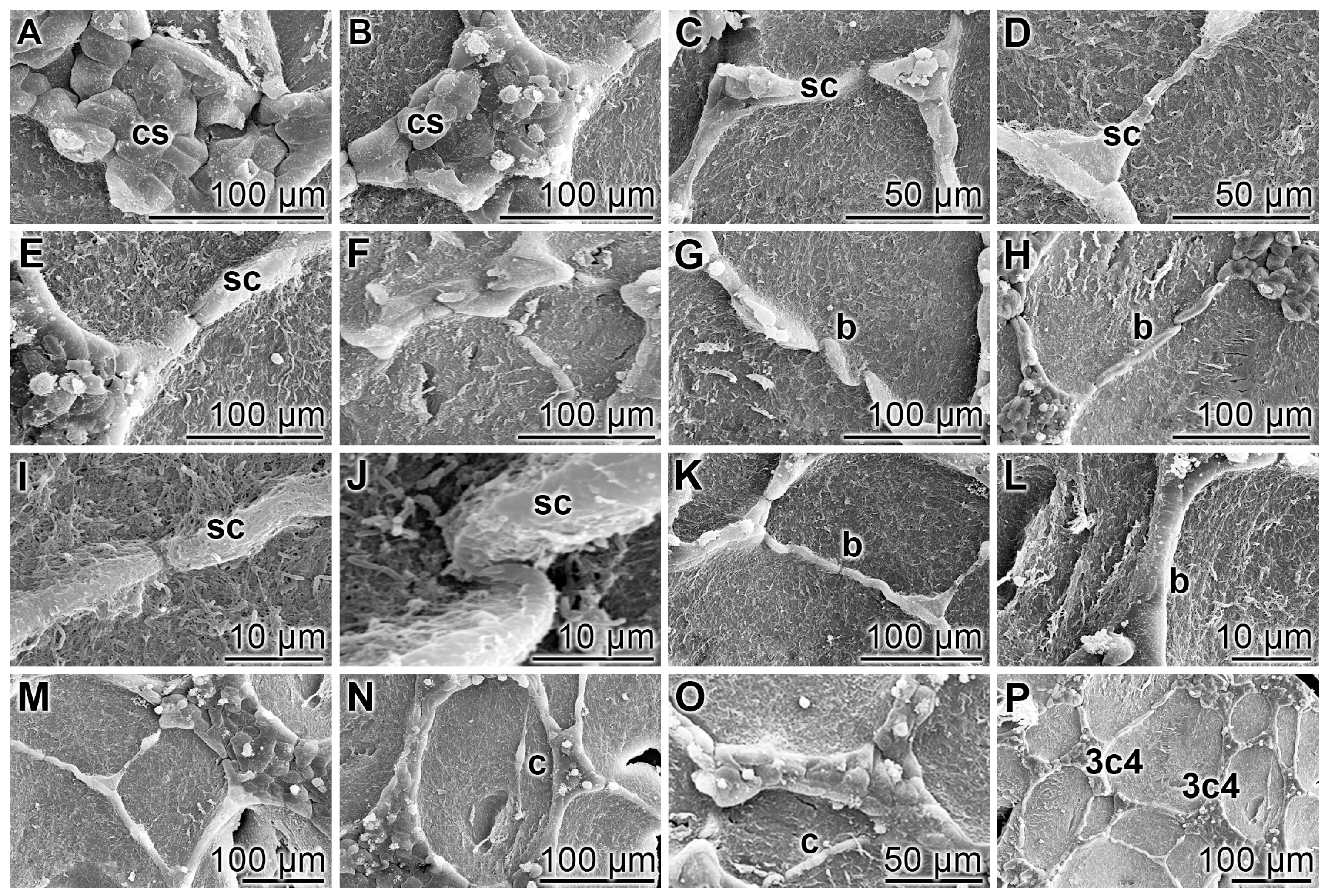
3.1. Endothelial Cell Tube Formation of HUVEC Cells onto Collagen/Basement Extract
3.2. Effect of Glucose Concentration on Tube Formation
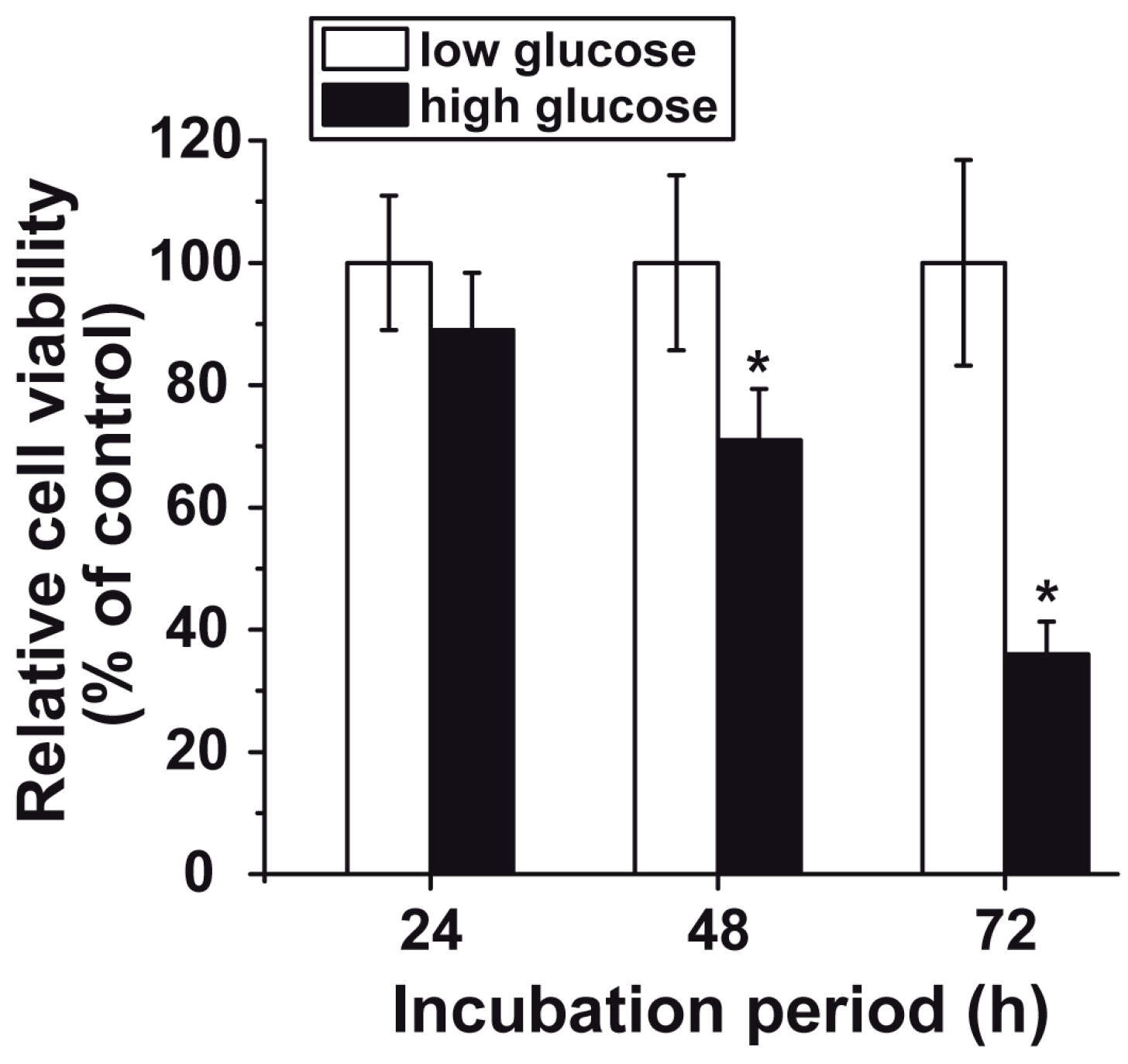
3.3. Reduced Cell Viability at “High Glucose”
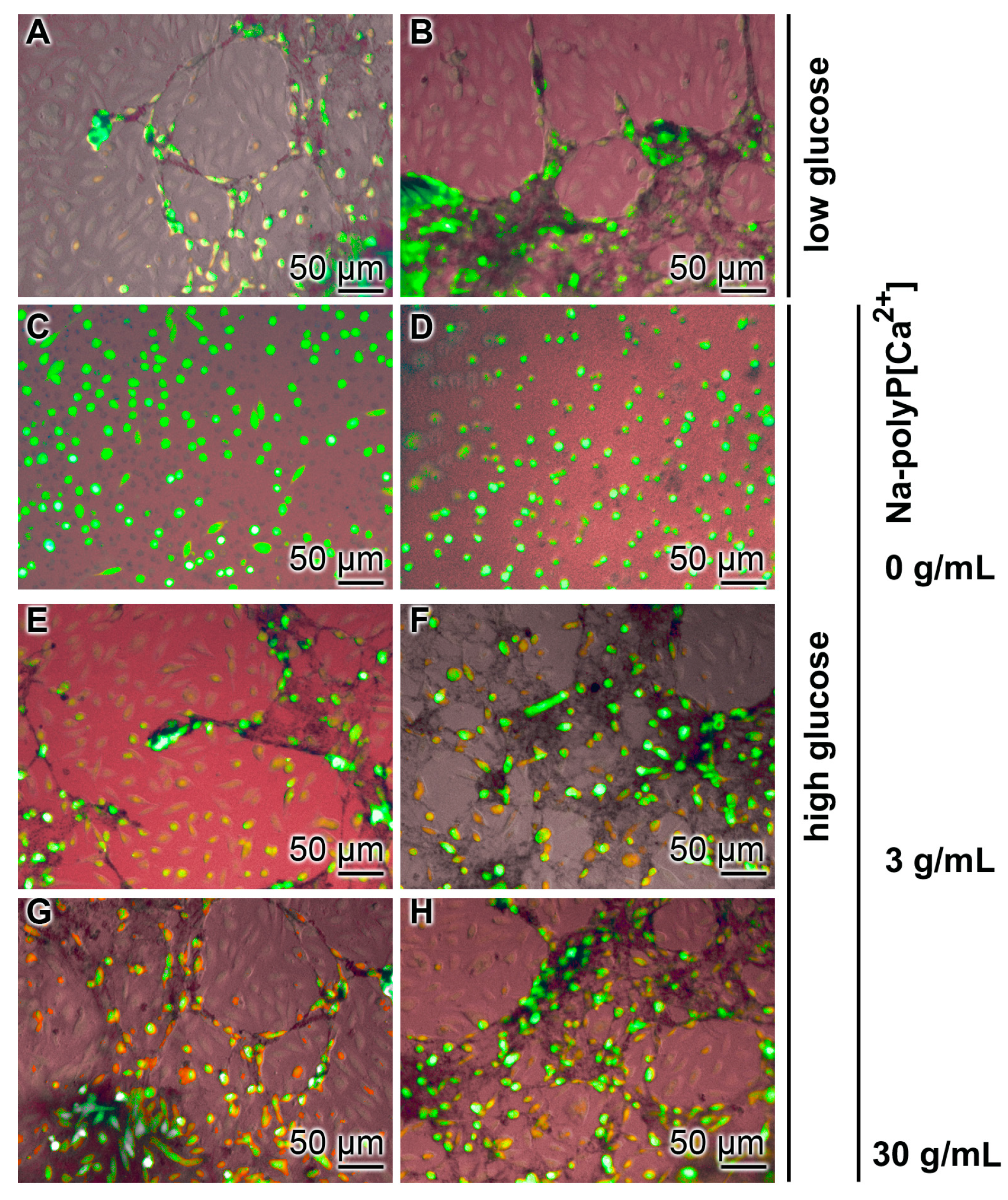
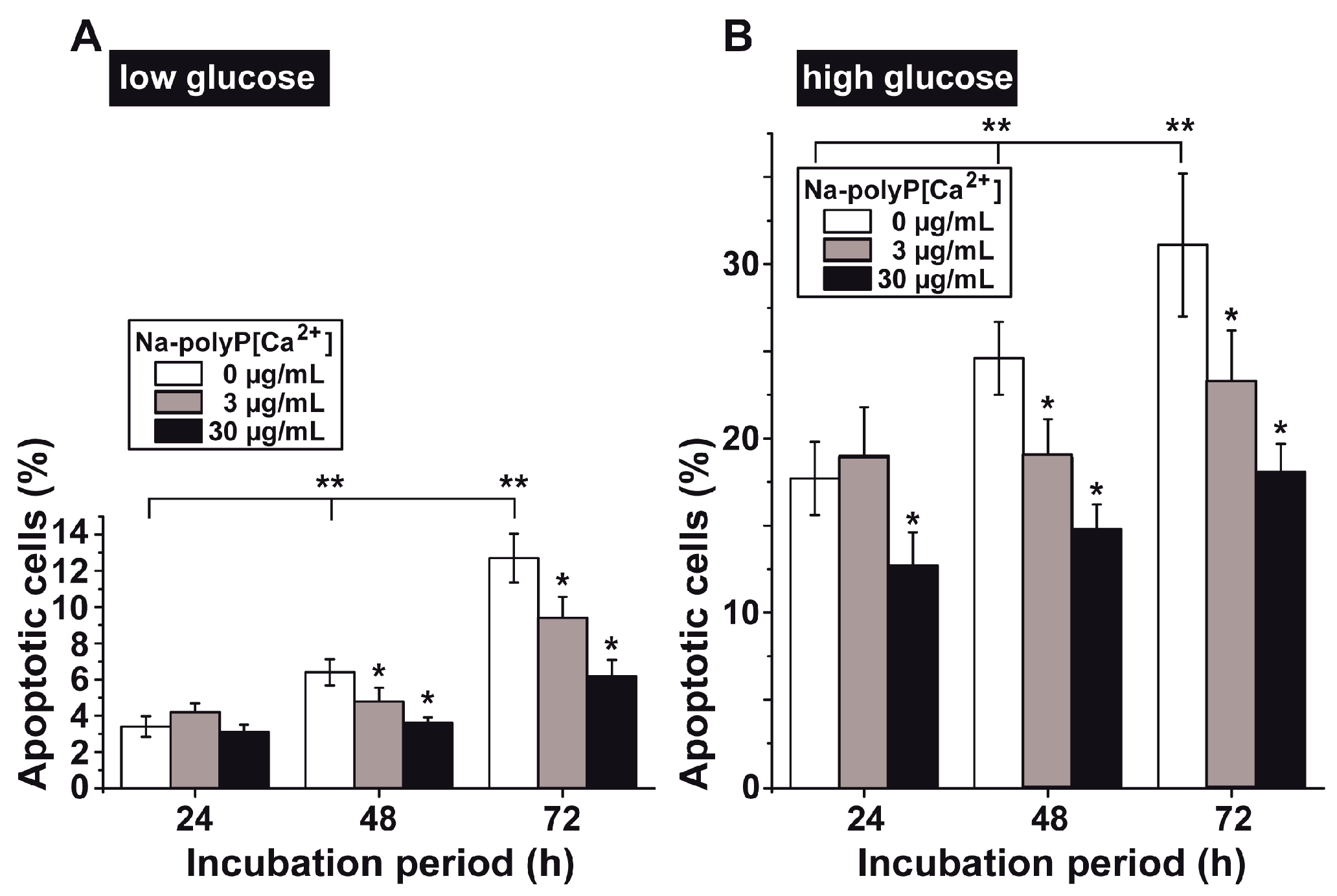
3.4. Determination of Impaired Cell Viability as Apoptosis
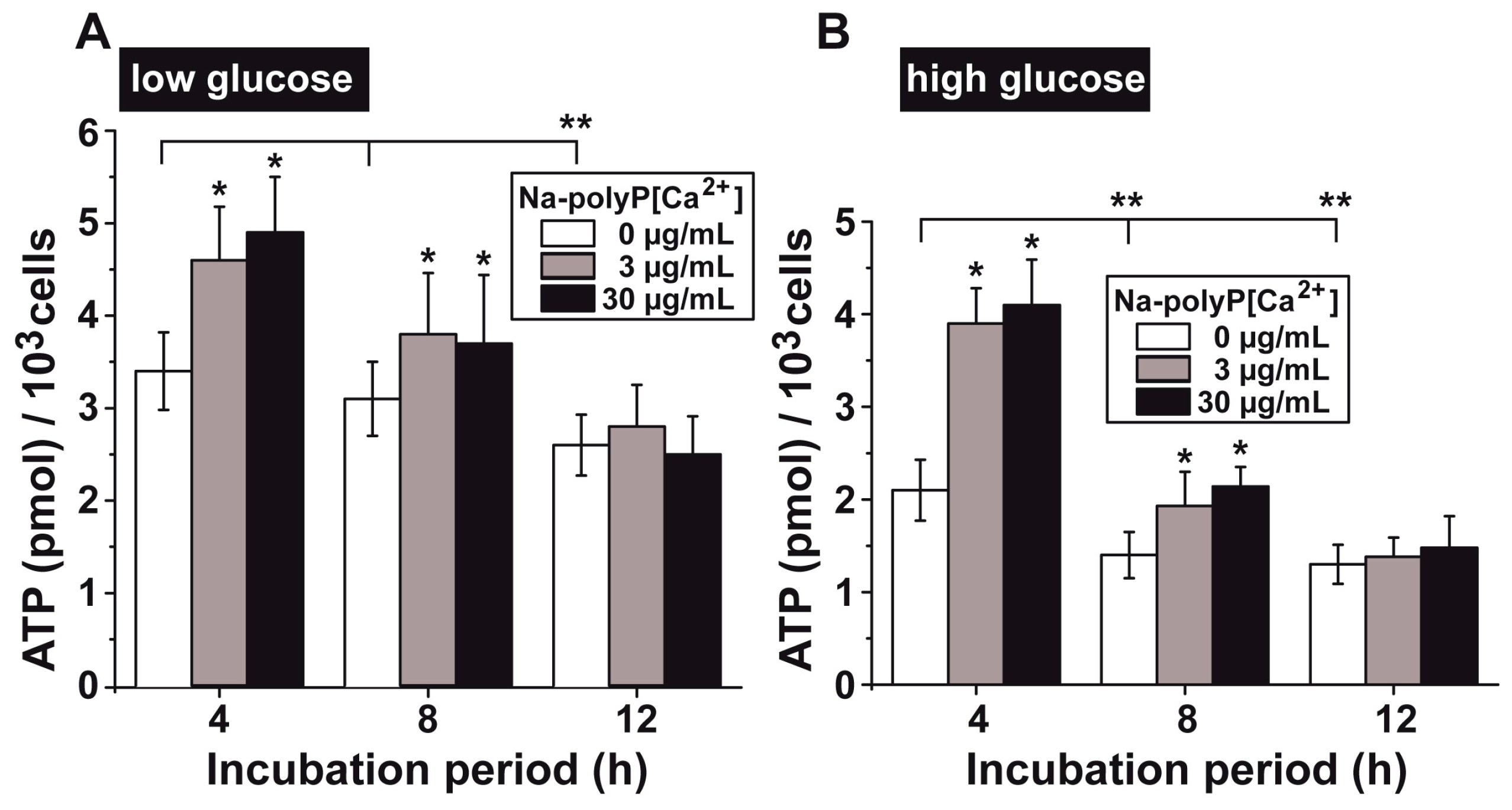
3.5. Upregulation of the ATP Pool in Cells by polyP
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Standards of medical care in diabetes-2016: Summary of revisions. Diabetes Care 2016, 39 (Suppl. 1), S4–S5.
- Orasanu, G.; Plutzky, J. The pathologic continuum of diabetic vascular disease. J. Am. Coll. Cardiol. 2009, 53 (Suppl. 5), S35–S42. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Chawla, R.; Jaggi, S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J. Endocrinol. Metab. 2016, 20, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Elrayah-Eliadarous, H.A.; Östenson, C.G.; Eltom, M.; Johansson, P.; Sparring, V.; Wahlström, R. Economic and social impact of diabetes mellitus in a low-income country: A case-control study in Sudan. J. Diabetes 2017. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation (IDF). IDF Diabetes Atlas 2015, 7th ed.; IDF: Brussels, Belgium, 2015. [Google Scholar]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical review of antidiabetic drugs: Implications for type 2 diabetes mellitus management. Front. Endocrinol. (Lausanne) 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, K. Über das Vorkommen und den Umsatz von Pyrophosphat in Zellen. I. Mitteilung: Nachweis und Isolierung des Pyrophosphates. Biochem. Z. 1928, 202, 466–493. [Google Scholar]
- Lohmann, K. Über die Pyrophosphatfraktion im Muskel. Naturwissenschaften 1929, 17, 624–625. [Google Scholar]
- Lane, A.N.; Fan, T.W. Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res. 2015, 43, 2466–2485. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic nerves. Pharmacol. Rev. 1972, 24, 509–581. [Google Scholar] [PubMed]
- Bennett, M.R. Autonomic neuromuscular transmission. Monogr. Physiol. Soc. 1972, 30, 1–271. [Google Scholar]
- Burnstock, G. Purine and pyrimidine receptors. Cell. Mol. Life Sci. 2007, 64, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Orriss, I.R.; Key, M.L.; Brandao-Burch, A.; Patel, J.J.; Burnstock, G.; Arnett, T.R. The regulation of osteoblast function and bone mineralisation by extracellular nucleotides: The role of p2x receptors. Bone 2012, 51, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Avanzato, D.; Genova, T.; Fiorio Pla, A.; Bernardini, M.; Bianco, S.; Bussolati, B.; Mancardi, D.; Giraudo, E.; Maione, F.; Cassoni, P.; et al. Activation of P2X7 and P2Y11 purinergic receptors inhibits migration and normalizes tumor-derived endothelial cells via cAMP signaling. Sci. Rep. 2016, 6, 32602. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.P.; Vulchanova, L.; Hargreaves, K.M.; Elde, R.; McCleskey, E.W. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature 1997, 387, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.R.; Baev, A.Y.; Berezhnov, A.V.; Abramov, A.Y. Role of inorganic polyphosphate in mammalian cells: From signal transduction and mitochondrial metabolism to cell death. Biochem. Soc. Trans. 2016, 44, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Tolba, E.; Schröder, H.C.; Wang, X. Polyphosphate: A morphogenetically active implant material serving as metabolic fuel for bone regeneration. Macromol. Biosci. 2015, 15, 1182–1197. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Schröder, H.C.; Müller, W.E.G. Polyphosphate as a metabolic fuel in Metazoa: A foundational breakthrough invention for biomedical applications. Biotechnol. J. 2016, 11, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, J.H.; Choi, S.H.; Smith, S.A. Polyphosphate: An ancient molecule that links platelets, coagulation, and inflammation. Blood 2012, 119, 5972–5979. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Ackermann, M.; Tolba, E.; Neufurth, M.; Wurm, F.; Feng, Q.; Wang, S.; Schröder, H.C.; Müller, W.E.G. Artificial cartilage bio-matrix formed of hyaluronic acid and Mg2+-polyphosphate. Eur. Cell. Mater. 2016, 32, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Lander, N.; Cordeiro, C.; Huang, G.; Docampo, R. Polyphosphate and acidocalcisomes. Biochem. Soc. Trans. 2016, 44, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Tolba, E.; Schröder, H.C.; Wang, S.; Glaßer, G.; Muñoz-Espí, R.; Link, T.; Wang, X.H. A new polyphosphate calcium material with morphogenetic activity. Mater. Lett. 2015, 148, 163–166. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Tolba, E.; Schröder, H.C.; Diehl-Seifert, B.; Wang, X.H. Retinol encapsulated into amorphous Ca2+ polyphosphate nanospheres acts synergistically in MC3T3-E1 cells. Eur. J. Pharm. Biopharm. 2015, 93, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, B.; Schröder, H.C. Mammalian intestinal alkaline phosphatase acts as highly active exopolyphosphatase. Biochim. Biophys. Acta 2001, 1547, 254–261. [Google Scholar] [CrossRef]
- Lippman, F. Metabolic generation and utilization of phosphate bond energy. Adv. Enzymol. 1941, 1, 99–162. [Google Scholar]
- Müller, W.E.G.; Tolba, E.; Feng, Q.; Schröder, H.C.; Markl, J.S.; Kokkinopoulou, M.; Wang, X.H. Amorphous Ca2+ polyphosphate nanoparticles regulate ATP level in bone-like SaOS-2 cells. J. Cell Sci. 2015, 128, 2202–2207. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Ackermann, M.; Wang, S.; Neufurth, M.; Muñoz-Espí, R.; Feng, Q.; Schröder, H.C.; Wang, X.H. Inorganic polyphosphate induces accelerated tube formation of HUVEC endothelial cells. Cell. Mol. Life Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Wang, S.; Neufurth, M.; Kokkinopoulou, M.; Feng, Q.; Schröder, H.C.; Wang, X.H. Polyphosphate as donor of high-energy phosphate for the synthesis of ADP and ATP. J. Cell Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Kubota, N.; Kumagai, H.; Yamaguchi, S.; Kozono, H.; Takahashi, T.; Inoue, M.; Itoh, S.; Takamoto, I.; Sasako, T.; et al. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab. 2011, 13, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.M.; Stella, N. Microglial cell migration stimulated by ATP and C5a involve distinct molecular mechanisms: Quantification of migration by a novel near-infrared method. Glia 2009, 57, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.M.; Stella, N. CB(2) receptor-mediated migration of immune cells: It can go either way. Br. J. Pharmacol. 2008, 153, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Joseph, E.K.; Green, P.G.; Levine, J.D. ATP release mechanisms of endothelial cell-mediated stimulus-dependent hyperalgesia. J. Pain 2014, 15, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, K.; Irino, Y.; Nakamura, Y.; Akazawa, C.; Inoue, K.; Kohsaka, S. Involvement of P2X4 and P2Y12 receptors in ATP-induced microglial chemotaxis. Glia 2007, 55, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, N.; Nagao, T.; Niki, R.; Toyofuku, A.; Tanaka, H.; Kuramoto, Y.; Emoto, Y.; Shibata, H.; Magota, K.; Higuti, T. Possible role of cell surface H+-ATP synthase in the extracellular ATP synthesis and proliferation of human umbilical vein endothelial cells. Mol. Cancer Res. 2003, 1, 931–939. [Google Scholar] [PubMed]
- Honda, S.; Sasaki, Y.; Ohsawa, K.; Imai, Y.; Nakamura, Y.; Inoue, K.; Kohsaka, S. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J. Neurosci. 2001, 21, 1975–1982. [Google Scholar] [PubMed]
- Ho, F.M.; Liu, S.H.; Liau, C.S.; Huang, P.J.; Lin-Shiau, S.Y. High glucose-induced apoptosis in human endothelial cells is mediated by sequential activations of c-Jun NH(2)-terminal kinase and caspase-3. Circulation 2000, 101, 2618–2624. [Google Scholar] [CrossRef] [PubMed]
- Ido, Y.; Carling, D.; Ruderman, N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: Inhibition by the AMP-activated protein kinase activation. Diabetes 2002, 51, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.H.; Wu, H.J. Methylglyoxal and high glucose co-treatment induces apoptosis or necrosis in human umbilical vein endothelial cells. J. Cell. Biochem. 2008, 103, 1144–1157. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Viana, R.; Aguado, C.; Esteban, I.; Moreno, D.; Viollet, B.; Knecht, E.; Sanz, P. Role of AMP-activated protein kinase in autophagy and proteasome function. Biochem. Biophys. Res. Commun. 2008, 369, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Wang, X.H.; Diehl-Seifert, B.; Kropf, K.; Schloßmacher, U.; Lieberwirth, I.; Glasser, G.; Wiens, M.; Schröder, H.C. Inorganic polymeric phosphate/polyphosphate as an inducer of alkaline phosphatase and a modulator of intracellular Ca2+ level in osteoblasts (SaOS-2 cells) in vitro. Acta Biomater. 2011, 7, 2661–2671. [Google Scholar] [CrossRef] [PubMed]
- DeCicco-Skinner, K.L.; Henry, G.H.; Cataisson, C.; Tabib, T.; Gwilliam, J.C.; Watson, N.J.; Bullwinkle, E.M.; Falkenburg, L.; O’Neill, R.C.; Morin, A.; et al. Endothelial cell tube formation assay for the in vitro study of angiogenesis. J. Vis. Exp. 2014, 91, e51312. [Google Scholar] [CrossRef] [PubMed]
- Esch, M.B.; Post, D.J.; Shuler, M.L.; Stokol, T. Characterization of in vitro endothelial linings grown within microfluidic channels. Tissue Eng. Part A 2011, 17, 2965–2971. [Google Scholar] [CrossRef] [PubMed]
- Muscari, C.; Gamberini, C.; Basile, I.; Bonafé, F.; Valgimigli, S.; Capitani, O.; Guarnieri, C.; Caldarera, C.M. Comparison between culture conditions improving growth and differentiation of blood and bone marrow cells committed to the endothelial cell lineage. Biol. Proced. Online 2010, 12, 9023. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner-Parzer, S.M.; Wagner, L.; Pettermann, M.; Grillari, J.; Gessl, A.; Waldhäusl, W. High-glucose-triggered apoptosis in cultured endothelial cells. Diabetes 1995, 44, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Potdar, S.; Kavdia, M. NO/peroxynitrite dynamics of high glucose-exposed HUVECs: Chemiluminescent measurement and computational model. Microvasc. Res. 2009, 78, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.Y.; Oh, Y.J. Glucose levels in culture medium determine cell death mode in MPP+-treated dopaminergic neuronal cells. Exp. Neurobiol. 2015, 24, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Weston, S.A.; Parish, C.R. New fluorescent dyes for lymphocyte migration studies. Analysis by flow cytometry and fluorescence microscopy. J. Immunol. Methods 1990, 133, 87–97. [Google Scholar] [CrossRef]
- Hou, Q.; Lei, M.; Hu, K.; Wang, M. The effects of high glucose levels on reactive oxygen species-induced apoptosis and involved signaling in human vascular endothelial cells. Cardiovasc. Toxicol. 2015, 15, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.B.; Nielsen, S.E.; Berg, K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods 1989, 119, 203–210. [Google Scholar] [CrossRef]
- Sen, T.; Sen, N.; Noordhuis, M.G.; Ravi, R.; Wu, T.C.; Ha, P.K.; Sidransky, D.; Hoque, M.O. OGDHL is a modifier of AKT-dependent signaling and NF-κB function. PLoS ONE 2012, 7, e48770. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Ahmad, A.; Ghosh, M.; Leslie, C.C.; White, C.W. Extracellular ATP-mediated signaling for survival in hyperoxia-induced oxidative stress. J. Biol. Chem. 2004, 279, 16317–16325. [Google Scholar] [CrossRef] [PubMed]
- Orriss, I.R.; Knight, G.E.; Utting, J.C.; Taylor, S.E.; Burnstock, G.; Arnett, T.R. Hypoxia stimulates vesicular ATP release from rat osteoblasts. J. Cell Physiol. 2009, 220, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Marcaida, G.; Miñana, M.D.; Grisolía, S.; Felipo, V. Determination of intracellular ATP in primary cultures of neurons. Brain Res. Brain Res. Protocol. 1997, 1, 75–78. [Google Scholar] [CrossRef]
- Moriwaki, T.; Kato, S.; Kato, Y.; Hosoki, A.; Zhang-Akiyama, Q.M. Extension of lifespan and protection against oxidative stress by an antioxidant herb mixture complex (KPG-7) in Caenorhabditis elegans. J. Clin. Biochem. Nutr. 2013, 53, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Petrie, A.; Watson, P. Statistics for Veterinary and Animal Science; Wiley-Blackwell: Oxford, UK, 2013; pp. 85–99. [Google Scholar]
- Hayashi, T.; Matsui-Hirai, H.; Miyazaki-Akita, A.; Fukatsu, A.; Funami, J.; Ding, Q.F.; Kamalanathan, S.; Hattori, Y.; Ignarro, L.J.; Iguchi, A. Endothelial cellular senescence is inhibited by nitric oxide: Implications in atherosclerosis associated with menopause and diabetes. Proc. Natl. Acad. Sci. USA 2006, 103, 17018–17023. [Google Scholar] [CrossRef] [PubMed]
- Hillen, F.; Griffioen, A.W. Tumour vascularization: Sprouting angiogenesis and beyond. Cancer Metastasis Rev. 2007, 26, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.B.; Savage, V.M.; West, G.B. A quantitative theory of solid tumor growth, metabolic rate and vascularization. PLoS ONE 2011, 6, e22973. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Komada, M.R.; Sane, D.C. Abnormal angiogenesis in diabetes mellitus. Med. Res. Rev. 2003, 23, 117–145. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Relkovic, D.; Ackermann, M.; Wang, S.; Neufurth, M.; Paravic-Radicevic, A.; Ushijima, H.; Schröder, H.C.; Wang, X.H. Enhancement of wound healing in normal and diabetic mice by topical application of amorphous polyphosphate—Superior effect of the host-guest composite material composed of collagen (host) and polyphosphate (guest). Polymers 2017, 9, 300. [Google Scholar] [CrossRef]
- Loots, M.A.; Lamme, E.N.; Mekkes, J.R.; Bos, J.D.; Middelkoop, E. Cultured fibroblasts from chronic diabetic wounds on the lower extremity (non-insulin-dependent diabetes mellitus) show disturbed proliferation. Arch. Dermatol. Res. 1999, 291, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Sivitz, W.I.; Yorek, M.A. Mitochondrial dysfunction in diabetes: From molecular mechanisms to functional significance and therapeutic opportunities. Antioxid. Redox Signal. 2010, 12, 537–577. [Google Scholar] [CrossRef] [PubMed]
- Sakowicz-Burkiewicz, M.; Grden, M.; Maciejewska, I.; Szutowicz, A.; Pawelczyk, T. High glucose impairs ATP formation on the surface of human peripheral blood B lymphocytes. Int. J. Biochem. Cell Biol. 2013, 45, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Dedkova, E.N. Inorganic polyphosphate in cardiac myocytes: From bioenergetics to the permeability transition pore and cell survival. Biochem. Soc. Trans. 2016, 44, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, C.; Saiardi, A. The new world of inorganic polyphosphates. Biochem. Soc. Trans. 2016, 44, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Kumble, K.D.; Kornberg, A. Inorganic polyphosphate in mammalian cells and tissues. J. Biol. Chem. 1995, 270, 5818–5822. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Lertwattanarak, R.; Lefort, N.; Molina-Carrion, M.; Joya-Galeana, J.; Bowen, B.P.; Garduno-Garcia Jde, J.; Abdul-Ghani, M.; Richardson, A.; DeFronzo, R.A.; et al. Reduction in reactive oxygen species production by mitochondria from elderly subjects with normal and impaired glucose tolerance. Diabetes 2011, 60, 2051–2060. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Elustondo, P.A.; Angelova, P.R.; Kawalec, M.; Michalak, M.; Kurcok, P.; Abramov, A.Y.; Pavlov, E.V. Polyhydroxybutyrate targets mammalian mitochondria and increases permeability of plasmalemmal and mitochondrial membranes. PLoS ONE 2013, 8, e75812. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Ackermann, M.; Neufurth, M.; Wang, S.; Li, Q.; Feng, Q.; Schröder, H.C.; Müller, W.E.G. Restoration of Impaired Metabolic Energy Balance (ATP Pool) and Tube Formation Potential of Endothelial Cells under “high glucose”, Diabetic Conditions by the Bioinorganic Polymer Polyphosphate. Polymers 2017, 9, 575. https://doi.org/10.3390/polym9110575
Wang X, Ackermann M, Neufurth M, Wang S, Li Q, Feng Q, Schröder HC, Müller WEG. Restoration of Impaired Metabolic Energy Balance (ATP Pool) and Tube Formation Potential of Endothelial Cells under “high glucose”, Diabetic Conditions by the Bioinorganic Polymer Polyphosphate. Polymers. 2017; 9(11):575. https://doi.org/10.3390/polym9110575
Chicago/Turabian StyleWang, Xiaohong, Maximilian Ackermann, Meik Neufurth, Shunfeng Wang, Qiang Li, Qingling Feng, Heinz C. Schröder, and Werner E. G. Müller. 2017. "Restoration of Impaired Metabolic Energy Balance (ATP Pool) and Tube Formation Potential of Endothelial Cells under “high glucose”, Diabetic Conditions by the Bioinorganic Polymer Polyphosphate" Polymers 9, no. 11: 575. https://doi.org/10.3390/polym9110575
APA StyleWang, X., Ackermann, M., Neufurth, M., Wang, S., Li, Q., Feng, Q., Schröder, H. C., & Müller, W. E. G. (2017). Restoration of Impaired Metabolic Energy Balance (ATP Pool) and Tube Formation Potential of Endothelial Cells under “high glucose”, Diabetic Conditions by the Bioinorganic Polymer Polyphosphate. Polymers, 9(11), 575. https://doi.org/10.3390/polym9110575








