Effects of Lateral and Terminal Chains of X-Shaped Bolapolyphiles with Oligo(phenylene ethynylene) Cores on Self-Assembly Behaviour. Part 1: Transition between Amphiphilic and Polyphilic Self-Assembly in the Bulk
Abstract
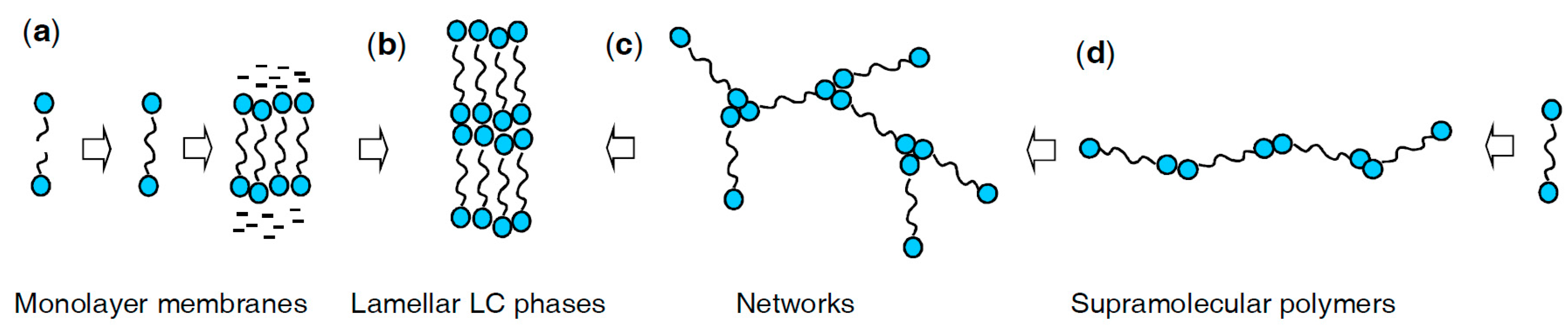
1. Introduction
2. Materials and Methods
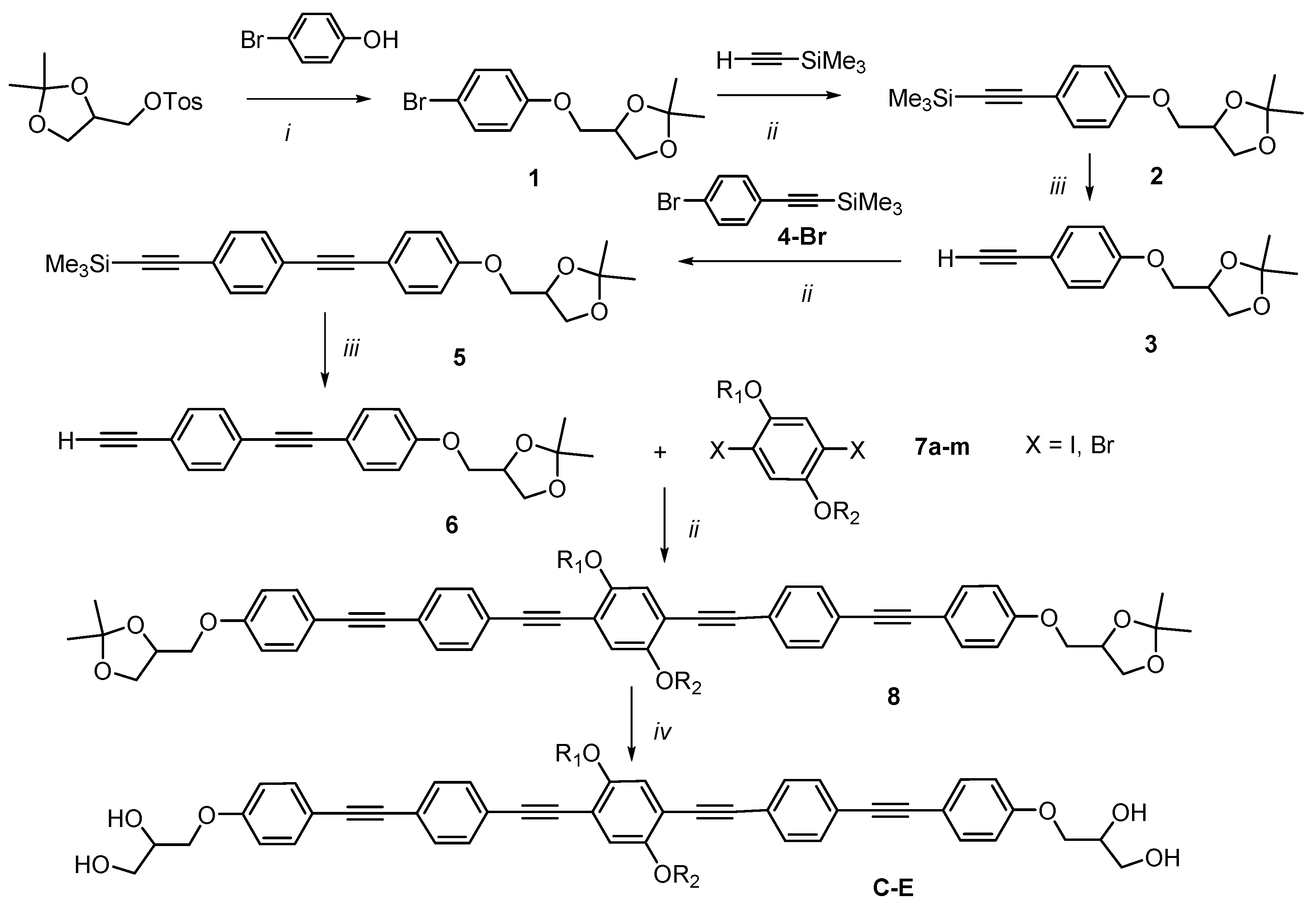
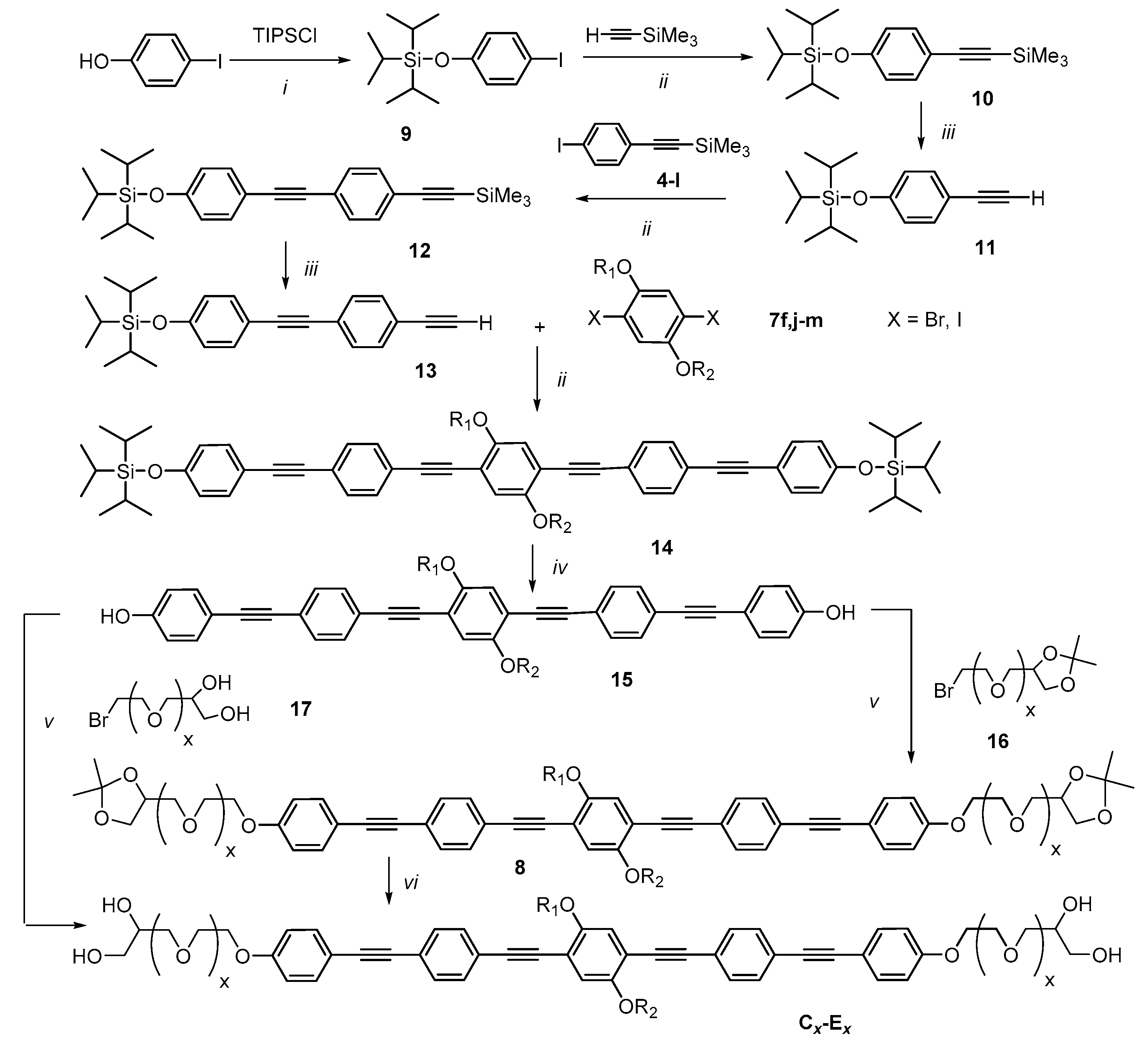
2.1. Synthesis
2.2. Methods
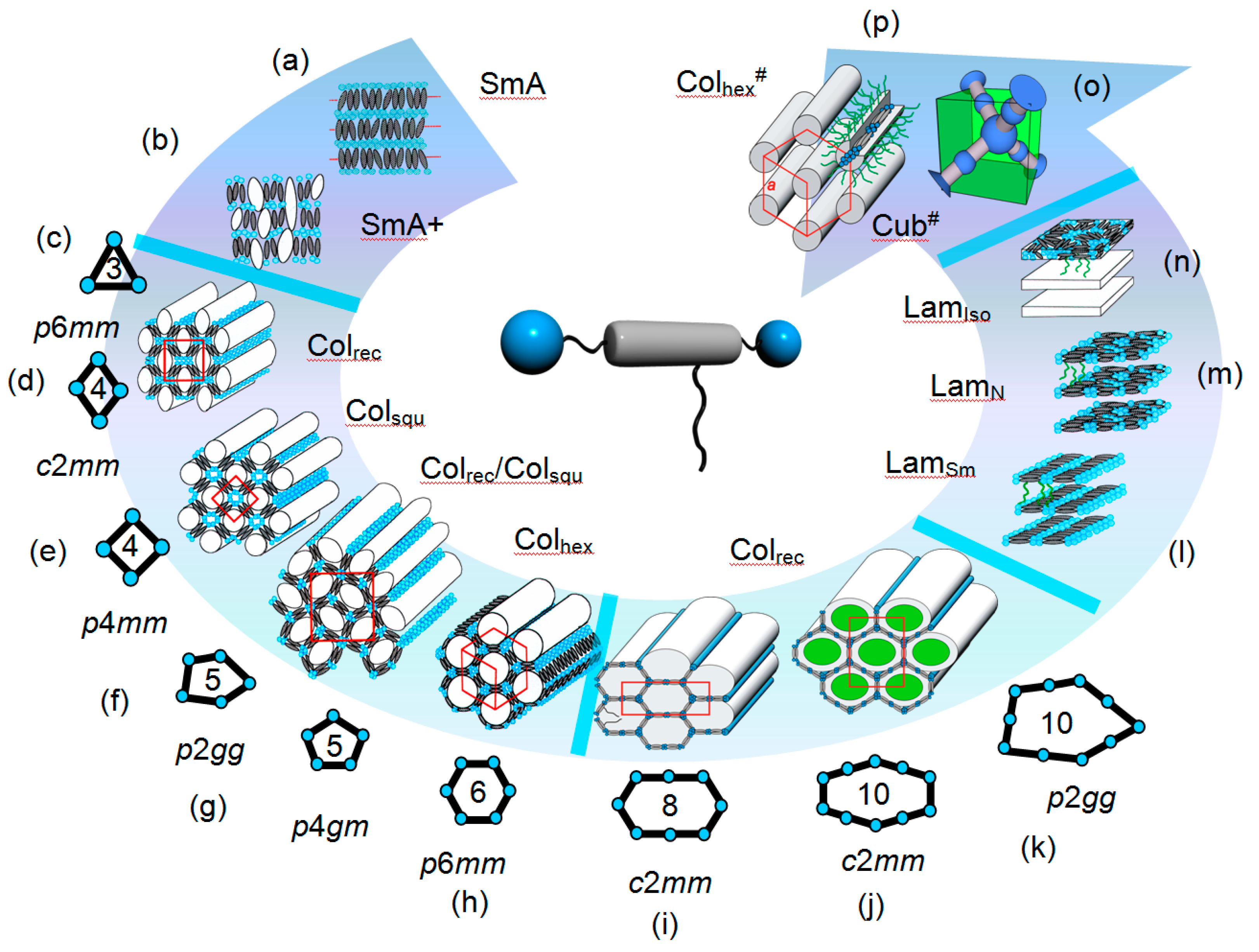
3. Results and Discussion
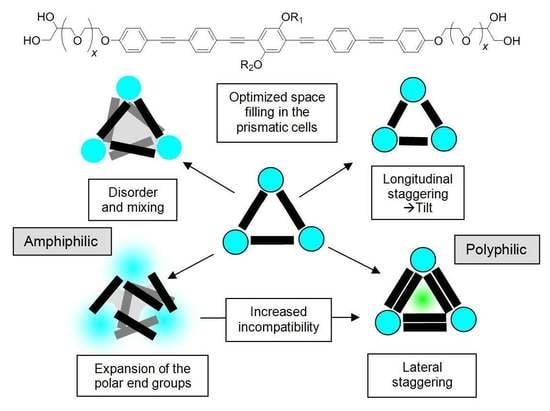
3.1. Effects of Variation of the Lipophilic Lateral Chains
3.2. Variation of the Terminal Polar Groups
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fuhrhop, J.H.; Wang, T. Bolaamphiphiles. Chem. Rev. 2004, 104, 2901–2937. [Google Scholar] [CrossRef] [PubMed]
- Benvegnu, T.; Lemiègre, L.; Cammas-Marion, S. Archaeal lipids: Innovative materials for biotechnological applications. Eur. J. Org. Chem. 2008, 2008, 4725–4744. [Google Scholar] [CrossRef]
- Brunsveld, L.; Folmer, B.J.B.; Meijer, E.W.; Sijbesma, R.P. Supramolecular polymers. Chem. Rev. 2001, 101, 4071–4097. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tan, X.; Wang, Z.; Zhang, X. Supramolecular polymers: Historical development, preparation, characterization, and functions. Chem. Rev. 2015, 115, 7196–7239. [Google Scholar] [CrossRef] [PubMed]
- Binder, W.H.; Zirbs, R. Supramolecular polymers and networks with hydrogen bonds in the main- and side-chain. Adv. Polym. Sci. 2007, 207, 1–78. [Google Scholar]
- Chen, S.; Binder, W.H. Dynamic ordering and phase segregation in hydrogen-bonded polymers. Acc. Chem. Res. 2016, 49, 1409–1420. [Google Scholar] [CrossRef] [PubMed]
- Tschierske, C.; Zaschke, H. Novel thermotropic and lyotropic double headed diol-based mesogens. Chem. Commun. 1990. [Google Scholar] [CrossRef]
- Hentrich, F.; Tschierske, C.; Zaschke, H. Bolaamphiphilic polyols, a novel class of amphotropic liquid crystals. Angew. Chem. Int. Ed. Engl. 1991, 30, 440–441. [Google Scholar] [CrossRef]
- Kato, T.; Mizoshita, N.; Kanie, K. Hydrogen-bonded liquid crystalline materials: Supramolecular polymeric assembly and the induction of dynamic function. Macromol. Rapid Commun. 2001, 22, 797–814. [Google Scholar] [CrossRef]
- Tschierske, C. Molecular self-organization of amphotropic liquid crystals. Prog. Polym. Sci. 1996, 21, 775–852. [Google Scholar] [CrossRef]
- Kölbel, M.; Beyersdorff, T.; Cheng, X.H.; Tschierske, C.; Kain, J.; Diele, S. Design of liquid crystalline block molecules with nonconventional mesophase morphologies: Calamitic bolaamphiphiles with lateral alkyl chains. J. Am. Chem. Soc. 2001, 123, 6809–6818. [Google Scholar] [CrossRef]
- Cheng, X.H.; Prehm, M.; Das, M.K.; Kain, J.; Baumeister, U.; Diele, S.; Leine, D.; Blume, A.; Tschierske, C. Calamitic bolaamphiphiles with (semi)perfluorinated lateral chains: Polyphilic block molecules with new liquid crystalline phase structures. J. Am. Chem. Soc. 2003, 125, 10977–10996. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, R.; Prehm, M.; Glettner, B.; Pelz, K.; Baumeister, U.; Liu, F.; Zeng, X.; Ungar, G.; Tschierske, C. X-shaped polyphilics: Liquid crystal honeycombs with single-molecule walls. Chem. Commun. 2008. [Google Scholar] [CrossRef] [PubMed]
- Tschierske, C. Liquid crystal engineering—New complex mesophase structures and their relations to polymer morphologies, nanoscale patterning and crystal engineering. Chem. Soc. Rev. 2007, 36, 1930–1970. [Google Scholar] [CrossRef] [PubMed]
- Tschierske, C.; Nürnberger, C.; Ebert, H.; Glettner, B.; Prehm, M.; Liu, F.; Zeng, X.B.; Ungar, G. Complex tiling patterns in liquid crystals. Interface Focus 2012, 2, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Ungar, G.; Tschierske, C.; Abetz, V.; Holyst, R.; Bates, M.A.; Liu, F.; Prehm, M.; Kieffer, R.; Zeng, X.; Walker, M.; et al. Self-assembly at different length scales: Polyphilic star-branched liquid crystals and miktoarm star copolymers. Adv. Funct. Mater. 2011, 21, 1296–1323. [Google Scholar] [CrossRef]
- Tschierske, C. Development of structural complexity by liquid-crystal self-assembly. Angew. Chem. Int. Ed. 2013, 52, 8828–8878. [Google Scholar] [CrossRef] [PubMed]
- Crane, A.J.; Martinez-Veracoechea, F.J.; Escobedo, F.A.; Muller, E.A. Molecular dynamics simulation of the mesophase behaviour of a model bolaamphiphilic liquid crystal with a lateral flexible chain. Soft Matter 2008, 4, 1820–1829. [Google Scholar] [CrossRef]
- Bates, M.A.; Walker, M. Dissipative particle dynamics simulation of T- and X-shaped polyphilic molecules exhibiting honeycomb columnar phases. Soft Matter 2009, 5, 346–353. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Glotzer, S.C. Reconfigurable assemblies of shape-changing nanorods. ACS Nano 2010, 4, 2585–2594. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, K.; Guo, H. Dissipative Particle dynamics simulation of the phase behavior of T-Shaped ternary amphiphiles possessing rodlike mesogens. J. Phys. Chem. B 2013, 117, 9106–9120. [Google Scholar] [CrossRef] [PubMed]
- Poppe, S.; Lehmann, A.; Scholte, A.; Prehm, M.; Zeng, X.; Ungar, G.; Tschierske, C. Zeolite-like liquid crystals. Nat. Commun. 2015, 6, 8637. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Kieffer, R.; Glettner, B.; Nürnberger, C.; Liu, F.; Pelz, K.; Prehm, M.; Baumeister, U.; Hahn, H.; Lang, H.; et al. Complex multicolor tilings and critical phenomena in tetraphilic liquid crystals. Science 2011, 331, 1302–1306. [Google Scholar] [CrossRef] [PubMed]
- Glettner, B.; Liu, F.; Zeng, X.; Prehm, M.; Baumeister, U.; Walker, M.; Bates, M.A.; Boesecke, P.; Ungar, G.; Tschierske, C. Liquid-crystalline kagome. Angew. Chem. Int. Ed. 2008, 47, 9063–9066. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Kieffer, R.; Zeng, X.; Pelz, K.; Prehm, M.; Ungar, G.; Tschierske, C. Arrays of giant octagonal and square cylinders by liquid crystalline self-assembly of X-shaped polyphilic molecules. Nat. Commun. 2012, 3, 1104. [Google Scholar] [CrossRef] [PubMed]
- Prehm, M.; Enders, C.; Yahyaee-Anzahaee, M.; Glettner, B.; Baumeister, U.; Tschierske, C. Distinct columnar and lamellar liquid crystalline phases formed by new bolaamphiphiles with linear and branched lateral hydrocarbon chains. Chem. Eur. J. 2008, 14, 6352–6368. [Google Scholar] [CrossRef] [PubMed]
- Prehm, M.; Liu, F.; Baumeister, U.; Zeng, X.; Ungar, G.; Tschierske, C. The giant-hexagon cylinder network—A liquid-crystalline organization formed by a T-shaped quaternary amphiphile. Angew. Chem. Int. Ed. 2007, 46, 7972–7975. [Google Scholar] [CrossRef] [PubMed]
- Prehm, M.; Cheng, X.-H.; Diele, S.; Das, M.-K.; Tschierske, C. New liquid crystalline phases with layerlike organization. J. Am. Chem. Soc. 2002, 124, 12072–12073. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.H.; Das, M.K.; Diele, S.; Tschierske, C. Novel liquid-crystalline phases with layerlike organization. Angew. Chem. Int. Ed. 2002, 41, 4031–4035. [Google Scholar] [CrossRef]
- Patel, N.M.; Rosenblatt, C.; Prehm, M.; Tschierske, C. Possible structures for the lamellar–isotropic (Lam-I) and lamellar–nematic (Lam-N) liquid crystalline phases. Liq. Cryst. 2005, 32, 55–61. [Google Scholar] [CrossRef]
- Chattham, N.; Zhu, C.; Cheng, X.-H.; Limtrakul, J.; Tschierske, C.; Maclennan, J.E.; Clark, N.A. Direct observation of two-dimensional nematic and smectic ordering in freely suspended films of a bolaamphiphilic liquid crystal. Soft Matter 2011, 7, 9978–9982. [Google Scholar] [CrossRef]
- Liu, F.; Prehm, M.; Zeng, X.; Tschierske, C.; Ungar, G. Skeletal cubic, lamellar, and ribbon phases of bundled thermotropic bolapolyphiles. J. Am. Chem. Soc. 2014, 136, 6846–6849. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Prehm, M.; Ungar, G.; Tschierske, C.; Liu, F. Formation of a double diamond cubic phase by thermotropic liquid crystalline self-assembly of bundled bolaamphiphiles. Angew. Chem. Int. Ed. 2016, 55, 8324–8327. [Google Scholar] [CrossRef] [PubMed]
- Prehm, M.; Liu, F.; Zeng, X.; Ungar, G.; Tschierske, C. Axial-bundle phases—New modes of 2D, 3D, and helical columnar self-assembly in liquid crystalline phases of bolaamphiphiles with swallow tail lateral chains. J. Am. Chem. Soc. 2011, 133, 4906–4916. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Prehm, M.; Zeng, X.; Ungar, C.; Tschierske, C. Two- and three-dimensional liquid-crystal phases from axial bundles of rodlike polyphiles: Segmented cylinders, crossed columns, and ribbons between sheets. Angew. Chem. Int. Ed. 2011, 50, 10599–10602. [Google Scholar] [CrossRef] [PubMed]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Guo, J.; Kim, J.; Ihee, H.; Jiang, D. A belt-shaped, blue luminescent, and semiconducting covalent organic framework. Angew. Chem. Int. Ed. 2008, 47, 8826–8830. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Wang, P.; Huang, N. Covalent organic frameworks: A materials platform for structural and functional designs. Nat. Rev. Mater. 2016, 1, 16068. [Google Scholar] [CrossRef]
- Urgel, J.I.; Écija, D.; Lyu, G.; Zhang, R.; Palma, C.A.; Auwärter, W.; Lin, N.; Barth, J.V. Quasicrystallinity expressed in two-dimensional coordination networks. Nat. Chem. 2016, 8, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Mali, K.S.; Pearce, N.; De Feyter, S.; Champness, N.R. Frontiers of supramolecular chemistry at solid surfaces. Chem. Soc. Rev. 2017, 46, 2520–2542. [Google Scholar] [CrossRef] [PubMed]
- Mahadevi, A.S.; Sastry, G.N. Cooperativity in noncovalent Interactions. Chem. Rev. 2016, 116, 2775–2825. [Google Scholar] [CrossRef] [PubMed]
- Tebben, L.; Meck-Lichtenfeld, C.; Fernandez, G.; Grimme, S.; Studer, A. From Additivity to cooperativity in chemistry: Can cooperativity be measured? Chem. Eur. J. 2017, 23, 5864–5873. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Ebert, H.; Lechner, B.-D.; Lange, F.; Achilles, A.; Bärenwald, R.; Poppe, S.; Blume, A.; Saalwächter, K.; Tschierske, C.; et al. Dendritic domains with hexagonal symmetry formed by X-shaped bolapolyphiles in lipid membranes. Chem. Eur. J. 2015, 21, 8840–8850. [Google Scholar] [CrossRef] [PubMed]
- Lechner, B.-D.; Ebert, H.; Prehm, M.; Werner, S.; Meister, A.; Hause, G.; Beerlink, A.; Saalwächter, K.; Bacia, K.; Tschierske, C.; et al. Temperature-dependent in-plane structure formation of an X-shaped bolapolyphile within lipid bilayers. Langmuir 2015, 31, 2839–2850. [Google Scholar] [CrossRef] [PubMed]
- Achilles, A.; Bärenwald, R.; Lechner, B.-D.; Werner, S.; Ebert, H.; Tschierske, C.; Blume, A.; Bacia, K.; Saalwächter, K. Self-assembly of X-shaped bolapolyphiles in lipid membranes: Solid-state NMR investigations. Langmuir 2016, 32, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Poppe, M.; Chen, C.; Ebert, H.; Poppe, S.; Prehm, M.; Kerzig, K.; Liu, F.; Tschierske, C. Transition from nematic to gyroid-type cubic soft self-assembly by side-chain engineering of π-conjugated sticky rods. Soft Matter 2017, 13, 4381–4392. [Google Scholar] [CrossRef] [PubMed]
- Poppe, M.; Chen, C.; Liu, F.; Prehm, M.; Poppe, S.; Tschierske, C. Emergence of tilt in square honeycomb liquid crystals. Soft Matter 2017, 13, 4676–4680. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Dong, X.; Wei, G.; Prehm, M.; Tschierske, C. Liquid-crystalline triangle honeycomb formed by a dithiophene-based X-shaped bolaamphiphile. Angew. Chem. Int. Ed. 2009, 48, 8014–8017. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Gao, H.; Tan, X.; Yang, X.; Prehm, M.; Ebert, H.; Tschierske, C. Transition between triangular and square tiling patterns in liquid-crystalline honeycombs formed by tetrathiophene-based bolaamphiphiles. Chem. Sci. 2013, 4, 3317–3331. [Google Scholar] [CrossRef]
- Chen, B.; Baumeister, U.; Diele, S.; Das, M.K.; Zeng, X.; Ungar, G.; Tschierske, C. A new type of square columnar liquid crystalline phases formed by facial amphiphilic triblock molecules. J. Am. Chem. Soc. 2004, 126, 8608–8609. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Kim, Y.; Lee, M. Intelligent supramolecular assembly of aromatic block molecules in aqueous solution. Nanoscale 2013, 5, 7711–7723. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, Y.; Hayashida, K.; Takano, A. Jewelry Box of morphologies with mesoscopic length scales—ABC star-shaped terpolymers. Macromol. Rapid Commun. 2010, 31, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Abetz, V.; Simon, P.F.W. Phase behaviour and morphologies of block copolymers. Adv. Polym. Sci. 2005, 189, 125–212. [Google Scholar]
- Meuler, A.J.; Hillmyer, M.A.; Bates, F.S. Ordered network mesostructures in block polymer materials. Macromolecules 2009, 42, 7221–7250. [Google Scholar] [CrossRef]
- Sioula, S.; Hadjichristidis, N.; Thomas, E.L. Direct evidence for confinement of junctions to lines in an 3 miktoarm star terpolymer microdomain structure. Macromolecules 1998, 31, 8429–8432. [Google Scholar] [CrossRef]
- Liu, F.; Chen, B.; Baumeister, U.; Zeng, X.; Ungar, G.; Tschierske, C. The triangular cylinder phase: A new mode of self-assembly in liquid-crystalline soft matter. J. Am. Chem. Soc. 2007, 129, 9578–9579. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zeng, X.; Baumeister, U.; Ungar, G.; Tschierske, C. Liquid crystalline networks composed of pentagonal, square, and triangular cylinders. Science 2005, 307, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Hädicke, A.; Blume, A. Interactions of Pluronic block copolymers with lipid vesicles depend on lipid phase and Pluronic aggregation state. Colloid Polym. Sci. 2014, 293, 267–276. [Google Scholar] [CrossRef]
- Schulz, M.; Werner, S.; Bacia, K.; Binder, W.H. Controlling molecular recognition with lipid/polymer domains in vesicle membranes. Angew. Chem. Int. Ed. 2013, 52, 1829–1833. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, T.; Kumar Allampally, N.; Gustavo Fernández, G.; Schacher, F.H. Controlling aqueous self-assembly mechanisms by hydrophobic interactions. Chem. Eur. J. 2014, 20, 13871–13875. [Google Scholar] [CrossRef] [PubMed]
- Bull, S.R.; Palmer, L.C.; Fry, N.J.; Greenfield, M.A.; Messmore, B.W.; Meade, T.J.; Stupp, S.I. A templating approach for monodisperse self-assembled organic nanostructures. J. Am. Chem. Soc. 2008, 130, 2742–2743. [Google Scholar] [CrossRef] [PubMed]
- Sakai, N.; Matile, S.; Mareda, J. Rigid-rod molecules in biomembrane models: From hydrogen-bonded chains to synthetic multifunctional pores. Acc. Chem. Res. 2005, 38, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Sakai, N.; Brennan, K.C.; Weiss, L.A.; Matile, S. Toward biomimetic ion channels formed by rigid-rod molecules: Length-dependent ion-transport activity of substituted oligo(p-phenylene)s. J. Am. Chem. Soc. 1997, 119, 8726–8727. [Google Scholar] [CrossRef]
- Dambenieks, A.K.; Vu, P.H.Q.; Fyles, T.M. Dissipative assembly of a membrane transport System. Chem. Sci. 2014, 5, 3396–3403. [Google Scholar] [CrossRef]
- Muraoka, T.; Shima, T.; Hamada, T.; Morita, M.; Takagi, M.; Tabata, K.V.; Noji, H.; Kinbara, K. Ion permeation by a folded multiblock amphiphilic oligomer achieved by hierarchical construction of self-assembled nanopores. J. Am. Chem. Soc. 2012, 134, 19788–19794. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, R.; Gokel, G.W. Membrane-length amphiphiles exhibiting structural simplicity and ion channel activity. Chem. Eur. J. 2009, 15, 10543–10553. [Google Scholar] [CrossRef] [PubMed]
- Nikolaus, J.; Czapla, S.; Möllnitz, K.; Höfer, C.T.; Herrmann, A.; Wessig, P.; Müller, P. New molecular rods —Characterization of their interaction with membranes. Biochim. Biophys. Acta 2011, 1808, 2781–2788. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Nikolaus, J.; Schiller, S.; Herrmann, A.; Möllnitz, K.; Czapla, S.; Wessig, P. Molecular rods with oligospiroketal backbones as anchors in biological membranes. Angew. Chem. Int. Ed. 2009, 48, 4433–4435. [Google Scholar] [CrossRef] [PubMed]
- Rolland, J.-P.; Santaella, C.; Monasse, B.; Vierling, P. Miscibility of binary mixtures of highly fluorinated double-chain glycerophosphocholines and 1,2-dipalmitoylphosphatidylcholine (DPPC). Chem. Phys. Lipids 1997, 85, 135–143. [Google Scholar] [CrossRef]
- Peschel, C.; Brehm, M.; Sebastiani, D. Polyphilic interactions as structural driving force investigated by molecular dynamics simulation (Project 7). Polymers 2017, 9, 445. [Google Scholar] [CrossRef]
- Sakai, N.; Matile, S. Synthetic ion channels. Langmuir 2013, 29, 9031–9040. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L. The Fluid—Mosaic model of membrane structure: Still relevant to understanding the structure, function and dynamics of biological membranes after more than 40 years. Biochim. Biophys. Acta 2014, 1838, 1451–1466. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, R.F.M.; Joly, E. Crystallization around solid-like nanosized docks can explain the specificity, diversity, and stability of membrane microdomains. Front. Plant Sci. 2014, 5, 72. [Google Scholar] [CrossRef] [PubMed]
- Bagatolli, L.A.; Ipsen, J.H.; Simonsen, A.C.B.; Mouritsen, O.G. An outlook on organization of lipids in membranes: Searching for a realistic connection with the organization of biological membranes. Prog. Lipid Res. 2010, 49, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.R.; Regen, S.L. The structural role of cholesterol in cell membranes: From condensed bilayers to lipid rafts. Acc. Chem. Res. 2014, 47, 3512–3521. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Trillo, F.; Grover, L.M.; Stephenson-Brown, A.; Harrison, P.; Mendes, P.M. Vesicles in nature and the laboratory: Elucidation of their biological properties and synthesis of increasingly complex synthetic vesicles. Angew. Chem. Int. Ed. 2017, 56, 3142–3160. [Google Scholar] [CrossRef] [PubMed]
- Goñi, F.M. Alpha-helices in channels and membrane proteins: The basic structure and dynamics of cell membranes: An update of the Singer–Nicolson model. Biochim. Biophys. Acta 2014, 1838, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Yeagle, P.L. Non-covalent binding of membrane lipids to membrane proteins. Biochim. Biophys. Acta 2014, 1838, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- Doval, D.A.; Areephong, J.; Bang, E.-K.; Bertone, L.; Charbonnaz, P.; Fin, A.; Lin, N.-T.; Lista, M.; Matile, S.; Montenegro, J.; et al. Recent progress with functional biosupramolecular systems. Langmuir 2011, 27, 9696–9705. [Google Scholar] [CrossRef] [PubMed]
- Arrhenius, T.S.; Blanchard-Desce, M.; Dvolaitzky, M.; Lehn, J.-M.; Malthete, J. Molecular devices: Caroviologens as an approach to molecular wires-synthesis and incorporation into vesicle membranes (carotenoids/bispyridinium polyenes/electron channel). Proc. Natl. Acad. Sci. USA 1986, 83, 5355–5359. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Ebenhan, J.; Poppe, M.; Poppe, S.; Ebert, H.; Tschierske, C.; Bacia, K. Effects of lateral and terminal chains of X-shaped bolapolyphiles with oligo(phenylene ethynylene) cores on self-assembly behavior. Part 2: Domain formation by self-assembly in lipid bilayer membranes. Polymers 2017, 9, 476. [Google Scholar] [CrossRef]
- Sonogashira, K.; Tohda, Y.; Hagihara, N. A convenient synthesis of acetylenes: Catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett. 1975, 50, 4467–4470. [Google Scholar] [CrossRef]
- Kölbel, M.; Beyersdorff, T.; Tschierske, C.; Diele, S.; Kain, J. Thermotropic an lyotropic crystalline phases of rigid aromatic amphiphiles. Chem. Eur. J. 2000, 6, 3821–3837. [Google Scholar] [CrossRef]
- Glettner, B.; Liu, F.; Zeng, X.; Prehm, M.; Baumeister, U.; Ungar, G.; Tschierske, C. Liquid-crystal engineering with anchor-shaped molecules: Honeycombs with hexagonal and trigonal symmetries formed by polyphilic bent-core molecules. Angew. Chem. Int. Ed. 2008, 47, 6080–6083. [Google Scholar] [CrossRef] [PubMed]
- Austin, W.; Bilow, N.; Kelleghan, W.; Lau, K. Facile synthesis of ethynylated benzoic acid derivatives and aromatic compounds via ethynyltrimethylsilane. J. Org. Chem. 1981, 46, 2280–2286. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Baldrighi, M.; Desper, J.; Metrangolo, P.; Resnati, G. Supramolecular hierarchy among halogen-bond donors. Chem. Eur. J. 2013, 19, 16240–16247. [Google Scholar] [CrossRef] [PubMed]
- Selvaraju, S.; Sachinthani, N.; Hopson, R.; MacFarland, F.; Guo, S.; Rheingold, A.; Nelson, T. Eumelanin-inspired core derived from vanillin: A new building block for organic semiconductors. Chem. Commun. 2015, 51, 2957–2959. [Google Scholar] [CrossRef] [PubMed]
- Masai, H.; Terao, J.; Seki, S.; Nakashima, S.; Kiguchi, M.; Okoshi, K.; Fujihara, T.; Tsuji, Y. Synthesis of one-dimensional metal-containing insulated molecular wire with versatile properties directed towards molecular electronics materials. J. Am. Chem. Soc. 2014, 136, 1742–1745. [Google Scholar] [CrossRef] [PubMed]
- van Rijsbergen, R.; Anteunis, M.-J.; de Bruyn, A. Selective removal of the isopropylidene group in 4-O-protected 1,6-anhydro-2,3-O-isopropylidene-β-d-mannopyranose and the conformational impact of it. J. Carbohydr. Chem. 2006, 2, 395–404. [Google Scholar] [CrossRef]
- Pokholenko, O.; Gissot, A.; Vialet, B.; Bathany, K.; Thiéry, A.; Barthélémy, P. Lipid oligonucleotide conjugates as responsive nanomaterials for drug delivery. J. Mater. Chem. B 2013, 1, 5329–5334. [Google Scholar] [CrossRef]
- Krapcho, A.P.; Weimaster, J.F.; Eldridge, J.M.; Jahngen, E.G.E., Jr.; Loves, A.J.; Stephens, W.P. Synthetic applications and mechanism studies of the decarbalkoxylations of germinal esters and related systems effected in dimethyl sulfoxide by water and/or by water with added salts. J. Org. Chem. 1978, 43, 138–147. [Google Scholar] [CrossRef]
- Nystrom, R.F.; Brown, W.G. Reduction of organic compounds by lithium aluminium hydride. I. Aldehydes, ketones, esters, acid chlorides and acid anhydrides. J. Am. Chem. Soc. 1947, 69, 1197–1199. [Google Scholar]
- Dakka, G.; Sasson, Y. Selective hydrobromination of branched alcohols using phase transfer catalysis. Tetrahedron Lett. 1987, 28, 1223–1224. [Google Scholar] [CrossRef]
- Williamson, A. Theory of etherification. Philos. Mag. 1850, 3, 350–356. [Google Scholar]
- Ishihara, T.; Kuroboshi, M.; Okada, Y. New efficient palladium-catalyzed perfluoroalkylation of carbon-carbon multiple bonds with F-alkyl iodides. An expedient route to f-alkylated alkyl and alkenyl iodides. Chem. Lett. 1986, 15, 1895–1896. [Google Scholar] [CrossRef]
- Hart, M.E.; Suchland, K.L.; Miyakawa, M.; Bunzow, J.R.; Grandy, D.K.; Scanlan, T.S. Trace Amine-associated receptor agonists: Synthesis and evaluation of thyronamines and related analogues. J. Med. Chem. 2006, 49, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Hacksell, U.; Doyle Daves, G. Facile synthesis of 2′-deoxy-3′-kezo- and 2′-deoxypseudouridine derivatives and analogues. Palladium(II)-mediated coupling reactions of furanoid glycals. J. Org. Chem. 1986, 51, 3093–3098. [Google Scholar] [CrossRef]
- Le Huérou, Y.; Doyon, J.; Grée, R.L. Stereocontrolled synthesis of key advanced intermediates toward simplified acetogenin analogues. J. Org. Chem. 1999, 64, 6782–6790. [Google Scholar] [CrossRef] [PubMed]
- Tamiakia, H.; Ogawaa, K.; Enomotob, K.; Takia, K.; Hottab, A.; Tomaa, K. Supramolecular gelation of alcohol and water by synthetic amphiphilic gallic acid derivatives. Tetrahedron 2010, 66, 1661–1666. [Google Scholar] [CrossRef]
- Bird, P.R.; Chadha, J.S. The synthesis of diacyl-l-glycerol bromohydrins. Tetrahedron Lett. 1966, 38, 4541–4546. [Google Scholar] [CrossRef]
- Wershofen, S.; Classen, A.; Scharf, H.D. Stereoselective acetalization of 1,1- and 1,2-disubstituted diols as common principle in the synthesis of the enantiomers of the bicyclic acetal pheromones endo- and exo-brevicomin and frontalin. Eur. J. Org. Chem. 1989, 1, 9–18. [Google Scholar] [CrossRef]
- Immirzi, A.; Perini, B. Prediction of density in organic crystals. Acta Crystallogr. Sect. A 1977, 33, 216–218. [Google Scholar] [CrossRef]
- Kitaigorodski, A.I. Molekülkristalle; Akademie Verlag: Berlin, Germany, 1979. [Google Scholar]
- Tschierske, C. Microsegregation: From basic concepts to complexity in liquid crystal self-assembly. Isr. J. Chem. 2012, 52, 935–959. [Google Scholar] [CrossRef]
- Tschierske, C. Fluorinated liquid crystals: Design of soft nanostructures and increased complexity of self-assembly by perfluorinated segments. Top. Curr. Chem. 2012, 318, 1–108. [Google Scholar] [PubMed]
- Tschierske, C. Microsegregation in liquid crystalline systems: Basic concepts. In Handbook of Liquid Crystals, 2nd ed.; Goodby, J.W., Collings, P.J., Kato, T., Tschierske, C., Gleeson, H.F., Raynes, P., Eds.; Wiley-VCH: Weinheim, Germany, 2014; Volume 5, pp. 1–43. [Google Scholar]
- Branca, C.; Magazù, S.; Maisano, G.; Migliardo, F.; Migliardo, P.; Romeo, G.; Vertessy, B. Conformational studies of poly(ethylene oxide) in crystalline, molten, and solution phase. Mol. Cryst. Liq. Cryst. 2001, 372, 17–23. [Google Scholar] [CrossRef]
- Tschierske, C. Non-conventional liquid crystals—The importance of micro-segregation for self-organisation. J. Mater. Chem. 1998, 8, 1485–1508. [Google Scholar] [CrossRef]
- Sinturel, C.; Bates, F.S.; Hillmyer, M.A. High χ−Low N block polymers: How far can we go? ACS Macro Lett. 2015, 4, 1044–1050. [Google Scholar] [CrossRef]
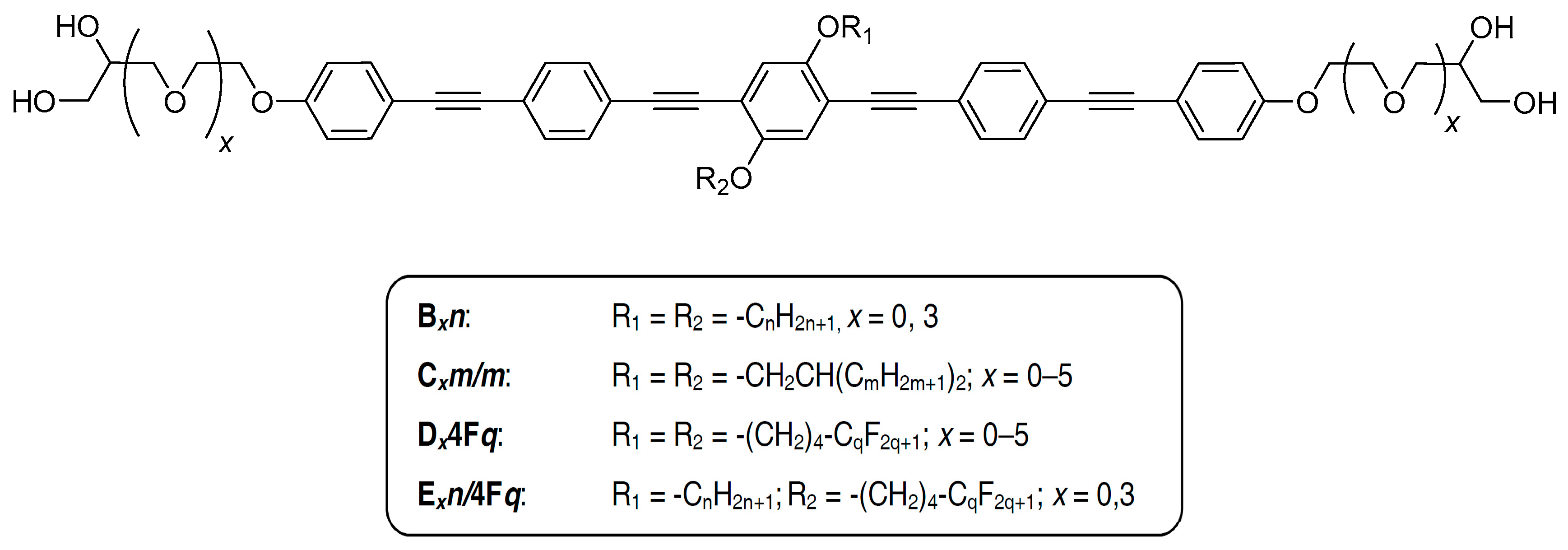
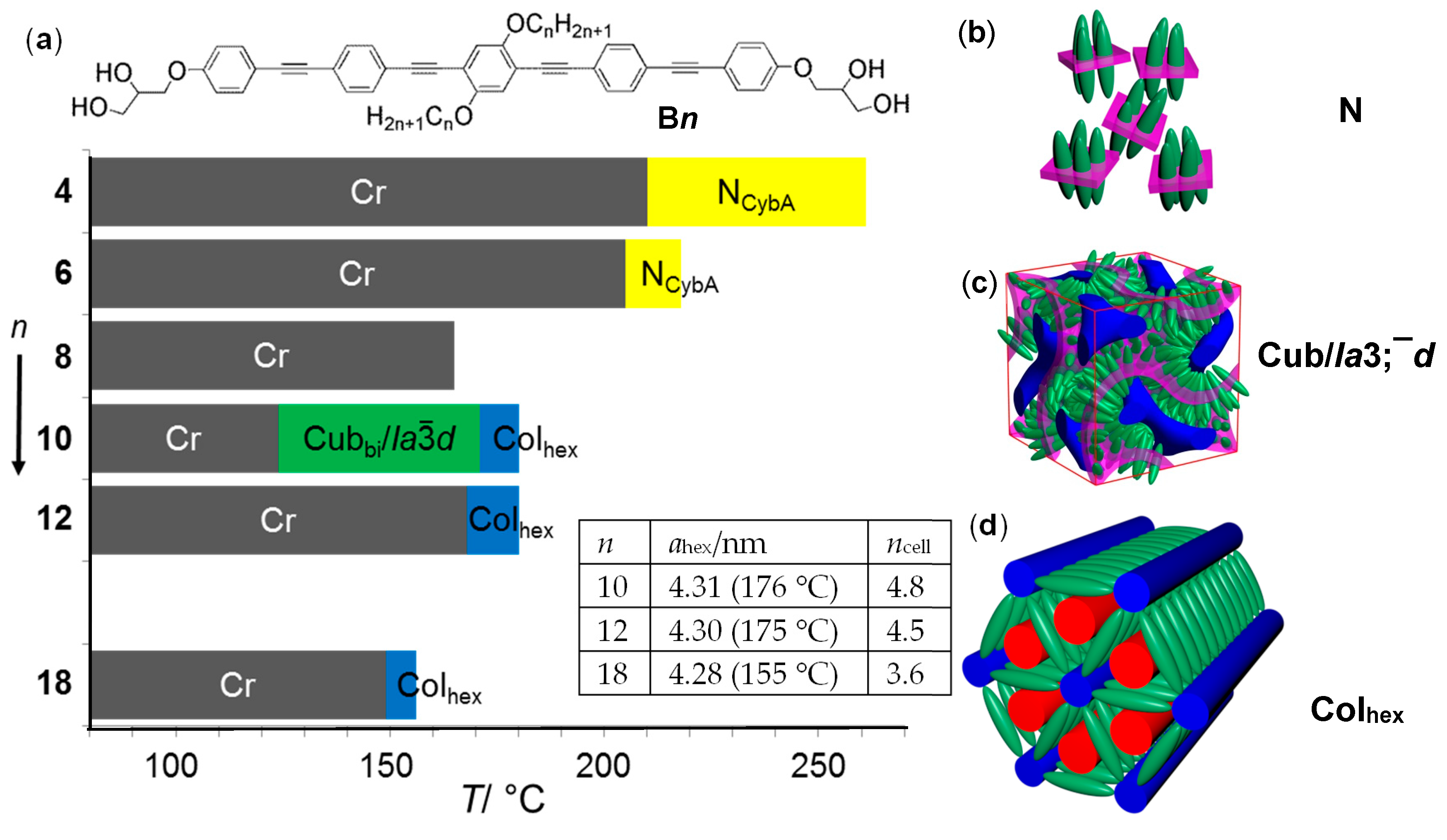
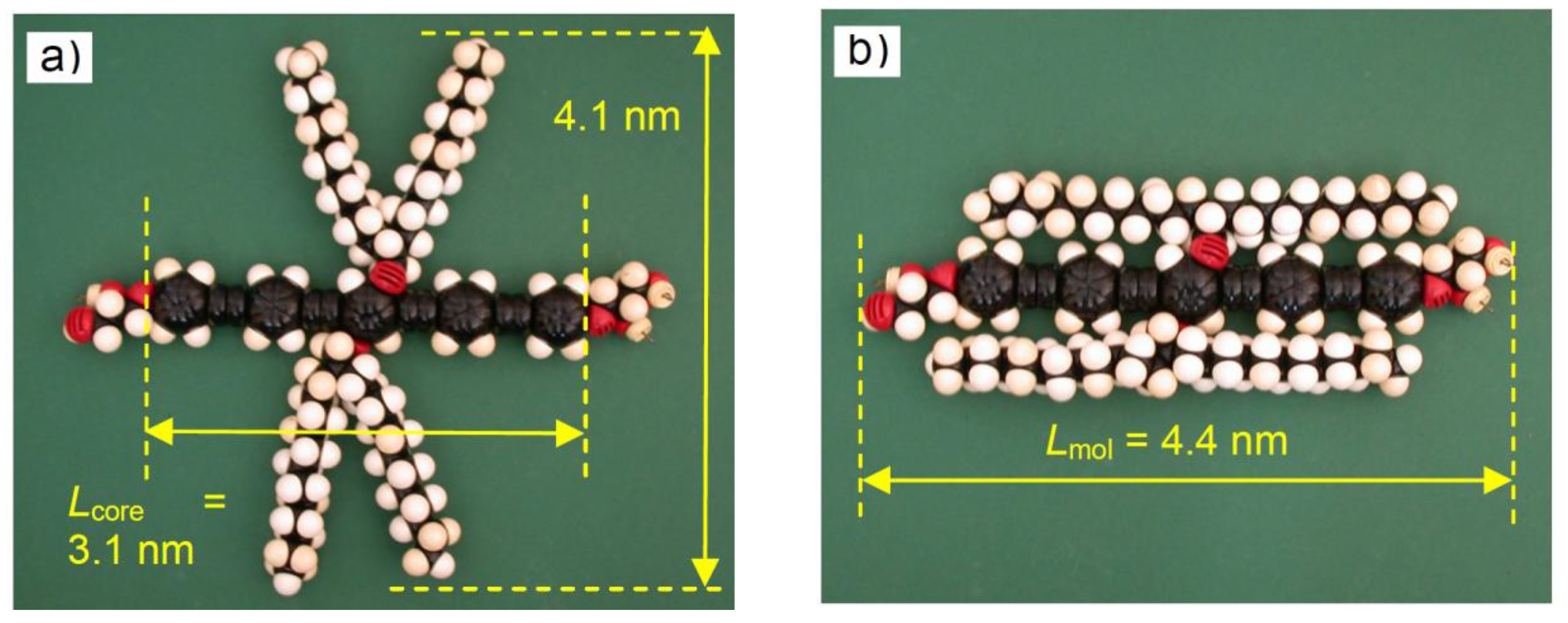
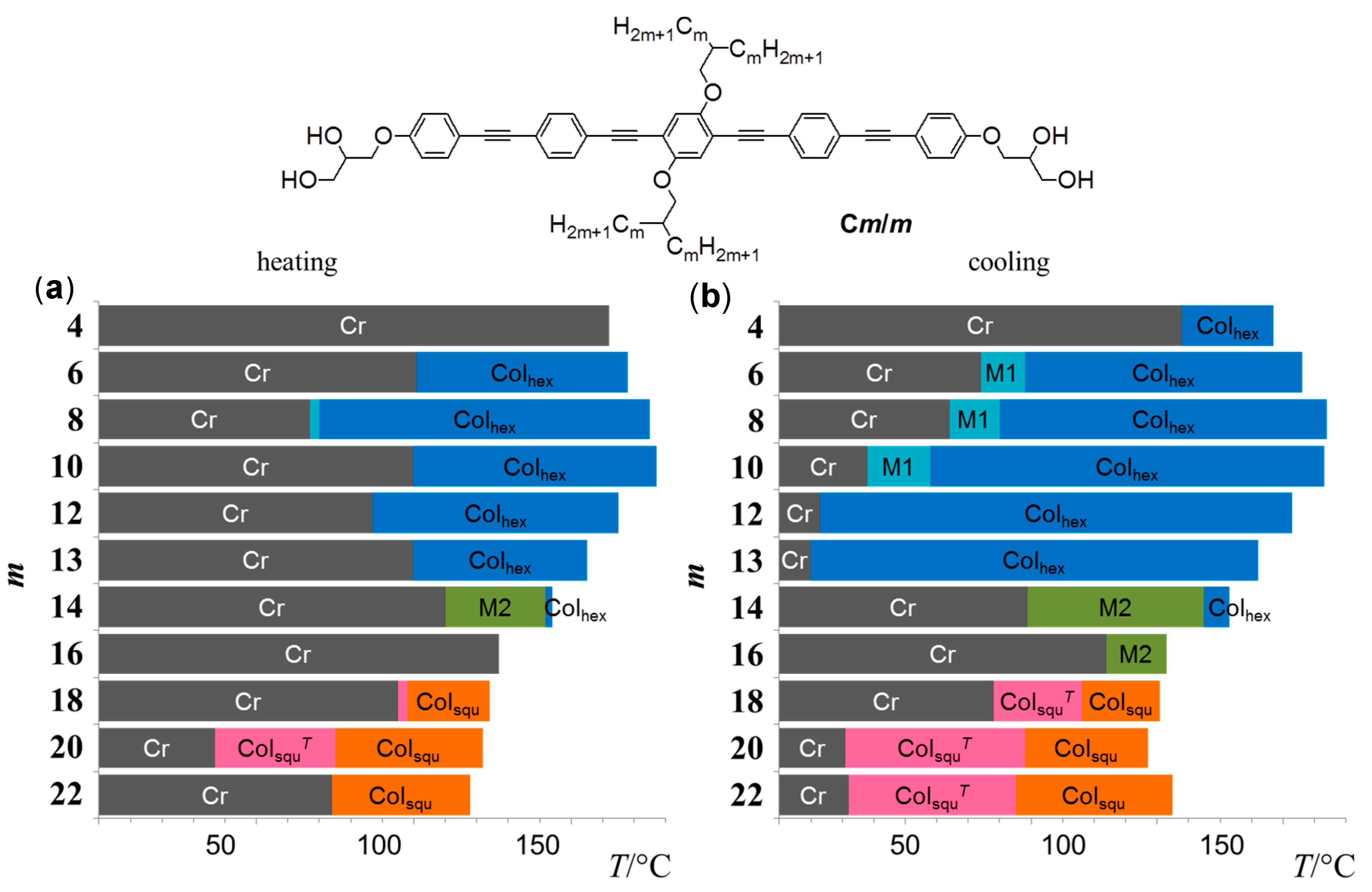
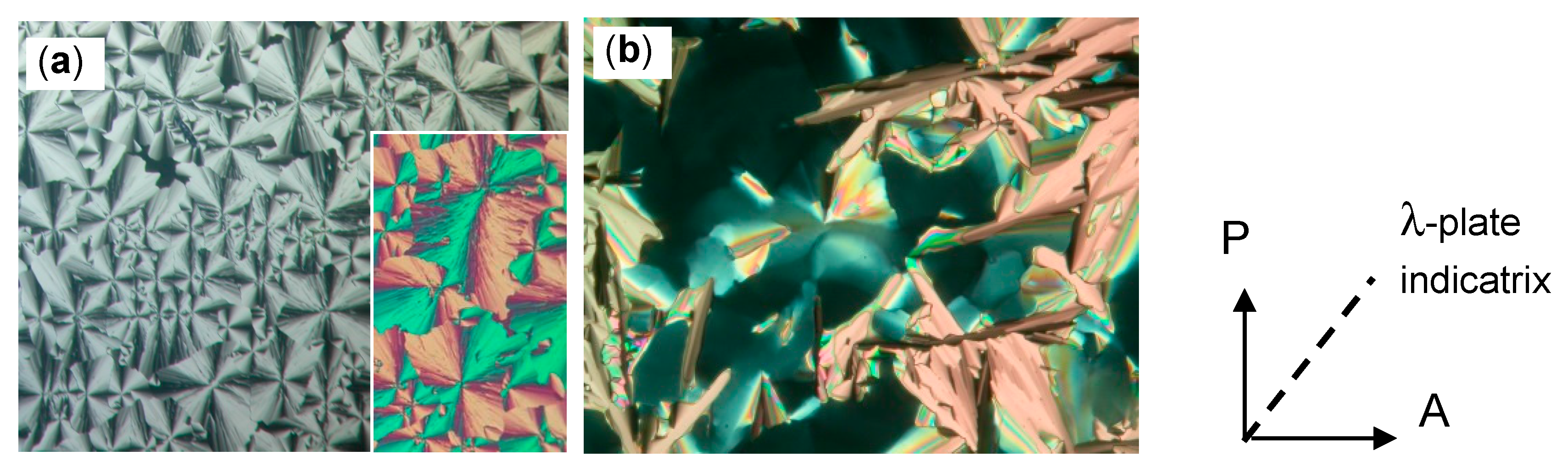
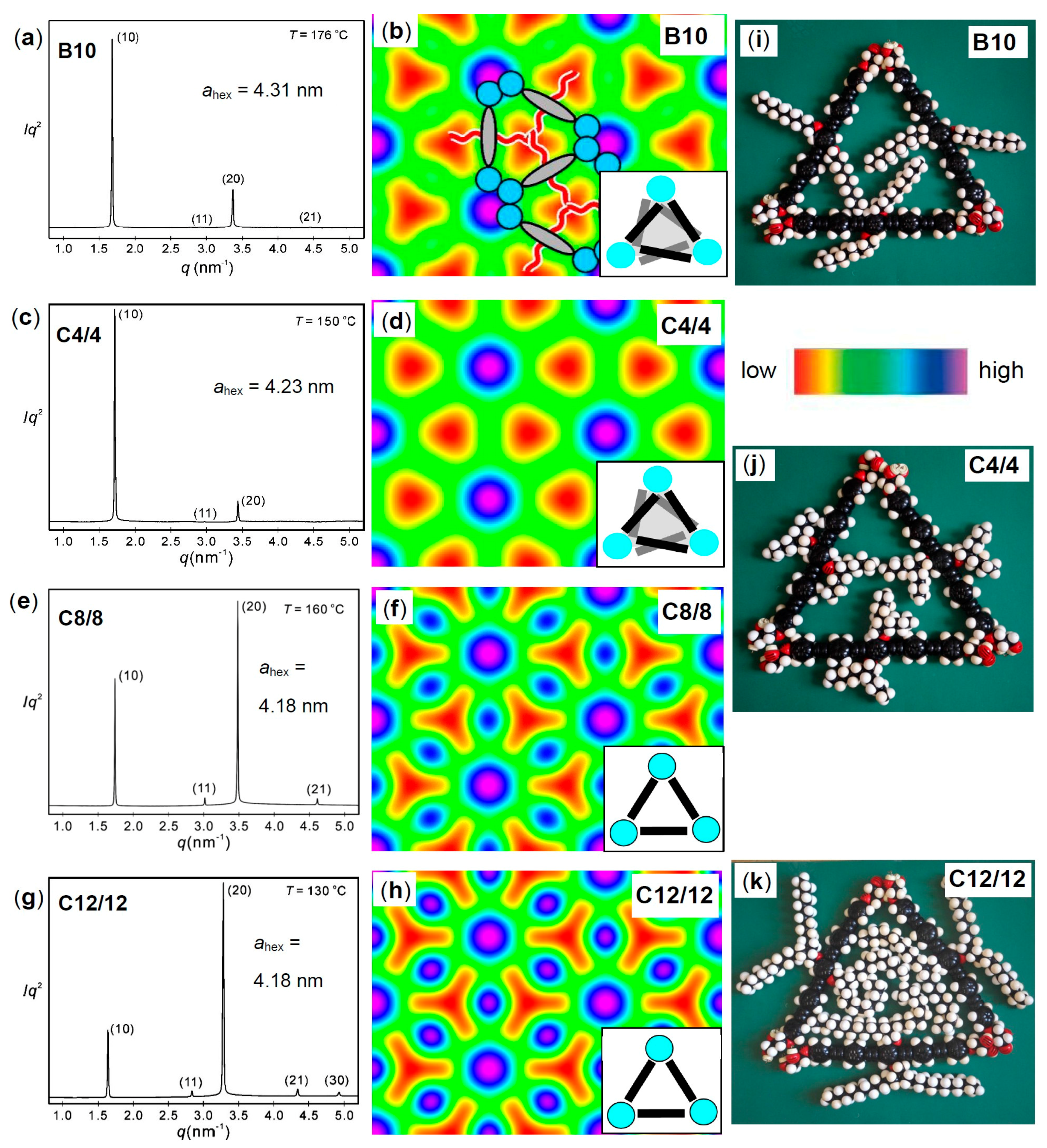
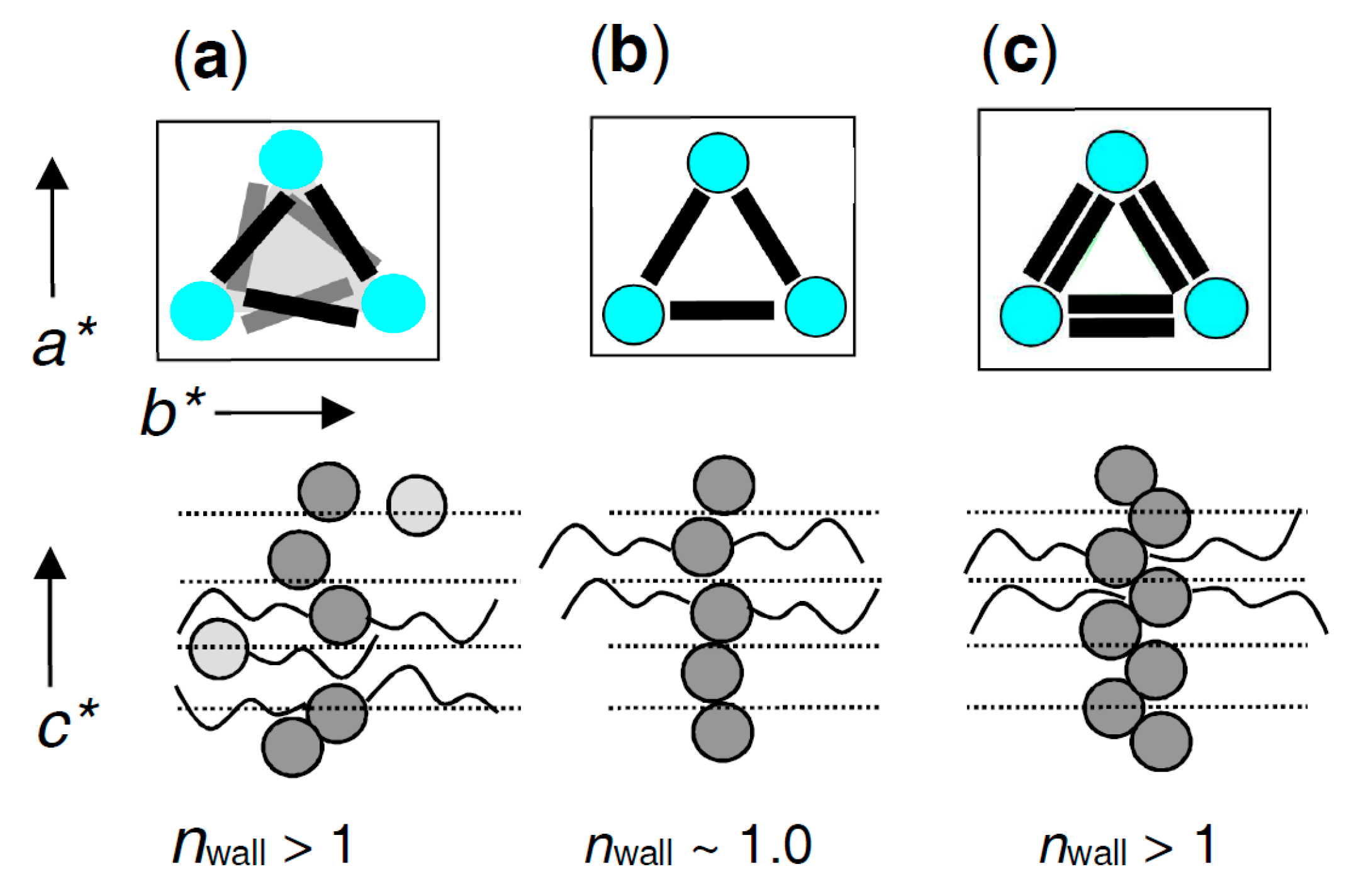
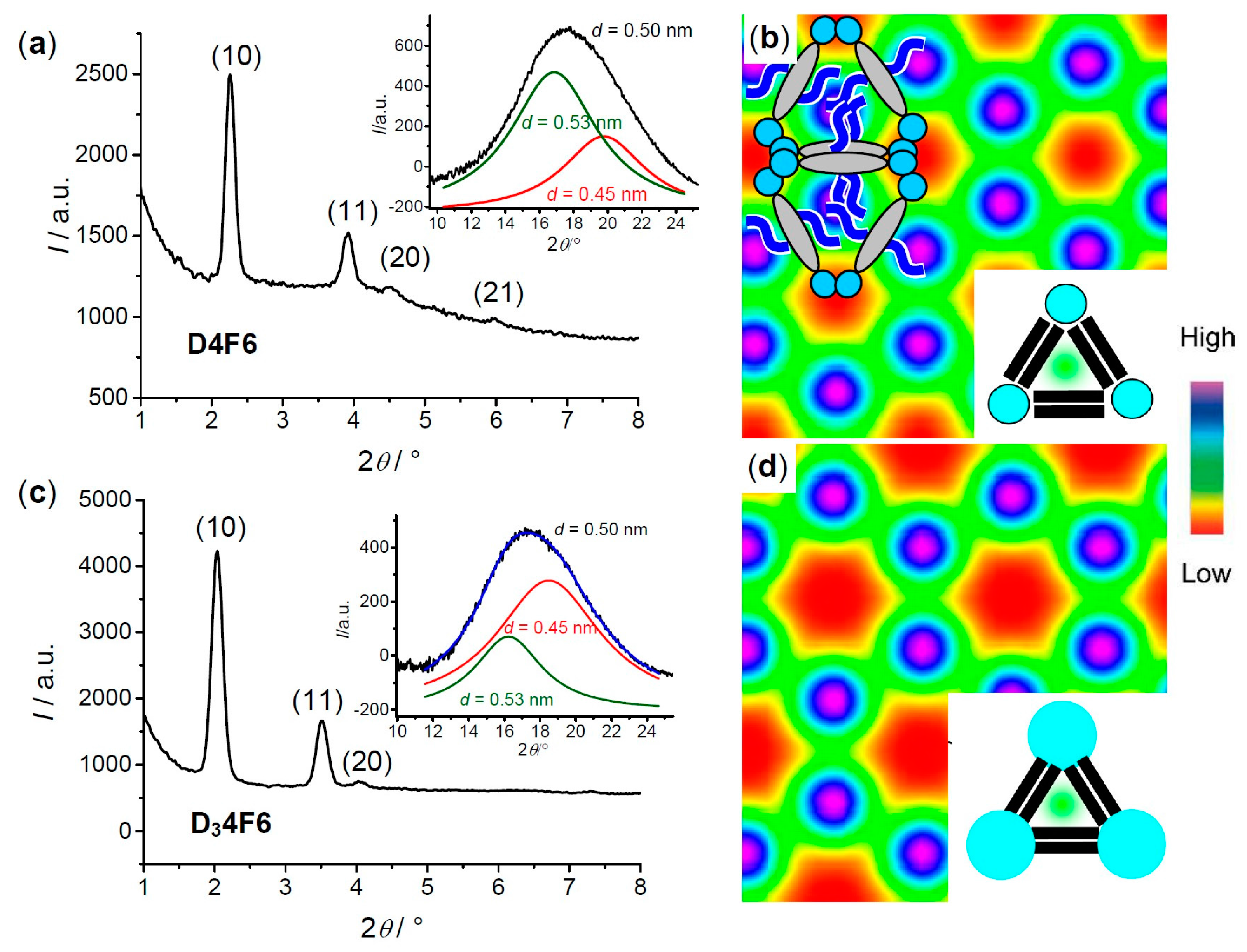
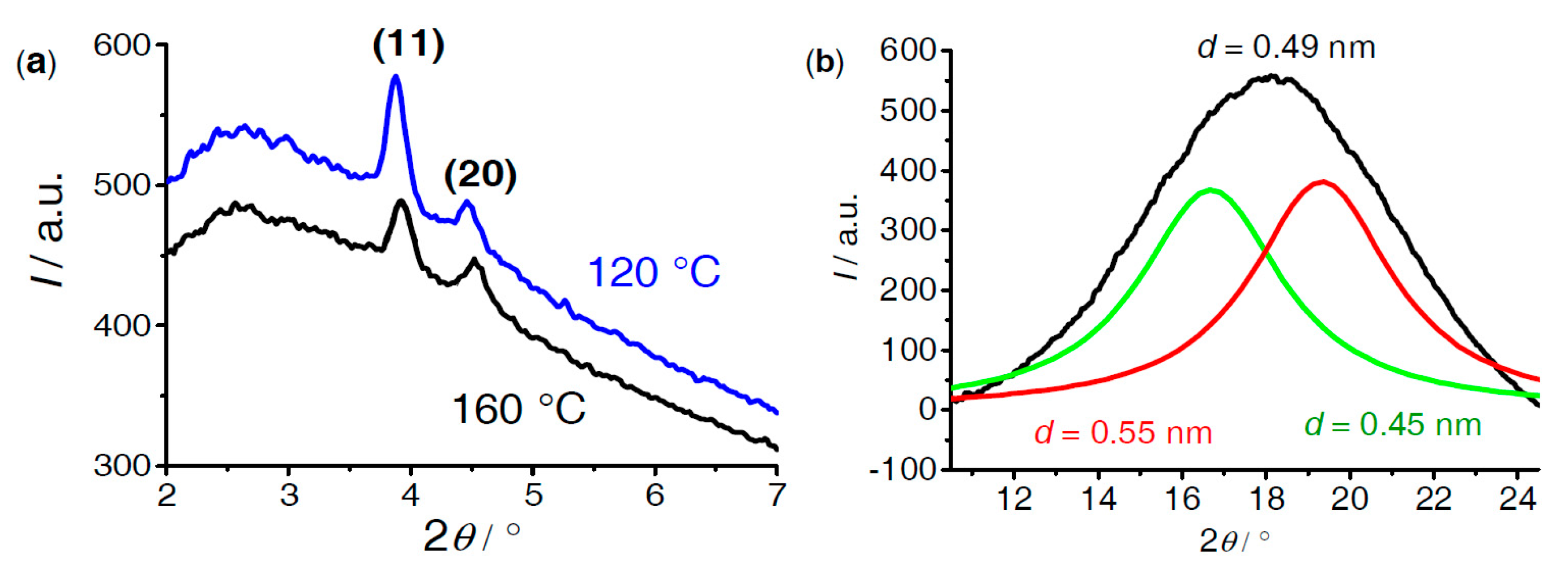
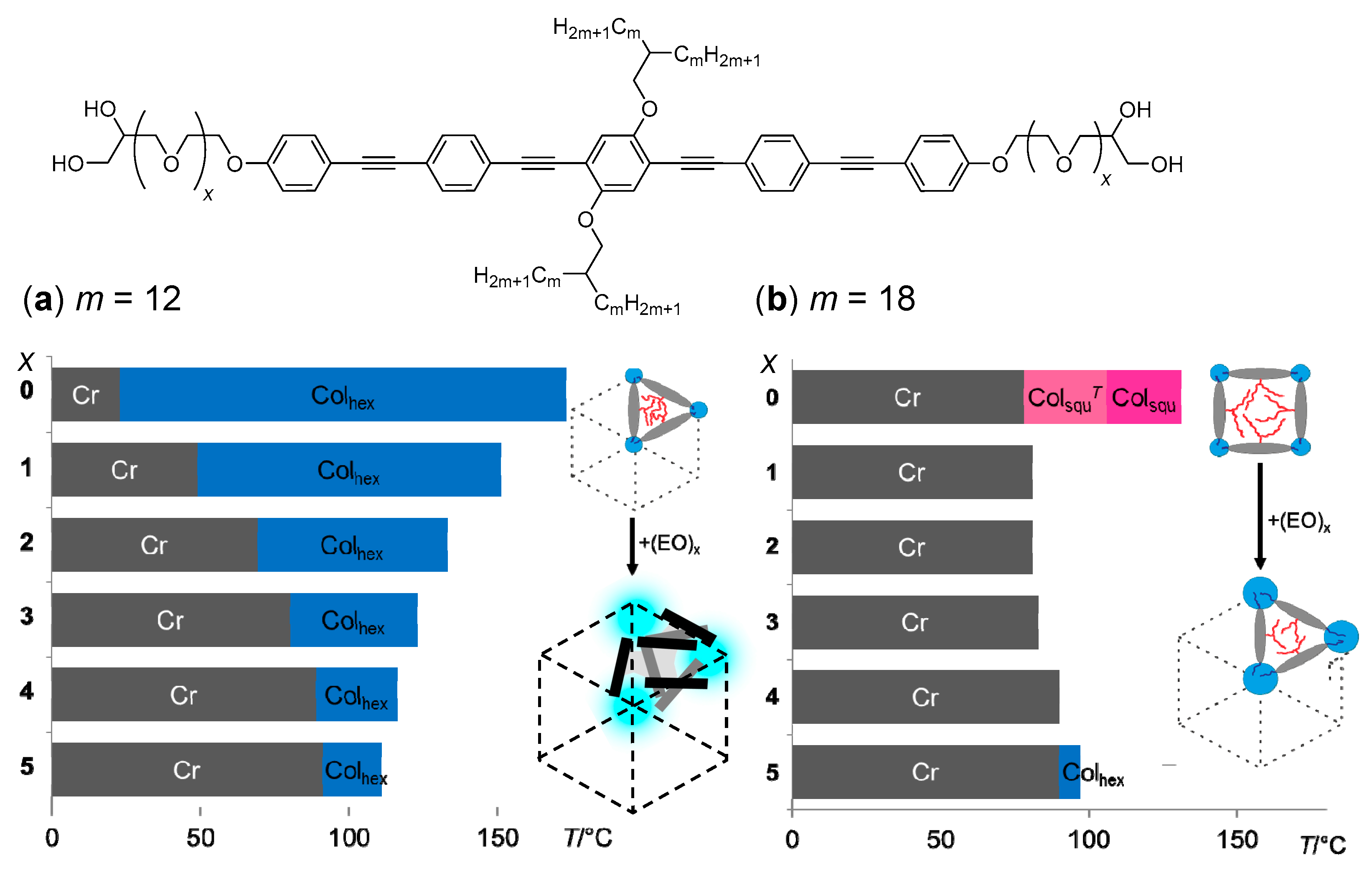
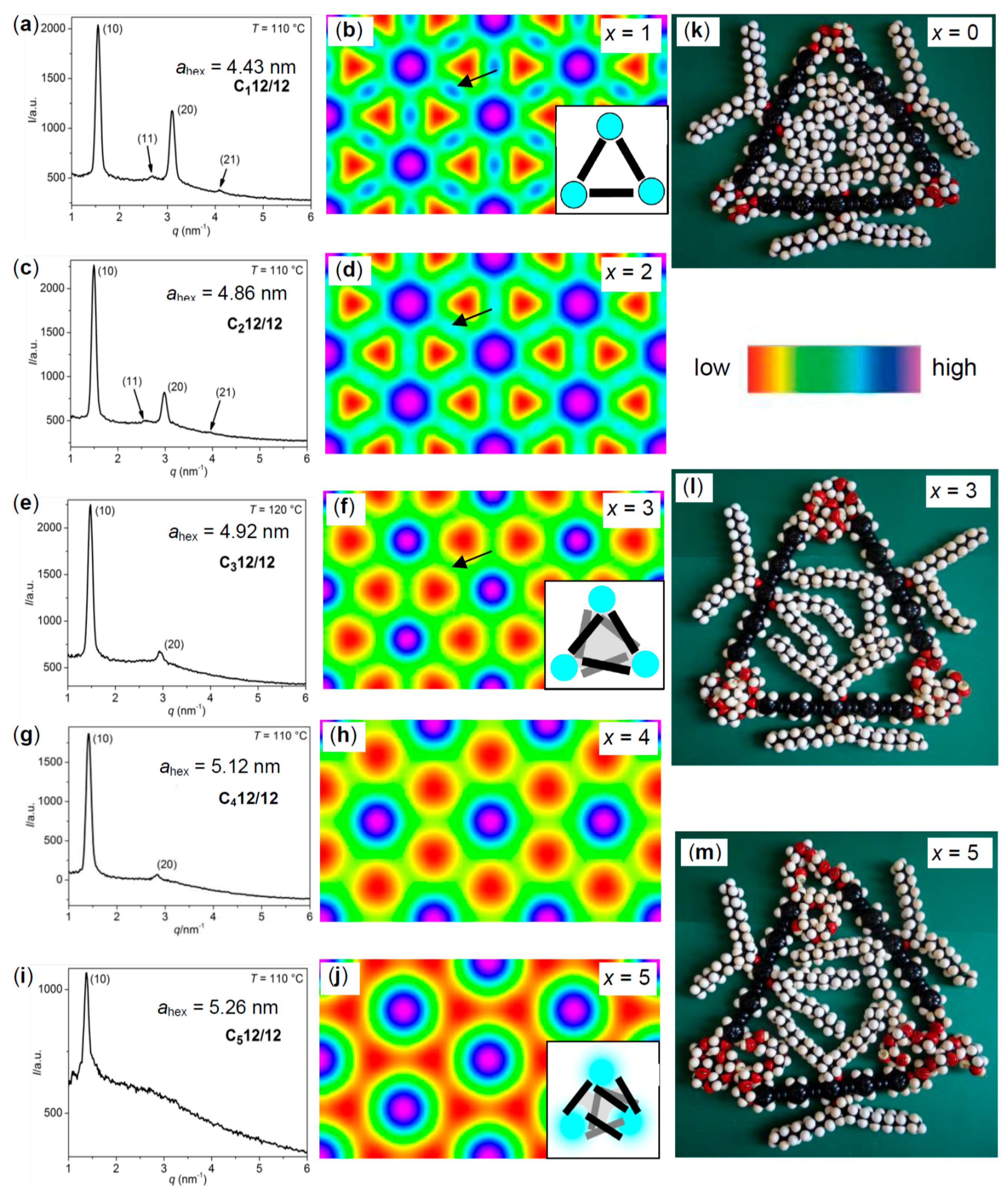
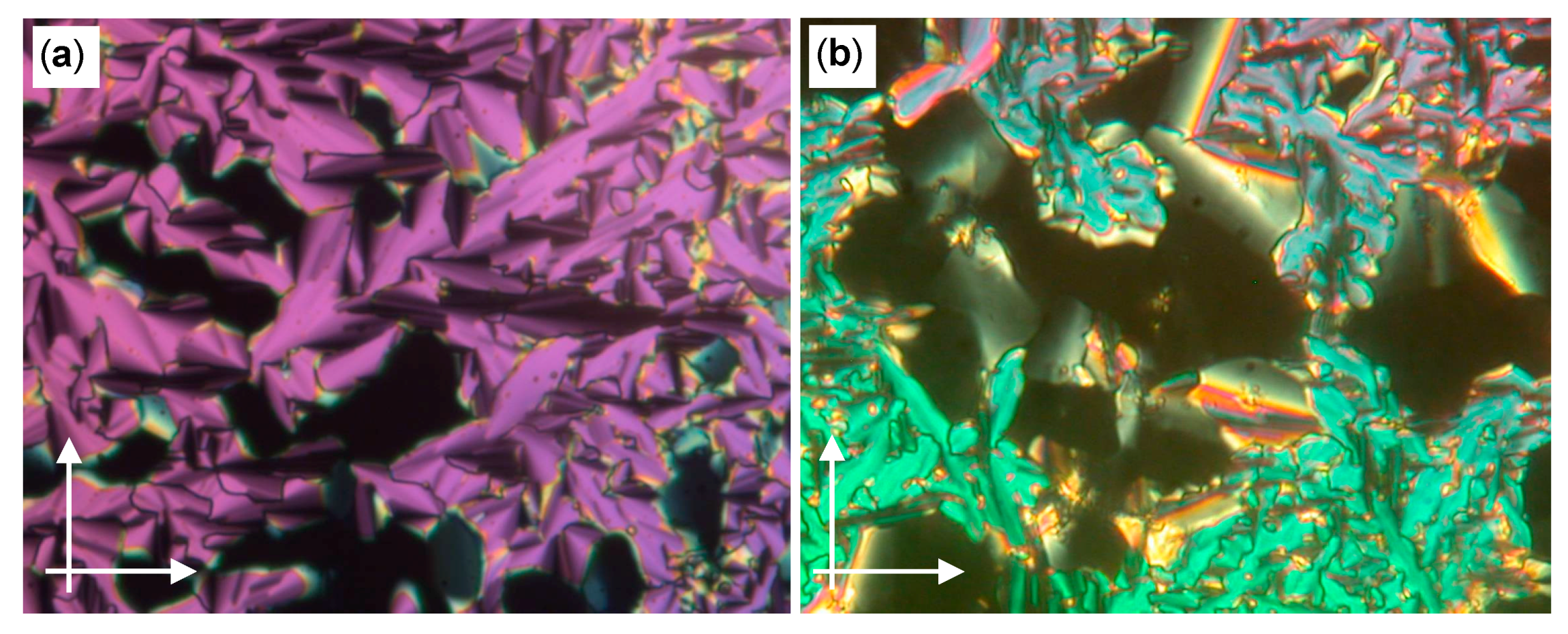
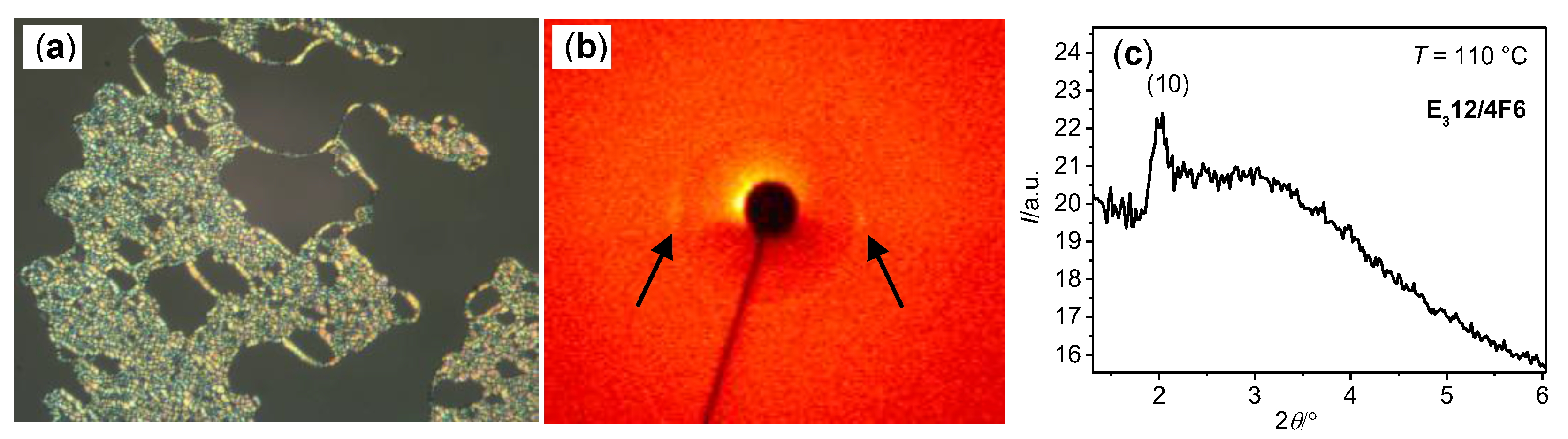
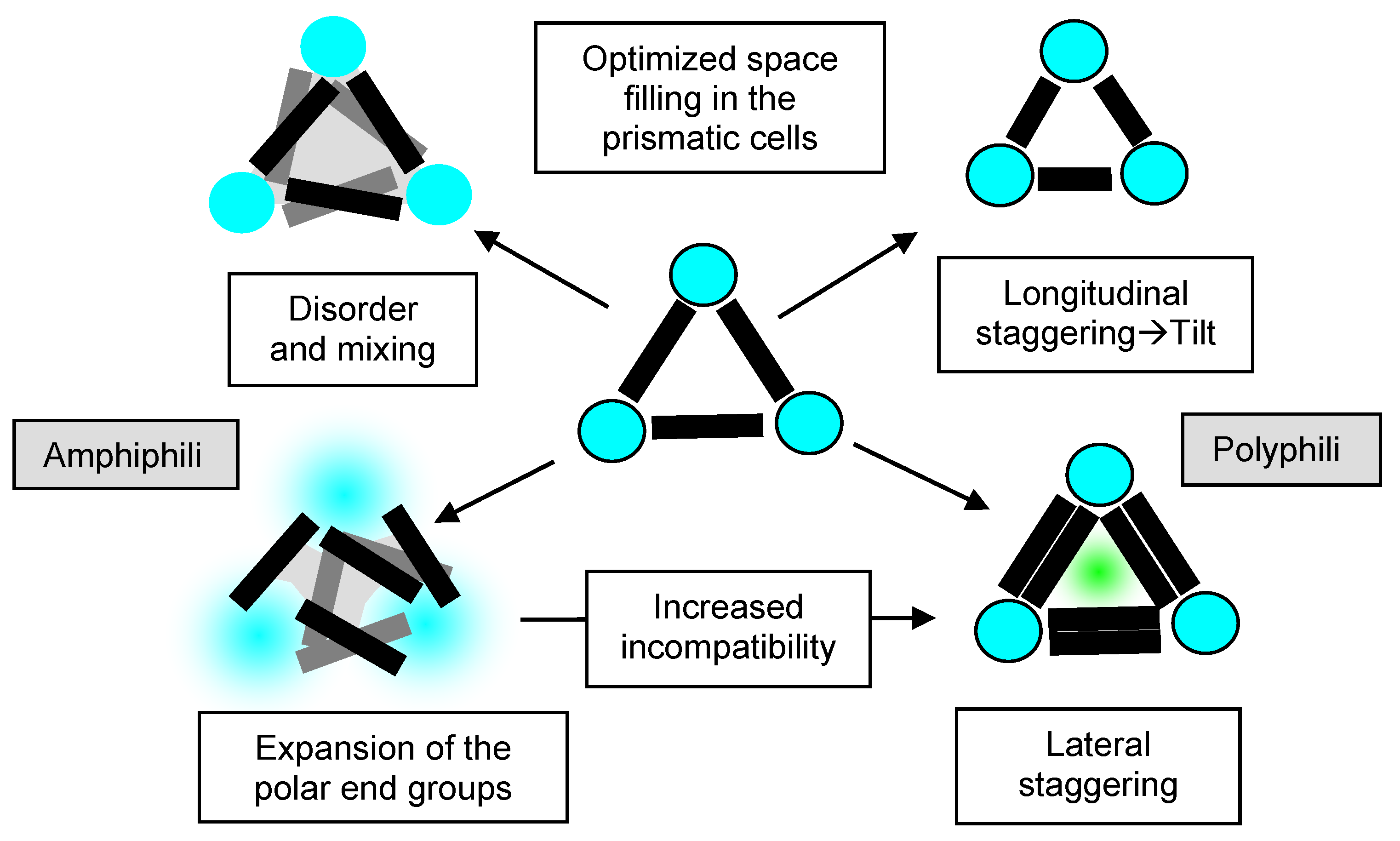
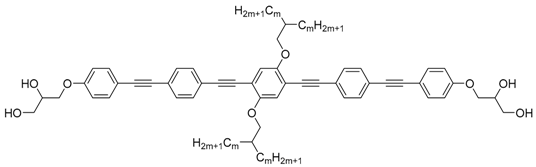
| Cm/m | Phase transitions | a (T/°C) | ncell | nwall |
|---|---|---|---|---|
| C2/2 | Cr 215 [57.2] Iso | - | - | - |
| Iso 205 [36.2] Cr | ||||
| C4/4 | Cr 172 [62.6] Iso | 4.23 (150 °C) | 4.68 | 1.56 |
| Iso 167 [3.1] Colhex [18.8] 138 Cr | ||||
| C6/6 | Cr 111 [27.0] Colhex 178 [4.5] Iso | 4.08 (150 °C) | 3.79 | 1.26 |
| Iso 176 [4.6] Colhex 88 [0.7] M1 74 [5.4] Cr | ||||
| C8/8 | Cr 77 [21.0] M1 80 [0.7] Colhex 185 [7.7] Iso | 4.16 (160 °C) | 3.51 | 1.17 |
| Iso 184 [7.8] Colhex 80 [0.7] M1 64 [16.3] Iso | ||||
| C10/10 | Cr 110 [19.6] Colhex 187 [11.0] Iso | 4.35 (130 °C) | 3.46 | 1.15 |
| Iso 183 [11.6] Colhex 58 [0.8] M1 38 [12.6] Cr | ||||
| C12/12 | Cr 97 [70.1] Colhex 175 [11.9] Iso | 4.43 (130 °C) | 3.24 | 1.08 |
| Iso 173 [12.4] Colhex 23 [18.9] Cr | ||||
| C13/13 | Cr 110 [68.1] Colhex 165 [9.3] Iso | 4.38 (130 °C) | 3.03 | 1.01 |
| Iso 162 [9.2] Colhex 20 [24.8] Cr | ||||
| C14/14 | Cr 120 [80.0] M2 152 [2.1] Colhex 154 [7.5] Iso | 4.24 (154 °C) | 2.73 | 0.91 |
| Iso 153 [7.6] Colhex 145 [1.6] M2 89 [103.6] Cr | ||||
| C16/16 | Cr 137 [103.1] Iso | - | - | - |
| Iso 133 [8.7] M2 114 [92.7] Cr | ||||
| C18/18 | Cr 105 [76.4] ColsquT 108 [2.7] Colsqu 134 [10.5] Iso | 4.05 (120 °C) | 2.5 | 1.23 |
| Iso 131 [10.4] Colsqu 106 [1.9] ColsquT 78 [68.6] Cr | 3.83 (100 °C) | |||
| C20/20 | Cr 47 [21.7] ColsquT 85 [1.1] Colsqu 132 [9.3] Iso | 4.09 (100 °C) | 2.3 | 1.17 |
| Iso 127 [10.5] Colsqu 88 [1.0] ColsquT 31 [30.0] Cr | 3.90 (80 °C) | |||
| C22/22 | Cr 84 [33.8] Colsqu 128 [12.7] Iso | 4.12 (110 °C) | 2.2 | 1.11 |
| Iso 125 [13.1] Colsqu 75 [1.2] ColsquT 32 [49.4] Cr | 4.00 (70 °C) |

| Comp. | R1 | R2 | Phase transitions | ahex (T/°C) | ncell | nwall |
|---|---|---|---|---|---|---|
| C12 [43,44,45] | –C12H25 | –C12H25 | Cr 178 [65.1] Colhex 181 [3.9] Iso | 4.30 (175) | 4.5 | 1.5 |
| Iso 180 [4.0] Colhex 168 [63.6] Cr | ||||||
| D4F6 | –(CH2)4C6F13 | –(CH2)4C6F13 | Cr 143 [26.0] Colhex 222 [7.2] Iso | 4.51 (160) | 4.7 | 1.6 |
| Iso 220 [7.0] Colhex 97 [16.4] Cr | ||||||
| E12/4F6 | –(CH2)4C6F13 | –C12H25 | Cr 112 [22.9] Colhex 197 [5.9] Iso | 4.50 (160) | 4.7 | 1.6 |
| Iso 195 [5.9] Colhex 92 [3.2] Cr | ||||||
| E18/4F6 | –(CH2)4C6F13 | –C18H37 | Cr 140 [51.8] Colhex 185 [5.1] Iso | 4.42 (150) | 4.1 | 1.4 |
| Iso 182 [4.9] Colhex 81 [19.0] Cr |
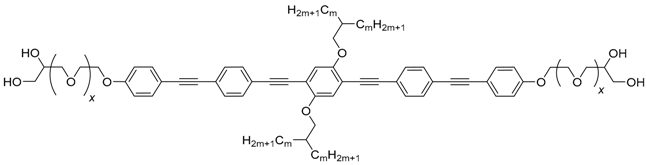
| Cm/m | x | Phase transitions | ahex (T/°C) | ncell | nwall | Lmol (nm) |
|---|---|---|---|---|---|---|
| C12/12 [43,44,45] | 0 | Cr 97 [70.1] Colhex 175 [11.9] Iso | 4.43 (130) | 3.23 | 1.08 | 4.4 |
| Iso 173 [12.4] Colhex 23 [18.9] Cr | ||||||
| C112/12 | 1 | Cr 92 [63.1] Colhex 152 [5.6] Iso | 4.67 (110) | 3.42 | 1.14 | 5.2 |
| Iso 150 [5.4] Colhex 44 [47.6] Cr | ||||||
| C212/12 | 2 | Cr 89 [56.3] Colhex 135 [3.2] Iso | 4.86 (110) | 3.44 | 1.15 | 5.9 |
| Iso 133 [3.4] Colhex 69 [57.6] Cr | ||||||
| C312/12 | 3 | Cr 96 [67.5] Colhex 125 [3.5] Iso | 4.92 (120) | 3.37 | 1.12 | 6.6 |
| Iso 123 [3.3] Colhex 80 [66.7] Cr | ||||||
| C412/12 | 4 | Cr 99 [69.9] Colhex 118 [2.5] Iso | 5.12 (110) | 3.56 | 1.19 | 7.3 |
| Iso 116 [2.6] Colhex 89 [70.7] Cr | ||||||
| C512/12 | 5 | Cr 100 [72.1] Colhex 113 [2.2] Iso | 5.26 (110) | 3.59 | 1.19 | 8.0 |
| Iso 111 [2.4] Colhex 91 [72.9] Cr | ||||||
| C18/18 [47] | 0 | Cr 105 [76.4] ColsquT 108 [2.7] Colsqu 134 [10.5] Iso | 4.05 (120) | - | - | 4.4 |
| Iso 131 [10.4] Colsqu 106 [1.9] ColsquT 78 [68.6] Cr | 3.83 (100) | |||||
| C118/18 | 1 | Cr 108 [80.0] Iso | - | - | - | 5.2 |
| Iso 81 [99.3] Cr | ||||||
| C218/18 | 2 | Cr 97 [88.3] Iso | - | - | - | 5.9 |
| Iso 81 [100.1] Cr | ||||||
| C318/18 | 3 | Cr 97 [95.1] Iso | - | - | - | 6.6 |
| Iso 83 [102.7] Cr | ||||||
| C418/18 | 4 | Cr 100 [79.9] Cr | - | - | - | 7.3 |
| Iso 90 [93.3] Cr | ||||||
| C518/18 | 5 | Cr 98 [92.3] Iso | 5.60 (95) | 3.27 | 1.09 | 8.0 |
| Iso 97 [-] Colhex 90 [96.6] Cr |
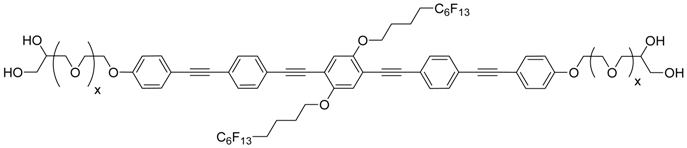
| Comp. | x | Phase transitions | ahex/nm (T/°C) | ncell | nwall |
|---|---|---|---|---|---|
| D4F6 | 0 | Cr 143 [26.0] Colhex 222 [7.2] Iso | 4.51(160) | 4.65 | 1.55 |
| Iso 220 [7.0] Colhex 97 [16.4] Cr | |||||
| D14F6 | 1 | Cr 138 [40.5] Colhex 203 [6.2] Iso | 4.78 (160) | 4.84 | 1.61 |
| Iso 200 [6.1] Colhex | |||||
| D24F6 | 2 | Cr 103 [23.0 Colhex 180 [4.3] Iso | 4.97 (140) | 4.89 | 1.63 |
| Iso 178 [4.6] Colhex 80 [18.2] Cr | |||||
| D34F6 | 3 | Cr 107 [23.9] Colhex 156 [2.3] Iso | 5.05 (140) | 4.73 | 1.58 |
| Iso 153 [2.5] Colhex 88 [20.8] Cr | 5.00 (80) | ||||
| D54F6 | 5 | Cr 99 [24.0] Colhex 128 [1.2] Iso | 5.35 (110) | 4.72 | 1.57 |
| Iso 125 [1.4] Colhex 86 [23.2] Cr |
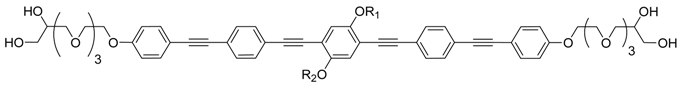
| Comp. | R1 | R2 | Phase transitions | d, ahex/nm (T/°C) | nwall |
|---|---|---|---|---|---|
| B312 | –C12H25 | –C12H25 | Cr 131 [57.9] (SmA+ 131) b Iso | - | - |
| E312/4F6 | –C12H25 | (CH2)4C6F13 | Cr 99 [39.7] SmA+ 124 [1.0] Iso | 4.38 (110) | - |
| D34F6 | (CH2)4C6F13 | (CH2)4C6F13 | Cr 107 [20.8] Colhex 156 [2.5] Iso | 5.05 (140) | 1.58 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poppe, S.; Poppe, M.; Ebert, H.; Prehm, M.; Chen, C.; Liu, F.; Werner, S.; Bacia, K.; Tschierske, C. Effects of Lateral and Terminal Chains of X-Shaped Bolapolyphiles with Oligo(phenylene ethynylene) Cores on Self-Assembly Behaviour. Part 1: Transition between Amphiphilic and Polyphilic Self-Assembly in the Bulk. Polymers 2017, 9, 471. https://doi.org/10.3390/polym9100471
Poppe S, Poppe M, Ebert H, Prehm M, Chen C, Liu F, Werner S, Bacia K, Tschierske C. Effects of Lateral and Terminal Chains of X-Shaped Bolapolyphiles with Oligo(phenylene ethynylene) Cores on Self-Assembly Behaviour. Part 1: Transition between Amphiphilic and Polyphilic Self-Assembly in the Bulk. Polymers. 2017; 9(10):471. https://doi.org/10.3390/polym9100471
Chicago/Turabian StylePoppe, Silvio, Marco Poppe, Helgard Ebert, Marko Prehm, Changlong Chen, Feng Liu, Stefan Werner, Kirsten Bacia, and Carsten Tschierske. 2017. "Effects of Lateral and Terminal Chains of X-Shaped Bolapolyphiles with Oligo(phenylene ethynylene) Cores on Self-Assembly Behaviour. Part 1: Transition between Amphiphilic and Polyphilic Self-Assembly in the Bulk" Polymers 9, no. 10: 471. https://doi.org/10.3390/polym9100471
APA StylePoppe, S., Poppe, M., Ebert, H., Prehm, M., Chen, C., Liu, F., Werner, S., Bacia, K., & Tschierske, C. (2017). Effects of Lateral and Terminal Chains of X-Shaped Bolapolyphiles with Oligo(phenylene ethynylene) Cores on Self-Assembly Behaviour. Part 1: Transition between Amphiphilic and Polyphilic Self-Assembly in the Bulk. Polymers, 9(10), 471. https://doi.org/10.3390/polym9100471