Preparation of Bio-Based Polyamide Elastomer by Using Green Plasticizers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
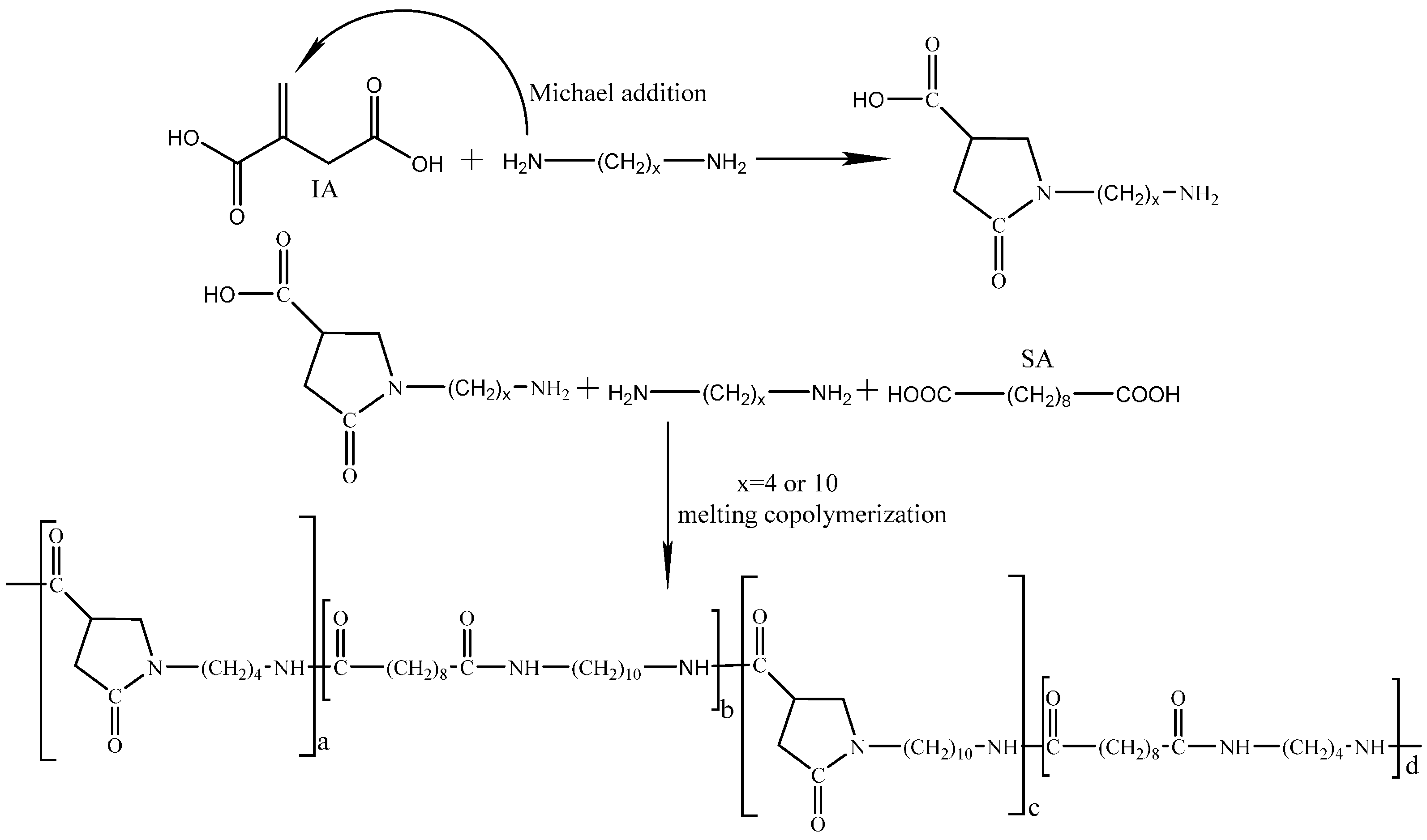
2.2. Synthesis of Bio-Based Polyamides
2.3. Plasticizing Experiments
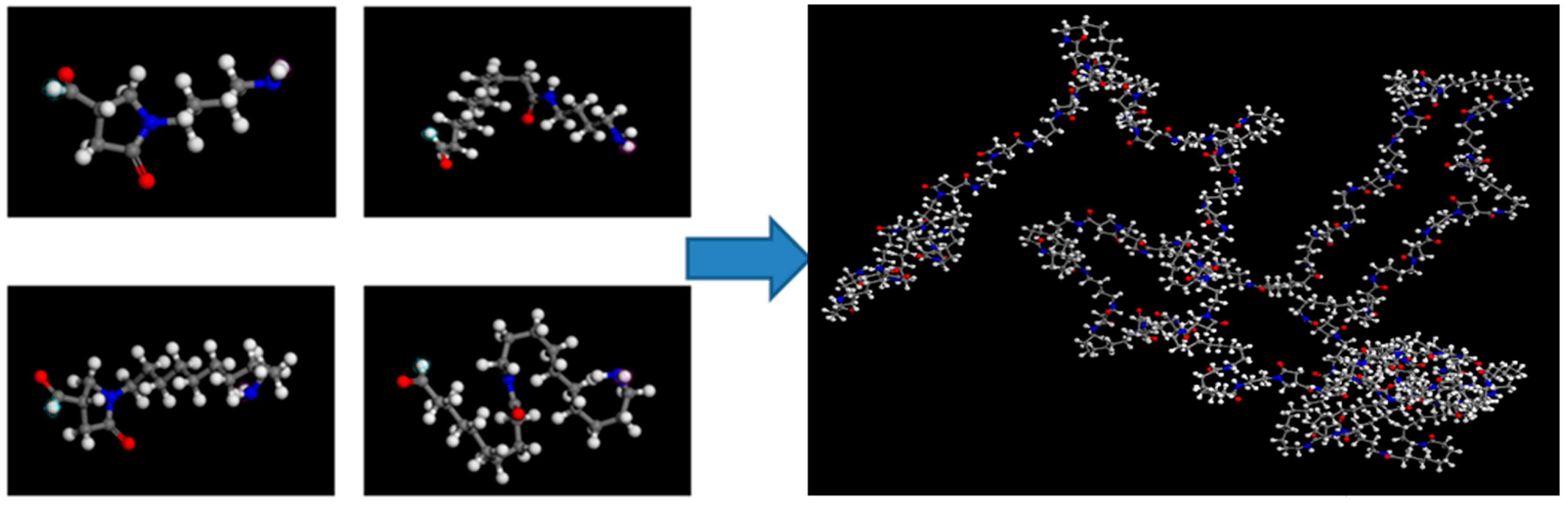
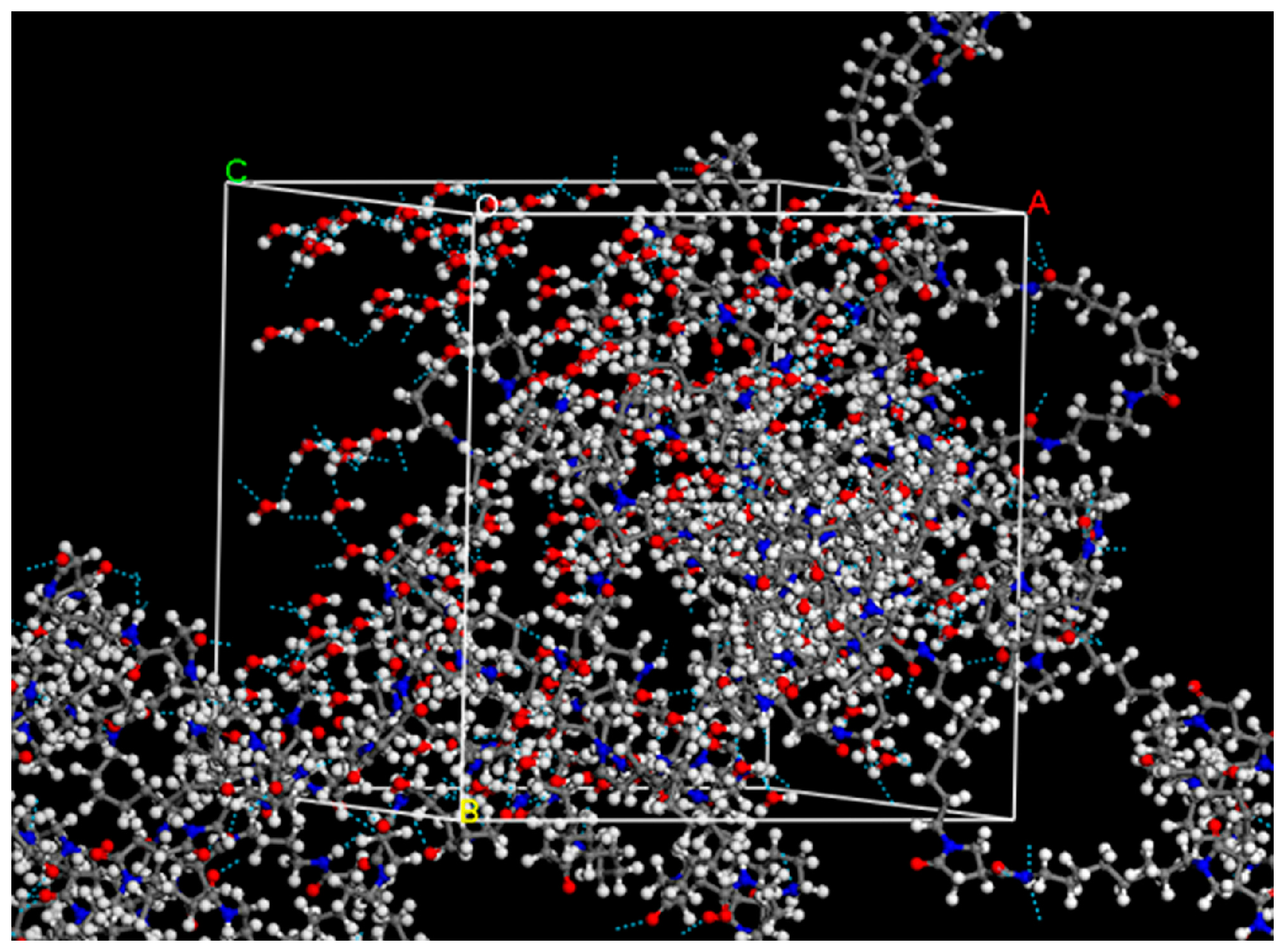
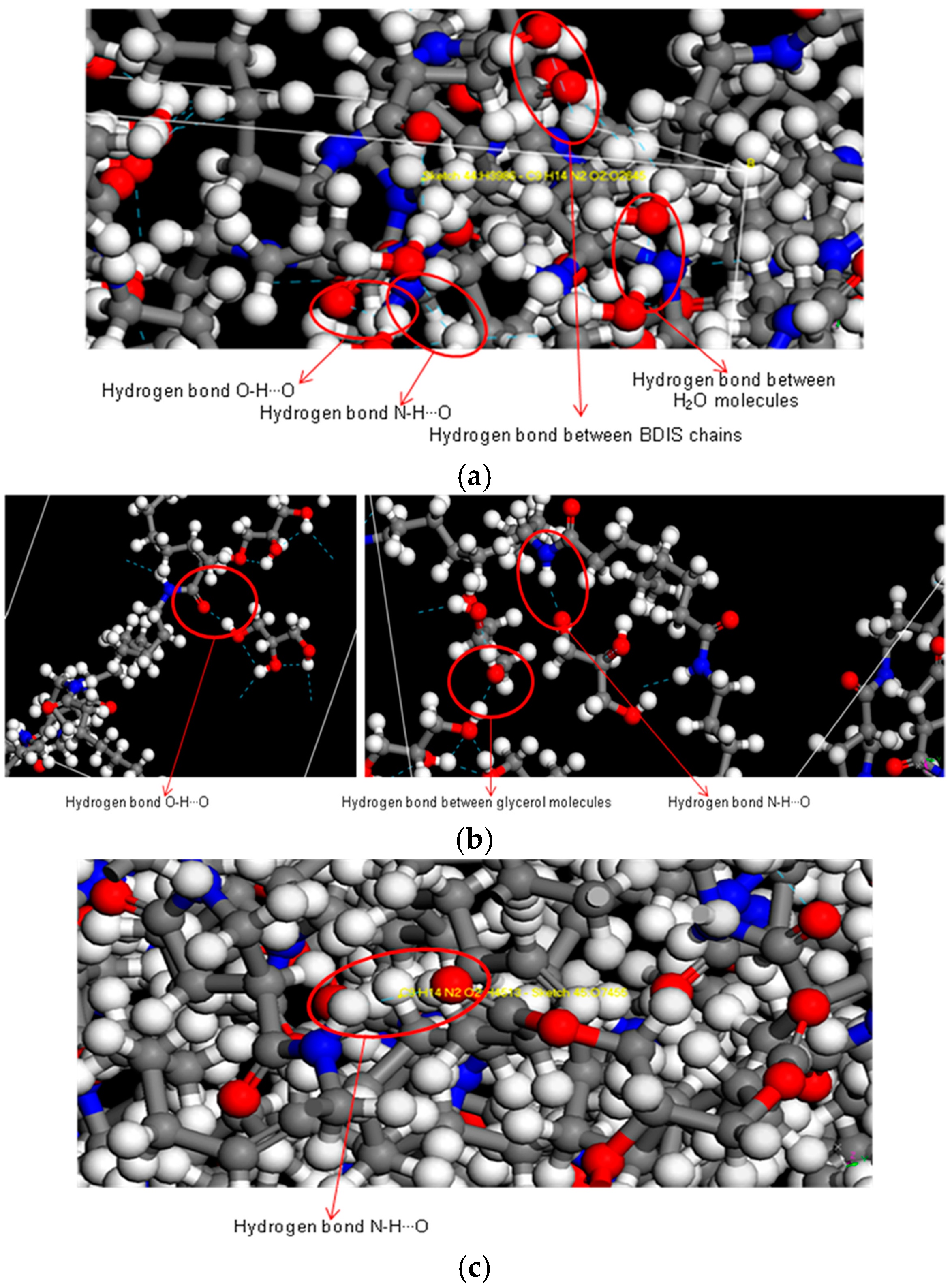
2.4. Determination of Hydrogen Bonding by Molecular Dynamics Simulation (MD)
2.5. Characterizations
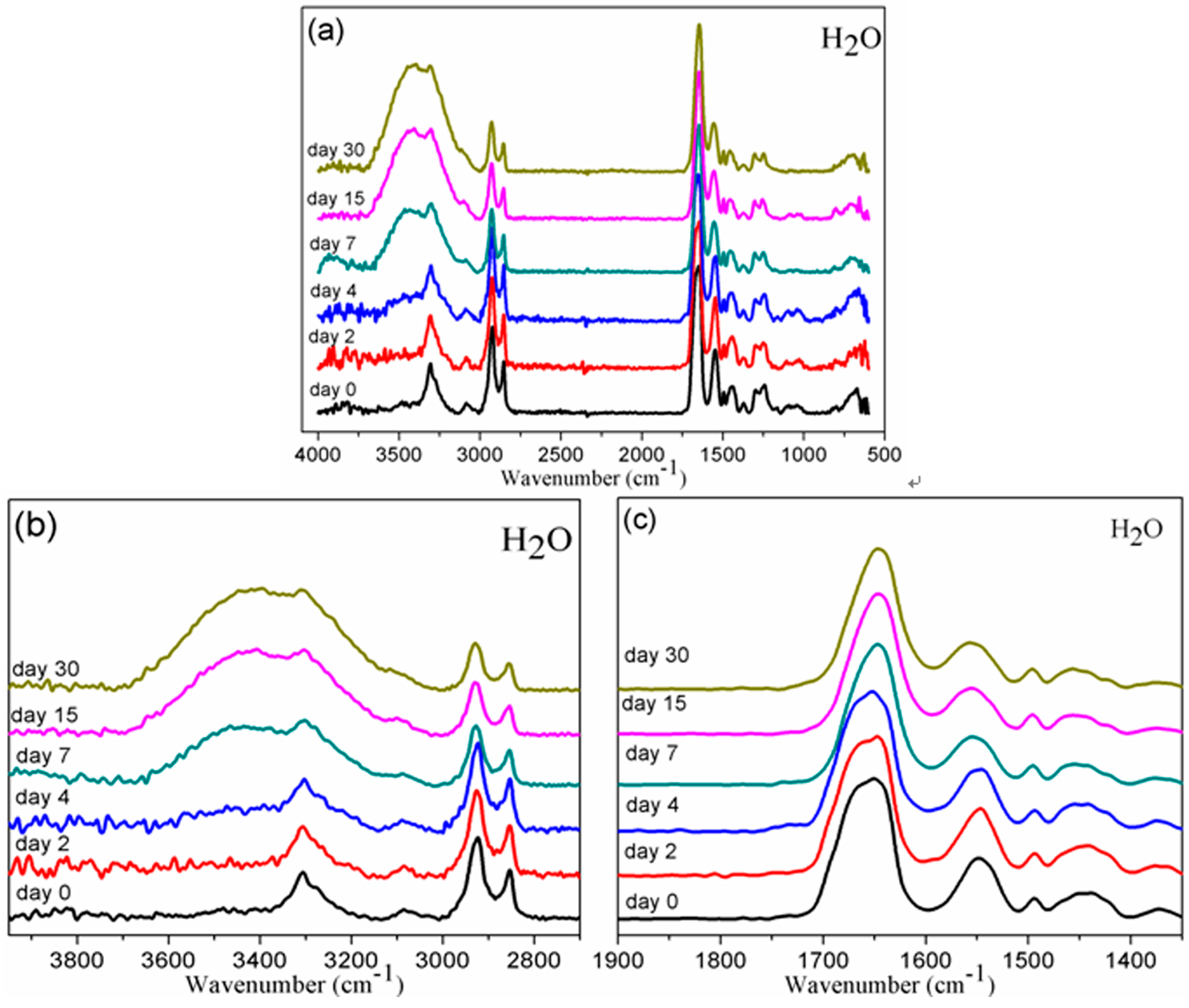
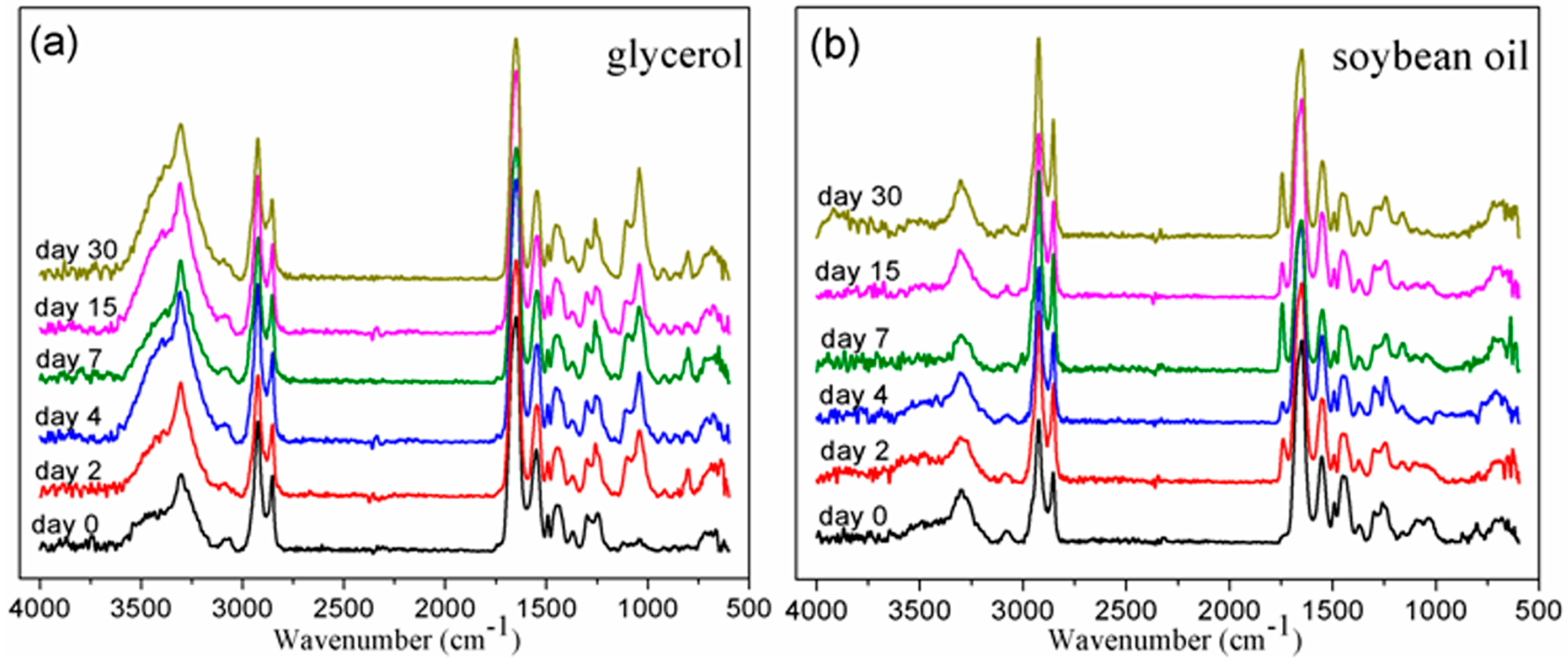
2.5.1. Fourier Transform Infrared Spectroscopy (FTIR)
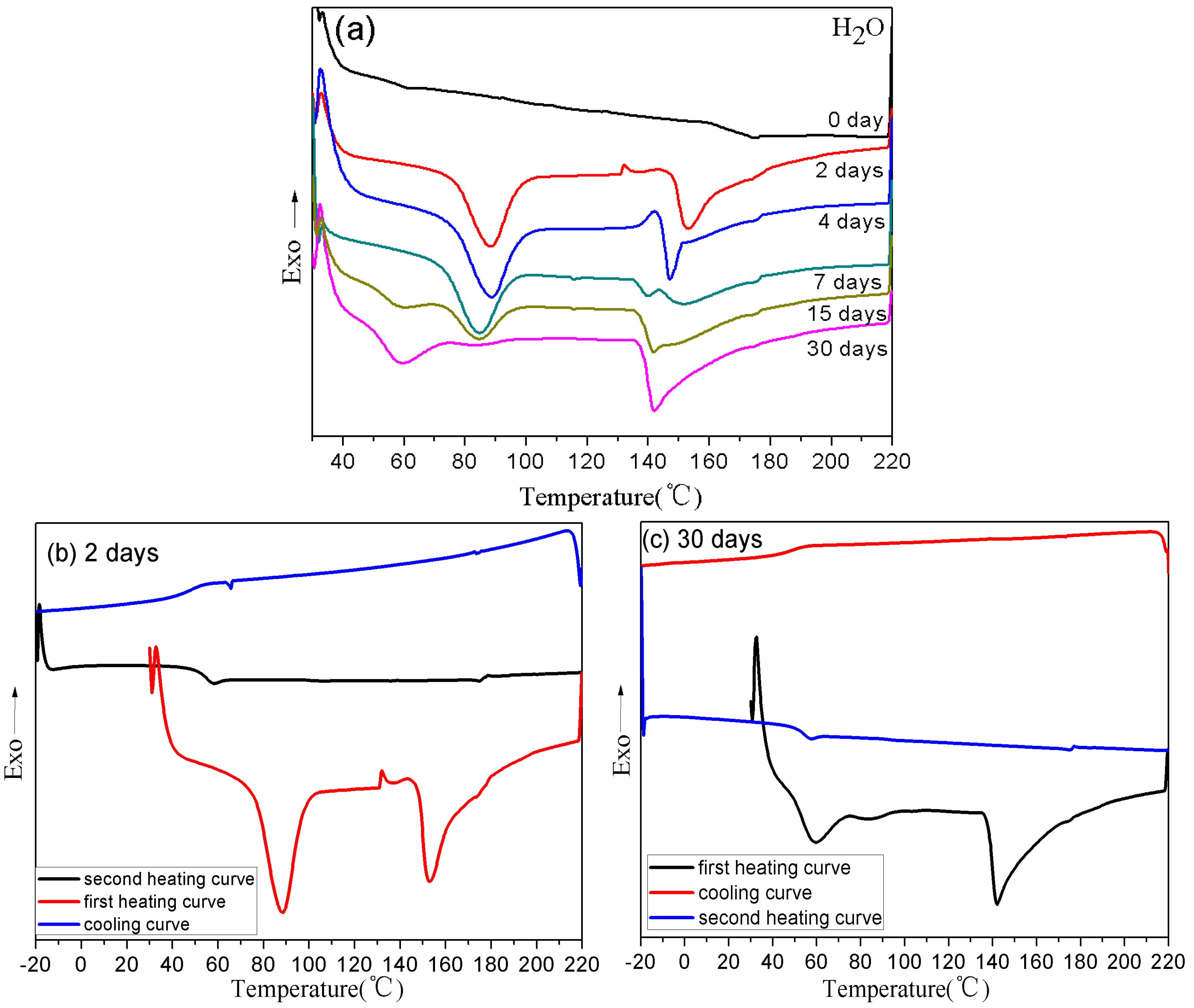
2.5.2. Differential Scanning Calorimetry (DSC)
2.5.3. Thermogravimetric Analysis (TGA)
2.5.4. X-Ray Diffraction (XRD)
2.5.5. Polarized Optical Microscopy (POM)
2.5.6. Mechanical Properties
3. Results and Discussion
3.1. Chemical Structure by FTIR
3.2. Solubility Parameters of BDIS (IA-80%) Polyamide and Plasticizers
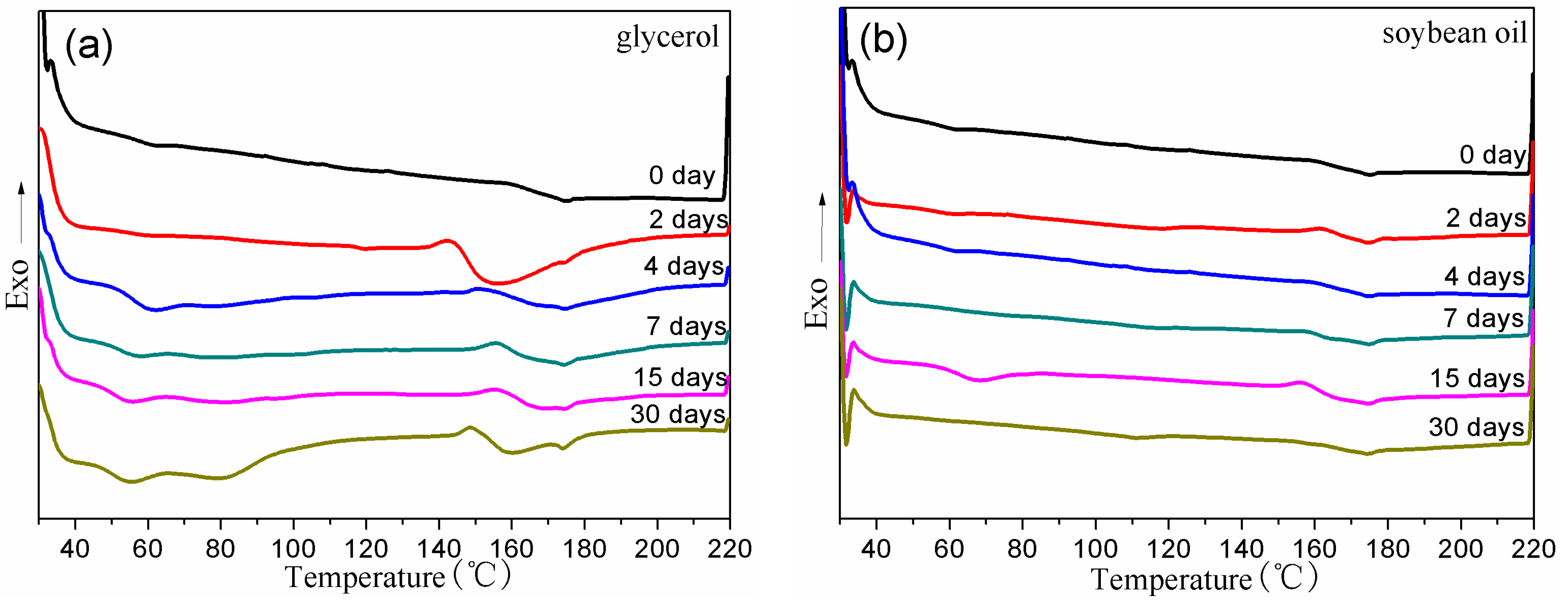
3.3. Thermal Properties of BDIS (IA-80%) Polyamide Plasticized with Different Plasticizers
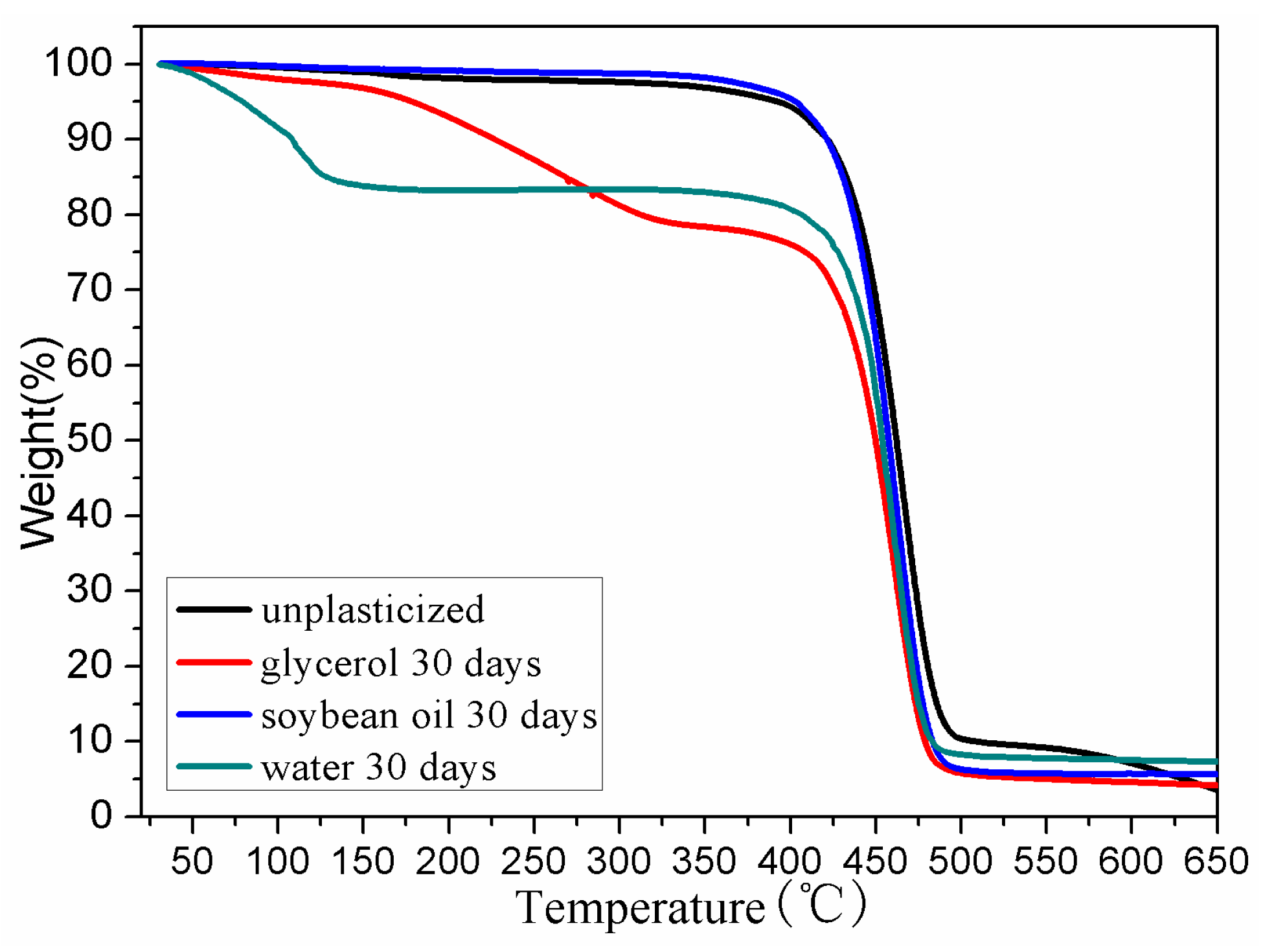
3.4. Thermal Stability
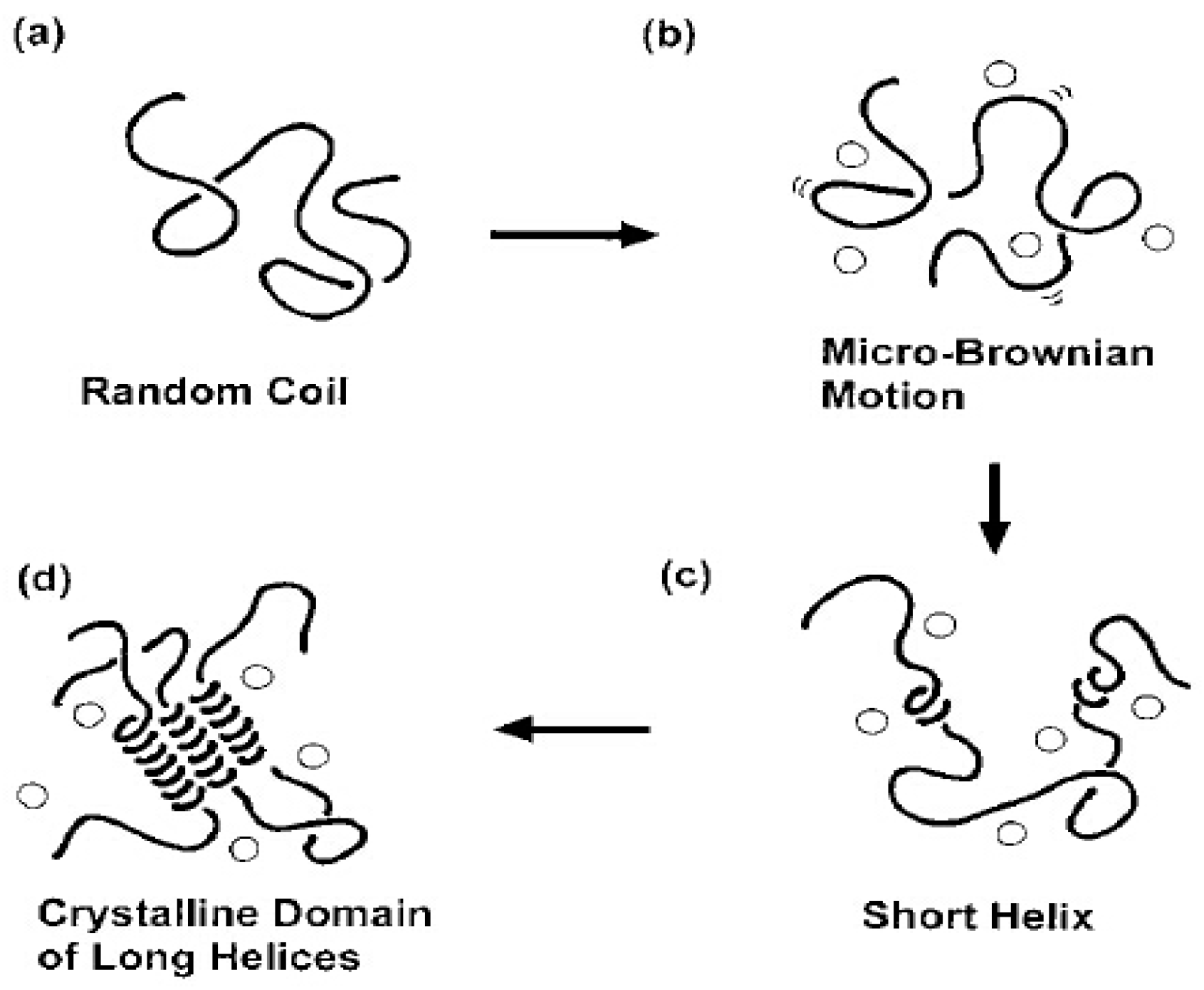
3.5. X-Ray Diffraction
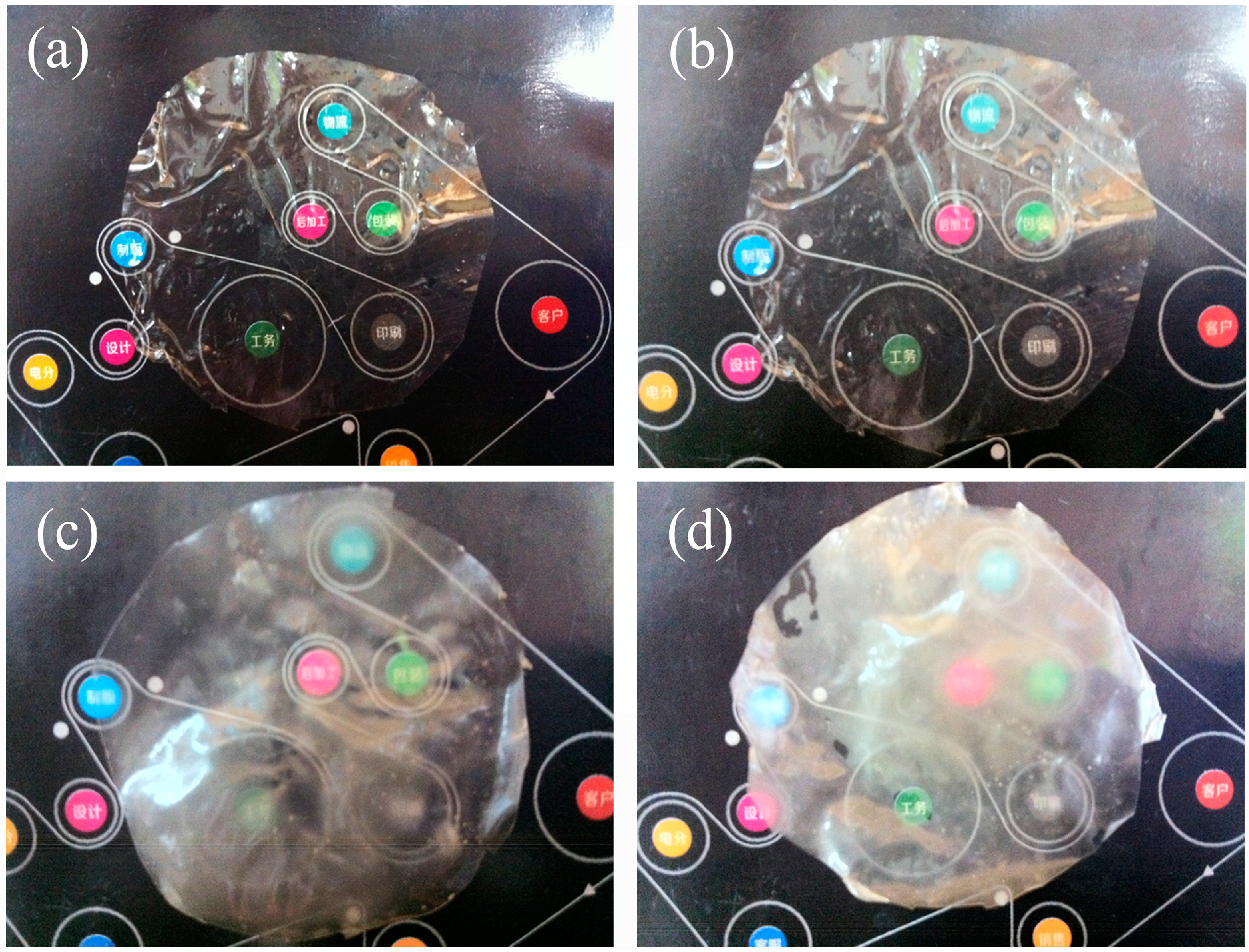
3.6. Polarized Optical Microscopy
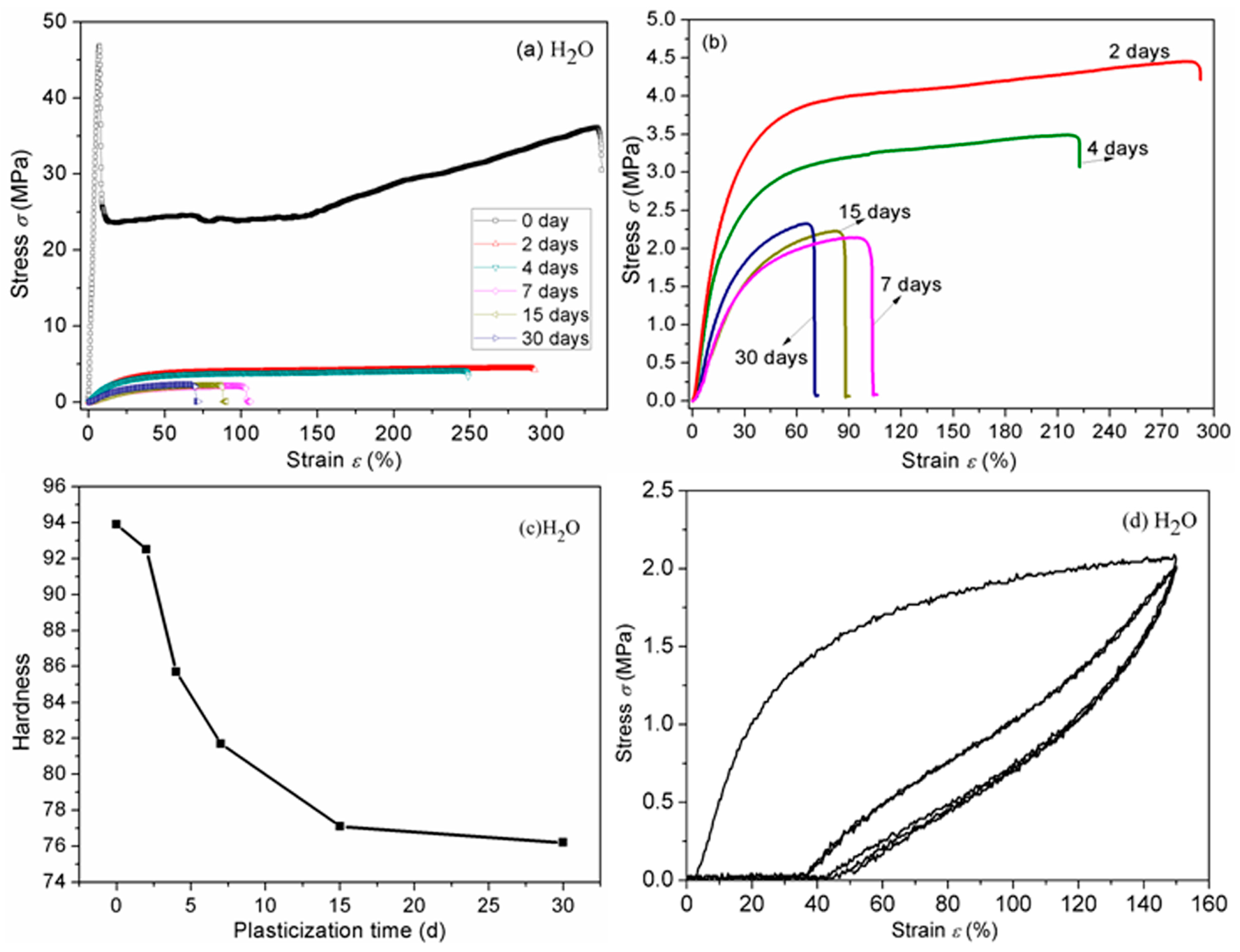
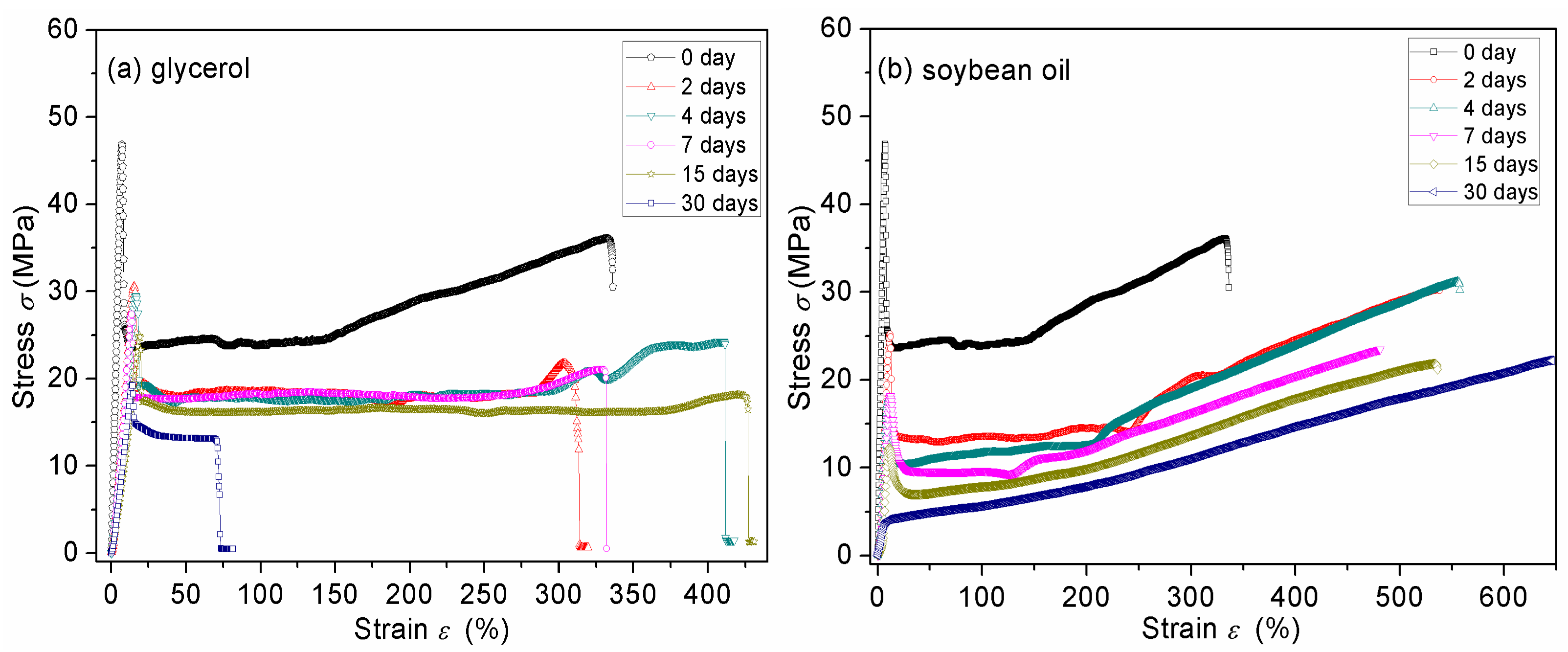
3.7. Mechanical Properties
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mouhmid, B.; Imad, A.; Benseddiq, N.; Benmedakhène, S.; Maazouz, A. A study of the mechanical behaviour of a glass fibre reinforced polyamide 6,6: Experimental investigation. Polym. Test. 2006, 25, 544–552. [Google Scholar] [CrossRef]
- Meng, H.; Sui, G.X.; Xie, G.Y.; Yang, R. Friction and wear behavior of carbon nanotubes reinforced polyamide 6 composites under dry sliding and water lubricated condition. Compos. Sci. Technol. 2009, 69, 606–611. [Google Scholar] [CrossRef]
- Tjong, S.; Meng, Y.; Xu, Y. Structure and properties of polyamide-6/vermiculite nanocomposites prepared by direct melt compounding. J. Polym. Sci. Part B. 2002, 40, 2860–2870. [Google Scholar] [CrossRef]
- Mark, J.E. Polymer Data Handbook; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Eslami, H.; Muller-Plathe, F. Molecular dynamics simulation of water influence on local structure of nanoconfined polyamide-6,6. J. Phys. Chem. B 2011, 115, 9720–9731. [Google Scholar] [CrossRef] [PubMed]
- Reuvers, N.; Huinink, H.; Adan, O. Water plasticizes only a small part of the amorphous phase in nylon-6. Macromol. Rapid Commun. 2013, 34, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Murthy, N.S. Hydrogen bonding, mobility, and structural transitions in aliphatic polyamides. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 1763–1782. [Google Scholar] [CrossRef]
- Litvinov, V.M.; Persyn, O.; Miri, V.; Lefebvre, J.M. Morphology, phase composition, and molecular mobility in polyamide films in relation to oxygen permeability. Macromolecules 2010, 43, 7668–7679. [Google Scholar] [CrossRef]
- Laurati, M.; Sotta, P.; Long, D.R.; Fillot, L.A.; Arbe, A.; Alegrı̀a, A.; Embs, J.P.; Unruh, T.; Schneider, G.J.; Colmenero, J. Dynamics of water absorbed in polyamides. Macromolecules 2012, 45, 1676–1687. [Google Scholar] [CrossRef]
- Litvinov, V.M.; Penning, J.P. Phase composition and molecular mobility in nylon 6 fibers as studied by proton nmr transverse magnetization relaxation. Macromol. Chem. Phys. 2004, 205, 1721–1734. [Google Scholar] [CrossRef]
- Rastogi, S.; Terry, A.E.; Vinken, E. Dissolution of hydrogen-bonded polymers in water: A study of Nylon-4,6. Macromolecules 2004, 37, 8825–8828. [Google Scholar] [CrossRef]
- Vinken, E.; Terry, A.E.; van Asselen, O.; Spoelstra, A.B.; Graf, R.; Rastogi, S. Role of superheated water in the dissolution and perturbation of hydrogen bonding in the crystalline lattice of polyamide 4,6. Langmuir 2008, 24, 6313–6326. [Google Scholar] [CrossRef] [PubMed]
- Harings, J.A.W.; Deshmukh, Y.S.; Hansen, M.R.; Graf, R.; Rastogi, S. Processing of polyamides in the presence of water via hydrophobic hydration and ionic interactions. Macromolecules 2012, 45, 5789–5797. [Google Scholar] [CrossRef]
- Deshmukh, Y.S.; Graf, R.; Hansen, M.R.; Rastogi, S. Dissolution and crystallization of polyamides in superheated water and concentrated ionic solutions. Macromolecules 2013, 46, 7086–7096. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, T.; Xue, X.; He, M.; Xue, J.; Song, M.; Wu, S.; Kang, H.; Zhang, L.; Jia, Q. Synthesis of fully bio-based polyamides with tunable properties by employing itaconic acid. Polymer 2014, 55, 4846–4856. [Google Scholar] [CrossRef]
- Duval, C.; Kébir, N.; Charvet, A.; Martin, A.; Burel, F. Synthesis and properties of renewable nonisocyanate polyurethanes (NIPUs) from dimethylcarbonate. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1351–1359. [Google Scholar] [CrossRef]
- Harmsen, P.F.; Hackmann, M.M.; Bos, H.L. Green building blocks for bio-based plastics. Biofuels Bioprod. Biorefining 2014, 8, 306–324. [Google Scholar] [CrossRef]
- Ali, M.A.; Tateyama, S.; Kaneko, T. Syntheses of rigid-rod but degradable biopolyamides from itaconic acid with aromatic diamines. Polym. Degrad. Stab. 2014, 109, 367–372. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, J.M. Plasticizer effect on mechanical properties of β-lactoglobulin films. J. Food Eng. 2001, 50, 149–155. [Google Scholar] [CrossRef]
- Suyatma, N.E.; Tighzert, L.; Copinet, A. Effects of hydrophilic plasticizers on mechanical, thermal, and surface properties of chitosan films. J. Agric. Food Chem. 2005, 53, 3950–3957. [Google Scholar] [CrossRef] [PubMed]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Effects of plasticizers on the properties of edible films from skin gelatin of bigeye snapper and brownstripe red snapper. Eur. Food Res. Technol. 2005, 222, 229–235. [Google Scholar] [CrossRef]
- Ali, M.A.; Tateyama, S.; Oka, Y.; Kaneko, D.; Okajima, M.K.; Kaneko, T. Syntheses of high-performance biopolyamides derived from itaconic acid and their environmental corrosion. Macromolecules 2013, 46, 3719–3725. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A.; Abe, A.; Bloch, D.R. Polymer Handbook; Wiley: New York, NY, USA, 1999; p. 89. [Google Scholar]
- Peng, F.; Pan, F.; Sun, H.; Lu, L.; Jiang, Z. Novel nanocomposite pervaporation membranes composed of poly(vinyl alcohol) and chitosan-wrapped carbon nanotube. J. Membr. Sci. 2007, 300, 13–19. [Google Scholar] [CrossRef]
- Khalili, M.; Liwo, A.; Rakowski, F.; Grochowski, P.; Scheraga, H.A. Molecular dynamics with the united-residue model of polypeptide chains. I. Lagrange equations of motion and tests of numerical stability in the microcanonical mode. J. Phys. Chem. B 2005, 109, 13785–13797. [Google Scholar] [CrossRef] [PubMed]
- Reuvers, N.J.W.; Huinink, H.P.; Fischer, H.R.; Adan, O.C.G. Quantitative water uptake study in thin nylon-6 films with nmr imaging. Macromolecules 2012, 45, 1937–1945. [Google Scholar] [CrossRef]
- Laurati, M.; Arbe, A.; Rios de Anda, A.; Fillot, L.A.; Sotta, P. Effect of polar solvents on the crystalline phase of polyamides. Polymer 2014, 55, 2867–2881. [Google Scholar] [CrossRef]
- Hablot, E.; Tisserand, A.; Bouquey, M.; Avérous, L. Accelerated artificial ageing of new dimer fatty acid-based polyamides. Polym. Degrad. Stab. 2011, 96, 1097–1103. [Google Scholar] [CrossRef]
- Galdeano, M.C.; Grossmann, M.V.E.; Mali, S.; Bello-Perez, L.A.; Garcia, M.A.; Zamudio-Flores, P.B. Effects of production process and plasticizers on stability of films and sheets of oat starch. Mater. Sci. Eng. C 2009, 29, 492–498. [Google Scholar] [CrossRef]
- Van Krevelen, D.W.; Te Nijenhuis, K. Properties of Polymers: Their Correlation with Chemical Structure, Their Numerical Estimation and Prediction from Additive Group Contributions; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Yang, J.; Dong, W.; Luan, Y.; Liu, J.; Liu, S.; Guo, X.; Zhao, X.; Su, W. Crystallization and crosslinking of polyamide-1010 under elevated pressure. J.Appl. Polym. Sci. 2002, 83, 2522–2527. [Google Scholar] [CrossRef]
- Tanaka, M.; Mochizuki, A. Effect of water structure on blood compatibility—Thermal analysis of water in poly(meth)acrylate. J. Biomed. Mater. Res. Part A 2004, 68, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.S.; Xu, Z.K.; Huang, X.J.; Wang, Z.G.; Ye, P. Hemocompatibility of poly(acrylonitrile-co-N-vinyl-2-pyrrolidone)s: Swelling behavior and water states. Macromol. Biosci. 2005, 5, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Ping, Z.; Nguyen, Q.; Chen, S.; Zhou, J.; Ding, Y. States of water in different hydrophilic polymers—DSC and FTIR studies. Polymer 2001, 42, 8461–8467. [Google Scholar] [CrossRef]
- GianchandaniI, J.; Spruiell, J.E.; Clark, E.S. Polymorphism and orientation development in melt spinning, drawing, and annealing of Nylon-6 filaments. J. Appl. Polym. Sci. 1982, 27, 3527–3551. [Google Scholar] [CrossRef]
- Tashiro, K.; Yoshioka, A. Molecular mechanism of solvent-induced crystallization of syndiotactic polystyrene glass. 2. Detection of enhanced motion of the amorphous chains in the induction period of crystallization. Macromolecules 2002, 35, 410–414. [Google Scholar] [CrossRef]
- Sato, S.; Gondo, D.; Wada, T.; Kanehashi, S.; Nagai, K. Effects of various liquid organic solvents on solvent-induced crystallization of amorphous poly(lactic acid) film. J. Appl. Polym. Sci. 2013, 129, 1607–1617. [Google Scholar] [CrossRef]
- Tashiro, K.; Ueno, Y.; Yoshioka, A.; Kobayashi, M. Molecular mechanism of solvent-induced crystallization of syndiotactic polystyrene glass. 1. Time-resolved measurements of infrared/Raman spectra and X-ray diffraction. Macromolecules 2001, 34, 310–315. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Qu, J.P. Mechanical and rheological properties of epoxidized soybean oil plasticized poly(lactic acid). J. Appl. Polym. Sci. 2009, 112, 3185–3191. [Google Scholar] [CrossRef]
- Xue, J.; He, M.; Liang, Y.; Crawford, A.; Coates, P.; Chen, D.; Shi, R.; Zhang, L. Fabrication and evaluation of electrospun PCL–gelatin micro-/nanofiber membranes for anti-infective GTR implants. J. Mater. Chem. B 2014, 2, 6867–6877. [Google Scholar] [CrossRef]

| Plasticizers | δd (MJ/m3)1/2 | δp (MJ/m3)1/2 | δh (MJ/m3)1/2 | δt (MJ/m3)1/2 |
|---|---|---|---|---|
| Water | 15.5 | 16.0 | 42.4 | 47.9 |
| Glycerol | 17.3 | 12.1 | 29.3 | 43.2 |
| Soybean oil | 17.1 | 0.5 | 2.8 | 17.3 |
| Samples | T5% (°C) | Tdmax (°C) | T95% (°C) | Residue mass (%) |
|---|---|---|---|---|
| BDIS (IA-80%) | 403 | 459 | 650 | 5.58 |
| Water 30 days | 79 | 458 | 601 | 7.28 |
| Glycerol 30 days | 179 | 460 | 538 | 4.14 |
| Soybean oil 30 days | 391 | 466 | 630 | 3.52 |
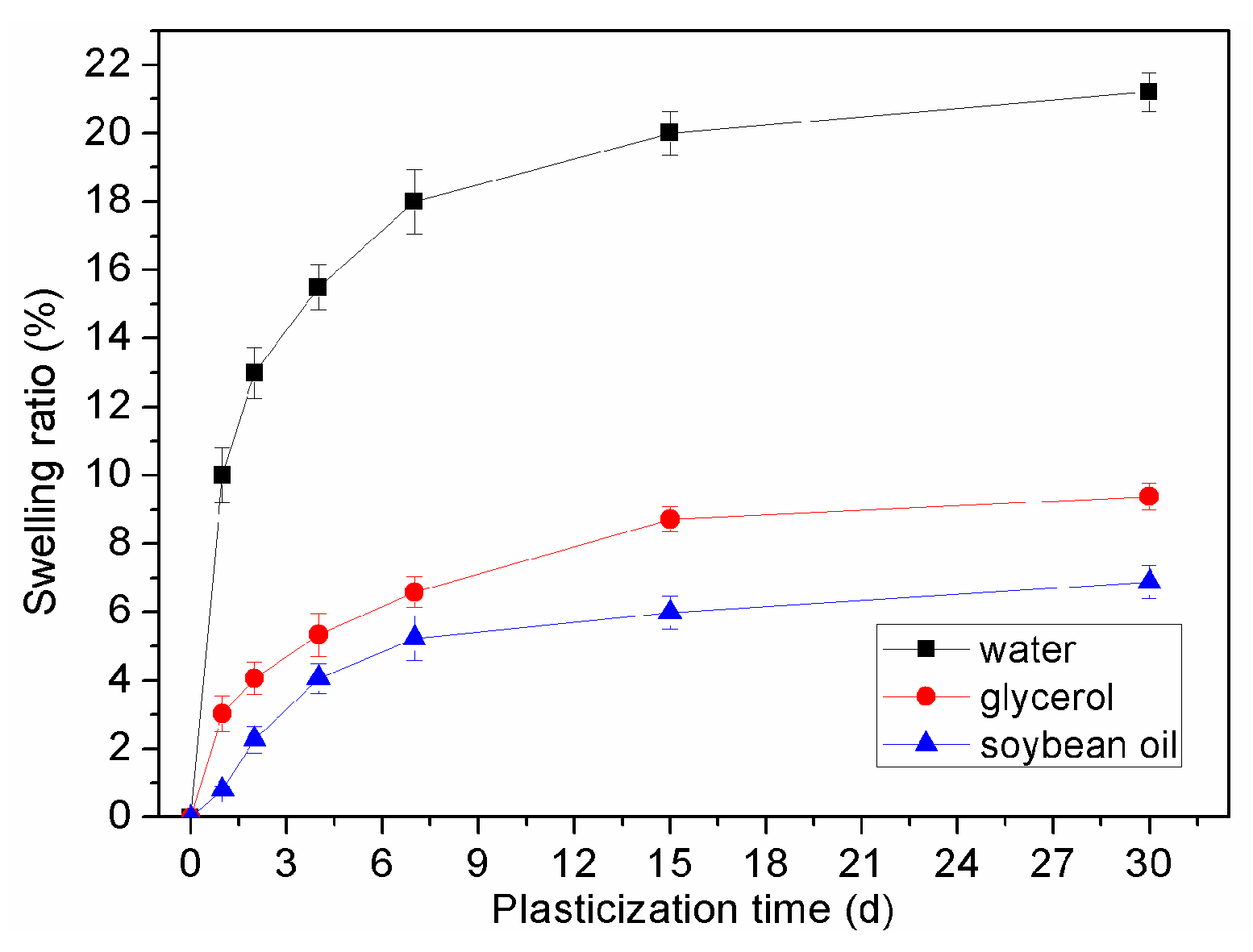
| Plasticizers | 2days | 4days | 7days | 15days | 30days |
|---|---|---|---|---|---|
| Water | 11.5 ± 0.8 | 15.0 ± 0.7 | 18.5 ± 0.7 | 20.9 ± 0.6 | 25.0 ± 0.6 |
| Glycerol | 4.0 ± 0.5 | 5.3 ± 0.6 | 6.6 ± 0.4 | 8.7 ± 0.4 | 9.4 ± 0.5 |
| Soybean oil | 2.3 ± 0.4 | 4.0 ± 0.4 | 5.2 ± 0.7 | 6.0 ± 0.5 | 6.9 ± 0.5 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.; Wang, Z.; Wang, R.; Zhang, L.; Jia, Q. Preparation of Bio-Based Polyamide Elastomer by Using Green Plasticizers. Polymers 2016, 8, 257. https://doi.org/10.3390/polym8070257
He M, Wang Z, Wang R, Zhang L, Jia Q. Preparation of Bio-Based Polyamide Elastomer by Using Green Plasticizers. Polymers. 2016; 8(7):257. https://doi.org/10.3390/polym8070257
Chicago/Turabian StyleHe, Miaomiao, Zhao Wang, Runguo Wang, Liqun Zhang, and Qingxiu Jia. 2016. "Preparation of Bio-Based Polyamide Elastomer by Using Green Plasticizers" Polymers 8, no. 7: 257. https://doi.org/10.3390/polym8070257
APA StyleHe, M., Wang, Z., Wang, R., Zhang, L., & Jia, Q. (2016). Preparation of Bio-Based Polyamide Elastomer by Using Green Plasticizers. Polymers, 8(7), 257. https://doi.org/10.3390/polym8070257






