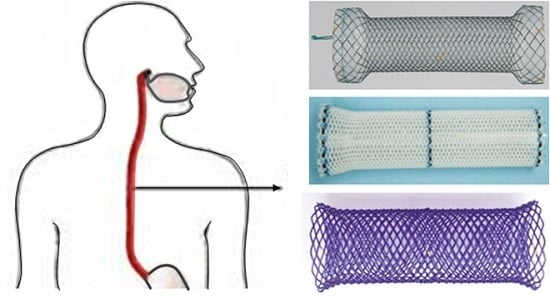
Polymeric Biodegradable Stent Insertion in the Esophagus
Abstract
:1. Introduction
2. Polymeric Biomaterials
3. Prerequisites for Polymeric Biodegradable Stents
4. Advantages of Polymeric Biodegradable Stents
5. History of Polymeric Biodegradable Stent Development
6. Preclinical Studies with Polymeric Biodegradable Stents
7. Preclinical Studies with Polymeric Biodegradable Esophageal Stents
8. Clinical Studies with Biodegradable Esophageal Stents
8.1. Benign Stricture
8.2. Malignant Stricture
8.3. Leaks or Perforations
9. Biodegradable Drug-Eluting Stents (DES)
Biodegradable Eluting Nanofiber-Covered Metal Stent
10. Complications of Biodegradable Esophageal Stents
11. Limitations of the Biodegradable Stents
12. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Siersema, P.D. Treatment options for esophageal strictures. Nat. Clin. Pract. Gastroenterol. Hepatol. 2008, 5, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Tokar, J.L.; Banerjee, S.; Barth, B.A.; Desilets, D.J.; Kaul, V.; Kethi, S.R.; Pedrosa, M.C.; Pfau, P.R.; Pleskow, D.K.; Varadarajulu, S. Drug-eluting/biodegradable stents. Gastrointest. Endosc. 2011, 74, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Fry, S.W.; Fleischer, D.E. Management of a refractory benign esophageal stricture with a new biodegradable stent. Gastrointest. Endosc. 1997, 45, 179–182. [Google Scholar] [CrossRef]
- Yu, X.J.; Wang, L.; Huang, M.; Gong, T.; Li, W.; Cao, Y.; Ji, D.; Wang, J.; Zhou, S. A shape memory stent of poly(ε-caprolactone-co-dl-lactide) copolymer for potential treatment of esophageal stenosis. J. Mater. Sci. Mater. Med. 2012, 23, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Freudenberg, S.; Rewerk, S.; Kaess, M.; Weiss, C.; Dorn-Beinecke, A.; Post, S. Biodegradation of absorbable sutures in body fluids and pH buffers. Eur. Surg. Res. 2004, 36, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Gunatillake, P.; Mayadunne, R.; Adhikari, R. Recent developments in biodegradable synthetic polymers. Biotechnol. Annu. Rev. 2006, 12, 301–347. [Google Scholar] [PubMed]
- Yang, J.; Kopeček, J. Polymeric biomaterials and nanomedicines. J. Drug Deliv. Sci. Technol. 2015, 30, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, W.; Pang, Y.; Yan, D. Hyperbranched polyphosphates: Synthesis, functionalization and biomedical applications. Chem. Soc. Rev. 2015, 44, 3942–3953. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Zhao, J.; Luan, S.; Yan, S.; Zheng, W.; Yin, J. Nuclease-functionalized poly(styrene-b-isobutylene-b-styrene) surface with anti-infection and tissue integration bifunctions. ACS Appl. Mater. Interfaces 2014, 6, 18078–18086. [Google Scholar] [CrossRef] [PubMed]
- Vasi, A.M.; Popa, M.I.; Tanase, E.C.; Butnaru, M.; Verestiuc, L. Poly(acrylic acid)–poly(ethylene glycol) nanoparticles designed for ophthalmic drug delivery. J. Pharm. Sci. 2014, 103, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Larrañaga, A.; Etxeberria, A.; Wang, W.; Sarasua, J.R. A new generation of poly(lactide/ε-caprolactone) polymeric biomaterials for application in the medical field. J. Biomed. Mater. Res. A 2014, 102, 3573–3584. [Google Scholar] [CrossRef] [PubMed]
- Tanzi, M.C.; Bozzini, S.; Candiani, G.; Cigada, A.; De Nardo, L.; Farè, S.; Ganataldi, D.; Levi, M.; Metranqolo, P.; Miqliavacca, F. Trends in biomedical engineering: Focus on smart bio-materials and drug delivery. J. Appl. Biomater. Biomech. 2011, 9, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Jaekel, D.J.; MacDonald, D.W.; Kurtz, S.M. Characterization of PEEK biomaterials using the small punch test. J. Mech. Behav. Biomed. Mater. 2011, 4, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [PubMed]
- Weiser, J.R.; Zawaneh, P.N.; Putnam, D. Poly(carbonate-ester)s of dihydroxyacetone and lactic acid as potential biomaterials. Biomacromolecules 2011, 12, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Erne, P.; Schier, M.; Resink, T.J. The road to bioabsorbable stents: Reaching clinical reality? Cardiovasc. Interv. Radiol. 2006, 29, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Ramcharitar, S.; Vaina, S.; Serruys, P.W. The next generation of drugeluting stents: What’s on the horizon? Am. J. Cardiovasc. Drugs 2007, 7, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Aoki, J.; Ong, A.T.; Rodriguez Granillo, G.A.; McFadden, E.P.; van Mieghem, C.A.; Valgimigi, M.; Tsuchida, K.; Sianos, G.; Regar, E.; de Jaegere, P.P. “Full metal jacket” (stented length > or = 64 mm) using drug-eluting stents for de novo coronary artery lesions. Am. Heart J. 2005, 150, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.; Mintz, G.S.; Popma, J.J.; Satler, L.F.; Pichard, A.D.; Kent, K.M.; Walsh, C.; Mackell, P.; Leon, M.B. Chronic arterial responses to stent implantation: A serial intravascular ultrasound analysis of Palmaz-Schatz stents in native coronary arteries. J. Am. Coll. Cardiol. 1996, 28, 1134–1139. [Google Scholar] [CrossRef]
- Spuentrup, E.; Ruebben, A.; Mahnken, A.; Kolker, C.; Nquyen, T.H.; Gunther, R.W.; Buecker, A. Artifact-free coronary magnetic resonance angiography and coronary vessel wall imaging in the presence of a new, metallic, coronary magnetic resonance imaging stent. Circulation 2005, 111, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Pinto Slottow, T.L.; Waksman, R. Overview of the 2006 food and drug administration circulatory system devices panel meeting on drug-eluting stent thrombosis. Catheter. Cardiovasc. Interv. 2007, 69, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Stack, R.E.; Califf, R.M.; Phillips, H.R.; Pryor, D.B.; Quigley, P.J.; Bauman, R.P.; Tcheng, J.E.; Greenfield, J.C., Jr. Interventional cardiac catheterization at Duke Medical Center. Am. J. Cardiol. 1988, 62, 3F–4F. [Google Scholar] [PubMed]
- Murphy, J.G.; Schwartz, R.S.; Huber, K.C.; Holmes, D.R., Jr. Polymeric stents: Modern alchemy or the future? J. Invasive Cardiol. 1991, 3, 144–148. [Google Scholar] [PubMed]
- Hastings, G.W. Cardiovascular Biomaterials; Springer-Verlag: London, UK, 1992. [Google Scholar]
- Ye, Y.W.; Landau, C.; Meidell, R.S.; Willard, J.E.; Moskowitz, A.; Aziz, S.; Carlisle, E.; Nelson, K.; Eberhart, R.C. Improved bioresorbable microporous intravascular stents for gene therapy. ASAIO J. 1996, 42, M823–M827. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, A.M.; Furst, J.G.; Ellis, S.G.; Tuch, R.J.; Topol, E.J. Sustained local delivery of dexamethasone by a novel intravascular eluting stent to prevent restenosis in the porcine coronary injury model. J. Am. Coll. Cardiol. 1997, 29, 808–816. [Google Scholar] [CrossRef]
- Tsuji, T.; Tamai, H.; Kyo, E. The effect of PLLA stent design on neointimal hyperplasia. J. Cardiol. 1998, 32, 235A. [Google Scholar]
- Eury, K.R. Multi-layered Biodegradable Stent and Method for Its Manufacture. EU Patent 604022 A1, 22 July 1994. [Google Scholar]
- Yamawaki, T.; Shimokawa, H.; Kozai, T.; Miyata, K.; Higo, T.; Tanaka, E.; Egashira, K.; Shiraishi, T.; Tamai, H.; Igaki, K.; et al. Intramural delivery of a specific tyrosine kinase inhibitor with biodegradable stent suppresses the restenotic changes of the coronary artery in pigs in vivo. J. Am. Coll. Cardiol. 1998, 32, 780–786. [Google Scholar] [CrossRef]
- Tamai, H.; Igaki, K.; Tsuji, T.; Kyo, E.; Kosuga, K.; Kawashima, A.; Matsui, S.; Komori, H.; Motohara, S.; Uehata, H.; et al. A biodegradable poly-l-lactic acid coronary stent in porcine coronary artery. J. Interv. Cardiol. 1999, 12, 443–450. [Google Scholar] [CrossRef]
- Hietala, E.M.; Salminen, U.S.; Stahls, A.; Välimaa, T.; Maasilta, P.; Törmälä, P.; Nieminen, M.S.; Harjula, A.L. Biodegradation of the copolymeric polylactide stent: Long-term follow-up in a rabbit aorta model. J. Vasc. Res. 2001, 38, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Vogt, F.; Stein, A.; Rettemeier, G.; Krott, N.; Hoffmann, R.; vom Dahl, J.; Bosserhoff, A.K.; Michaeli, W.; Hanrath, P.; Weber, C.; et al. Long-term assessment of a novel biodegradable paclitaxel-eluting coronary polylactide stent. Eur. Heart J. 2004, 25, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Van der Giessen, W.J.; Lincoff, A.M.; Schwartz, R.S.; van Beusekom, H.M.; Serruys, P.W.; Holmes, D.R., Jr.; Ellis, S.G.; Topol, E.J. Marked inflammatory sequelae to implantation of biodegradable and nonbiodegradable polymers in porcine coronary arteries. Circulation 1996, 94, 1690–1697. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, S.; Poh, T.L.; Vinalia, T.; Mak, K.H.; Boey, F. Collapse pressures of biodegradable stents. Biomaterials 2003, 24, 2105–2011. [Google Scholar] [CrossRef]
- Uurto, I.; Juuti, H.; Parkkinen, J.; Kellomäki, M.; Keski-Nisula, L.; Nevalainen, T.; Törmälä, P.; Salenius, J.P. Biodegradable self-expanding poly-l/d-lactic acid vascular stent: A pilot study in canine and porcine iliac arteries. J. Endovasc. Ther. 2004, 11, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Asplund, B.; Sperens, J.; Mathisen, T.; Hilborn, J. Effects of hydrolysis on a new biodegradable co-polymer. J. Biomater. Sci. Polym. Ed. 2006, 17, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.W.; Landau, C.; Willard, J.E.; Rajasubramanian, G.; Moskowitz, A.; Aziz, S.; Meidell, R.S.; Eberhart, R.C. Bioresorbable microporous stents deliver recombinant adenovirus gene transfer vectors to the arterial wall. Ann. Biomed. Eng. 1998, 26, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Kemppainen, E.; Talja, M.; Riihelä, M.; Pohjonen, T.; Törmälä, P.; Alfthan, O. A bioresorbable urethral stent. An experimental study. Urol. Res. 1993, 21, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Rejchrt, S.; Kopacova, M.; Bartova, J.; Bures, J. Intestinal biodegradable stents: Initial experience in the Czech Republic. Folia Gastroenterol. Hepatol. 2009, 7, 7–11. [Google Scholar]
- Battersby, I.; Doyle, R. Use of a biodegradable self-expanding stent in the management of a benign oesophageal stricture in a cat. J. Small Anim. Pract. 2010, 51, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Pauli, E.M.; Schomisch, S.J.; Furlan, J.P.; Marks, A.S.; Chak, A.; Lash, R.H.; Ponsky, J.L.; Marks, J.M. Biodegradable esophageal stent placement does not prevent high-grade stricture formation after circumferential mucosal resection in a porcine model. Surg. Endosc. 2012, 26, 3500–3508. [Google Scholar] [CrossRef] [PubMed]
- Lam, N.; Weisse, C.; Berent, A.; Murphy, S.; Radlinsky, M.; Richter, K.; Dunn, M.; Gingerich, K. Esophageal stenting for treatment of refractory benign esophageal strictures in dogs. J. Vet. Intern. Med. 2013, 27, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, P.G.; Vleggaar, F.P.; Siersema, P.D. Biodegradable stent placement in the esophagus. Expert Rev. Med. Devices 2013, 10, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.K.; Pani, K.C.; Neuman, C.; Leonard, F. Polylactic acid for surgical implants. Arch. Surg. 1966, 93, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Schakenraad, J.M.; Dijkstra, P.J. Biocompatibility of poly (dl-lactic acid/glycine) copolymers. Clin. Mater. 1991, 7, 253–269. [Google Scholar] [CrossRef]
- Saito, Y.; Minami, K.; Kobayashi, M.; Nakao, Y.; Omiya, H.; Imamura, H.; Sakaida, N.; Okamura, A. New tubular bioabsorbable knitted airway stent: Biocompatibility and mechanical strength. J. Thorac. Cardiovasc. Surg. 2002, 123, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Goldin, E.; Fiorini, A.; Ratan, Y.; Keter, A.; Loshakove, O.; Globerman, M.; Beyar, M. A new biodegradable and self-expandable stent for benign esophageal strictures. Gastrointest. Endosc. 1996, 43, 294. [Google Scholar] [CrossRef]
- Tanaka, T.; Takahashi, M.; Nitta, N.; Furukawa, A.; Andoh, A.; Saito, Y.; Fujiyama, Y.; Murata, K. Newly developed biodegradable stents for benign GI tract stenoses: A preliminary clinical trial. Digestion 2006, 74, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Tanaka, T.; Andoh, A.; Minematsu, H.; Hata, K.; Tsujikawa, T.; Nitta, N.; Murata, K.; Fujiyama, Y. Usefulness of biodegradable stents constructed of poly-l-lactic acid monofilaments in patients with benign esophageal stenosis. World J. Gastroenterol. 2007, 13, 3977–3980. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Tanaka, T.; Andoh, A.; Minematsu, H.; Hata, K.; Tsujikawa, T.; Nitta, N.; Murata, K.; Fujiyama, Y. Novel biodegradable stents for benign esophageal strictures following endoscopic submucosal dissection. Dig. Dis. Sci. 2008, 53, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.; Topping, J.H.; Johns, E.; O’Neill, D. Biodegradable stents in refractory benign oesophageal strictures first report of 4 patients from, UK. Gastrointest. Endosc. 2009, 69, AB254. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Hauser, B.; Devreker, T.; Urbain, D.; Reynaert, H. A biodegradable esophageal stent in the treatment of a corrosive esophageal stenosis in a child. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Orive-Calzada, A.; Alvarez-Rubio, M.; Romero-Izquierdo, S.; Cobo Martin, M.; Juanmartiñena, J.F.; Ogueta-Fernández, M.; Molina-Alvarez, E.; Eraña-Ledesma, L. Severe epithelial hyperplasia as a complication of a novel biodegradable stent. Endoscopy 2009, 41, 137–138. [Google Scholar] [CrossRef] [PubMed]
- Bychkova, O.V.; Lazyuk, I.I.; Averin, V. Biodegradable stents new approach to the treatment of caustic stenoses in children. Folia Gastroenterol. Hepatol. 2009, 7, 2–5. [Google Scholar]
- Stivaros, S.M.; Williams, L.R.; Senger, C.; Wilbraham, L.; Laasch, H.U. Woven polydioxanone biodegradable stents: A new treatment option for benign and malignant oesophageal strictures. Eur. Radiol. 2010, 20, 1069–1072. [Google Scholar] [CrossRef] [PubMed]
- Viedma, B.L.; Lorente, R.; Domper, F.; de la Santa, E. Preliminary experience with biodegradable stents in the treatment of refractary benign GI strictures. Gastrointest. Endosc. 2010, 71, AB234. [Google Scholar] [CrossRef]
- Repici, A.; Vleggaar, F.P.; Hassan, C.; van Boeckel, P.G.; Romeo, F.; Pagano, N.; Malesci, A.; Siersema, P.D. Efficacy and safety of biodegradable stents for refractory benign esophageal strictures: The BEST (Biodegradable Esophageal Stent) study. Gastrointest. Endosc. 2010, 72, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; vandermeeren, A.; van Maele, V.; Deprez, P. Belgian multicenter experience with biodegradable Ella-stent in benign strictures of the digestive tract. Endoscopy 2010, 42, 259. [Google Scholar]
- Jung, G.E.; Sauer, P.; Schaible, A. Tracheoesophageal fistula following implantation of a biodegradable stent for a refractory benign esophageal stricture. Endoscopy 2010, 42, E338–E339. [Google Scholar] [CrossRef] [PubMed]
- Güitrón-Cantú, A.; Adalid-Martínez, R.; Gutiérrez-Bermúdez, J.A.; Meza Mata, E.; Segura López, F.K.; García Vázquez, A. Foreign body reaction to biodegradable esophageal prosthesis. Rev. Gastroenterol. Mex. 2010, 75, 203–207. [Google Scholar]
- Hair, C.S.; Devonshire, D.A. Severe hyperplastic tissue stenosis of a novel biodegradable esophageal stent and subsequent successful management with high-pressure balloon dilation. Endoscopy 2010, 42, E132–E133. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, P.G.; Vleggaar, F.P.; Siersema, P.D. A comparison of temporary self-expanding plastic and biodegradable stents for refractory benign esophageal strictures. Clin. Gastroenterol. Hepatol. 2011, 9, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Van Hooft, J.E.; van Berge Henegouwen, M.I.; Rauws, E.A.; Bergman, J.J.; Busch, O.R.; Fockens, P. Endoscopic treatment of benign anastomotic esophagogastric strictures with a biodegradable stent. Gastrointest. Endosc. 2011, 73, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Bausch, D.; Baier, P.; Braun, A.; Richter-Schrag, H. Risk of biodegradable stent-induced hypergranulation causing restenosis of a gastric conduit after esophageal resection. Endoscopy 2012, 44, E125. [Google Scholar] [PubMed]
- Canena, J.M.; Liberato, M.J.; Rio-Tinto, R.A.; Pinto-Marques, P.M.; Romão, C.M.; Coutinho, A.V.; Neves, B.A.; Santos-Silva, M.F. A comparison of the temporary placement of 3 different self expanding stents for the treatment of refractory benign esophageal strictures: A prospective multicentre study. BMC Gastroenterol. 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, E.A.; Gregory, C.J.; Pursnani, K.G.; Ward, J.B.; Stockwell, R.C. The use of biodegradable (SX-ELLA) oesophageal stents to treat dysphagia due to benign and malignant oesophageal disease. Surg. Endosc. 2012, 26, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Hirdes, M.M.; Siersema, P.D.; van Boeckel, P.G.; Vleggaar, F.P. Single and sequential biodegradable stent placement for refractory benign esophageal strictures: A prospective follow up study. Endoscopy 2012, 44, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Hirdes, M.M.; van Hooft, J.E.; Wijrdeman, H.K.; Hulshof, M.C.; Fockens, P.; Reerink, O.; van Oijen, M.G.; van der Tweel, I.; Vleggaar, F.P.; Siersema, P.D. Combination of biodegradable stent placement and single-dose brachytherapy is associated with an unacceptably high complication rate in the treatment of dysphagia from esophageal cancer. Gastrointest. Endosc. 2012, 76, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Dumoulin, F.L.; Plassmann, D. Tissue hyperplasia following placement of a biodegradable stent for a refractory esophageal stricture: Treatment with argon plasma coagulation. Endoscopy 2012, 44, E356. [Google Scholar] [CrossRef] [PubMed]
- Karakan, T.; Utku, O.G.; Dorukoz, O.; Sen, I.; Colak, B.; Erdal, H.; Karatay, E.; Tahtaci, M.; Cengiz, M. Biodegradable stents for caustic esophageal strictures: A new therapeutic approach. Dis. Esophagus 2013, 26, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Krokidis, M.; Burke, C.; Spiliopoulos, S.; Gkoutzios, P.; Hynes, O.; Ahmed, I.; Dourado, R.; Sabharwal, T. The use of biodegradable stents in malignant oesophageal strictures for the treatment of dysphagia before neoadjuvant treatment or radical radiotherapy: A feasibility study. Cardiovasc. Interv. Radiol. 2013, 36, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Muñoz, D.; Ortiz-Moyano, C.; Gómez-Rodríguez, B. Resolution of a refractory anastomotic stricture with a novel biodegradable esophageal stent. Clin. Gastroenterol. Hepatol. 2013, 11, e63. [Google Scholar] [CrossRef] [PubMed]
- Okata, Y.; Hisamatsu, C.; Bitoh, Y.; Yokoi, A.; Nishijima, E.; Maeda, K.; Yoshida, M.; Ishida, T.; Azuma, T.; Kutsumi, H. Efficacy and histopathological esophageal wall damage of biodegradable esophageal stents for treatment of severe refractory esophageal anastomotic stricture in a child with long gap esophageal atresia. Clin. J. Gastroenterol. 2014, 7, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Martín Cano, F.; Rodríguez Vargas, J.; Velasco Sánchez, B.; Herrera Montes, I. Use of self-expandable prosthesis in esophageal stenosis in children. Cir. Pediatr. 2012, 25, 207–210. [Google Scholar] [PubMed]
- Van den Berg, M.W.; Walter, D.; de Vries, E.M.; Vleggaar, F.P.; van Berge Henegouwen, M.I.; van Hillegersberg, R.; Siersema, P.D.; Fockens, P.; van Hooft, J.E. Biodegradable stent placement before neoadjuvant chemoradiotherapy as a bridge to surgery in patients with locally advanced esophageal cancer. Gastrointest. Endosc. 2014, 80, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Van Hagen, P.; Hulshof, M.C.; van Lanschot, J.J.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.; Richel, D.J.; Nieuwenhuijzen, G.A.; Hospers, G.A.; Bonenkamp, J.J.; et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Adler, D.G.; Fang, J.; Wong, R.; Wills, J.; Hilden, K. Placement of Polyflex stents in patients with locally advanced esophageal cancer is safe and improves dysphagia during neoadjuvant therapy. Gastrointest. Endosc. 2009, 70, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Langer, F.B.; Schoppmann, S.F.; Prager, G.; Tomaselli, F.; Pluschnig, U.; Hejna, M.; Schmid, R.; Zacherl, J. Temporary placement of selfexpanding oesophageal stents as bridging for neo-adjuvant therapy. Ann. Surg. Oncol. 2010, 17, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Pellen, M.G.; Sabri, S.; Razack, A.; Gilani, S.Q.; Jain, P.K. Safety and efficacy of self-expanding removable metal esophageal stents during neoadjuvant chemotherapy for resectable esophageal cancer. Dis. Esophagus 2012, 25, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.A.; Sarkar, A.; Beltz, S.; Lewis, J.; Loren, D.; Kowalski, T.; Fang, J.; Hilden, K.; Adler, D.G. Placement of fully covered self-expandable metal stents in patients with locally advanced esophageal cancer before neoadjuvant therapy. Gastrointest. Endosc. 2012, 76, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, Y.; Saito, Y.; Tanaka, T.; Nitta, N.; Yamada, H.; Tsujikawa, T.; Murata, K.; Fujiyama, Y.; Andoh, A. Endoscopic submucosal dissection combined with the placement of biodegradable stents for recurrent esophageal cancer after chemoradiotherapy. J. Gastrointest. Cancer 2012, 43, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, A.; Bârla, R.; Hoara, P.; Yokoi, A.; Nishijima, E.; Maeda, K.; Yoshida, M.; Ishida, T.; Azuma, T.; Kutsumi, H. Endoscopic palliation of advanced esophageal cancer. J. Med. Life 2015, 8, 193–201. [Google Scholar] [PubMed]
- Černá, M.; Köcher, M.; Válek, V.; Aujeský, R.; Neoral, Č.; Andrašina, T.; Pánek, J.; Mahathmakanthi, S. Covered biodegradable stent: New therapeutic option for the management of esophageal perforation or anastomotic leak. Cardiovasc. Interv. Radiol. 2011, 34, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, K.; Miyazawa, M.; Aikawa, M.; Akimoto, N.; Koyama, I.; Ikada, Y.; Kitam, H. Experimental trial for perforation caused by esophageal endoscopic submucosal dissection using a biodegradable polymer stent in an animal model. Dig. Endosc. 2012, 24, 286. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Q.; Cui, W.G.; Cheng, Y.S.; Chang, J.; Chen, N.W.; Yan, L.; Li, M.H. Biodegradable rapamycin-eluting nano-fiber membrane-covered metal stent placement to reduce fibroblast proliferation in experimental stricture in a canine model. Endoscopy 2013, 45, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Q.; Hu, C.M.; Li, B.; Cheng, Y.S.; Cui, W.G. A highly flexible paclitaxel-loaded poly(e-caprolactone) electrospunfibrous-membrane-covered stent for benign cardia stricture. Acta Biomater. 2013, 9, 8328–8336. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Q.; Cui, W.G.; Cheng, Y.S.; Chang, J.; Chen, N.W.; Yan, L. Evaluation of biodegradable paclitaxel-eluting nanofibre-covered metal stents for the treatment of benign cardia stricture in an experimental model. Br. J. Surg. 2013, 100, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, M.; Choudhury, N.R.; Knott, R.; Garg, S. Engineering stent based delivery system for esophageal cancer using docetaxel. Mol. Pharm. 2015, 12, 2305–2317. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, L.W.; Wright, J.C.; Wang, Y. Evolution of implantable and insertable drug delivery systems. J. Control. Release 2014, 181, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Van Halsema, E.E.; van Hooft, J.E. Clinical outcomes of self-expandable stent placement for benign esophageal diseases: A pooled analysis of the literature. World J. Gastrointest. Endosc. 2015, 7, 135–153. [Google Scholar]
- Chaput, U.; Heresbach, D.; Audureau, E.; Vanbiervliet, G.; Gaudric, M.; Bichard, P.; Bauret, P.; Coumaros, D.; Ponchon, T.; Fumex, F.; et al. Comparison of a standard fully covered stent with a super-thick silicone-covered stent for the treatment of refractory esophageal benign strictures: A prospective multicenter study. United Eur. Gastroenterol. J. 2013, 1, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Holm, A.N.; de la Mora Levy, J.G.; Gostout, C.J.; Topazian, M.D.; Baron, T.H. Self-expanding plastic stents in treatment of benign esophageal conditions. Gastrointest. Endosc. 2008, 67, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Song, H.Y.; Choi, E.K.; Kim, K.R.; Shin, J.H.; Lim, J.O. Temporary metallic stent placement in the treatment of refractory benign esophageal strictures: Results and factors associated with outcome in 55 patients. Eur. Radiol. 2009, 19, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Bakken, J.C.; Song, L.M.W.K.; de Groen, P.C.; Baron, T.H. Use of a fully covered self-expandable metal stent for the treatment of benign esophageal diseases. Gastrointest. Endosc. 2010, 72, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Senousy, B.E.; Gupte, A.R.; Draganov, P.V.; Forsmark, C.E.; Wagh, M.S. Fully covered Alimaxx esophageal metal stents in the endoscopic treatment of benign esophageal diseases. Dig. Dis. Sci. 2010, 55, 3399–3403. [Google Scholar] [CrossRef] [PubMed]
- Hirdes, M.M.; Vleggaar, F.P.; de Beule, M.; Siersema, P.D. In vitro evaluation of the radial and axial force of self-expanding esophageal stents. Endoscopy 2013, 45, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Dan, D.T.; Gannavarapu, B.; Lee, J.G.; Chang, K.; Muthusamy, V.R. Removable esophageal stents have poor efficacy for the treatment of refractory benign esophageal strictures (RBES). Dis. Esophagus 2014, 27, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Tse, A.; Basu, S.; Ali, H.; Hamouda, A. Black necrotic oesophagus following the use of biodegradable stent for benign oesophageal stricture. J. Surg. Case Rep. 2015, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Rejchrt, S.; Douda, T.; Kopáčová, M.; Široký, M.; Repák, R.; Nožička, J.; Špaček, J.; Bureš, J. Acute oesophageal necrosis (black oesophagus)—Report of three cases. Folia Gastroenterol. Hepatol. 2004, 2, 87–91. [Google Scholar]
- Katsanos, K.; Sabharwal, T.; Koletsis, E.; Fotiadis, N.; Roy-Choudhury, S.; Dougenis, D.; Adam, A. Direct erosion and prolapsed of oesophageal stents into the tracheobronchial tree leading to life-threatening airway compromise. J. Vasc. Interv. Radiol. 2009, 20, 1491–1495. [Google Scholar] [CrossRef] [PubMed]
- Nogales Rincon, O.; Huerta Madrigal, A.; Merino Rodriguez, B.; Gonzalez Asanza, C.; Cos Arregui, E.; Menchen Fernandez-Pacheco, P. Esophageal obstruction due to a collapsed biodegradable esophageal stent. Endoscopy 2011, 43, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Ron, W.; Rajbabu, P. Biodegradable and bioabsorbable Stents. Curr. Pharm. Des. 2010, 16, 4041–4051. [Google Scholar]
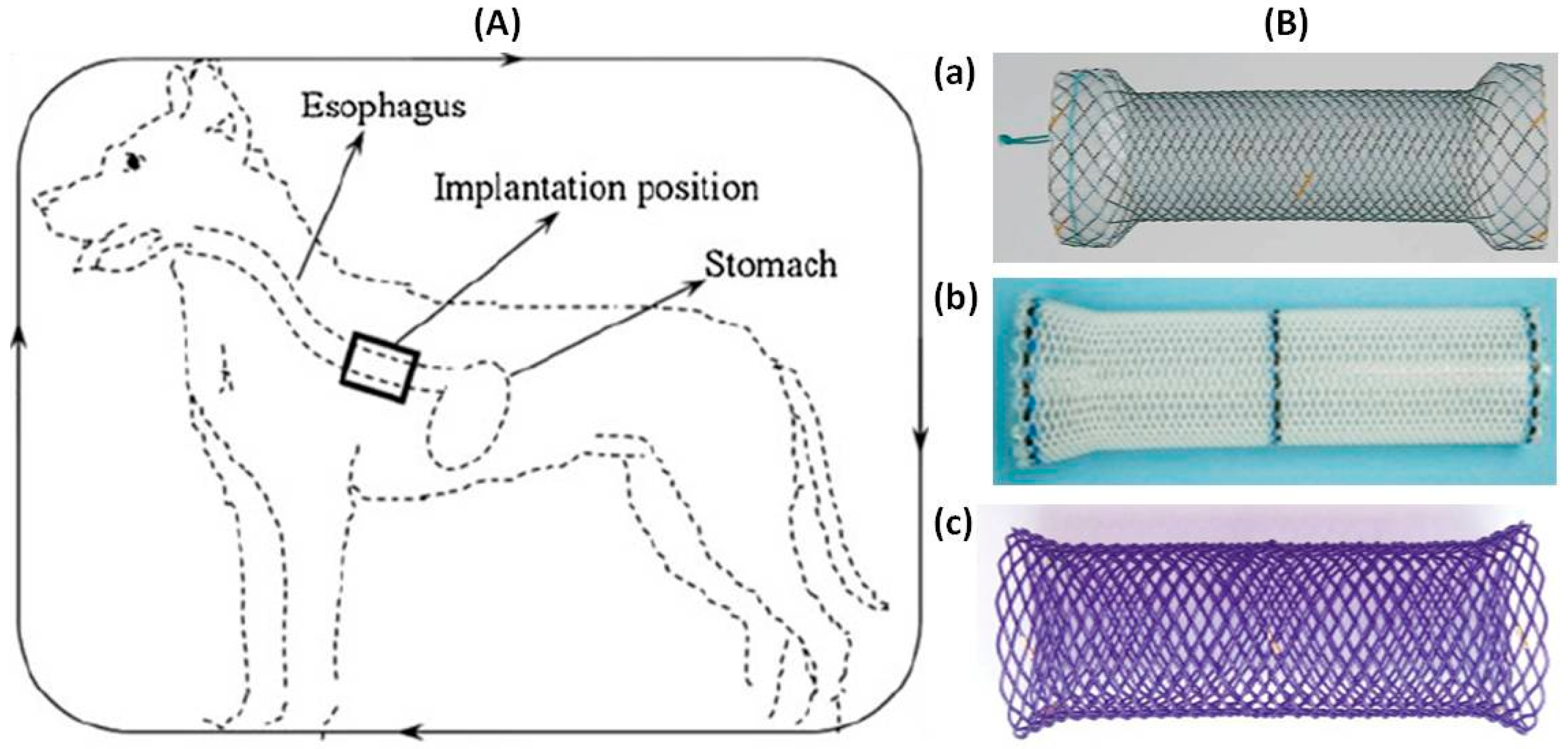
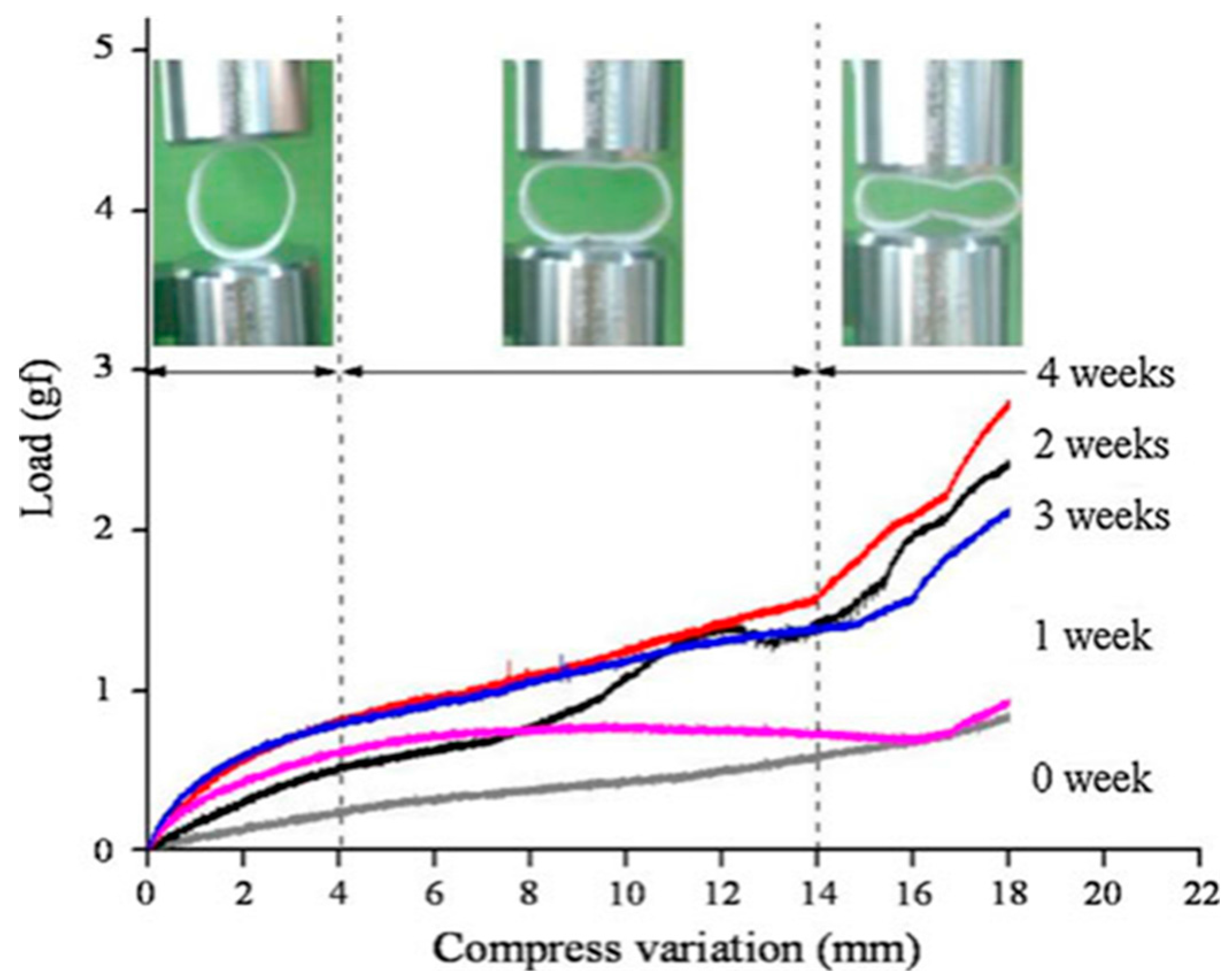
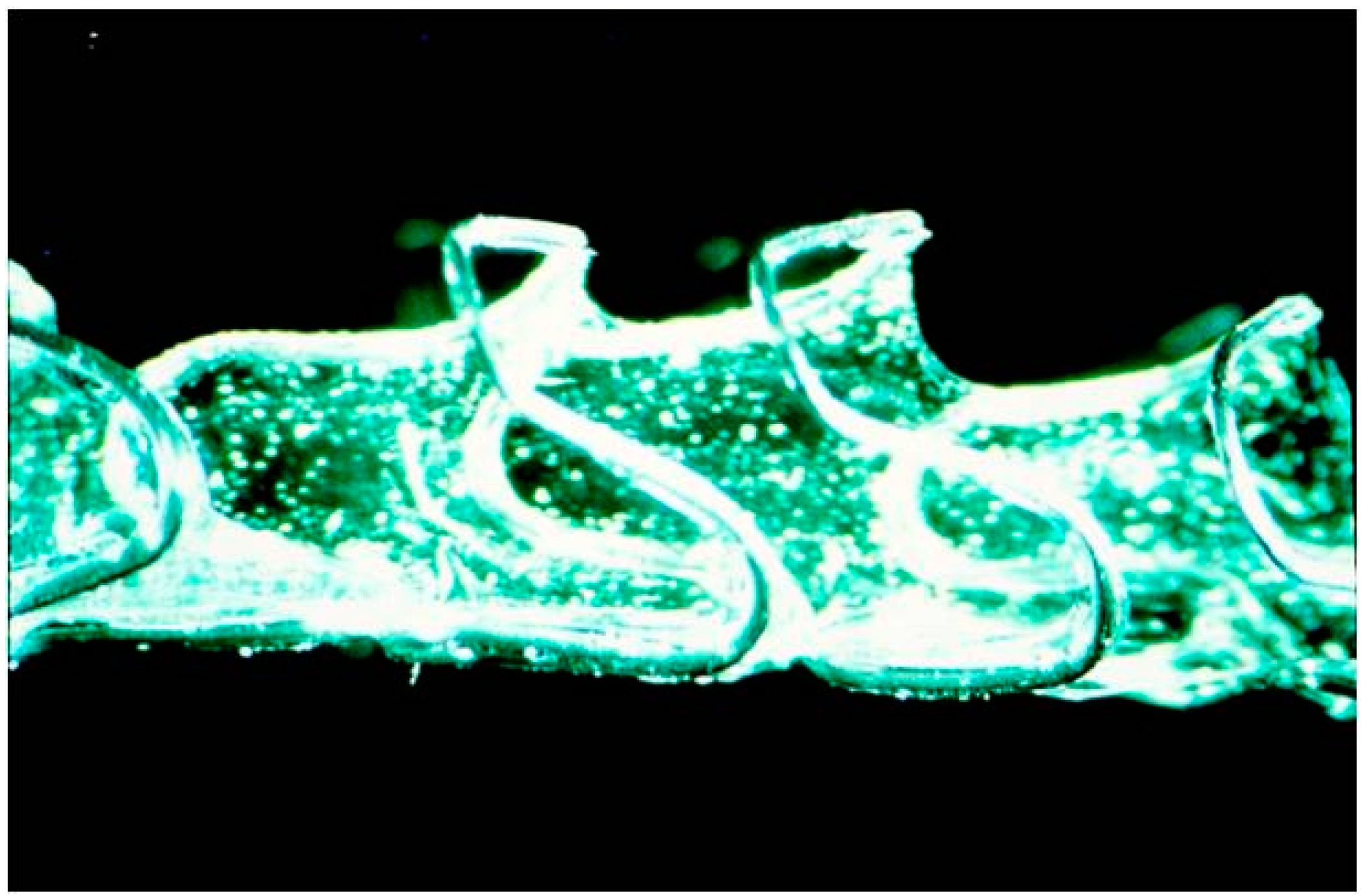
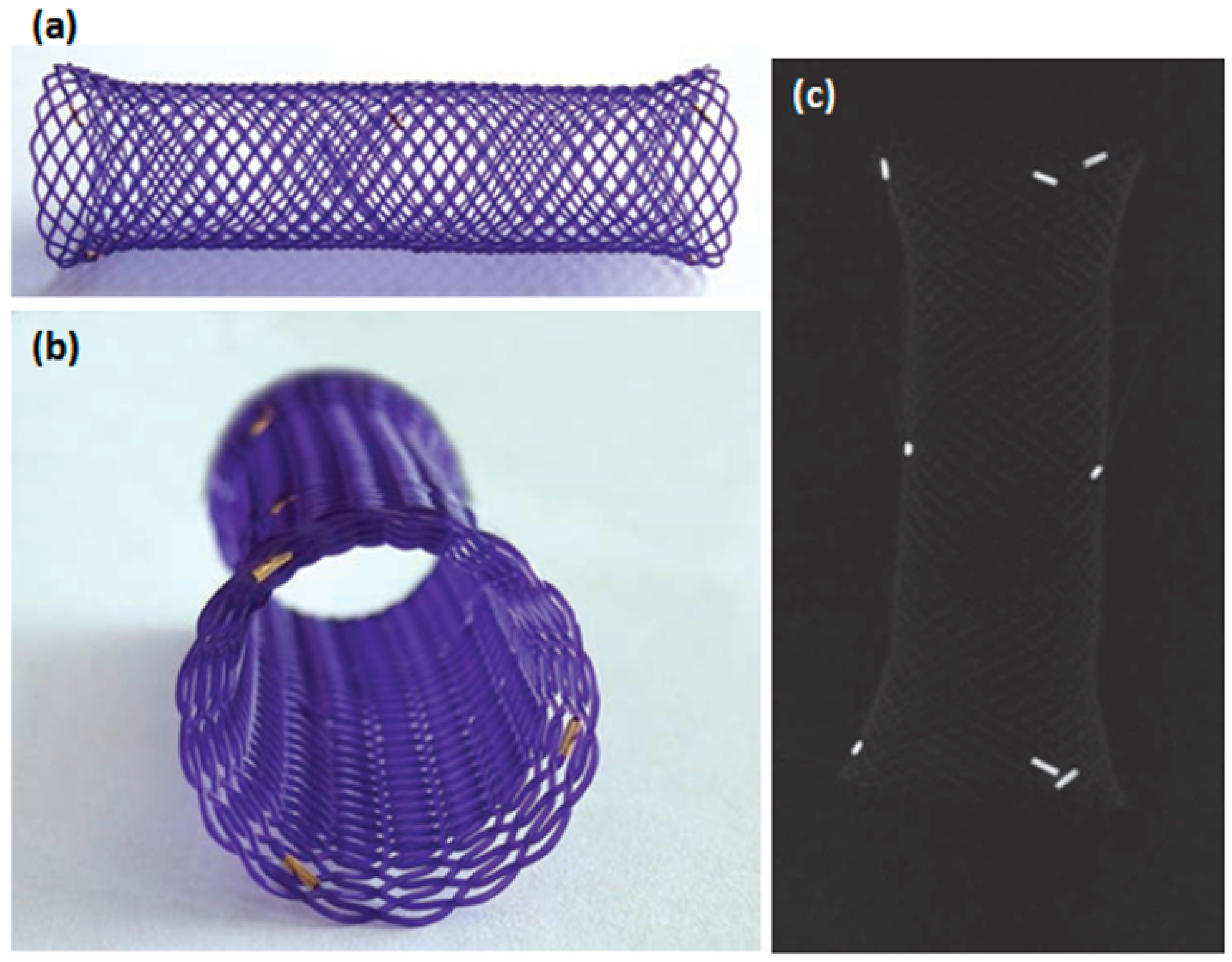
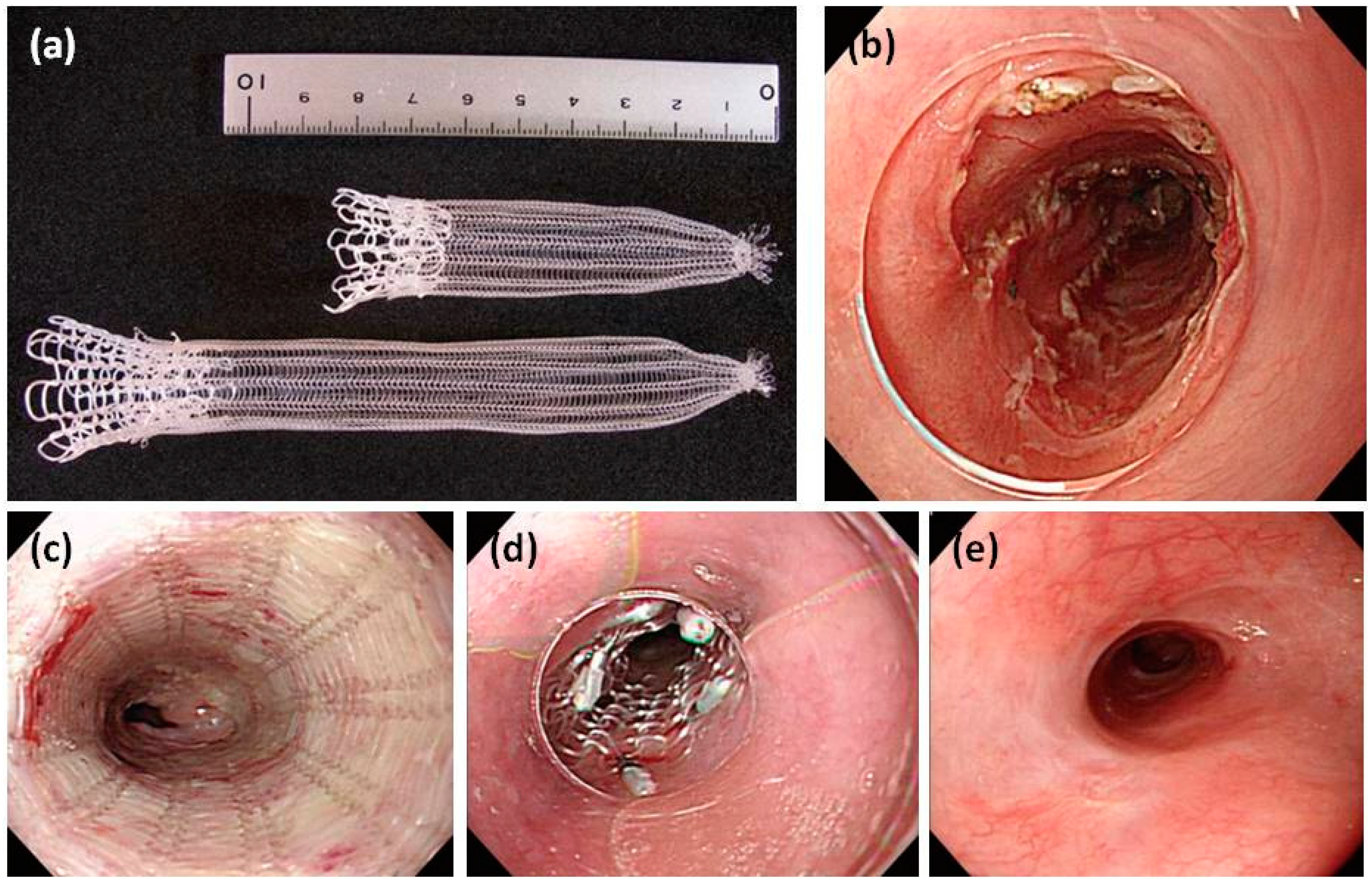

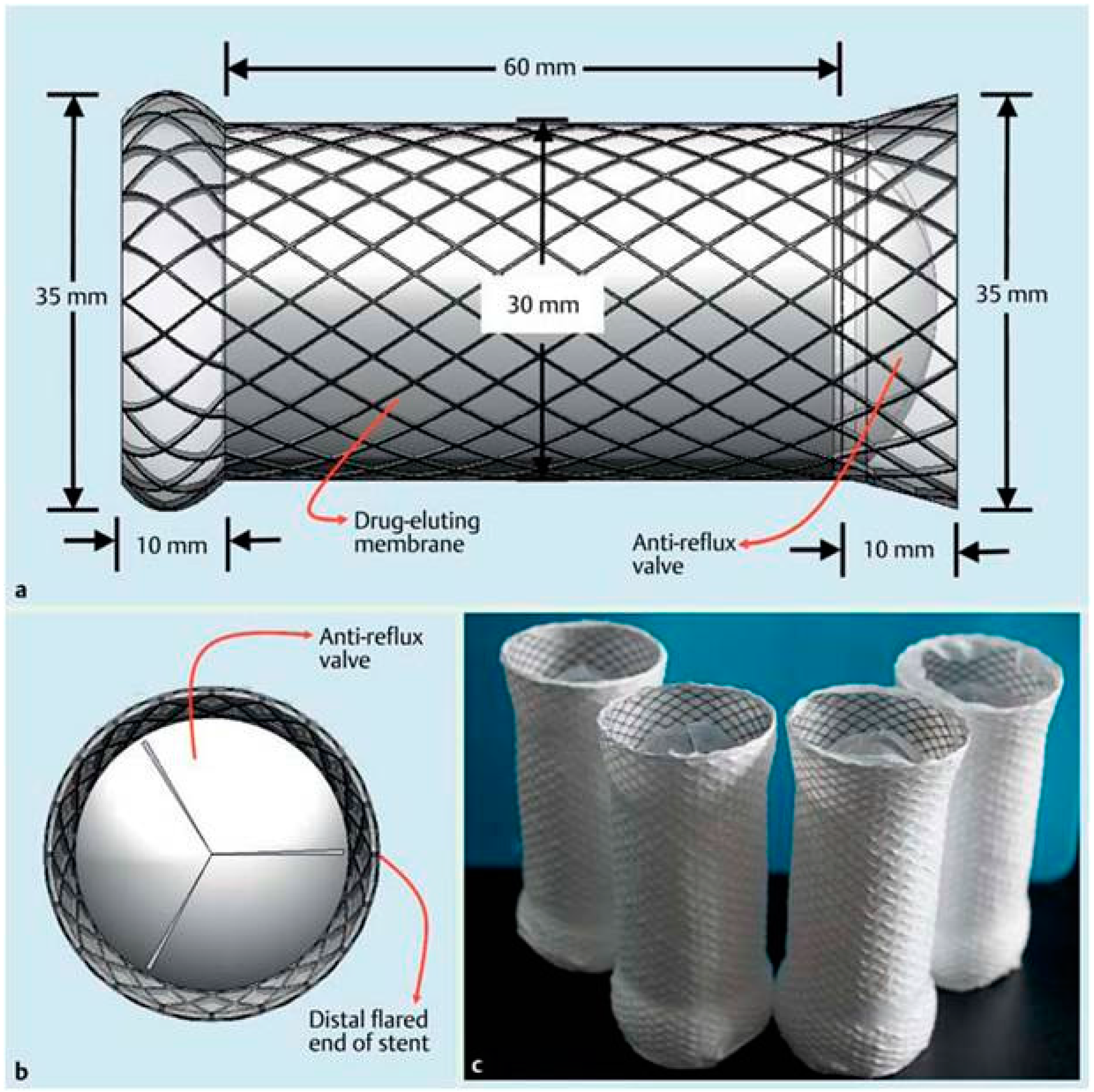

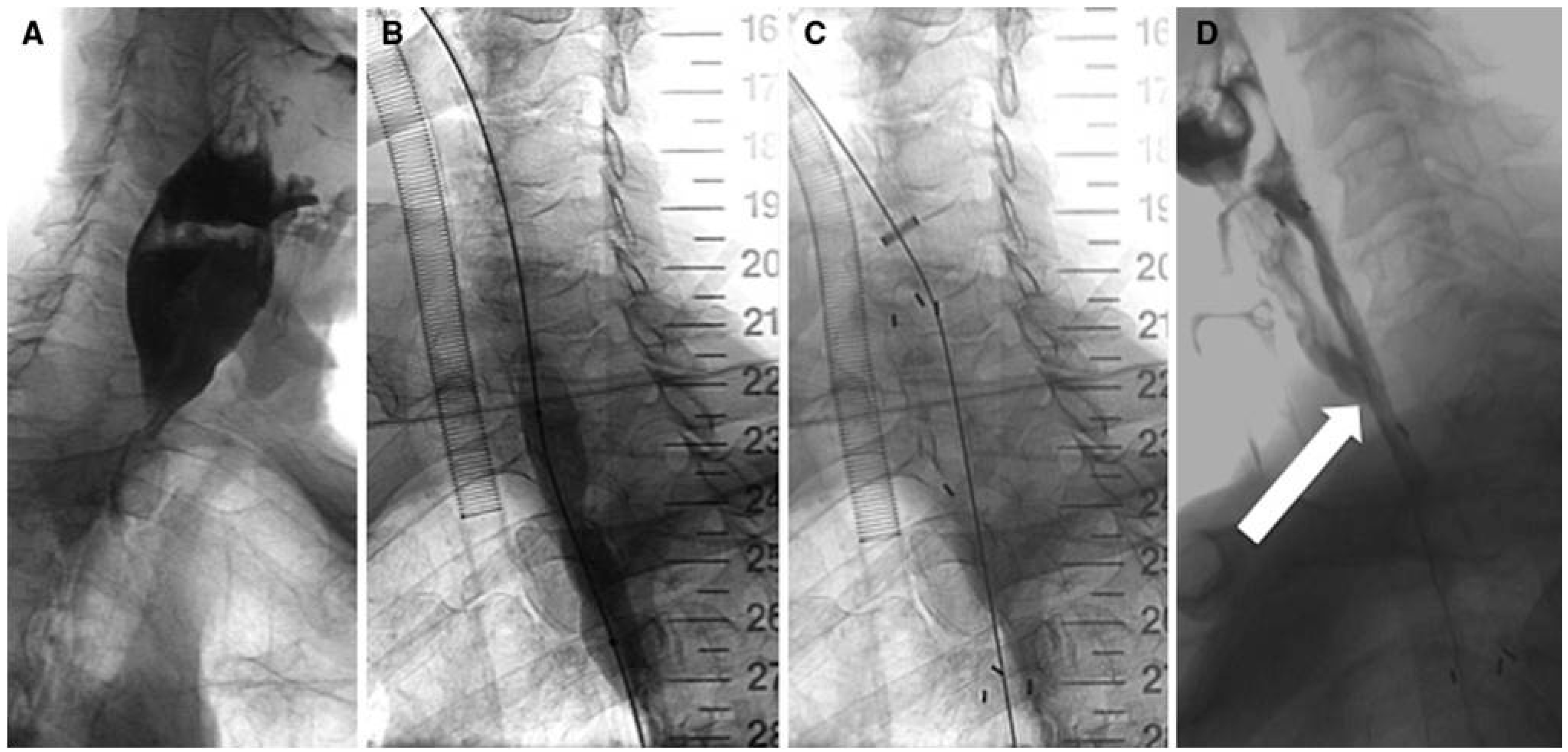
| Study (Year) | Year | Stent Type | Study design | n | Indication | Follow-up | Relief dysphagia | Migration (%) | Hyperplasia (%) | Complication (%) | Clinical success (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Goldin et al. [47] | 1996 | PLLA Instent Improve Instent | Retrospective | 3 | BES | 2–3 weeks | 3(100) | 3(100) | 0 | 0 | |
| 2 | 2 months | 2(100) | 0 | 0 | 0 | 2(100) | |||||
| Fry et al. [3] | 1997 | PLLA Instent | Case report | 1 | BES | 6 weeks | 1(100) | 0 | 0 | Proximal stent collapsed, removed | 1(100) |
| Tanaka et al. [48] | 2006 | Tanaka-Marui Stent PLLA | Retrospective | 2 | BES | 6 months | 2(100) | 0 | 0 | 0 | 2(100) |
| Satio et al. [49] | 2007 | Tanaka-Marui Stent PLLA | Retrospective | 13 | 6 BES 7 RBES | 7–24 months | 13(100) | 10(77) 10–21 days | 0 | 0 | 13(100) |
| Satio et al. [50] | 2008 | Tanaka-Marui Stent PLLA | Retrospective | 2 | Cancer | 7–24 months | 2(100) | 0 | 0 | 0 | 2(100) |
| Dhar et al. [51] | 2009 | ELLA | Prospective | 4 | RBES | 4–17 weeks | 4(100) | 0 | 0 | 1(25) | 4(100) |
| Vandenplas et al. [52] | 2009 | ELLA | Caes report | 1 | BES | 10 months | 1 | 0 | 0 | Pain, vomit | 1(100) |
| Orive-Calzada et al. [53] | 2009 | ELLA | Case report | 1 | BES | 2 months | 1(100) | 0 | 1(100) | 0 | 0 |
| Bychkova et al. [54] | 2009 | ELLA | Case report | 1 | BES | 6 months | 0 | 0 | 0 | 0 | 1(100) |
| Stivaros et al. [55] | 2010 | ELLA | Retrospective | 2 | 1RBES 1 RT | 3–4 months | 2(100) | 2(100) | 0 | 1(50) Pain(1) | 2(100) |
| Viedma et al. [56] | 2010 | ELLA | Prospective | 4 | RBES | mean 24 weeks | 4(100) | 0 | 3(75) | 0 | 3(75) |
| Repici et al. [57] | 2010 | ELLA | Prospective | 21 | RBES | 25–88 weeks | 9/20(45) | 2(9.5) 7weeks | 1(4.7) | 4(18.7) Pain (3) Bleed (1) | 9(43) |
| Ibrahim et al. [58] | 2010 | ELLA | Retrospective | 20 | RBES | mean 90 days | 20(100) | 0 | 12(60) | 8(40) | |
| Jung et al. [59] | 2010 | ELLA | Case report | 1 | RBES | 4 months | 1(100) 4 weeks | 0 | 0 | Tracheoesophageal fistula 4 weeks later | 0 |
| Güitrón-Cantú et al. [60] | 2010 | ELLA | Case report | 1 | RBES | 1(100) | 0 | 1 | 1(100) | ||
| Hair et al. [61] | 2010 | ELLA | Case repot | 1 | achalasia | 8 weeks | 1(100) | 0 | 1(100) | stent collapse (Surgical resection) | 0 |
| Van Boeckel et al. [62] | 2011 | ELLA | Prospective | 18 | RBES | 21–559 days | 6(33) | 4(22) | 2(11) | 4(22) Pain (2) Hematemesis (2) | 6(33) |
| van Hooft et al. [63] | 2011 | ELLA | Prospective | 10 | RBES | 6 months | 6(60) | 2(20) | 2(20) | 0 | 6(60) |
| Fischer et al. [64] | 2012 | ELLA | Retrospective | 2 | BES | 12 months | 1(50) | 0 | 1(50) | 0 | 1(50) |
| Canena et al. [65] | 2012 | ELLA | Retrospective | 10 | BES | 11–21 months | 10(100) | 2(20) | 3(30) | 2(20) Pain (1) Hematemesis (1) | 3(30) |
| Griffiths et al. [66] | 2012 | ELLA | Prospective | 24 | 7 RBES + 17 cancer | 6–8 months | 17/22 (77) 12 weeks | 2(8.3) | 0 | 3(12.5) Bleed(1) vomiting(1) Hematemesis (1) | 18(75) |
| Hirdes et al. [67] | 2012 | ELLA | Prospective | 19 | cancer | 51–140 days | 17(89) | 0 | 2(11) | 8(42) Hematemesis (1) pain (6) | 17(89) |
| Hirdes et al. [68] | 2012 | ELLA | Prospective | 28 | RBES | 14–618 days | 15(54) | 3(11) | 0 | 11(40) | 7(25) |
| Dumoulin et al. [69] | 2012 | ELLA | Case report | 1 | RBES | 18 months | 1(100) 4 month | 0 | 1(100) | 1(100) Pain 2 days | 1(100) |
| Karakan et al. [70] | 2013 | ELLA | Retrospective | 7 | BES | 36–102 weeks | 7(100) | 0 | 4(57) | 0 | 7(100) |
| Krokidis et al. [71] | 2013 | ELLA | Retrospective | 11 | cancer | 32–210 days | 8(72) 32–201 days | 3(27) 42–84 days | 3(27) 111–201 days | 1(9) aspiration 30 days | 11(100) |
| Sanchez et al. [72] | 2013 | ELLA | Case report | 1 | RBES | 20 months | 1(100) | 0 | 0 | 0 | 1(100) |
| Okata et al. [73] | 2014 | ELLA | Case report | 1 | RBES Four sessions | 4 BDS the age of 5–8 years | 1(100) 4–7 months | 0 | 0 | 0 | 1(100) |
| Martín et al. [74] | 2012 | PLLA | Case report | 1 | Cancer ESD | 7 months | 1(100) 1 month | 0 | 0 | food stuck in the stents | 1(100) |
| Van den Berg et al. [75] | 2014 | ELLA | Retrospective | 10 | cancer | 93–166 days | 8(80) | 0 | 2(20) | Pain 6(60) 1–57 days | 10(100) |
| PLLA stent (Marui Textile Machinery, Japan) | ELLA stent (Hradec Kralove, Czech Republic) | |
|---|---|---|
| Materials | Polyglycoside (knitted poly-l-lactic acid monofilaments) | Polydioxanone (a semicrystalline, degradable polymer) |
| Bioabsorption period | 3–6 months | 2–3 months |
| Length and diameter | Designed according to esophageal lesion | Size: 18, 20, 23, 25 mm Length: 60, 80, 100, 135 mm |
| Setting | Fitted over an endoscope | Delivery system |
| Other features | One end is reduced to a diameter of 5 mm by tying with silk sutures | Manual loading is needed |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Ling, C.; Yuan, T.; Zhu, Y.; Cheng, Y.; Cui, W. Polymeric Biodegradable Stent Insertion in the Esophagus. Polymers 2016, 8, 158. https://doi.org/10.3390/polym8050158
Yang K, Ling C, Yuan T, Zhu Y, Cheng Y, Cui W. Polymeric Biodegradable Stent Insertion in the Esophagus. Polymers. 2016; 8(5):158. https://doi.org/10.3390/polym8050158
Chicago/Turabian StyleYang, Kai, Christopher Ling, Tianwen Yuan, Yueqi Zhu, Yingsheng Cheng, and Wenguo Cui. 2016. "Polymeric Biodegradable Stent Insertion in the Esophagus" Polymers 8, no. 5: 158. https://doi.org/10.3390/polym8050158
APA StyleYang, K., Ling, C., Yuan, T., Zhu, Y., Cheng, Y., & Cui, W. (2016). Polymeric Biodegradable Stent Insertion in the Esophagus. Polymers, 8(5), 158. https://doi.org/10.3390/polym8050158