The Effect of Temperature Treatment on the Structure of Polyelectrolyte Multilayers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Layer-by-Layer Deposition
2.3. Ellipsometry
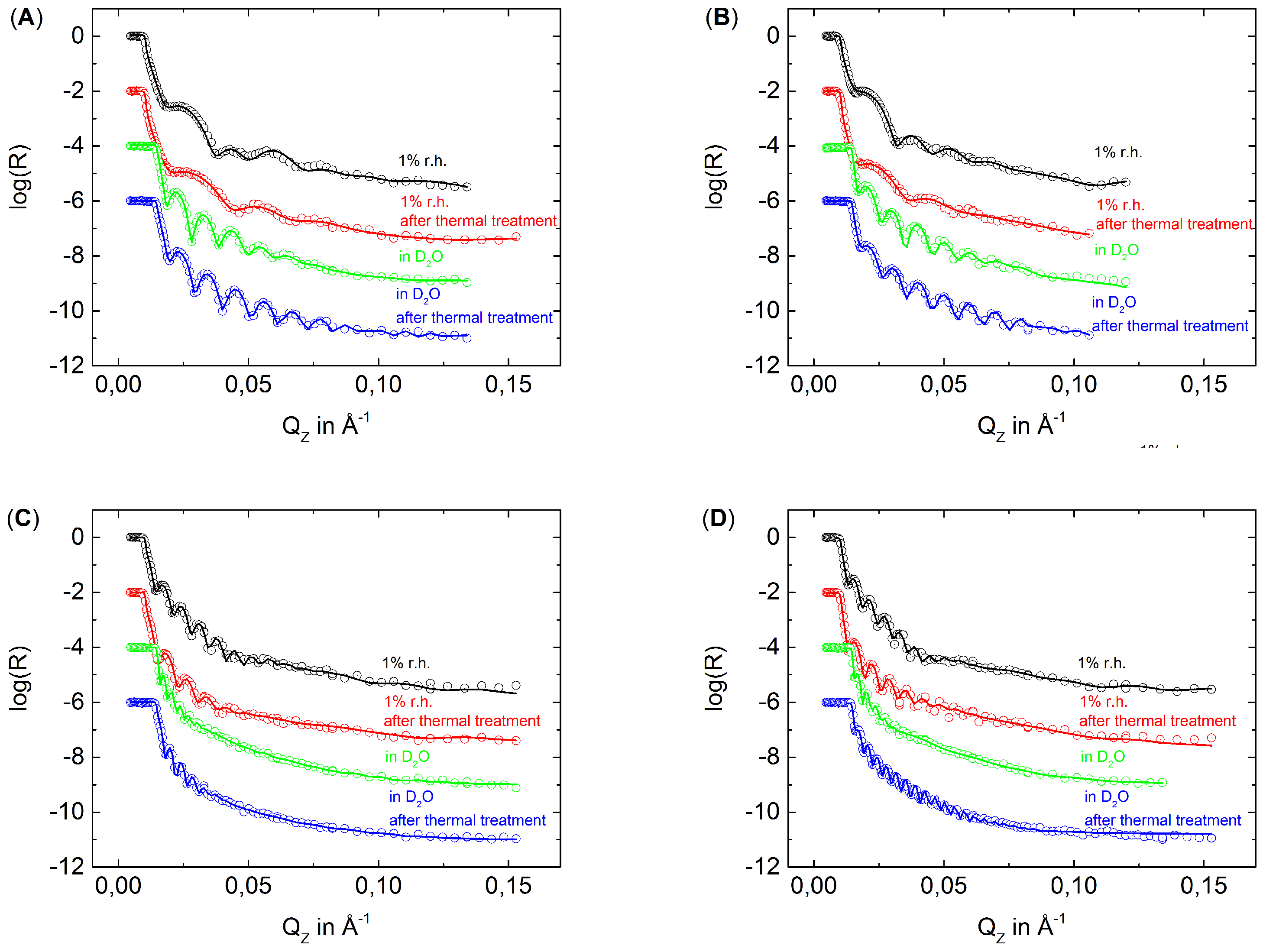
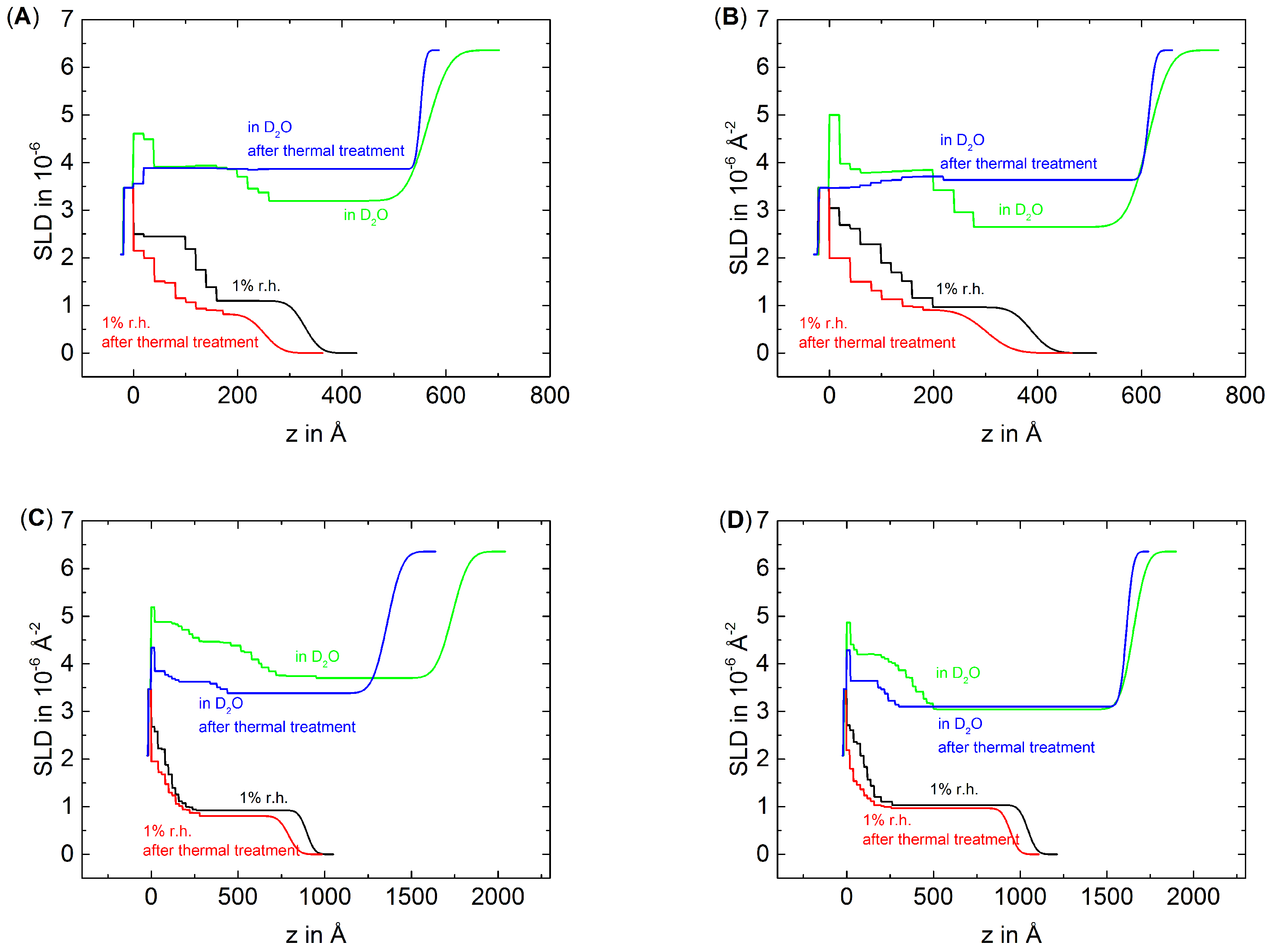
2.4. Neutron Reflectometry
3. Results
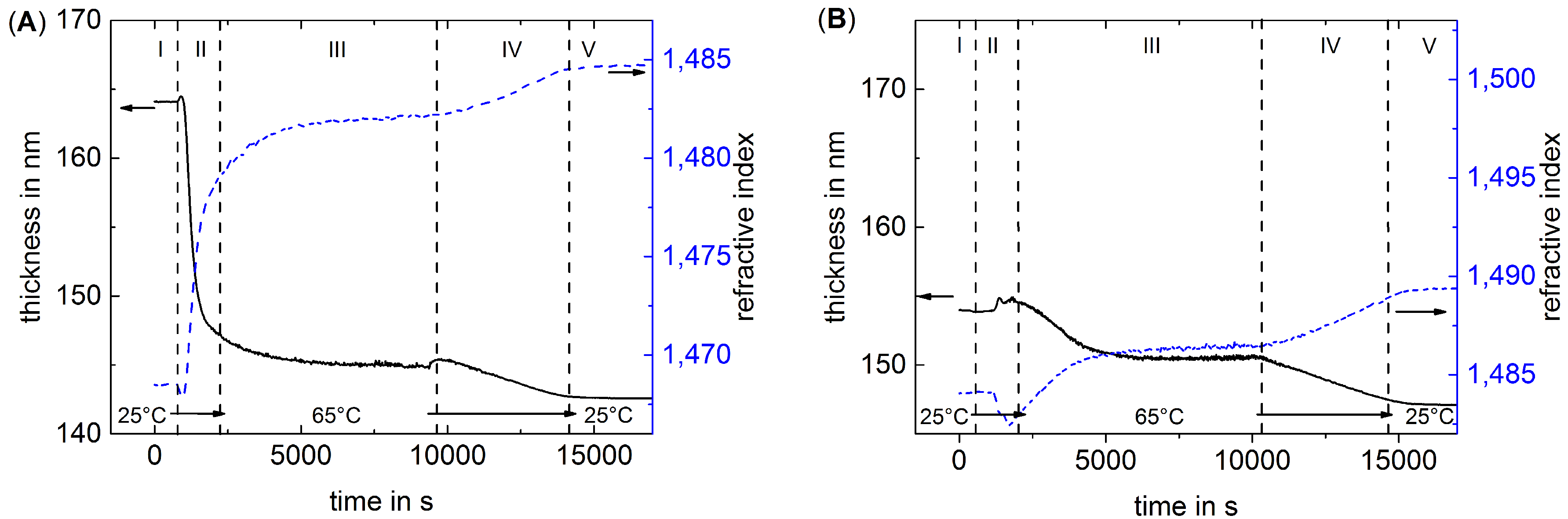
3.1. The Effect of Thermal Treatment on the Average Structure of PEMs
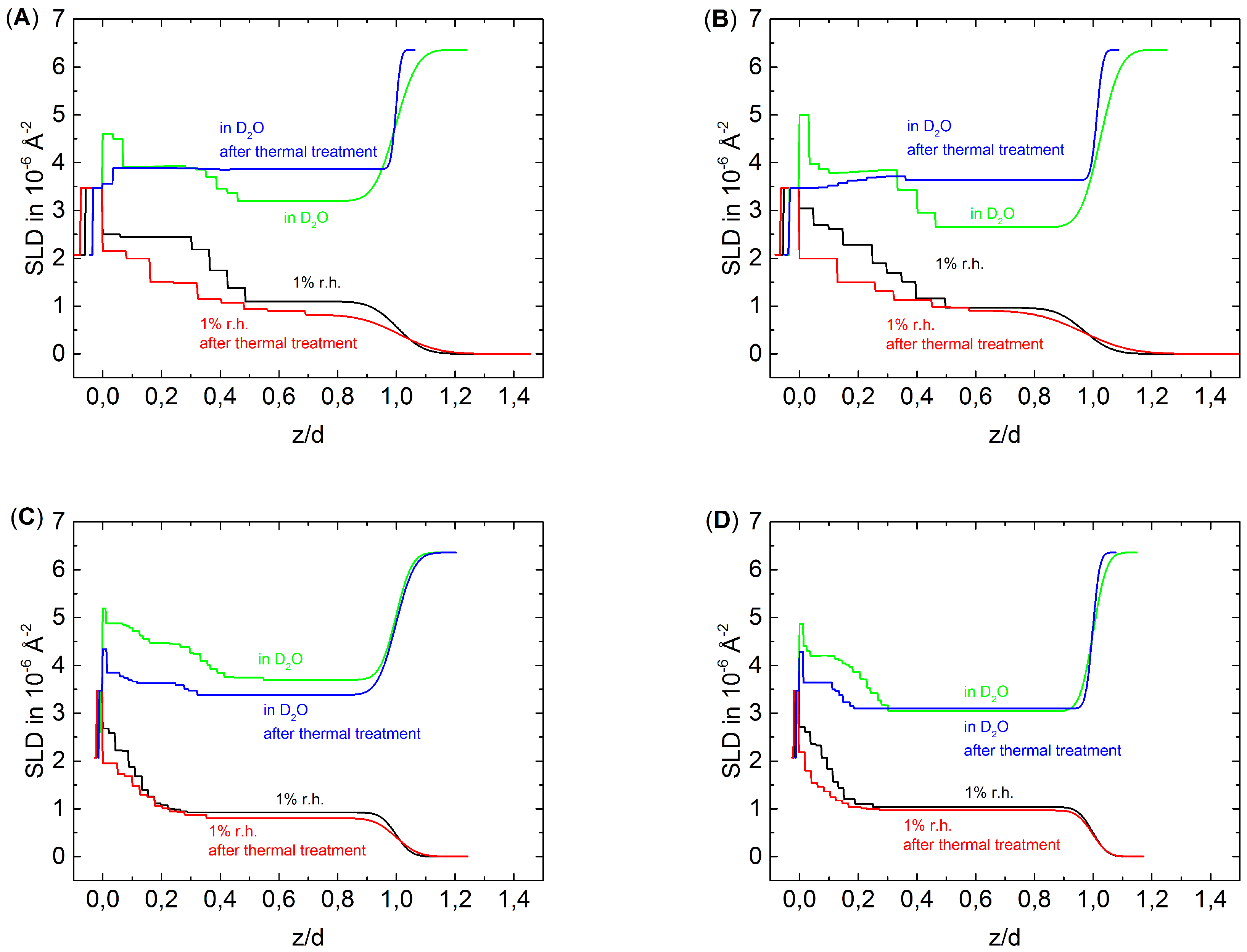
3.2. The Effect of Thermal Treatment on the Internal Structure of PEMs
4. Discussion
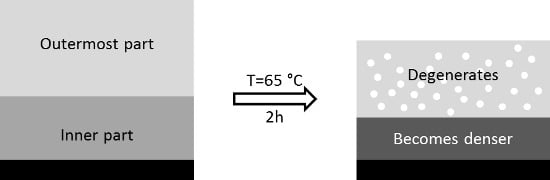
4.1. Degradation and Densification of the PEMs
4.2. The Weakened Odd-Even Effect
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PE | polyelectrolyte |
| PSS | polystyrene sodium sulfonate |
| d-PSS | deuterated polystyrene sodium sulfonate |
| PEI | polyethyleneimine |
| PDADMAC | poly(diallyldimethylammoniumchloride) |
| NR | neutron reflectometry |
| SLD | scattering length density |
| n | refractive index |
| d | thickness |
References
- Ariga, K.; Yamauchi, Y.; Rydzek, G.; Ji, Q.; Yonamine, Y.; Wu, K.C.W.; Hill, J.P. Layer-by-layer nanoarchitectonics: Invention, innovation, and evolution. Chem. Lett. 2014, 43, 36–68. [Google Scholar] [CrossRef]
- Richardson, J.J.; Bjornmalm, M.; Caruso, F. Technology-driven layer-by-layer assembly of nanofilms. Science 2015, 348, aaa2491. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.; Mano, J.F. Molecular interactions driving the layer-by-layer assembly of multilayers. Chem. Rev. 2014, 114, 8883–8942. [Google Scholar] [CrossRef] [PubMed]
- Büscher, K.; Graf, K.; Ahrens, H.; Helm, C.A. Influence of adsorption conditions on the structure of polyelectrolyte multilayers. Langmuir 2002, 18, 3585–3591. [Google Scholar] [CrossRef]
- Tan, H.L.; Mcmurdo, M.J.; Pan, G.; Patten, P.G.V. Temperature dependence of polyelectrolyte multilayer assembly. Langmuir 2003, 19, 9311–9314. [Google Scholar] [CrossRef]
- Salomäki, M.; Vinokurov, I.A.; Kankare, J. Effect of temperature on the buildup of polyelectrolyte multilayers. Langmuir 2005, 21, 11232–11240. [Google Scholar] [CrossRef] [PubMed]
- Gopinadhan, M.; Ahrens, H.; Gu, J.U.; Steitz, R.; Helm, C.A. Approaching the precipitation temperature of the deposition solution and the effects on the internal order of polyelectrolyte multilayers. Macromolecules 2005, 38, 5228–5235. [Google Scholar] [CrossRef]
- Nestler, P.; Block, S.; Helm, C.A. Temperature-induced transition from odd–even to even–odd effect in polyelectrolyte multilayers due to interpolyelectrolyte interactions. J. Phys. Chem. B 2012, 116, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Köhler, K.; Möhewald, H.; Sukhorukow, G.B. Thermal behavior of polyelectrolyte multilayer microcapsules: 2. Insight into molecular mechanisms for the PDADMAC/PSS System. J. Phys. Chem. B 2006, 110, 24002–24010. [Google Scholar] [CrossRef] [PubMed]
- Köhler, K.; Shchukin, D.G.; Möhwald, H.; Sukhorukov, G.B. Thermal behavior of polyelectrolyte multilayer microcapsules. 1. The effect of odd and even layer number. J. Phys. Chem. B 2005, 109, 18250–18259. [Google Scholar] [CrossRef] [PubMed]
- Steitz, R.; Leiner, V.; Tauer, K.; Khrenov, V.; von Klitzing, R. Temperature-induced changes in polyelectrolyte films at the solid-liquid interface. Appl. Phys. A 2002, 521, 519–521. [Google Scholar] [CrossRef]
- Vidyasagar, A.; Sung, C.; Gamble, R.; Lutkenhaus, J.L. Thermal transitions in dry and hydrated layer-by-layer assemblies exhibiting linear and exponential growth. ACS Nano 2012, 6, 6174–6184. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Fan, T.H.; Vinogradova, O.I. Thermal softening of superswollen polyelectrolyte microcapsules. Soft Matter 2011, 7, 2705–2708. [Google Scholar] [CrossRef]
- Ma, J.; Yang, S.; Li, Y.; Xu, X.; Xu, J. Effect of temperature on the build-up and post hydrothermal processing of hydrogen-bonded PVPON/PAA film. Soft Matter 2011, 7, 9435–9443. [Google Scholar] [CrossRef]
- Vidyasagar, A.; Sung, C.; Losensky, K.; Lutkenhaus, J.L. pH-Dependent thermal transitions in hydrated layer-by-layer assemblies containing weak polyelectrolytes. Macromolecules 2012, 45, 9169–9176. [Google Scholar] [CrossRef]
- Sung, C.; Vidyasagar, A.; Hearn, K.; Lutkenhaus, J.L. Effect of thickness on the thermal properties of hydrogen-bonded LbL assemblies. Langmuir 2012, 28, 8100–8109. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.; Gruenwald, T.; Decher, G.; Pershan, P.S.; Kjaer, K.; Lösche, M. Internal structure of layer-by-layer adsorbed polyelectrolyte films: A neutron and X-ray reflectivity study. Macromolecules 1993, 26, 7058–7063. [Google Scholar] [CrossRef]
- Tanchak, O.M.; Yager, K.G.; Fritzsche, H.; Harroun, T.; Katsaras, J.; Barrett, C.J. Water distribution in multilayers of weak polyelectrolytes. Langmuir 2006, 22, 5137–5143. [Google Scholar] [CrossRef] [PubMed]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Jomaa, H.W.; Schlenoff, J.B. Salt-induced polyelectrolyte interdiffusion in multilayered films: A neutron reflectivity study. Macromolecules 2005, 38, 8473–8480. [Google Scholar] [CrossRef]
- De Vos, W.M.; Mears, L.L.E.; Richardson, R.M.; Cosgrove, T.; Barker, R.; Prescott, S.W. Nonuniform hydration and odd–even effects in polyelectrolyte multilayers under a confining pressure. Macromolecules 2013, 46, 1027–1034. [Google Scholar] [CrossRef]
- Soltwedel, O.; Ivanova, O.; Nestler, P.; Mueller, M.; Koehler, R.; Helm, C.A. Interdiffusion in polyelectrolyte multilayers. Macromolecules 2010, 43, 7288–7293. [Google Scholar] [CrossRef]
- Dautzenberg, H.; Görnitz, E.; Jaeger, W. Synthesis and characterization of poly(diallyldimethylammonium chloride) in a broad range of molecular weight. Macromol. Chem. Phys. 1998, 199, 1561–1571. [Google Scholar] [CrossRef]
- Kolasinska, M.; Krastev, R.; Warszynski, P. Characteristics of polyelectrolyte multilayers: Effect of PEI anchoring layer and posttreatment after deposition. J. Colloid Interface Sci. 2007, 305, 46–56. [Google Scholar] [CrossRef] [PubMed]
- McCrackin, F.; Passaglia, E.; Stromberg, R.R.; Steinberg, H. Measurement of the thickness and refractive index of very thin films and the optical properties of surfaces by ellipsometry. J. Res. Natl. Bur. Stand. Sect. A 1963, 67A, 363–377. [Google Scholar] [CrossRef]
- Herzinger, C.M.; Johs, B.; McGahan, W.A.; Woollam, J.A.; Paulson, W. Ellipsometric determination of optical constants for silicon and thermally grown silicon dioxide via a multi-sample, multi-wavelength, multi-angle investigation. J. Appl. Phys. 1998, 83, 3323–3336. [Google Scholar] [CrossRef]
- Benjamins, J.W.; Joensson, B.; Thuresson, K.; Nylander, T. New experimental setup to use ellipsometry to study liquid–liquid and liquid–solid Interfaces. Langmuir 2002, 18, 6437–6444. [Google Scholar] [CrossRef]
- Mezei, F.; Golub, R.; Klose, F.; Toews, H. Focussed beam reflectometer for solid and liquid surfaces. Phys. B 1995, 213–214, 898–900. [Google Scholar] [CrossRef]
- Nelson, A. Co-refinement of multiple-contrast neutron/X-ray reflectivity data using MOTOFIT. J. Appl. Cryst. 2006, 39, 273–276. [Google Scholar] [CrossRef]
- Ghostine, R.A.; Markarian, M.Z.; Schlenoff, J.B. Asymmetric growth in polyelectrolyte multilayers. J. Am. Chem. Soc. 2013, 135, 7636–7646. [Google Scholar] [CrossRef] [PubMed]
- Zerball, M.; Laschewsky, A.; von Klitzing, R. Swelling of polyelectrolyte multilayers: The relation between, surface and bulk characteristics. J. Phys. Chem. B 2015, 119, 11879–11886. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, B.; Schönhoff, M. Surface potential driven swelling of polyelectrolyte multilayers. Langmuir 2002, 250, 2964–2966. [Google Scholar] [CrossRef]
- McCormick, M.; Smith, R.N.; Graf, R.; Barrett, C.J.; Reven, L.; Spiess, H.W. NMR Studies of the effect of adsorbed water on polyelectrolyte multilayer films in the solid state. Macromolecules 2003, 36, 3616–3625. [Google Scholar] [CrossRef]
- Wong, J.E.; Rehfeldt, F.; Ha, P.; Tanaka, M.; von Klitzing, R. Swelling behavior of polyelectrolyte multilayers in saturated water vapor. Macromolecules 2004, 37, 7285–7289. [Google Scholar] [CrossRef]
- Dodoo, S.; Steitz, R.; Laschewsky, A.; von Klitzing, R. Effect of ionic strength and type of ions on the structure of water swollen polyelectrolyte multilayers. Phys. Chem. Chem. Phys. 2011, 13, 10318–10325. [Google Scholar] [CrossRef] [PubMed]
- Köhler, R.; Chevigny, C.; von Klitzing, R. Neutron reflectometry at polyelectrolyte multilayers. In Multilayer Thin Films: Sequential Assembly of Nanocomposite Materials, 2nd ed.; Decher, G., Schlenoff, J.B., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; pp. 219–268. [Google Scholar]
- Köhler, R.; Steitz, R.; von Klitzing, R. About different types of water in swollen polyelectrolyte multilayers. Adv. Colloid Interface Sci. 2014, 207, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Szyk-Warszyńska, L.; Piekoszewska, J.; Warszyński, P. Formation and stability of poly-l-lysine/casein multilayers. Adsorption 2010, 16, 241–248. [Google Scholar] [CrossRef]
- Porcel, C.; Lavalle, P.; Ball, V.; Decher, G.; Senger, B.; Voegel, J.C.; Schaaf, P. From exponential to linear growth in polyelectrolyte multilayers. Langmuir 2006, 22, 4376–4383. [Google Scholar] [CrossRef] [PubMed]
- Volodkin, D.; von Klitzing, R. Competing mechanisms in polyelectrolyte multilayer formation and swelling: Polycation-polyanion pairing vs. polyelectrolyte-ion pairing. Curr. Opin. Colloid Interface Sci. 2014, 19, 25–31. [Google Scholar] [CrossRef]
- Kovacevic, D.; van der Burgh, S.; de Keizer, A.; Cohen Stuart, M.A. Kinetics of formation and dissolution of weak polyelectrolyte multilayers: Role of salt and free polyions. Langmuir 2002, 18, 5607–5612. [Google Scholar] [CrossRef]
- Kharlampieva, E.; Sukhishvili, S.A. Polyelectrolyte multilayers of weak polyacid and cationic copolymer: Competition of hydrogen-bonding and electrostatic interactions. Macromolecules 2003, 36, 9950–9956. [Google Scholar] [CrossRef]
- Von Klitzing, R. Internal structure of polyelectrolyte multilayer assemblies. Phys. Chem. Chem. Phys. 2006, 8, 5012–5033. [Google Scholar] [CrossRef] [PubMed]
- Micciulla, S.; Dodoo, S.; Chevigny, C.; Laschewsky, A.; von Klitzing, R. Short versus long chain polyelectrolyte multilayers: A direct comparison of self-assembly and structural properties. Phys. Chem. Chem. Phys. 2014, 16, 21988–21998. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.E.; Zastrow, H.; Jaeger, W.; von Klitzing, R. Specific ion versus electrostatic effects on the construction of polyelectrolyte multilayers. Langmuir 2009, 25, 14061–14070. [Google Scholar] [CrossRef] [PubMed]
- Neff, P.A.; Naji, A.; Ecker, C.; Nickel, F.; von Klitzing, R.; Bausch, A. Electrical detection of self-assembled polyelectrolyte multilayers by a thin film resistor. Macromolecules 2006, 39, 463–466. [Google Scholar] [CrossRef] [Green Version]
- Neff, P.A.; Wunderlich, B.K.; Klitzing, R.V.; Bausch, A.R. Formation and dielectric properties of polyelectrolyte multilayers studied by a silicon-on-insulator based thin film resistor. Langmuir 2007, 23, 4048–4052. [Google Scholar] [CrossRef] [PubMed]
| Conditions | 20 Layers | |||
| (nm) | (nm) | (10 Å) | ||
| 1% b.t. | 31 ± 1 | 1.54 ± 0.01 | 32.8 ± 0.7 | 1.55 ± 0.03 |
| water b.t. | 55 ± 3 | 1.468 ± 0.005 | 56.6 ± 0.9 | 3.6 ± 0.1 |
| 1% a.t. | 29 ± 1 | 1.52 ± 0.01 | 24.9 ± 0.5 | 1.13 ± 0.02 |
| water a.t. | 53 ± 2 | 1.462 ± 0.004 | 54.1 ± 0.8 | 3.9 ± 0.1 |
| Conditions | 21 Layers | |||
| (nm) | (nm) | (10 Å) | ||
| 1% b.t. | 37 ± 1 | 1.55 ± 0.01 | 40.0 ± 0.8 | 1.71 ± 0.04 |
| water b.t. | 60 ± 3 | 1.488 ± 0.004 | 59.8 ± 0.9 | 3.3 ± 0.1 |
| 1% a.t. | 34 ± 1 | 1.52 ± 0.01 | 31.2 ± 0.6 | 1.12 ± 0.02 |
| water a.t. | 59 ± 3 | 1.472 ± 0.003 | 60.6 ± 0.9 | 3.7 ± 0.1 |
| Conditions | 36 Layers | |||
| (nm) | (nm) | (10 Å) | ||
| 1% b.t. | 92 ± 4 | 1.553 ± 0.003 | 90 ± 2 | 1.11 ± 0.02 |
| water b.t. | 164 ± 6 | 1.470 ± 0.002 | 174 ± 4 | 4.02 ± 0.07 |
| 1% a.t. | 89 ± 4 | 1.544 ± 0.003 | 79 ± 2 | 0.97 ± 0.02 |
| water a.t. | 142 ± 5 | 1.486 ± 0.002 | 136 ± 3 | 3.47 ± 0.08 |
| Conditions | 37 Layers | |||
| (nm) | (nm) | (10 Å) | ||
| 1% b.t. | 94 ± 3 | 1.559 ± 0.002 | 105 ± 3 | 1.21 ± 0.02 |
| water b.t. | 154 ± 4 | 1.483 ± 0.001 | 165 ± 5 | 3.3 ± 0.1 |
| 1% a.t. | 91 ± 3 | 1.553 ± 0.001 | 94 ± 2 | 1.06 ± 0.02 |
| water a.t. | 147 ± 4 | 1.488 ± 0.001 | 162 ± 5 | 3.2 ± 0.1 |
| Conditions | 20 Layers | 21 Layers | ||
| DO b.t. | 0.42 ± 0.04 | 0.02 ± 0.01 | 0.33 ± 0.04 | 0.03 ± 0.01 |
| DO a.t. | 0.54 ± 0.05 | 0.00 ± 0.01 | 0.49 ± 0.04 | 0.01 ± 0.01 |
| Conditions | 36 Layers | 37 Layers | ||
| DO b.t. | 0.48 ± 0.04 | 0.06 ± 0.01 | 0.38 ± 0.04 | 0.03 ± 0.01 |
| DO a.t. | 0.42 ± 0.05 | 0.03 ± 0.01 | 0.42 ± 0.04 | 0.00 ± 0.01 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zerball, M.; Laschewsky, A.; Köhler, R.; Von Klitzing, R. The Effect of Temperature Treatment on the Structure of Polyelectrolyte Multilayers. Polymers 2016, 8, 120. https://doi.org/10.3390/polym8040120
Zerball M, Laschewsky A, Köhler R, Von Klitzing R. The Effect of Temperature Treatment on the Structure of Polyelectrolyte Multilayers. Polymers. 2016; 8(4):120. https://doi.org/10.3390/polym8040120
Chicago/Turabian StyleZerball, Maximilian, André Laschewsky, Ralf Köhler, and Regine Von Klitzing. 2016. "The Effect of Temperature Treatment on the Structure of Polyelectrolyte Multilayers" Polymers 8, no. 4: 120. https://doi.org/10.3390/polym8040120
APA StyleZerball, M., Laschewsky, A., Köhler, R., & Von Klitzing, R. (2016). The Effect of Temperature Treatment on the Structure of Polyelectrolyte Multilayers. Polymers, 8(4), 120. https://doi.org/10.3390/polym8040120