Multilayer Graphene/Carbon Black/Chlorine Isobutyl Isoprene Rubber Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the CIIR Compounds
2.3. Characterization
- Initiation: AIBN ➔ r• + RH ➔ R•
- Propagation: R• + O2 ➔ RO2• + RH ➔ ROOH + R•
- Termination: 2 RO2• ➔ inactive products
- (RH = cumene, R• = cumylalkyl radical, RO2• = cumylperxoy radical, ROOH = cumylhydroperoxide).
3. Results
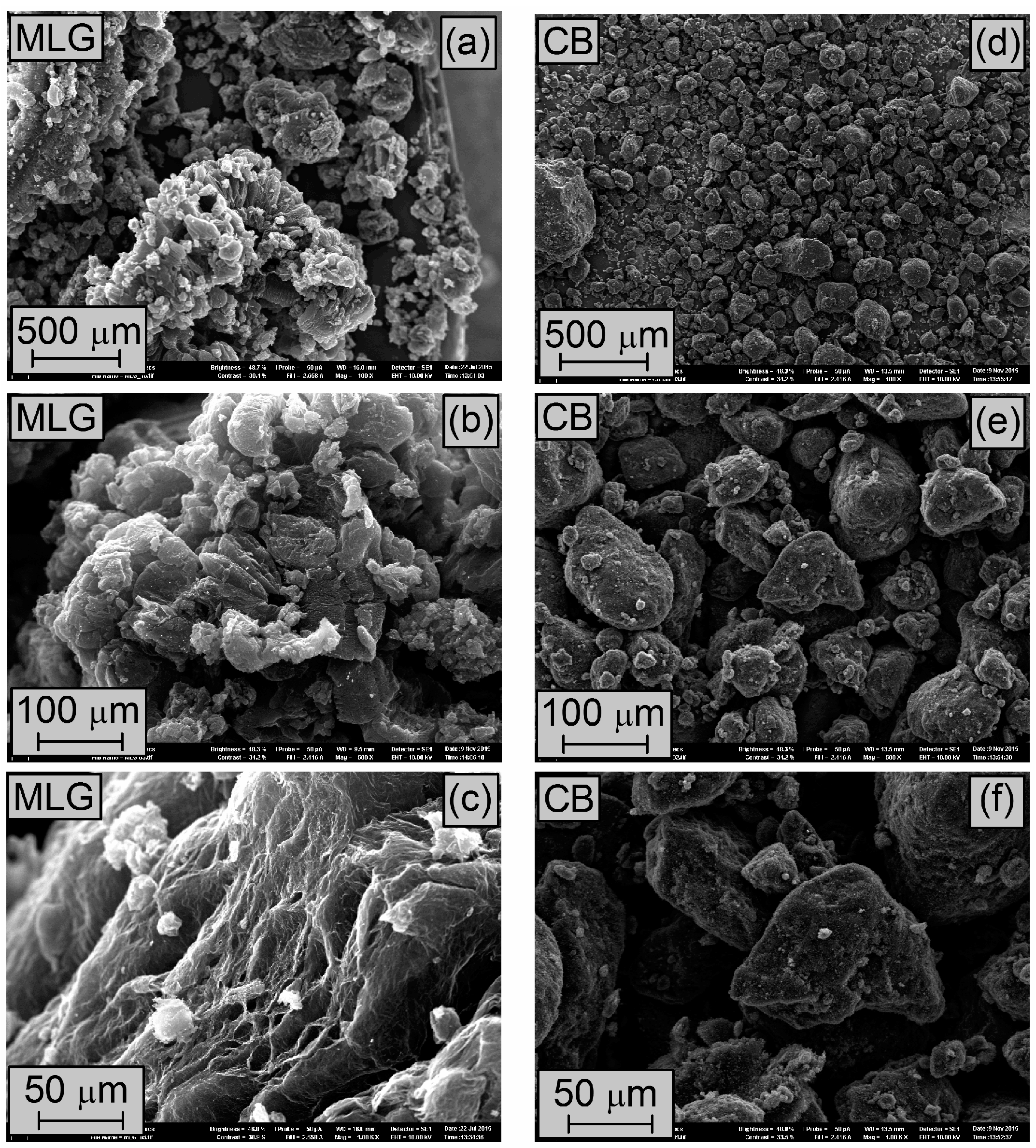
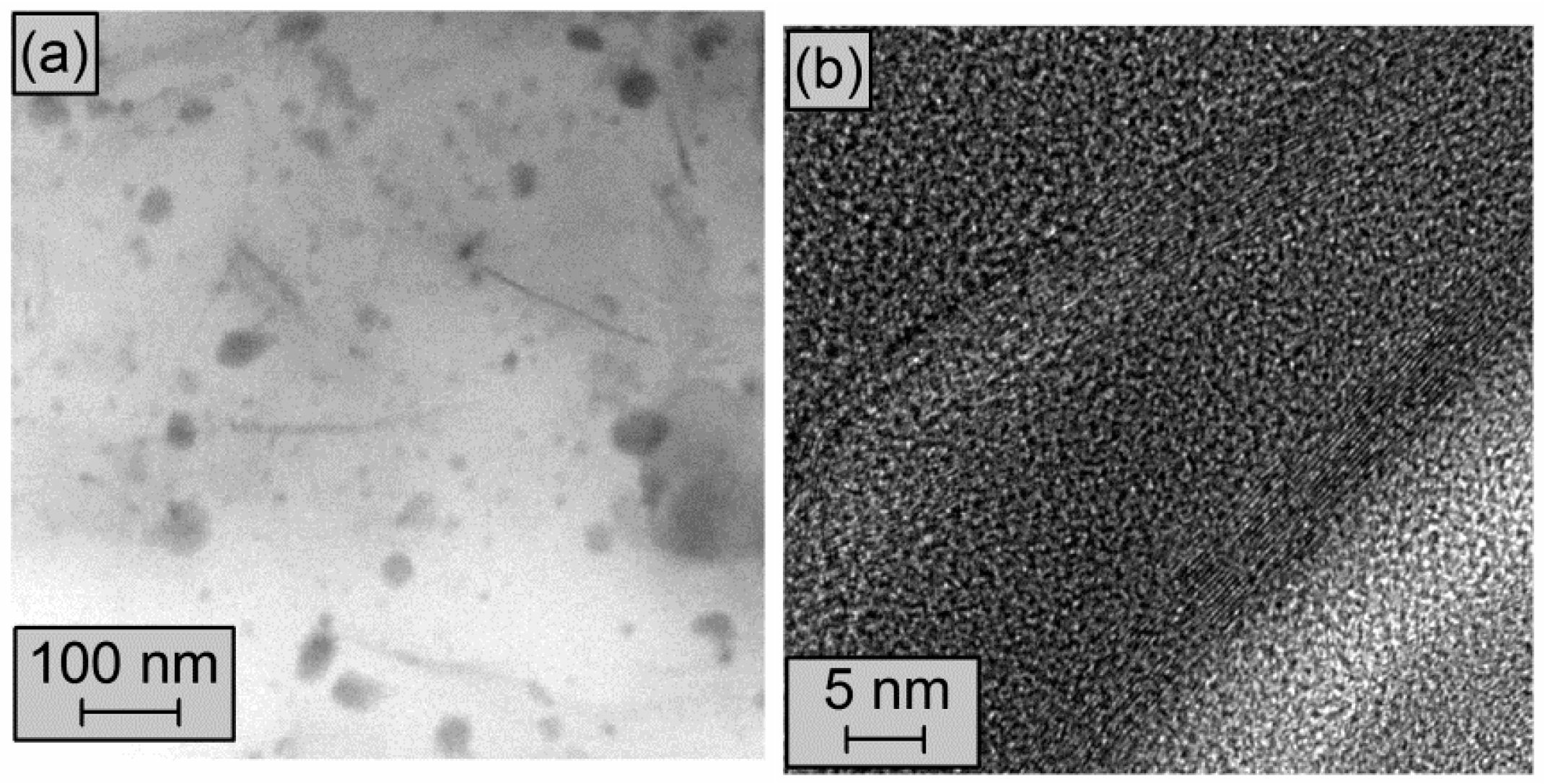
3.1. Characterization of MLG and CB
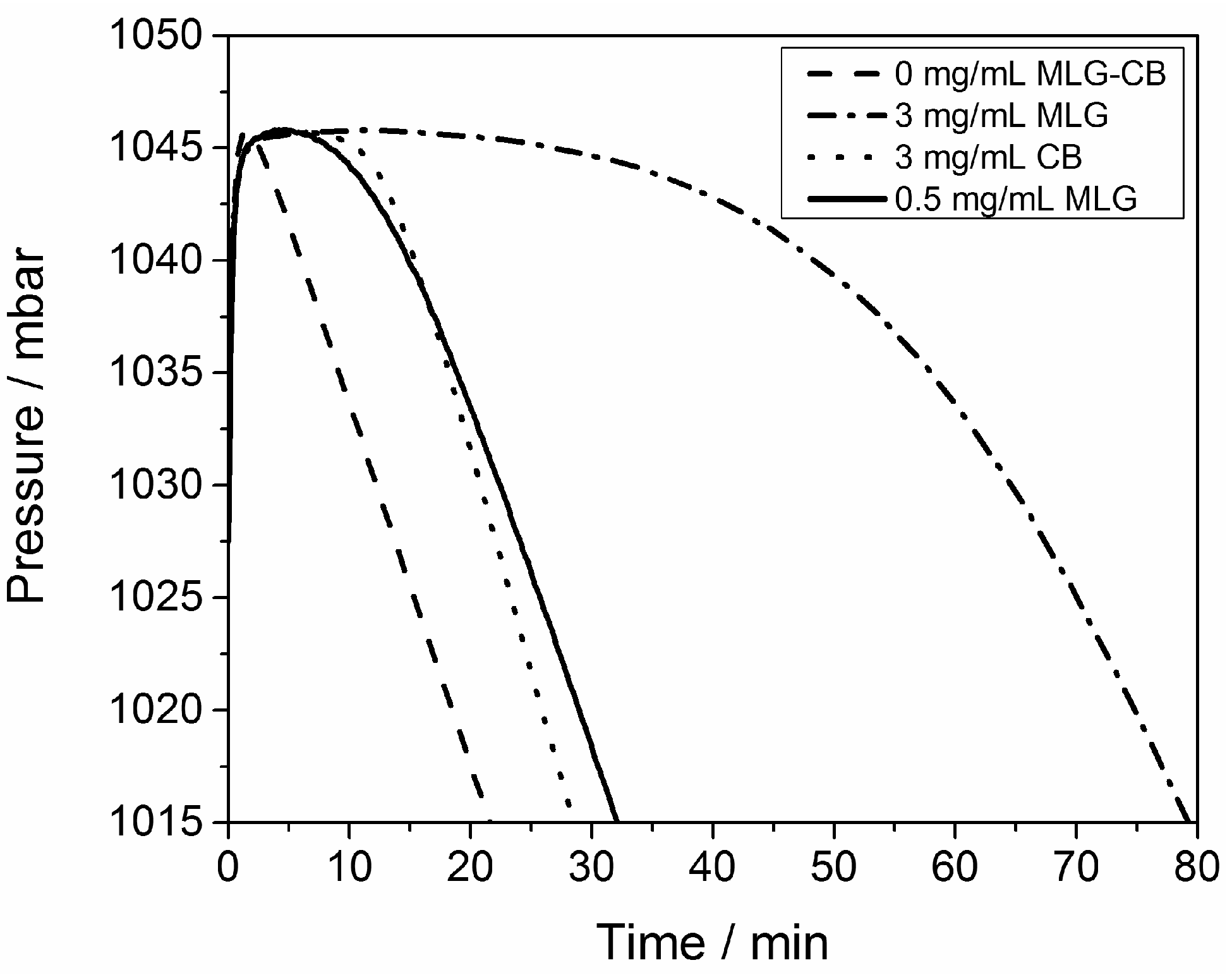
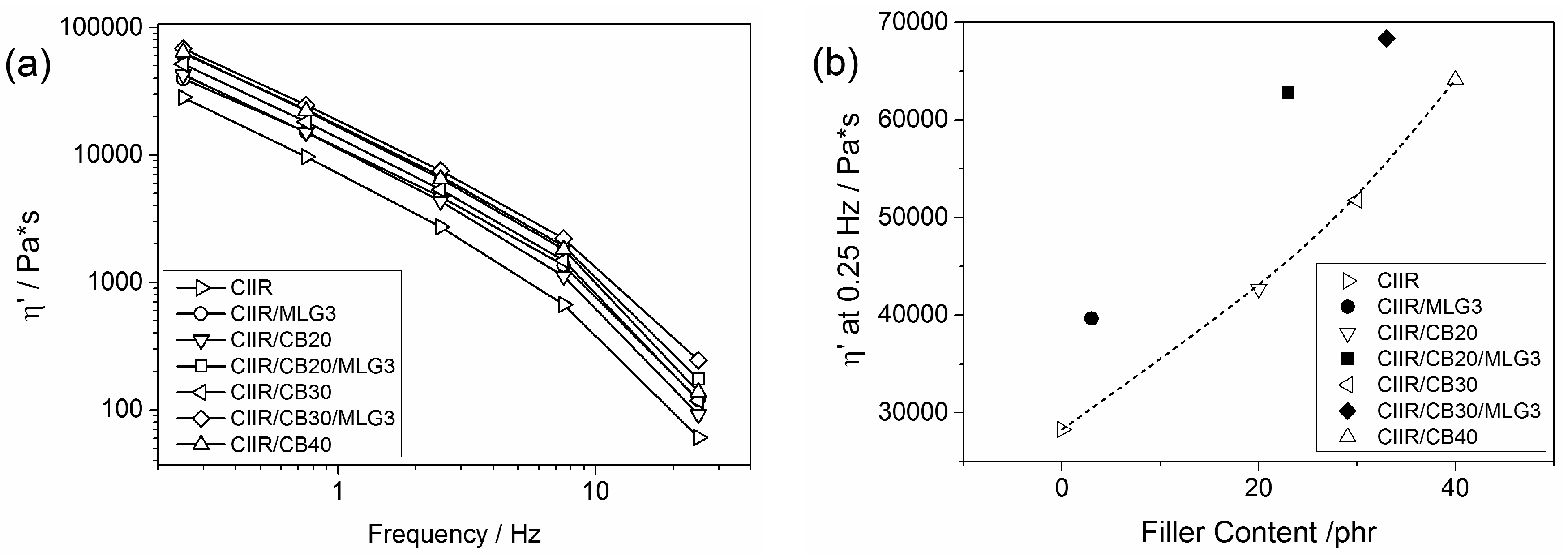
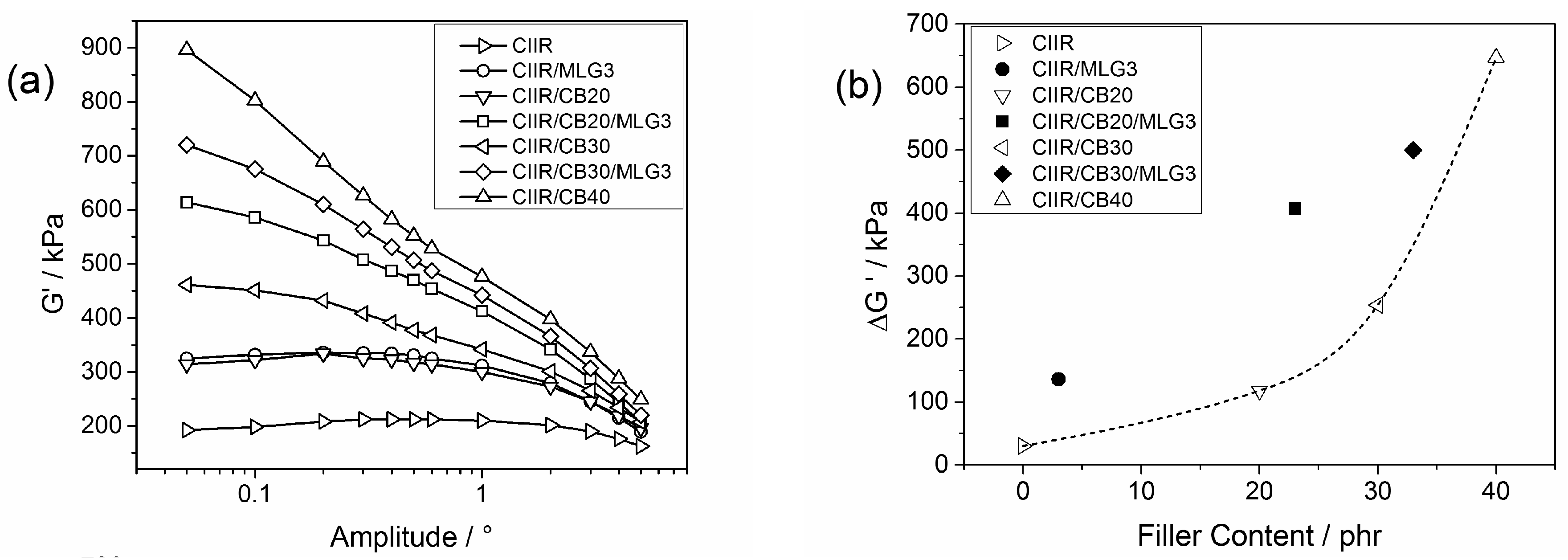
3.2. Rheological Properties of the Uncured Systems
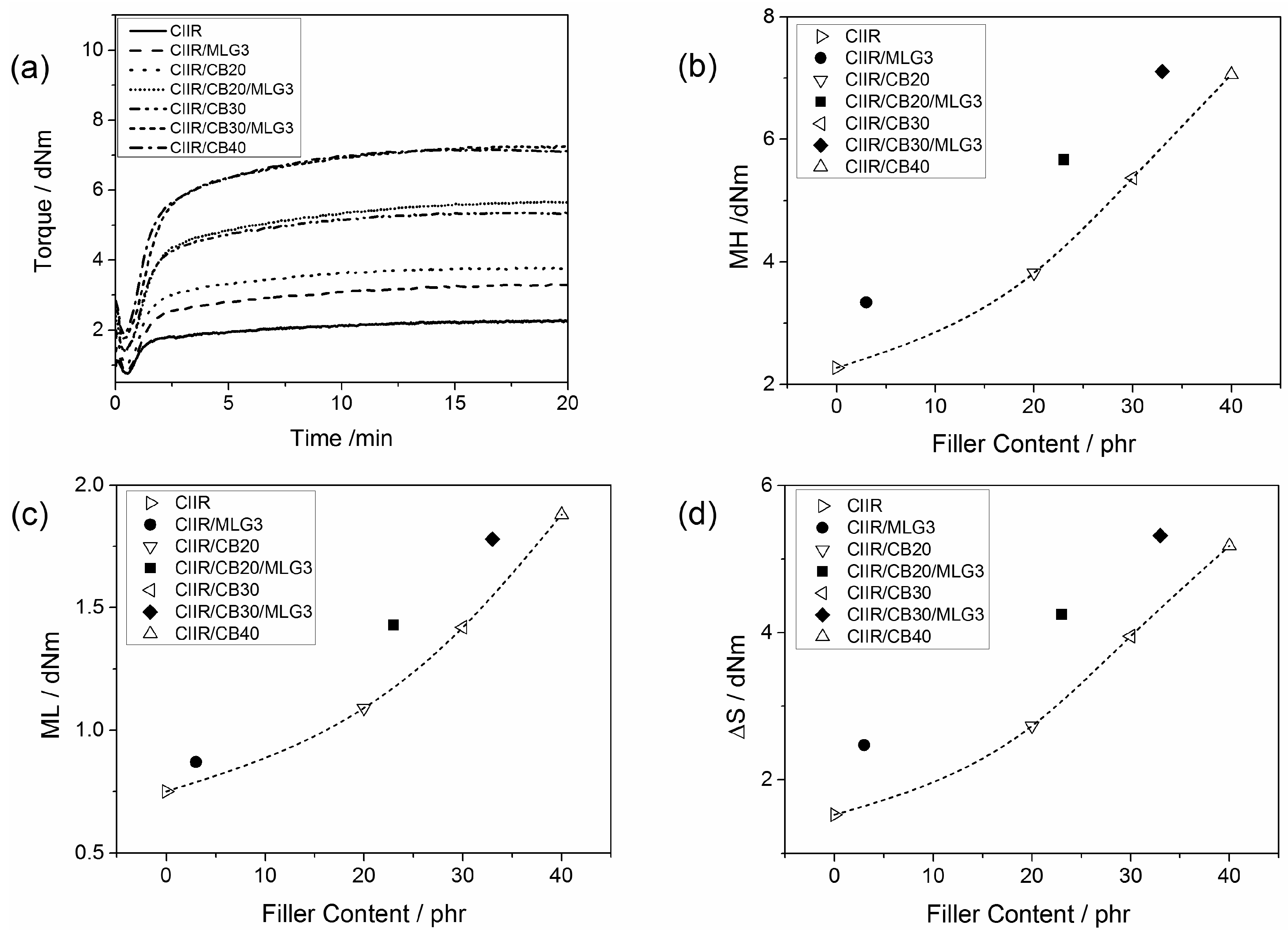
3.3. Curing Properties
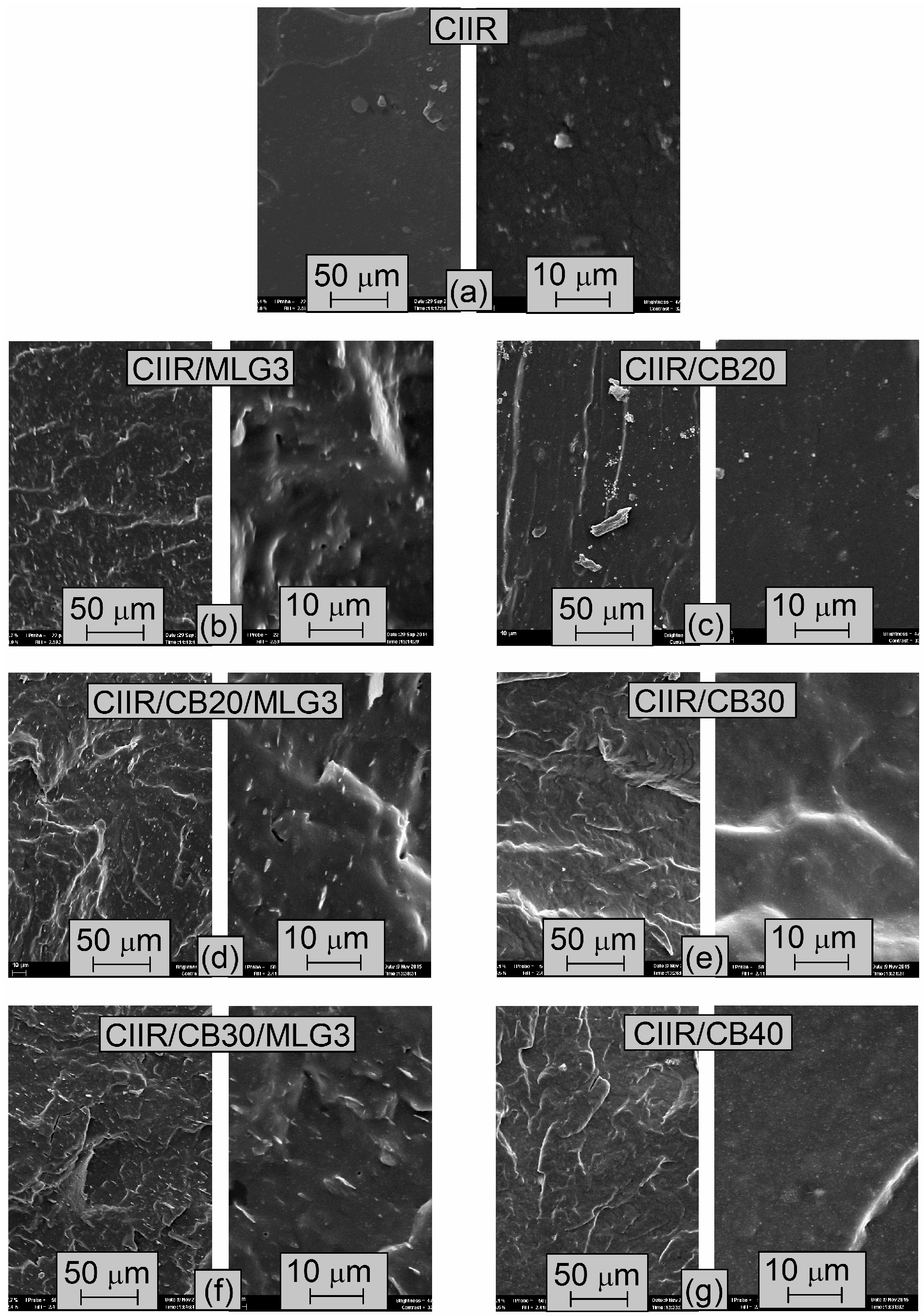
3.4. Morphology of the CIIR and its Composites
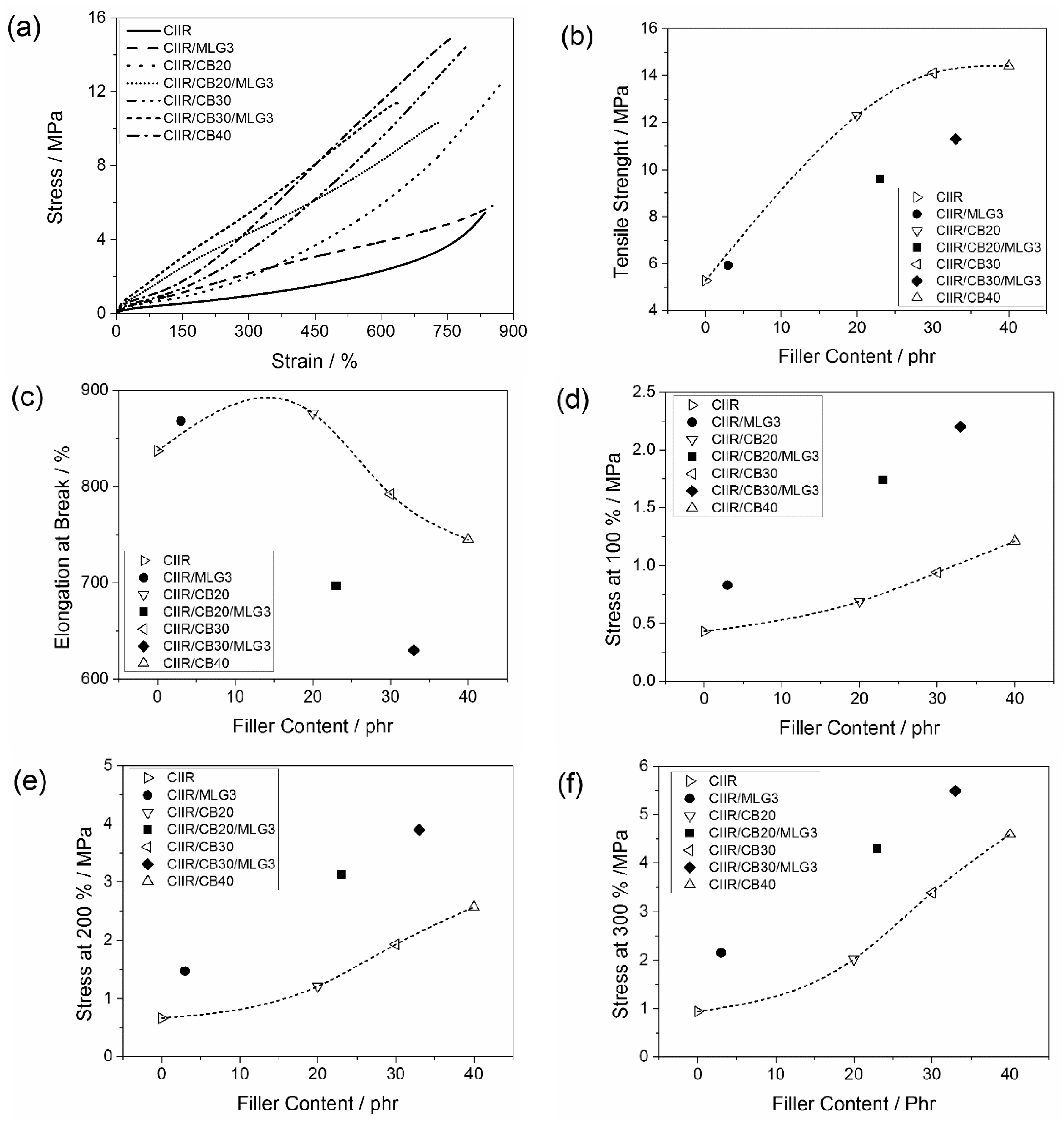
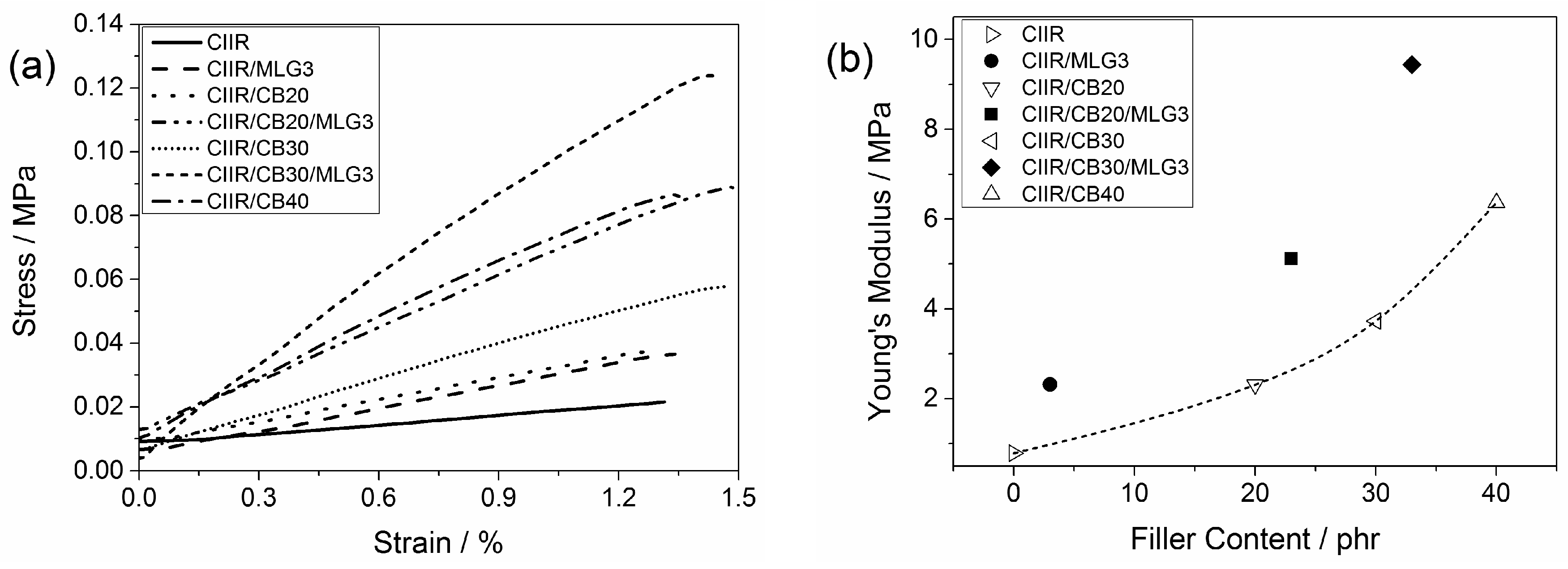
3.5. Mechanical Properties
3.6. Dynamic Mechanical Properties
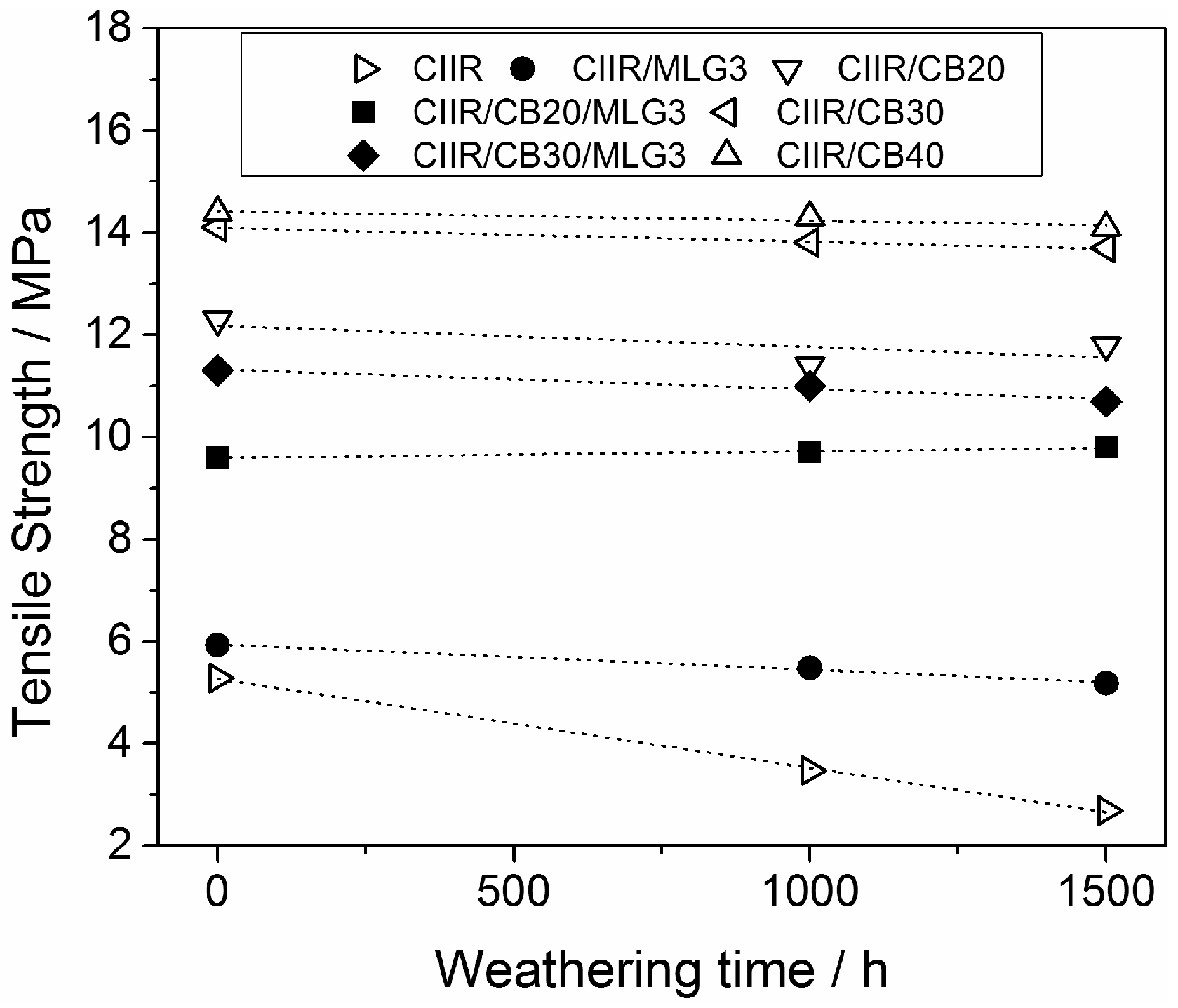
3.7. Durability of Mechanical Properties Against Weathering Exposure
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Araby, S.; Meng, Q.; Zhang, L.; Zaman, I.; Mejewski, P.; Ma, J. Elastomeric composites based on carbon nanomaterials. Nanotechnology 2015, 26, 112001. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S. Chemical aspects of rubber reinforcement by fillers. Rubber Chem. Technol. 1996, 69, 325–346. [Google Scholar] [CrossRef]
- Dannenberg, E.M. The effects of surface chemical interactions on the properties of filler-reinforced rubbers. Rubber Chem. Technol. 1975, 48, 410–444. [Google Scholar] [CrossRef]
- Usuki, A.; Kojima, Y.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T.; Kamigaito, O. Synthesis of nylon 6-clay hybrid. J. Mater. Res. 1993, 8, 1179–1184. [Google Scholar] [CrossRef]
- Arroyo, M.; López-Manchado, M.A.; Herrero, B. Organo-montmorillonite as substitute of carbon black in natural rubber compounds. Polymer 2003, 44, 2447–2453. [Google Scholar] [CrossRef]
- Conzatti, L.; Stagnaro, P.; Colucci, G.; Bongiovanni, R.; Priola, A.; Lostritto, A.; Galimberti, M. The clay mineral modifier as the key to steer the properties of rubber nanocomposites. Appl. Clay Sci. 2012, 61, 14–21. [Google Scholar] [CrossRef]
- Peng, C.-C.; Göpfert, A.; Drechsler, M.; Abetz, V. “Smart” silica-rubber nanocomposites in virtue of hydrogen bonding interaction. Polym. Adv. Technol. 2005, 16, 770–782. [Google Scholar] [CrossRef]
- Gallo, E.; Schartel, B.; Schmaucks, G.; Von der Ehe, K.; Böhning, M. Effect of well dispersed amorphous silicon dioxide in flame retarded styrene butadiene rubber. Plast. Rubber Compos. 2013, 42, 34–42. [Google Scholar] [CrossRef]
- Peddini, S.K.; Bosnyak, C.P.; Henderson, N.M.; Ellison, C.J.; Paul, D.R. Nanocomposites from styrene-butadiene rubber (SBR) and multiwall carbon nanotubes (MWCNT) part 1: Morphology and rheology. Polymer 2014, 55, 258–270. [Google Scholar] [CrossRef]
- Das, A.; Stöckelhuber, K.W.; Jurk, R.; Saphiannikova, M.; Fritzsche, J.; Lorenz, H.; Klüppel, M.; Heinrich, G. Modified and unmodified multiwalled carbon nanotubes in high performance solution-styrene-butadiene and butadiene rubber blends. Polymer 2008, 49, 5276–5283. [Google Scholar] [CrossRef]
- Galimberti, M.; Coombs, M.; Cipolletti, V.; Spatola, A.; Guerra, G.; Lostritto, A.; Giannini, L.; Pandini, S.; Riccò, T. Delaminated and intercalated organically modified montmorillonite in poly(1,4-cis-isoprene) matrix. Indications of counterintuitive dynamic-mechanical behavior. Appl. Clay Sci. 2014, 97–98, 8–16. [Google Scholar] [CrossRef]
- Galimberti, M.; Coombs, M.; Cipolletti, V.; Giannini, L.; Conzatti, L. The origin of synergism between an organoclay and carbon black. Appl. Clay Sci. 2013, 83, 449–456. [Google Scholar] [CrossRef]
- Galimberti, M.; Coombs, M.; Cipolletti, V.; Riccio, P.; Riccò, T.; Pandini, S.; Conzatti, L. Enhancement of mechanical reinforcement due to hybrid filler networking promoted by an organoclay in hydrocarbon-based nanocomposites. Appl. Clay Sci. 2012, 65, 57–66. [Google Scholar] [CrossRef]
- Forouharshad, M.; Gardella, L.; Furfaro, D.; Galimberti, M.; Monticelli, O. A low-environmental-impact approach for novel bio-composites based on PLLA/PCL blends and high surface area graphite. Eur. Polym. J. 2015, 70, 28–36. [Google Scholar] [CrossRef]
- Lapa, V.L.D.C.; de Oliveira, P.D.; Visconte, L.L.Y.; Nunes, R.C.R. Investigation of NBR-cellulose ii nanocomposites by rheometric and equilibrium swelling properties. Polym. Bull. 2008, 60, 281–290. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Schopp, S.; Thomann, R.; Ratzsch, K.F.; Kerling, S.; Altstädt, V.; Mülhaupt, R. Functionalized graphene and carbon materials as components of styrene-butadiene rubber nanocomposites prepared by aqueous dispersion blending. Macromol. Mater. Eng. 2014, 299, 319–329. [Google Scholar] [CrossRef]
- Sadasivuni, K.K.; Ponnamma, D.; Thomas, S.; Grohens, Y. Evolution from graphite to graphene elastomer caposites. Prog. Polym. Sci. 2014, 39, 749–780. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, D.W.; Makotchenko, V.G.; Nazarov, A.S.; Fedorov, V.E.; Yoo, J.H.; Yu, S.M.; Choi, J.-Y.; Kim, J.M.; Ji-Beom, Y. The superior dispersion of easily soluble graphite. Small 2010, 6, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Boehm, H.P.; Clauss, A.; Fischer, G.O.; Hofmann, U. Das adsorptionsverhalten sehr dünner kohlenstoff-folien. Z. Anorg. Allg. Chem. 1962, 316, 119–127. [Google Scholar] [CrossRef]
- Steurer, P.; Wissert, R.; Thomann, R.; Mülhaupt, R. Functionalized graphenes and thermoplastic nanocomposites based upon expanded graphite oxide. Macromol. Rapid Commun. 2009, 30, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, B.; Wartig, K.A.; Hofmann, D.; Mülhaupt, R.; Schartel, B. The influence of layered, spherical, and tubular carbon nanomaterials’ concentration on the flame retardancy of polypropylene. Polym. Compos. 2015, 36, 1230–1241. [Google Scholar] [CrossRef]
- Dittrich, B.; Wartig, K.A.; Mülhaupt, R.; Schartel, B. Flame-retardancy properties of intumescent ammonium poly(phosphate) and mineral filler magnesium hydroxide in combination with graphene. Polymers 2014, 6, 2875–2895. [Google Scholar] [CrossRef]
- Dittrich, B.; Wartig, K.A.; Hofmann, D.; Mülhaupt, R.; Schartel, B. Carbon black, multiwall carbon nanotubes, expanded graphite and functionalized graphene flame retarded polypropylene nanocomposites. Polym. Adv. Technol. 2013, 24, 916–926. [Google Scholar] [CrossRef]
- Dittrich, B.; Wartig, K.-A.; Hofmann, D.; Mülhaupt, R.; Schartel, B. Flame retardancy through carbon nanomaterials: Carbon black, multiwall nanotubes, expanded graphite, multi-layer graphene and graphene in polypropylene. Polym. Degrad. Stab. 2013, 98, 1495–1505. [Google Scholar] [CrossRef]
- Frasca, D.; Schulze, D.; Böhning, M.; Krafft, B.; Schartel, B. Multilayer graphene chlorine isobutyl isoprene rubber nanocomposites: Influence of the multilayer graphene concentration on physical and flame-retardants properties. Rubber Chem. Technol. 2016, in press. [Google Scholar] [CrossRef]
- Frasca, D.; Schulze, D.; Wachtendorf, V.; Huth, C.; Schartel, B. Multifunctional multilayer graphene/elastomer nanocomposites. Eur. Polym. J. 2015, 71, 99–113. [Google Scholar] [CrossRef]
- Wu, J.; Huang, G.; Pan, Q.; Zheng, J.; Zhu, Y.; Wang, B. An investigation on the molecular mobility through the glass transition of chlorinated butyl rubber. Polymer 2007, 48, 7653–7659. [Google Scholar] [CrossRef]
- Frasca, D.; Schulze, D.; Wachtendorf, V.; Morys, M.; Schartel, B. Multilayer graphene/chlorune-isobutene-isoprene rubber nanocomposites: Effect of the dispersion. Polym. Adv. Technol. 2016, in press. [Google Scholar] [CrossRef]
- Mertz, G.; Hassouna, F.; Leclère, P.; Dahoun, A.; Toniazzo, V.; Ruch, D. Correlation between (nano)-mechanical and chemical changes occurring during photo-oxidation of filled vulcanised styrene butadiene rubber (SBR). Polym. Degrad. Stab. 2012, 97, 2195–2201. [Google Scholar] [CrossRef]
- Schröder, H.; Zeynalov, E.; Bahr, H.; Rybak, T. Analysing the content of antioxidants in pp materials. Polym. Polym. Compos. 2002, 10, 73–82. [Google Scholar]
- Mensah, B.; Kumar, D.; Lim, D.-K.; Kim, S.G.; Jeong, B.-H.; Nah, C. Preparation and properties of acrylonitrile–butadiene rubber–graphene nanocomposites. J. Appl. Polym. Sci. 2015, 132, 42457. [Google Scholar] [CrossRef]
- Lai, Q.; Zhu, S.; Luo, X.; Zou, M.; Huang, S. Ultraviolet-visible spectroscopy of graphene oxides. Advances 2012, 2, 032146. [Google Scholar] [CrossRef]
- Jäger, C.; Henning, T.; Schlögl, R.; Spillecke, O. Spectral properties of carbon black. J. Non-Cryst. Solids 1999, 258, 161–179. [Google Scholar] [CrossRef]
- Zeynalov, E.B.; Allen, N.S.; Salmanova, N.I. Radical scavengering efficiency of different fullerenes c60–c70 and fullerene soot. Polym. Degrad. Stab. 2009, 94, 1183–1189. [Google Scholar] [CrossRef]
- Kumar, V.; Hanel, T.; Giannini, L.; Galimberti, M.; Giese, U. Graphene reinforced synthetic isoprene rubber nanocomposites. KGK 2014, 10. [Google Scholar]
- Payne, A.R. The dynamic properties of carbon black-loaded natural rubber vulcanizates. J. Appl. Polym. Sci. 1962, 6, 57–63. [Google Scholar] [CrossRef]
- Fritzsche, J.; Lorenz, H.; Klüppel, M. CNT based elastomer-hybrid-nanocomposites with promising mechanical and electrical properties. Macromol. Mater. Eng. 2009, 294, 551–560. [Google Scholar] [CrossRef]
- Teh, P.L.; Mohd Ishak, Z.A.; Hashim, A.S.; Karger-Kocsis, J.; Ishiaku, U.S. Effects of epoxidized natural rubber as a compatibilizer in melt compounded natural rubber–organoclay nanocomposites. Eur. Polym. J. 2004, 40, 2513–2521. [Google Scholar] [CrossRef]
- Malas, A.; Pal, P.; Das, C.K. Effect of expanded graphite and modified graphite flakes on the physical and thermo-mechanical properties of styrene butadiene rubber/polybutadiene rubber (SBR/BR) blends. Mater. Des. 2014, 55, 664–673. [Google Scholar] [CrossRef]
- Ismail, H.; Chia, H.H. The effects of multifunctional additive and epoxidation in silica filled natural rubber compounds. Polym. Test. 1998, 17, 199–210. [Google Scholar] [CrossRef]
- Ismail, H.; Chia, H.H. The effects of multifunctional additive and vulcanization systems on silica filled epoxidized natural rubber compounds. Eur. Polym. J. 1998, 34, 1857–1863. [Google Scholar] [CrossRef]
- Xing, W.; Wu, J.; Huang, G.; Li, H.; Tang, M.; Fu, X. Enhanced mechanical properties of graphene/natural rubber nanocomposites at low content. Polym. Int. 2014, 63, 1674–1681. [Google Scholar] [CrossRef]
- Kang, H.; Zuo, K.; Wang, Z.; Zhang, L.; Liu, L.; Guo, B. Using a green method to develop graphene oxide/elastomers nanocomposites with combination of high barrier and mechanical performance. Compos. Sci. Technol. 2014, 92, 1–8. [Google Scholar] [CrossRef]
- Menes, O.; Cano, M.; Benedito, A.; Giménez, E.; Castell, P.; Maser, W.K.; Benito, A.M. The effect of ultra-thin graphite on the morphology and physical properties of thermoplastic polyurethane elastomer composites. Compos. Sci. Technol. 2012, 72, 1595–1601. [Google Scholar] [CrossRef]
- Das, A.; Kasaliwal, G.R.; Jurk, R.; Boldt, R.; Fischer, D.; Stöckelhuber, K.W.; Heinrich, G. Rubber composites based on graphene nanoplatelets, expanded graphite, carbon nanotubes and their combination: A comparative study. Compos. Sci. Technol. 2012, 72, 1961–1967. [Google Scholar] [CrossRef]
- Poikelispää, M.; Das, A.; Dierkes, W.; Vuorinen, J. The effect of partial replacement of carbon black by carbon nanotubes on the properties of natural rubber/butadiene rubber compound. J. Appl. Polym. Sci. 2013, 130, 3153–3160. [Google Scholar] [CrossRef]



| Ingredients | CIIR | CIIR/MLG3 | CIIR/CB20 | CIIR/CB20/MLG3 | CIIR/CB30 | CIIR/CB30/MLG3 | CIIR/CB40 |
|---|---|---|---|---|---|---|---|
| CIIR | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Zinc oxide | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Stearic acid | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| CB660 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Struktol | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 |
| Sulfur | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| MBTS | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| CB | - | - | 20 | 20 | 30 | 30 | 40 |
| MLG | - | 3 | - | 3 | - | 3 | - |
| Time/h | Temperature/°C | Humidity | |
|---|---|---|---|
| 4 | 25 | Rain | |
| 4 | 80 | <10% | |
| 4 | 25 | Rain | |
| 4 | 80 | <10% | |
| 4 | 25 | Rain | |
| 4 | −10 | <10% | |
| ML (dNm) | MH (dNm) | ΔS (dNm) | |
|---|---|---|---|
| CIIR | 0.75 ± 0.01 | 2.27 ± 0.01 | 1.52 ± 0.02 |
| CIIR/MLG3 | 0.87 ± 0.03 | 3.34 ± 0.02 | 2.47 ± 0.05 |
| CIIR/CB20 | 1.09 ± 0.06 | 3.82 ± 0.06 | 2.73 ± 0.01 |
| CIIR/CB20/MLG3 | 1.43 ± 0.01 | 5.67 ± 0.01 | 4.25 ± 0.01 |
| CIIR/CB30 | 1.42 ± 0.01 | 5.37 ± 0.01 | 3.95 ± 0.02 |
| CIIR/CB30/MLG3 | 1.79 ± 0.10 | 7.11 ± 0.21 | 5.32 ± 0.11 |
| CIIR/CB40 | 1.88 ± 0.04 | 7.06 ± 0.16 | 5.18 ± 0.20 |
| Stress 100%/MPa | Stress 200%/MPa | Stress 300%/MPa | Tensile strength/MPa | |
| CIIR | 0.43 ± 0.01 | 0.66 ± 0.03 | 0.94 ± 0.04 | 5.28 ± 0.89 |
| CIIR/MLG3 | 0.83 ± 0.01 | 1.47 ± 0.02 | 2.15 ± 0.03 | 5.93 ± 0.29 |
| CIIR/CB20 | 0.69 ± 0.01 | 1.21 ± 0.02 | 2.02 ± 0.04 | 12.30 ± 0.51 |
| CIIR/CB20/MLG3 | 1.74 ± 0.03 | 3.13 ± 0.05 | 4.30 ± 0.05 | 9.62 ± 0.81 |
| CIIR/CB30 | 0.94 ± 0.02 | 1.93 ± 0.04 | 3.39 ± 0.08 | 14.10 ± 0.68 |
| CIIR/CB30/MLG3 | 2.20 ± 0.06 | 3.90 ± 0.08 | 5.49 ± 0.96 | 11.30 ± 0.74 |
| CIIR/CB40 | 1.21 ± 0.01 | 2.57 ± 0.04 | 4.60 ± 0.06 | 14.40 ± 0.28 |
| Elongation at break/% | Young’s modulus/MPa | Hardness/Shore A | ||
| CIIR | 837 ± 29 | 0.79 ± 0.22 | 22.6 ± 0.6 | |
| CIIR/MLG3 | 868 ± 26 | 2.32 ± 0.08 | 34.3 ± 0.9 | |
| CIIR/CB20 | 876 ± 20 | 2.31 ± 0.11 | 37.5 ± 0.3 | |
| CIIR/CB20/MLG3 | 697 ± 46 | 5.12 ± 0.18 | 53.1 ± 0.4 | |
| CIIR/CB30 | 792 ± 25 | 3.73 ± 0.05 | 45.4 ± 0.4 | |
| CIIR/CB30/MLG3 | 630 ± 38 | 9.44 ± 0.43 | 59.4 ± 0.5 | |
| CIIR/CB40 | 745 ± 16 | 6.37 ± 0.25 | 54.1 ± 0.6 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frasca, D.; Schulze, D.; Wachtendorf, V.; Krafft, B.; Rybak, T.; Schartel, B. Multilayer Graphene/Carbon Black/Chlorine Isobutyl Isoprene Rubber Nanocomposites. Polymers 2016, 8, 95. https://doi.org/10.3390/polym8030095
Frasca D, Schulze D, Wachtendorf V, Krafft B, Rybak T, Schartel B. Multilayer Graphene/Carbon Black/Chlorine Isobutyl Isoprene Rubber Nanocomposites. Polymers. 2016; 8(3):95. https://doi.org/10.3390/polym8030095
Chicago/Turabian StyleFrasca, Daniele, Dietmar Schulze, Volker Wachtendorf, Bernd Krafft, Thomas Rybak, and Bernhard Schartel. 2016. "Multilayer Graphene/Carbon Black/Chlorine Isobutyl Isoprene Rubber Nanocomposites" Polymers 8, no. 3: 95. https://doi.org/10.3390/polym8030095
APA StyleFrasca, D., Schulze, D., Wachtendorf, V., Krafft, B., Rybak, T., & Schartel, B. (2016). Multilayer Graphene/Carbon Black/Chlorine Isobutyl Isoprene Rubber Nanocomposites. Polymers, 8(3), 95. https://doi.org/10.3390/polym8030095