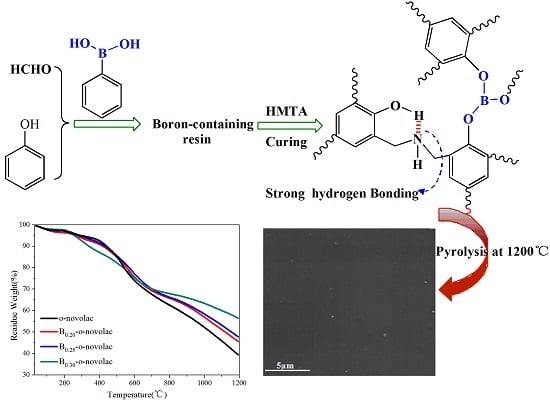
The Effect of Introducing B and N on Pyrolysis Process of High Ortho Novolac Resin
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
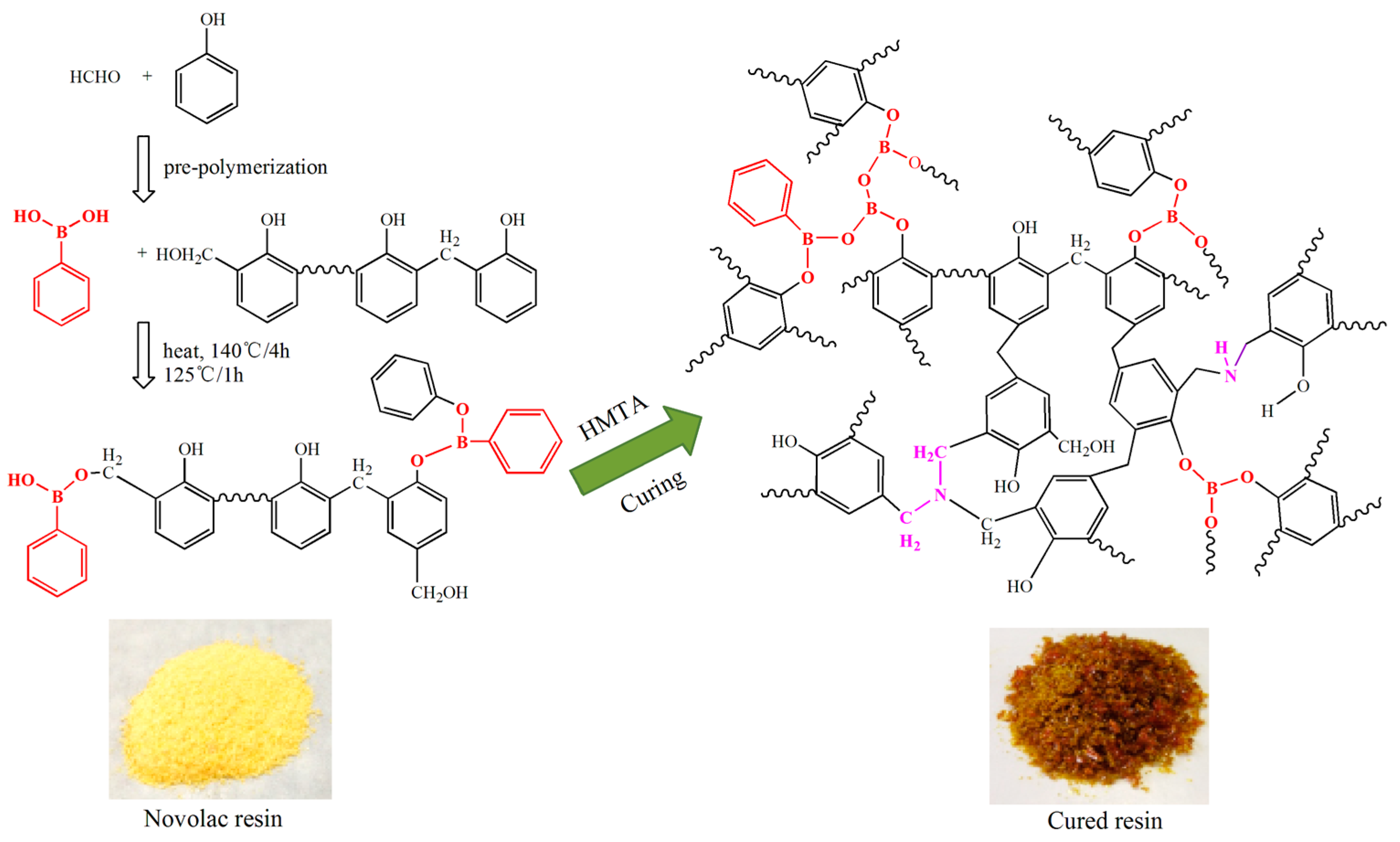
2.2. Synthesis of B-o-novolacs
2.3. Synthesis of o-Novolac
2.4. Preparation of the Cured B-o-novolac
2.5. Preparation of Carbonized Samples
2.6. Characterization
3. Results and Discussion
3.1. Characterization of B-o-novolac and Cured B-o-novolac
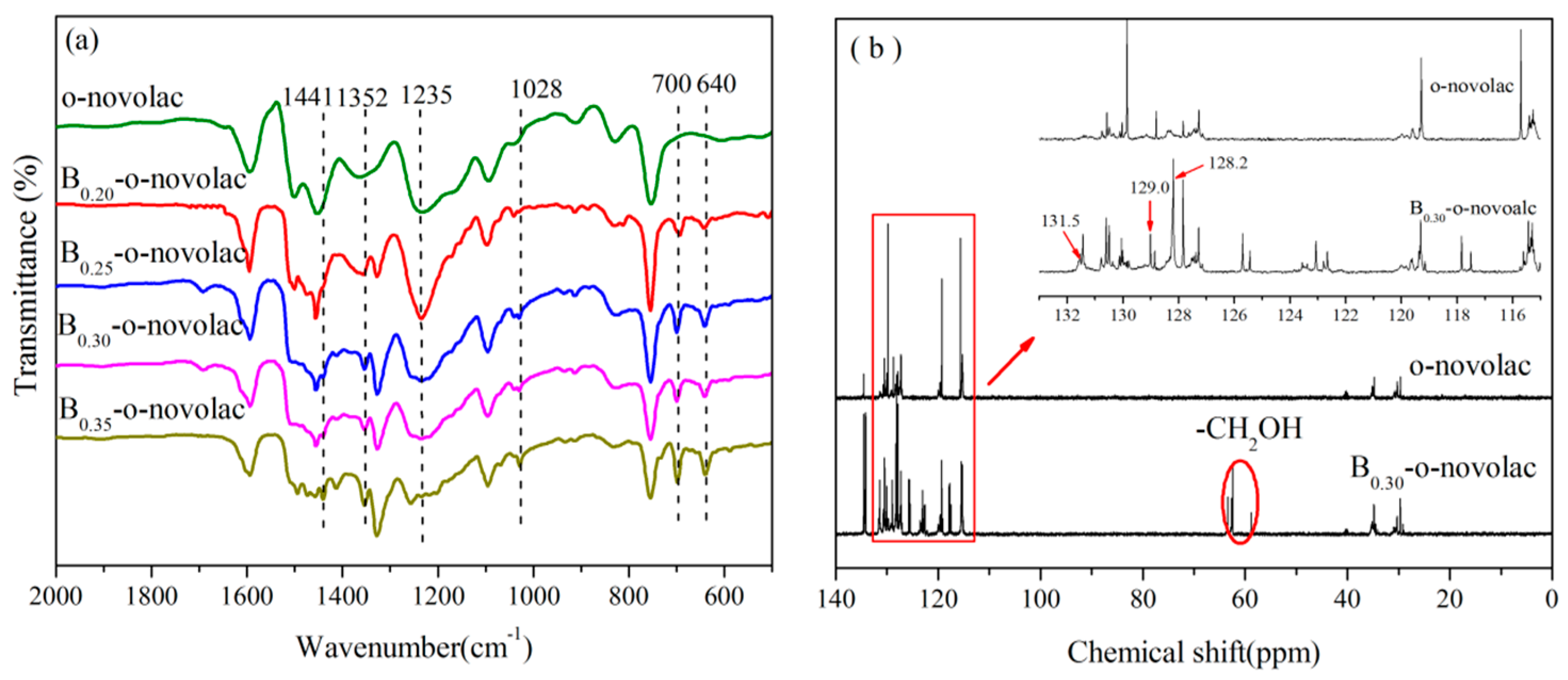
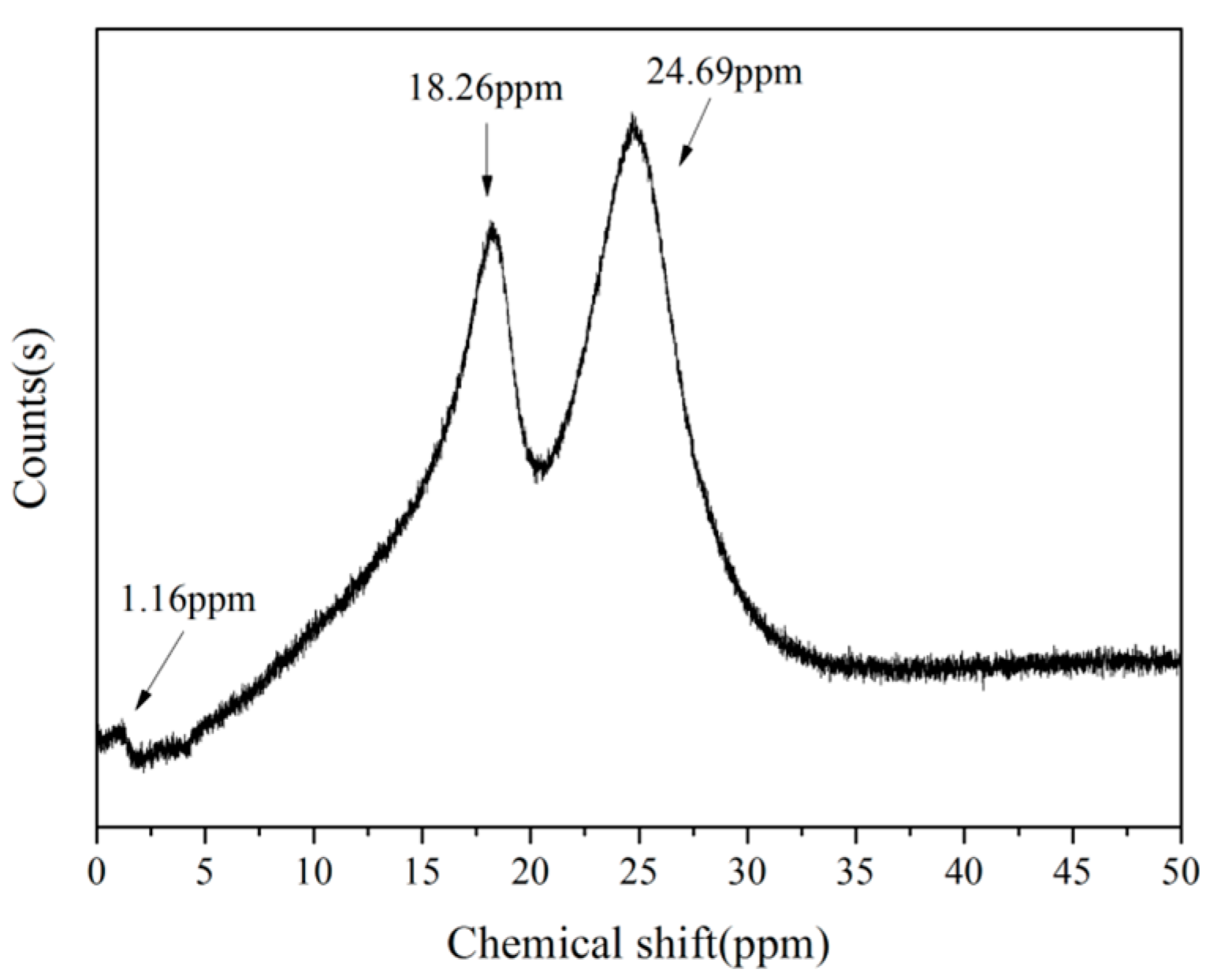
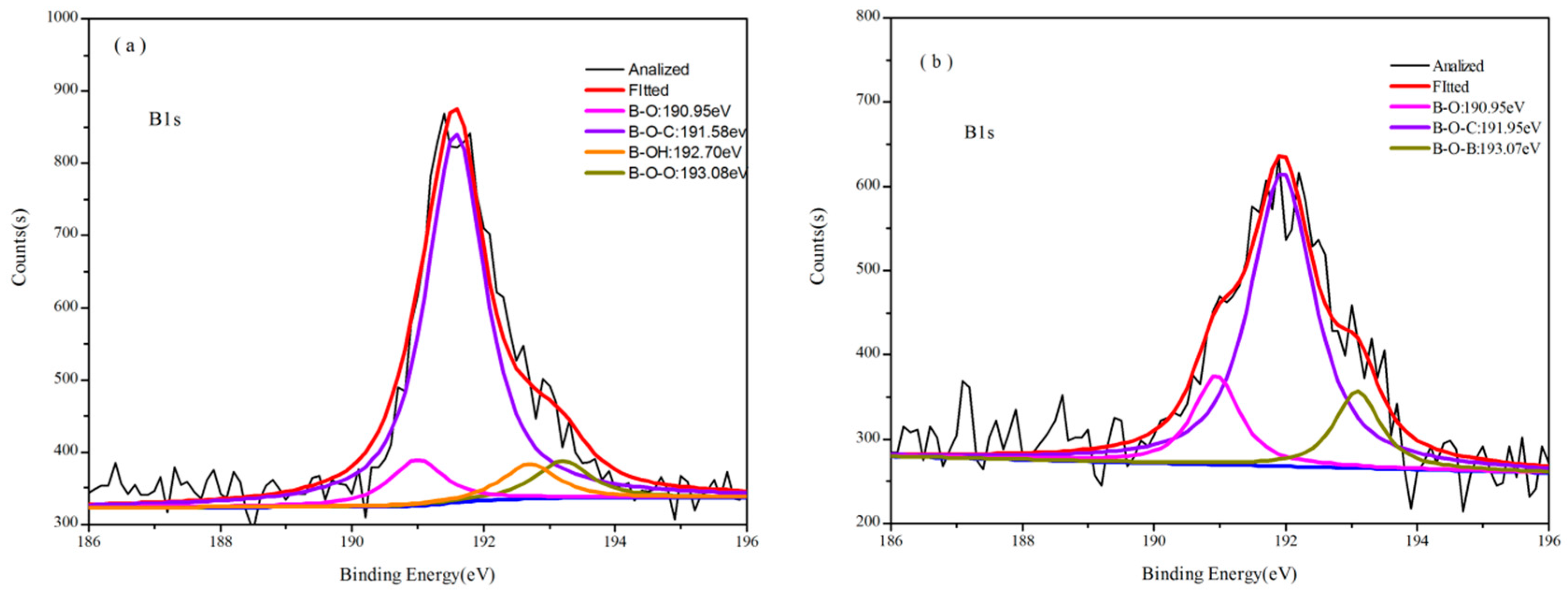
3.2. Thermal Properties of B-o-novolacs
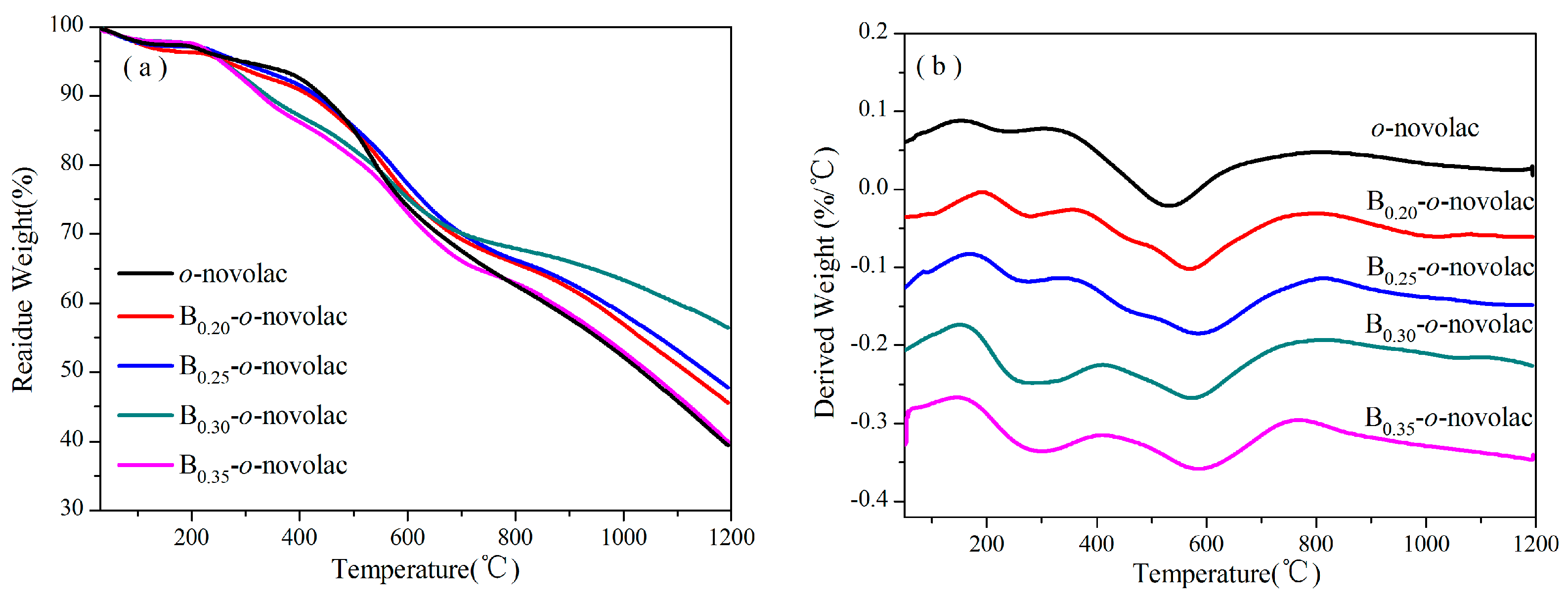
| Resin | T5%/°C | T10%/°C | Tmax/°C | C800/% | C1000/% | C1200/% | Weigh Loss in Stage 1 (%) |
|---|---|---|---|---|---|---|---|
| B0.20-o-novolac | 260.93 | 419.61 | 568.82 | 65.76 | 56.94 | 45.54 | 5.39 |
| B0.25-o-novolac | 286.43 | 431.56 | 584.53 | 66.20 | 58.40 | 47.75 | 5.58 |
| B0.30-o-novolac | 255.02 | 340.05 | 574.86 | 67.89 | 63.34 | 56.41 | 10.01 |
| B0.35-o-novolac | 253.82 | 329.01 | 584.39 | 62.88 | 52.92 | 39.85 | 11.38 |
| o-novolac | 291.38 | 441.87 | 531.39 | 62.57 | 52.21 | 39.39 | 4.55 |
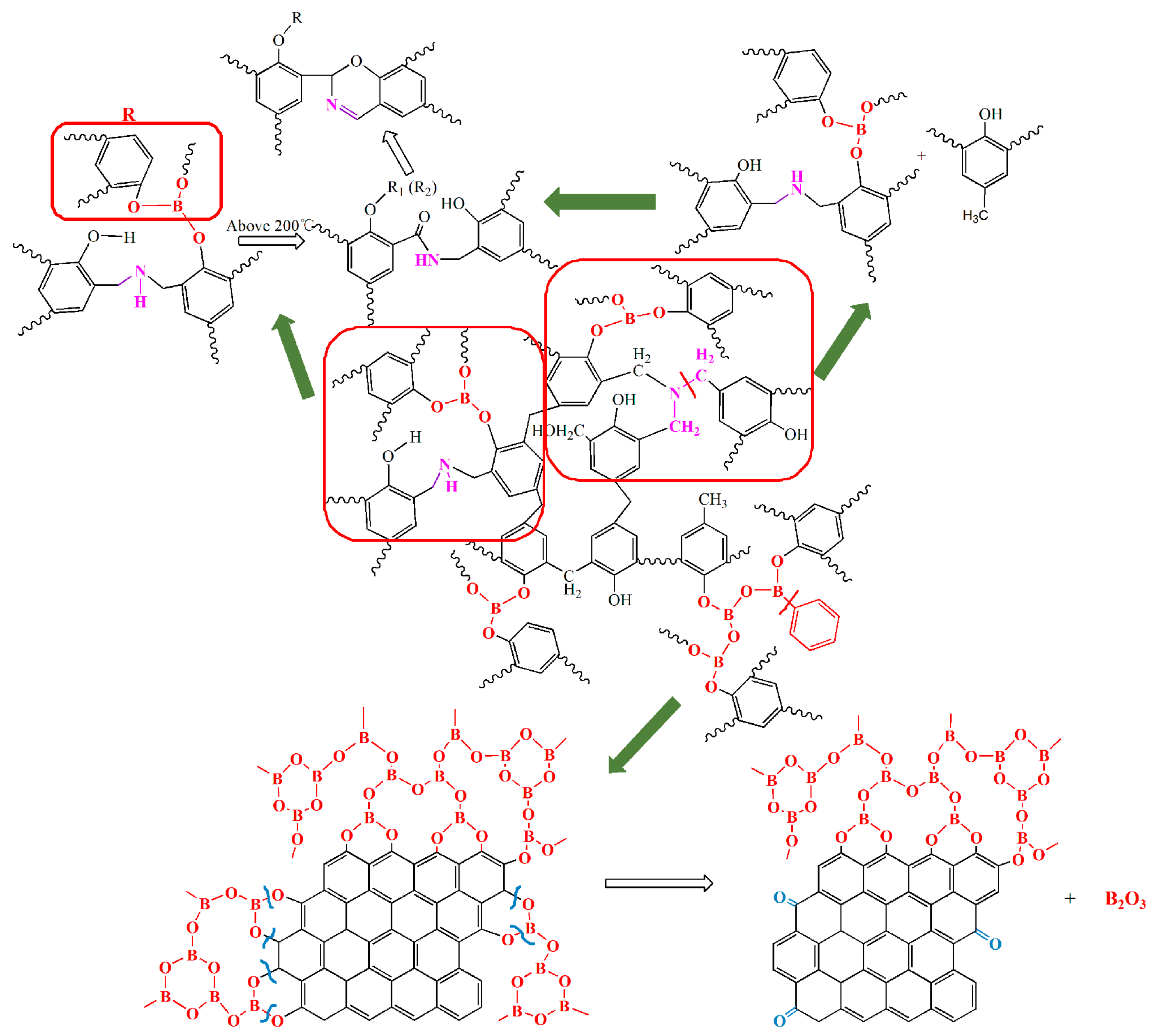
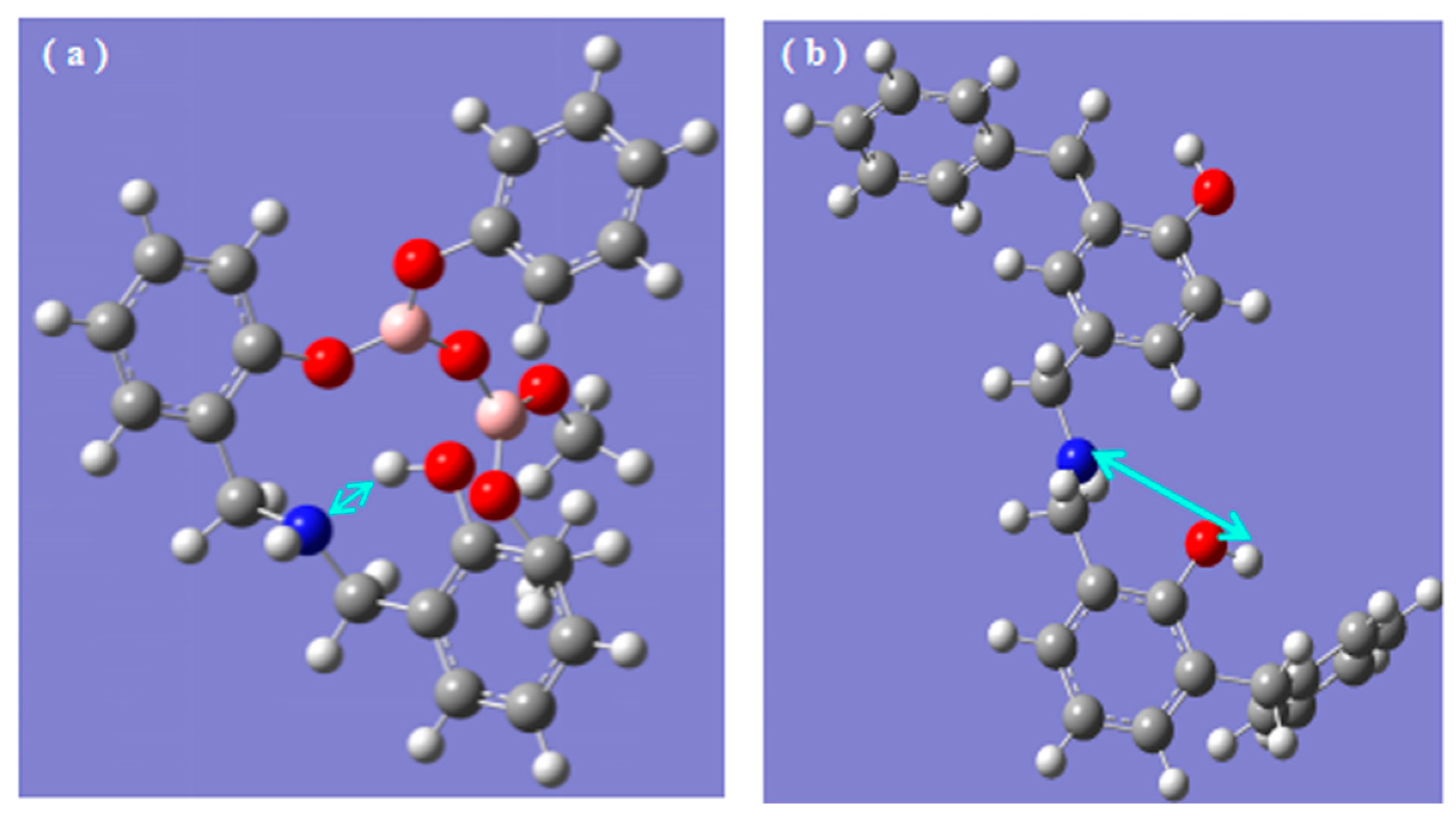
3.3. Chemical State of B and N during Pyrolysis Process
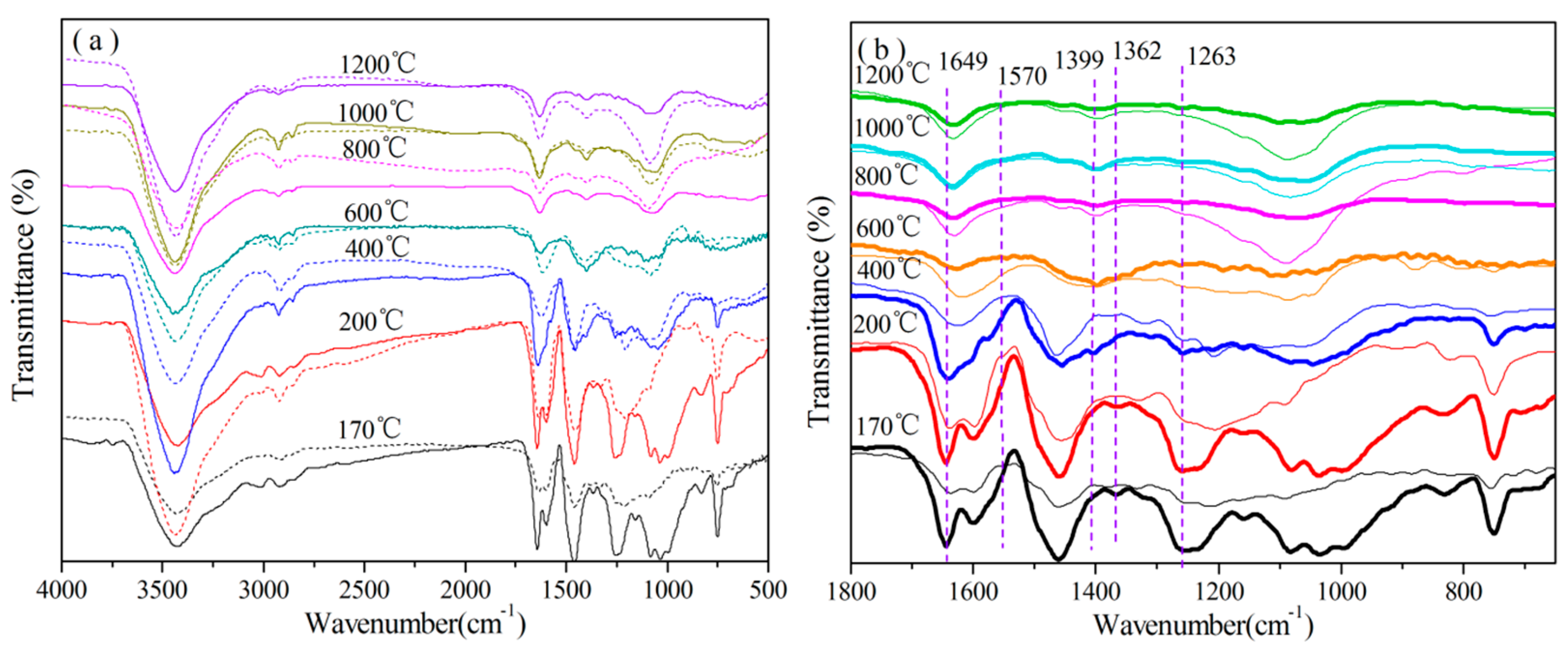
3.3.1. Analysis of FT-IR
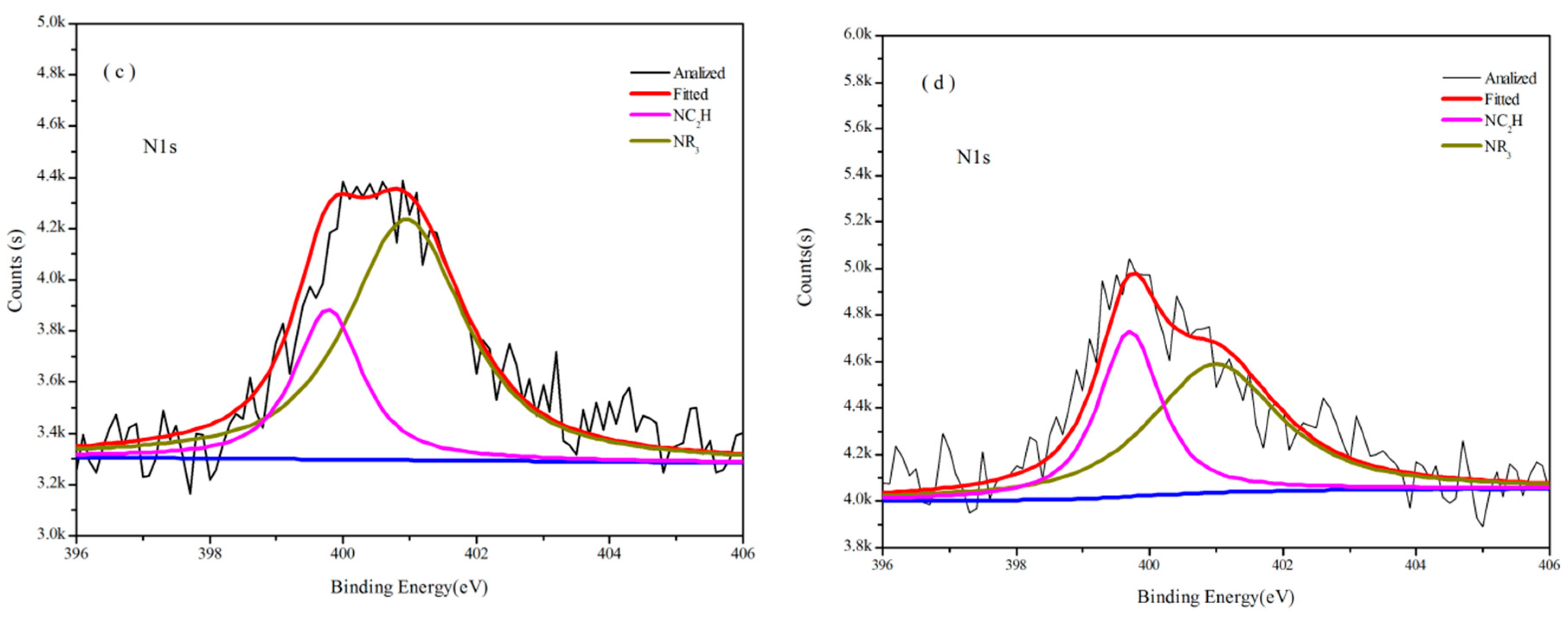
3.3.2. Analysis of XPS
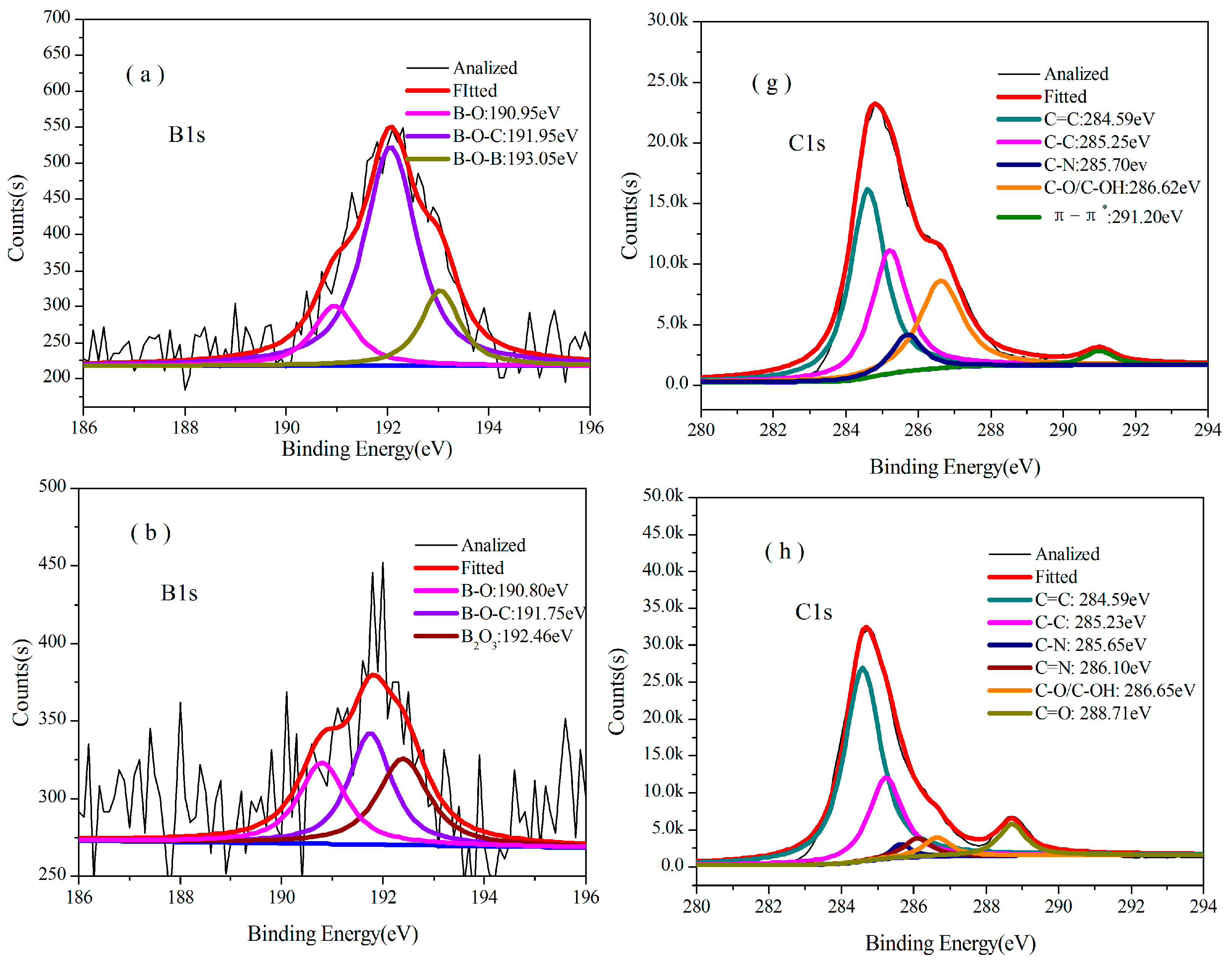
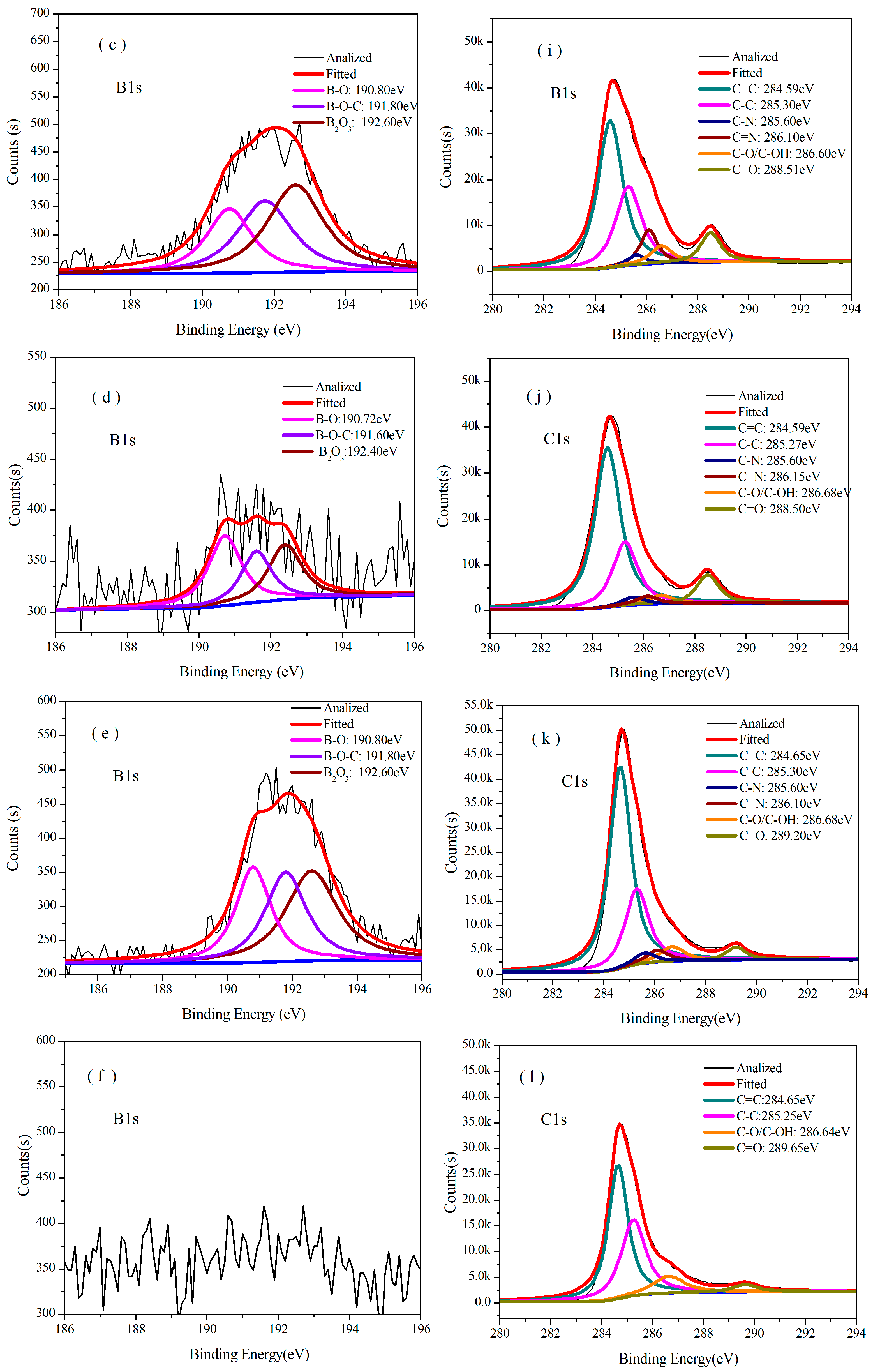
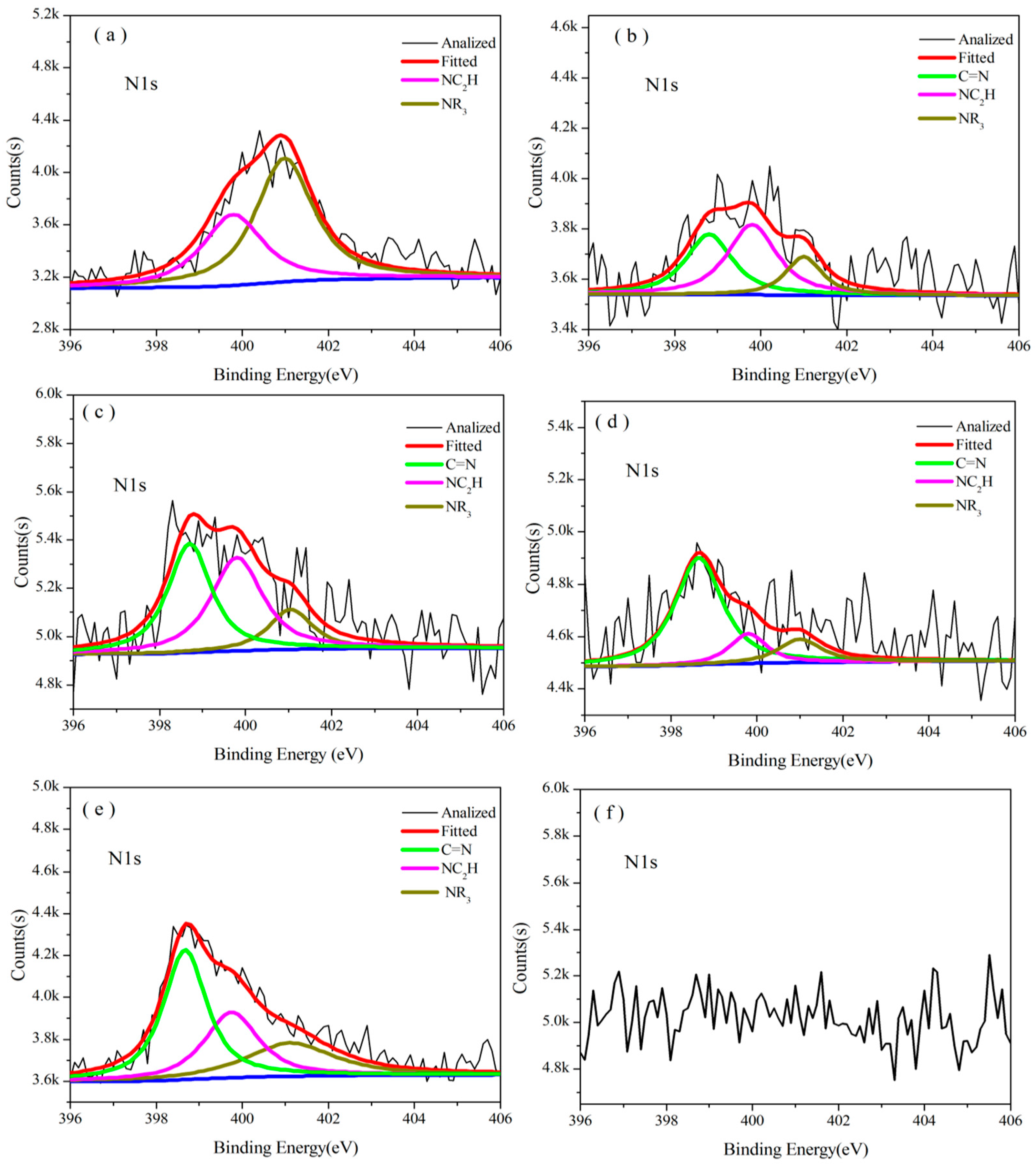
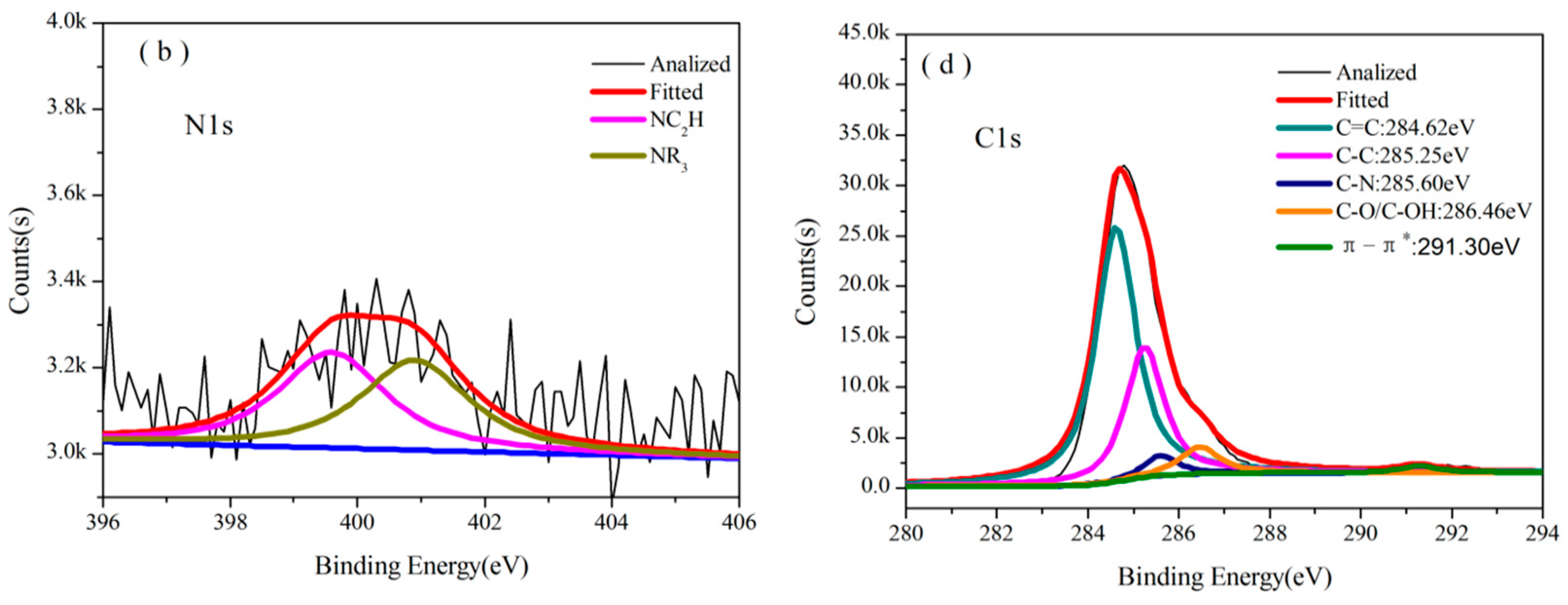
| Temperature/°C | Atom ratio/% | ||||||
|---|---|---|---|---|---|---|---|
| Cured B0.30-o-novolac | Cured o-novolac | ||||||
| C | O | B | N | C | O | N | |
| 170 | 75.09 | 19.23 | 2.27 | 3.40 | 77.91 | 19.79 | 2.31 |
| 200 | 75.21 | 19.83 | 2.18 | 2.78 | 79.35 | 18.97 | 1.69 |
| 400 | 75.32 | 22.64 | 1.00 | 1.04 | 82.55 | 15.93 | 1.52 |
| 600 | 75.86 | 21.75 | 1.46 | 0.93 | 82.05 | 17.95 | 0 |
| 800 | 75.86 | 22.52 | 0.60 | 1.03 | 94.08 | 5.92 | 0 |
| 1,000 | 83.26 | 12.34 | 2.34 | 2.06 | 91.99 | 8.01 | 0 |
| 1,200 | 76.32 | 23.68 | 0 | 0 | 89.62 | 10.38 | 0 |
| Temperature/°C | Resin | |||||
|---|---|---|---|---|---|---|
| Component | Cured B0.30-o-novolac | Cured o-novolac | ||||
| NR3 | NC2H | C=N | NR3 | NC2H | ||
| 170 | Binding Energy/eV | 400.98 | 399.78 | -- | 400.98 | 399.70 |
| Area/% | 73.77 | 26.23 | -- | 61.99 | 38.01 | |
| 200 | Binding Energy/eV | 400.98 | 399.78 | -- | 400.80 | 399.80 |
| Area/% | 62.34 | 37.66 | -- | 33.78 | 66.22 | |
| 400 | Binding Energy/eV | 401.00 | 399.80 | 398.80 | 400.90 | 399.60 |
| Area/% | 18.11 | 45.07 | 36.82 | 48.50 | 51.50 | |
| 600 | Binding Energy/eV | 401.05 | 399.80 | 398.70 | -- | -- |
| Area/% | 15.38 | 42.49 | 42.13 | -- | -- | |
| 800 | Binding Energy/eV | 401.00 | 399.80 | 398.65 | -- | -- |
| Area/% | 14.16 | 15.12 | 70.72 | -- | -- | |
| 1,000 | Binding Energy/eV | 401.10 | 399.75 | 398.68 | -- | -- |
| Area/% | 27.34 | 29.26 | 43.40 | -- | -- | |
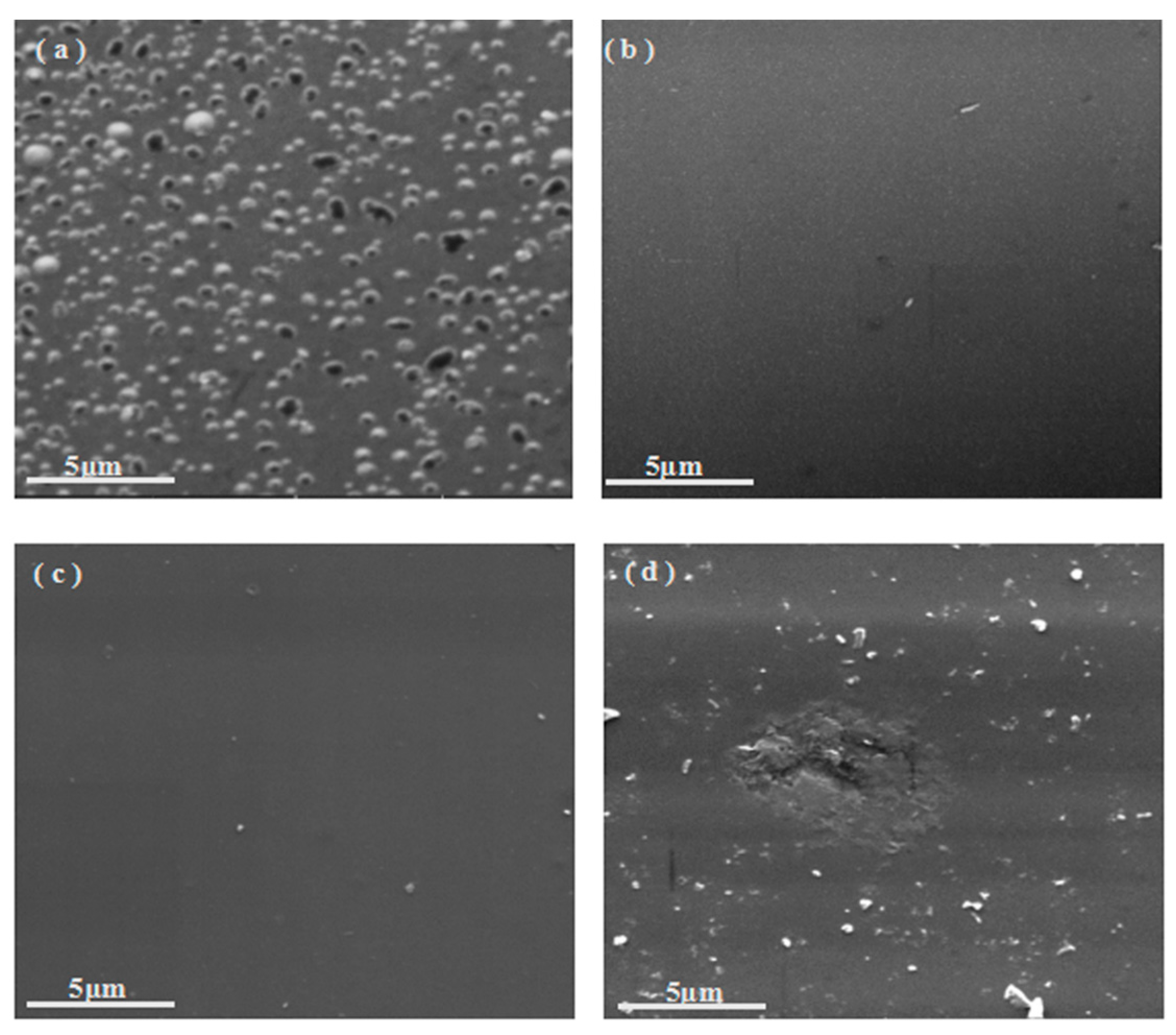
3.3.3. Analysis of SEM
3.3.4. Analysis of XRD
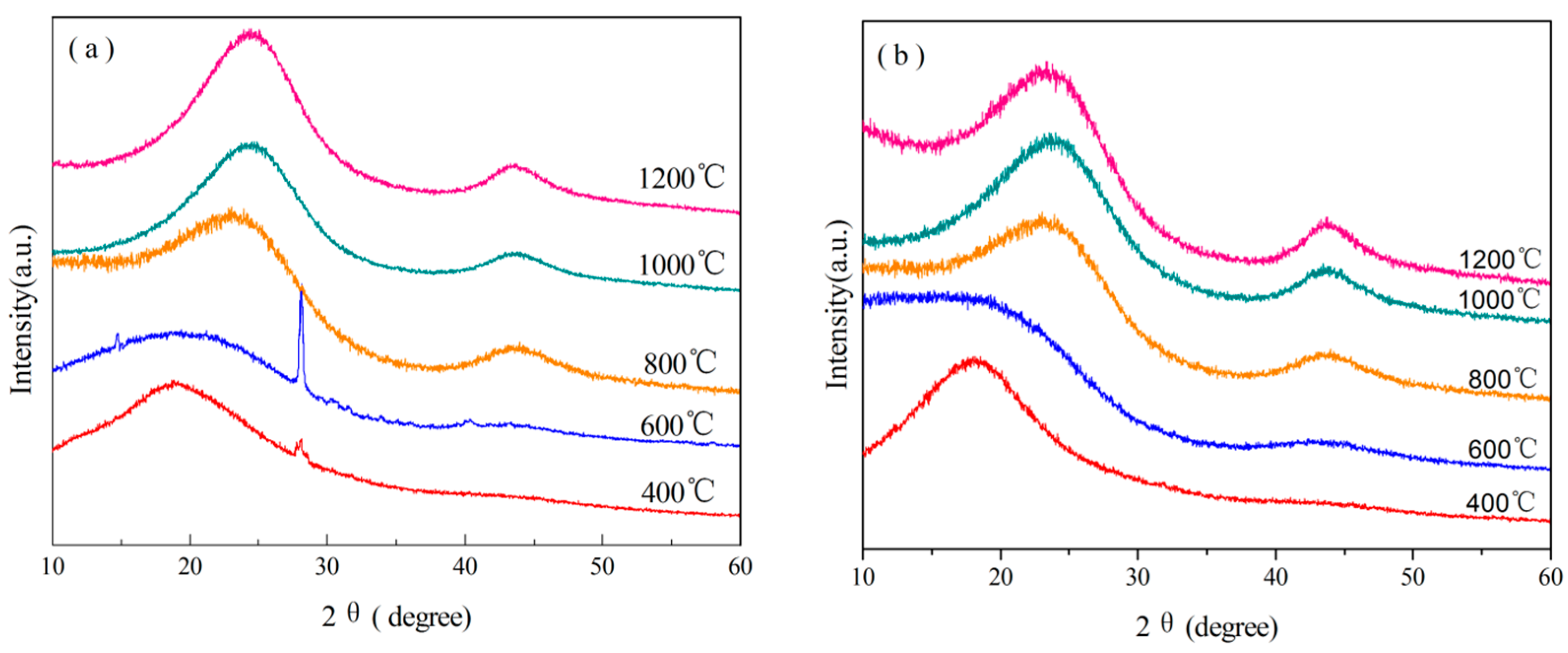
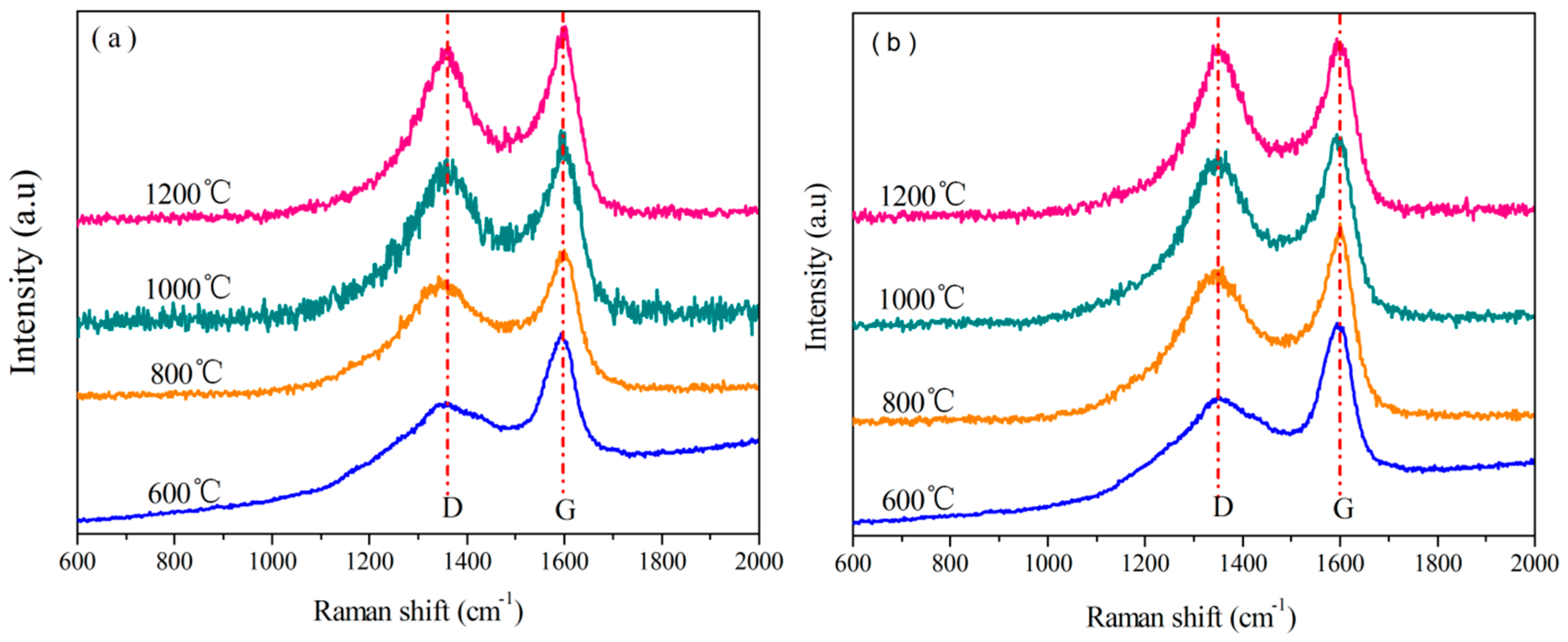
| Resin | Temperature/°C | 2θ(002)/° | d002-spacing/nm | D-band/cm−1 | G-band/cm−1 | R |
|---|---|---|---|---|---|---|
| o-novolac | 800 | 23.06 | 0.3853 | 1,338 | 1,601 | 2.262 |
| 1,000 | 23.65 | 0.3759 | 1,339 | 1,595 | 2.060 | |
| 1,200 | 23.38 | 0.3801 | 1,345 | 1,597 | 1.846 | |
| B0.30-o-novolac | 800 | 24.13 | 0.3685 | 1,347 | 1,598 | 2.038 |
| 1,000 | 24.18 | 0.3677 | 1,350 | 1,601 | 1.944 | |
| 1,200 | 24.50 | 0.3603 | 1,350 | 1,598 | 1.747 |
3.3.5. Analysis of Raman Spectra
4. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| HMTA | hexamethylenetetramine |
| DFT | Density functional theory |
| PF | Phenolic resin |
| BPF | boron-modified resin |
| B-o-novolac | boron-containing high ortho novolac resin |
| o-novolac | high ortho novalac resin |
| NMR | nuclear magnetic resonance |
| FT-IR | Fourier transform infrared |
| TGA | Thermalgravimetric analysis |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopic |
References
- Choi, M.H.; Byun, H.Y.; Chung, I.J. The effect of chain length of flexible diacid on morphology and mechanical property of modified phenolic resin. Polymer 2002, 43, 4437–4438. [Google Scholar] [CrossRef]
- Choi, M.H.; Chung, I.J.; Lee, J.D. Morphology and curing behaviors of phenolic resin-layered silicate nanocomposites prepared by melt intercalation. Chem. Mater. 2000, 12, 2977–2983. [Google Scholar] [CrossRef]
- Choi, M.H.; Jeon, B.H.; Chung, I. The effect of coupling agent on electrical and mechanical properties of carbon fiber/phenolic resin composites. Polymer 2000, 41, 3243–3252. [Google Scholar] [CrossRef]
- Ma, H.; Wei, G.; Liu, Y.; Zhang, X.; Gao, J.; Huang, F.T.; Tan, B.; Song, Z.; Qiao, J. Effect of elastomeric nanoparticles on properties of phenolic resin. Polymer 2005, 46, 10568–10573. [Google Scholar] [CrossRef]
- Cherian, A.B.; Varghese, L.A.; Thachil, E.T. Epoxy-modified, unsaturated polyester hybrid networks. Eur. Polymer J. 2007, 43, 1460–1469. [Google Scholar] [CrossRef]
- Chiang, C.-L.; Ma, C.-C.M. Synthesis, characterization, thermal properties and flame retardance of novel phenolic resin/silica nanocomposites. Polymer Degrad. Stabil. 2004, 83, 207–214. [Google Scholar] [CrossRef]
- Zhang, Y.; Lee, S.; Yoonessi, M.; Liang, K.; Pittman, U.C. Phenolic resin-trisilanophenyl polyhedral oligomeric silsesquioxane (POSS) hybrid nanocomposites: Structure and properties. Polymer 2006, 47, 2984–2996. [Google Scholar] [CrossRef]
- Wang, D.-C.; Chang, G.-W.; Chen, Y. Preparation and thermal stability of boron-containing phenolic resin/clay nanocomposites. Polymer Degrad. Stabil. 2008, 93, 125–133. [Google Scholar] [CrossRef]
- Daniel, C.; Marianne, B.; Martin, M. 100 years of bakelite, the material of a 1000 uses. Angew. Chem. Int. Edit. 2007, 47, 3322–3328. [Google Scholar]
- Ida, P.; Matjaž, K. Characterization of phenol-formaldehyde prepolymer resins by in line FT-IR spectroscopy. Acta Chim. Slov. 2005, 52, 238–244. [Google Scholar]
- Mohamed, O.A.; Adriane, L.; Temisha, M. Boron-modified phenolic resins for high performance applications. Polymer 2003, 44, 7353–7359. [Google Scholar]
- Wang, H.; Guo, Q.; Yang, J.; Liu, Z.; Zhao, Y.; Lia, J.; Feng, Z.; Liua, L. Microstructural evolution and oxidation resistance of polyacrylonitrile-based carbon fibers doped with boron by the decomposition of B4C. Carbon 2013, 56, 296–308. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, N.; Jiang, H. Micro-structural evolution of phenol-formaldehyde resin modified by boron-carbide at elevated temperatures. Mater. Chem. Phys. 2010, 120, 187–192. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Q.; Liu, L.; Song, J. The preparation and performance of high-temperature adhesives for graphite bonding. Int. J. Adhes. Adhes. 2005, 24, 495–501. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, N.; Jiang, H. Effect of the evolution of phenol-formaldehyde resin on the high-temperature bonding. Int. J. Adhes. Adhes. 2009, 29, 718–723. [Google Scholar] [CrossRef]
- Wang, S.; Jing, X.; Wang, Y.; Si, J. High char yield of aryl boron-containing phenolic resins: The effect of phenylboronic acid on the thermal stability and carbonization of phenolic resins. Polymer Degrad. Stabil. 2014, 99, 1–11. [Google Scholar] [CrossRef]
- Cameron, G.C.; James, E. Oxidative and hydrolytic stability of boron nitride-a new approach to improving the oxidation resistance of carbonaceous structures. Carbon 1995, 33, 389–395. [Google Scholar]
- Gao, J.; Jiang, C.; Su, X. Synthesis and thermal properties of boron-nitrogen containing phenol formaldehyde resin/MMT nanocomposites. Int. J. Polymer Mater. 2010, 59, 544–552. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Y.; Yang, L. Thermal stability of boron-containing phenol formaldehyde resin. Polymer Degrad. Stabil. 1999, 63, 19–22. [Google Scholar] [CrossRef]
- Fan, D.B.; Li, G.Y.; Qin, T.F.; Chu, F.-X. Synthesis and structure characterization of phenol-urea-formaldehyde resins in the presence of magnesium oxide as catalyst. Polymers 2014, 6, 2221–2231. [Google Scholar] [CrossRef]
- Hu, Y.; Geng, W.; You, H.; Wang, Y.; Loy, D.A. Modification of a phenolic resin with eoxy- and methacrylate-functionalized silica sols to improve the ablation resistance of their glass fiber-reinforced composites. Polymers 2014, 6, 105–113. [Google Scholar] [CrossRef]
- Wang, S.; Jing, X.; Wang, Y. Synthesis and characterization of novel phenolic resins containing aryl-boron backbone and their utilization in polymeric composites with improved thermal and mechanical properties. Polymer Adv. Technol. 2014, 25, 152–159. [Google Scholar] [CrossRef]
- Endo, M.; Kim, C.; Karaki, T.; Nishimura, Y.; Matthews, M.J.; Brown, S.D.M.; Dresselhaus, M.S. Structural analysis of the B-doped mesophase pitch-based graphite fibers by Raman spectroscopy. Phys. Rev. B 1998, 58, 8991–8996. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koening, J.L. Ranman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Gouin, X.; Grange, P.; Bois, L. Characterization of the nitridation process of phenylboronic acid. J. Alloys Compd. 1995, 224, 22–28. [Google Scholar] [CrossRef]
- Odom, J.D.; Moore, T.F. Nuclear magnetic resonance studies of boron compounds: XVI. Carbon-13 studies of organoboranes: Phenylboranes and boron-substituted aromatic heterocycles. J. Organomet. Chem. 1979, 173, 15–32. [Google Scholar] [CrossRef]
- Joseph, W.E.; Weiss, D.L.B. A solid-State 11B NMR and computational study of boron electric field gradient and chemical shift tensors in boronic acids and boronic esters. J. Phys. Chem. A 2010, 114, 5119–5131. [Google Scholar]
- Wang, S.; Wang, Y.; Bian, C.; Zhonga, Y.; Jing, X. The thermal stability and pyrolysis mechanism of boron-containing phenolic resins: The effect of phenyl borates on the char formation. Appl. Surf. Sci. 2015, 331, 519–529. [Google Scholar] [CrossRef]
- Bois, L.; L’Haridon, P.; Laurenta, Y.; Gouin, X.; Grange, P.; Létard, J.-F.; Birot, M.; Pillot, J.-P.; Dunoguès, J. Characterization of a boro-silicon oxynitride prepared by thermal nitridation of a polyborosiloxane. J. Alloys Compd. 1996, 232, 244–253. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Bian, C. Effects of chemical structure and cross-link density on the heat resistance of phenolic rensin. Polymer Degrad. Stabil. 2015, 111, 239–246. [Google Scholar] [CrossRef]
- Wang, D.; Li, B.; Zhang, Y.; Lu, Z. Triazine-containing benzoxazine and its high-performance polymer. J. Appl. Polymer Sci. 2013, 127, 516–522. [Google Scholar] [CrossRef]
- Burgess, J.S.; Acharya, C.K.; Lizarazo, J.; Yancey, N.; Flowers, B.; Kwon, G.; Klein, T.; Weaver, M.; Lane, A.M.; Turnerb, C.H.; et al. Boron-doped carbon powders formed at 1000 °C and one atmosphere. Carbon 2008, 46, 1711–1717. [Google Scholar] [CrossRef]
- Wang, B.; Huang, Y.; Liu, L. Effect of solvents on adsorption of phenolic resin onto -aminopropyl-triethoxysilane treated silica fiber during resin transfermolding. J. Mater. Sci. 2006, 41, 1243–1246. [Google Scholar] [CrossRef]
- Iwazaki, T.; Obinata, R.; Sugimoto, W. High oxygen-reduction activity of silk-derived activated carbon. Electrochem. Commun. 2009, 11, 376–378. [Google Scholar] [CrossRef]
- Jackson, W.M.; Conley, R.T. High temperature oxidative degradation of phenol-formaldehyde polycondensates. J. Appl. Polym. Sci. 1964, 8, 2163–2219. [Google Scholar] [CrossRef]
- Bessire, B.K.; Lahankar, S.A.; Minton, T.K. Pyrolysis of phenolic impregnated carbon ablator. ACS Appl. Mater. Inter. 2015, 7, 1383–1395. [Google Scholar] [CrossRef] [PubMed]
- Thilagar, P.; Murillo, D.; Chen, J.; Jäkle, F. Synthesis and supramolecular assembly of the bifunctional borinic acid [1, 2-fcB(OH)]2. Dalton Trans. 2013, 42, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, J.; Wu, S.; Yuan, Z.; Hu, Z.; Wu, R.; Liu, Q. The pyrolysis mechanism of phenol formaldehyde resin. Polym. Degrad. Stabil. 2012, 97, 1527–1533. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, N.; Feng, M.W. Thermal degradation characteristics of phenol–formaldehyde resins derived from beetle infested pine barks. Thermochim. Acta 2013, 555, 46–52. [Google Scholar] [CrossRef]
- Hall, D.G. Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials; Wiley: New York, NY, USA, 2012. [Google Scholar]
- William, C.; Thomas, E.P.; Carina, O. Synthesis and characterization of boron-doped carbons. Carbon 1995, 33, 367–374. [Google Scholar]
- Ardelean, P.P. Comparative vibrational study of xFe2O3(1−x) [3B2O3MO](MOCaO or CaF2) glass systems. Mater. Lett. 2004, 58, 3499–3502. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, J.; Jin, Y.; Deng, D.; Xiao, F. Synthesis and properties of highly cross-linked thermosetting resins of benzocyclobutene-functionalized benzoxa-zine. Macromolecules 2012, 45, 4085–4091. [Google Scholar] [CrossRef]
- Kashiwakura, S.; Takahashi, T.; Maekawa, H.; Tetsuya, N. Applica-tion of 11B MAS-NMR to the characterization of boron in coal fly ash generated from Nantun coal. Fuel 2010, 89, 1006–1011. [Google Scholar] [CrossRef]
- Anja, W.-B.; Doris, M.; Dimitrios, P. Structure and properties of orthoborate glasses in the Eu2O3-(Sr,Eu)O-B2O3 quaternary. J. Phys. Chem. B 2015, 119, 3259–3272. [Google Scholar]
- Wu, H.; Wei, Q.; He, H.; Yang, B.-F.; Zhang, Q.; Yang, G.-Y. A new acentric metal borate Mg [B6O9(OH)2]·4H2O: Synthesis, structure and optical property. Inorg. Chem. Commun. 2014, 46, 69–72. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, X.; Tang, Y.; Su, K.; Kong, J. Hierarchically porous silicon-carbon-nitrogen hybrid materials towards highly efficient and selective adsorption of organic dyes. Sci. Rep. 2015, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Agag, T.; Liu, J.; Graf, R.; Spiess, H.W.; Ishida, H. Benzoxazole resin: A novel class of thermoset polymer via smart benzoxazine resin. Macromolecules 2012, 45, 8991–8997. [Google Scholar] [CrossRef]
- Kim, S.-K.; Choi, S.-W.; Jeon, W.S.; Park, J.O.; Ko, T.; Chang, H. Cross-linked benzoxazine-benzimidazole copolymer electrolyte membranes for fuel cells at elevated temperature. Macromolecules 2012, 45, 1438–1446. [Google Scholar] [CrossRef]
- Ashourirad, B.; Sekizkardes, A.K.; Altarawneh, S. Exceptional gas adsorption properties by nitrogen-doped porous carbons derived from benzimidazole-linked polymers. Chem. Mater. 2015, 27, 1349–1358. [Google Scholar] [CrossRef]
- Guo, J.; Rao, Q.; Xu, Z. Effects of particle size of fiberglass-resin powder from PCBs on the properties and volatile behavior of phenolic molding compound. J. Hazard. Mater. 2010, 175, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Mark, G.L.; David, H.S. The chemistry of novolac resins: 3. 13C and 15N n.m.r, studies of curing with hexamethylenetetramine. Polymer 1997, 38, 5835–5848. [Google Scholar] [CrossRef]
- Zhang, X.; Alan, C.P.; David, H.S. The chemistry of novolac resins-V. Reactions of benzoxazine intermediates. Polymer 1998, 39, 399–404. [Google Scholar] [CrossRef]
- Lytle, C.A.; Bertsch, W.; McKinley, M. Determination of novolac resin thermal decomposition products by pyrolysis-gas chromatography-mass spectrometry. J. Anal. Appl. Pyrol. 1998, 45, 121–131. [Google Scholar] [CrossRef]
- Costa, A.C., Jr.; Ramos, J.M.; Téllez Soto, C.A.; Martin, A.A.; Raniero, L.; Ondar, G.F.; Versiane, O.; Moraes, L.S. Fourier transform infrared and raman spectra, DFT:B3LYP/6–311G(d,p) calculations and structural properties of bis(diethyldithiocarbamate) copper(II). Spectrochim. Acta A 2013, 105, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Serpell Christopher, J.; Kilah Nathan, L.; Costa Paulo, J.; Felix, V.; Beer, P.D. Halogen bond anion templated assembly of an imidazolium pseudorotaxane. Angew. Chem. Int. Edit. 2010, 49, 5322–5326. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Radovic, R.L. Inhibition of catalytic oxidation of carbon/carbon composites by boron-doping. Carbon 2005, 43, 1768–1777. [Google Scholar] [CrossRef]
- Norio, I.; Chong, R.P.; Hiroyuki, F. Specification for a standard procedure of X-ray diffraction measurements on carbon materials. Carbon 2004, 42, 701–714. [Google Scholar]
- Lyu, S.C.; Han, J.H.; Shin, K.W. Synthesis of boron-doped double-walled carbon nanotubes by the catalytic decomposition of tetrahydrofuran and triisopropyl borate. Carbon 2011, 49, 1532–1541. [Google Scholar] [CrossRef]
- Wang, H.; Han, T.; Yang, J.; Tao, Z.; Guo, Q.; Liu, Z.; Feng, Z.; Liu, L. Structural evolution of rayon-based carbon fibers induced by doping boron. RSC Adv. 2014, 4, 59150–59156. [Google Scholar] [CrossRef]
- Zhong, D.H.; Sano, H.; Uchiyama, Y.; Kobayashi, K. Effect of low-level boron doping on oxidation behavior of polyimide-derived carbon films. Carbon 2000, 38, 1199–1206. [Google Scholar] [CrossRef]
- Matthews, M.J.; PimentM, A.; Dresselhaus, G. Origin of dispersive effects of the Raman D band in carbon materials. Phys. Rev. B 1999, 59, 6585–6588. [Google Scholar] [CrossRef]
- Nikiel, L.; Jagodzinski, P.W. Raman spectroscopic characterization of graphites: A re-evalution of spectra/structure correlation. Carbon 1993, 31, 1313–1317. [Google Scholar] [CrossRef]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, J.; Chen, L.; Zhang, X.; Feng, J.; Liu, L. The Effect of Introducing B and N on Pyrolysis Process of High Ortho Novolac Resin. Polymers 2016, 8, 35. https://doi.org/10.3390/polym8030035
Yun J, Chen L, Zhang X, Feng J, Liu L. The Effect of Introducing B and N on Pyrolysis Process of High Ortho Novolac Resin. Polymers. 2016; 8(3):35. https://doi.org/10.3390/polym8030035
Chicago/Turabian StyleYun, Jin, Lixin Chen, Xiaofei Zhang, Junjun Feng, and Linlin Liu. 2016. "The Effect of Introducing B and N on Pyrolysis Process of High Ortho Novolac Resin" Polymers 8, no. 3: 35. https://doi.org/10.3390/polym8030035
APA StyleYun, J., Chen, L., Zhang, X., Feng, J., & Liu, L. (2016). The Effect of Introducing B and N on Pyrolysis Process of High Ortho Novolac Resin. Polymers, 8(3), 35. https://doi.org/10.3390/polym8030035