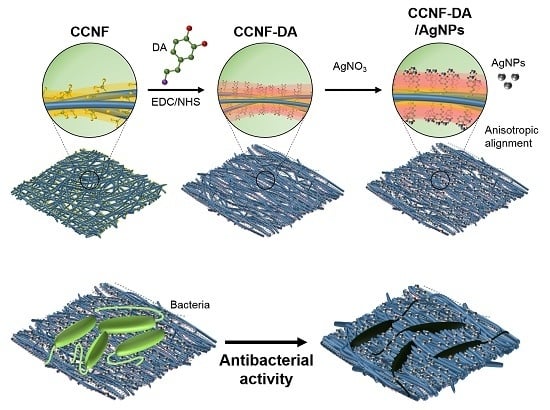
Mussel-Inspired Anisotropic Nanocellulose and Silver Nanoparticle Composite with Improved Mechanical Properties, Electrical Conductivity and Antibacterial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Carboxylated Cellulose Nanofibers (CCNF)
2.3. Preparation of CCNF-Dopamine (DA)
2.4. Preparation of CCNF-DA/Silver Nanoparticles (AgNPs)
2.5. Characterization of CCNF-DA/AgNPs Film
2.6. Characterization of AgNPs
2.7. Mechanical Properties and Conductivity Test
2.8. Antibacterial Activity Assay
2.9. Sustainability Test for Antibacterial Activity
3. Results and Discussions
3.1. Anisotropic Structure of the CCNF-DA/AgNPs Film
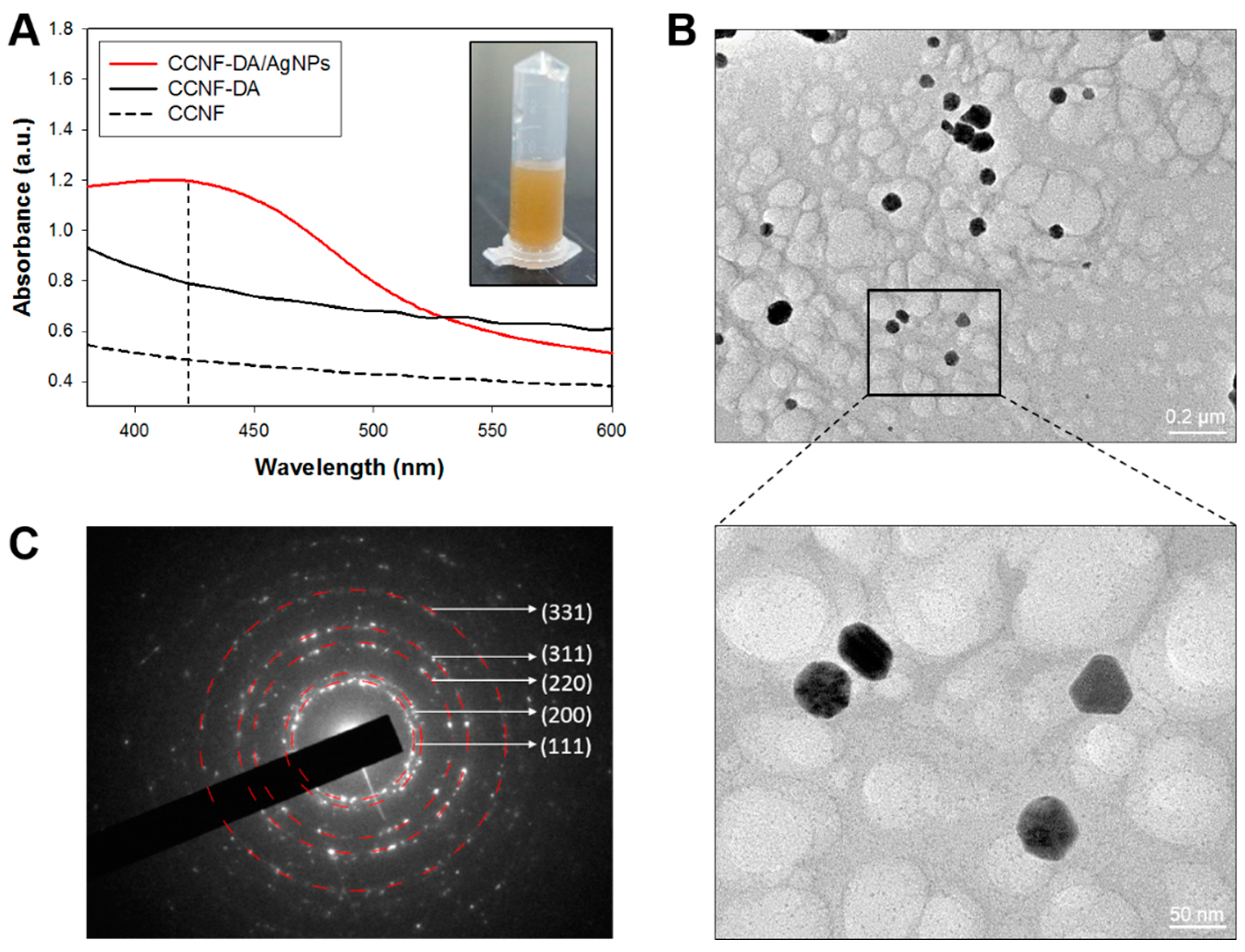
3.2. AgNPs in the CCNF Composite
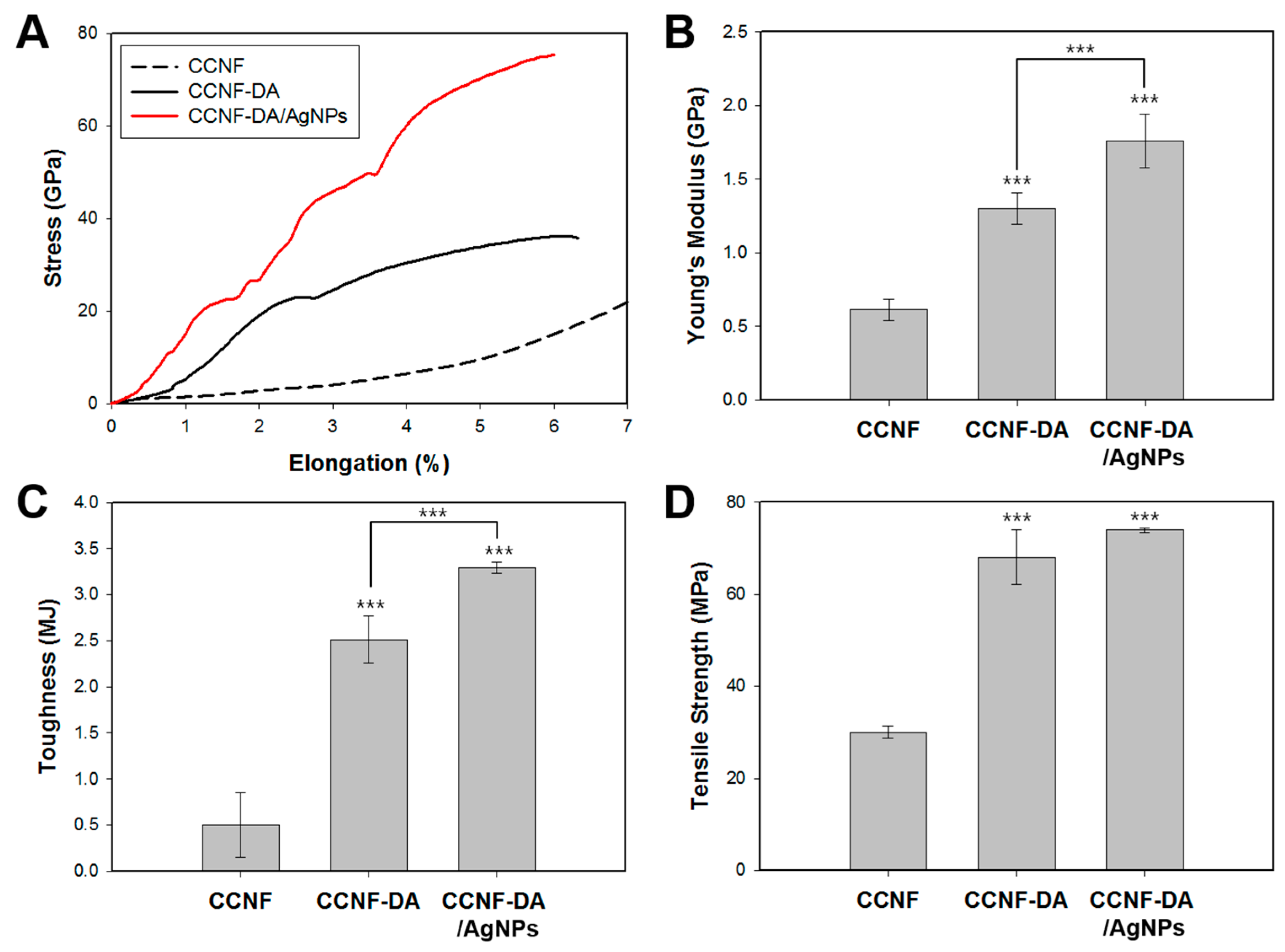
3.3. Mechanical Tests
3.4. Conductivity Test
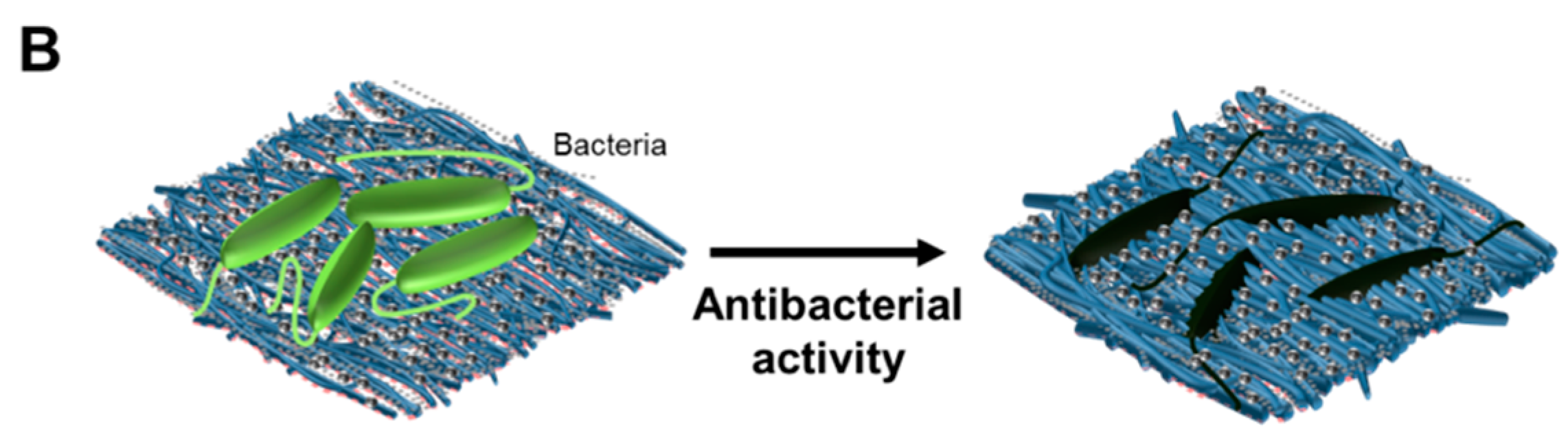
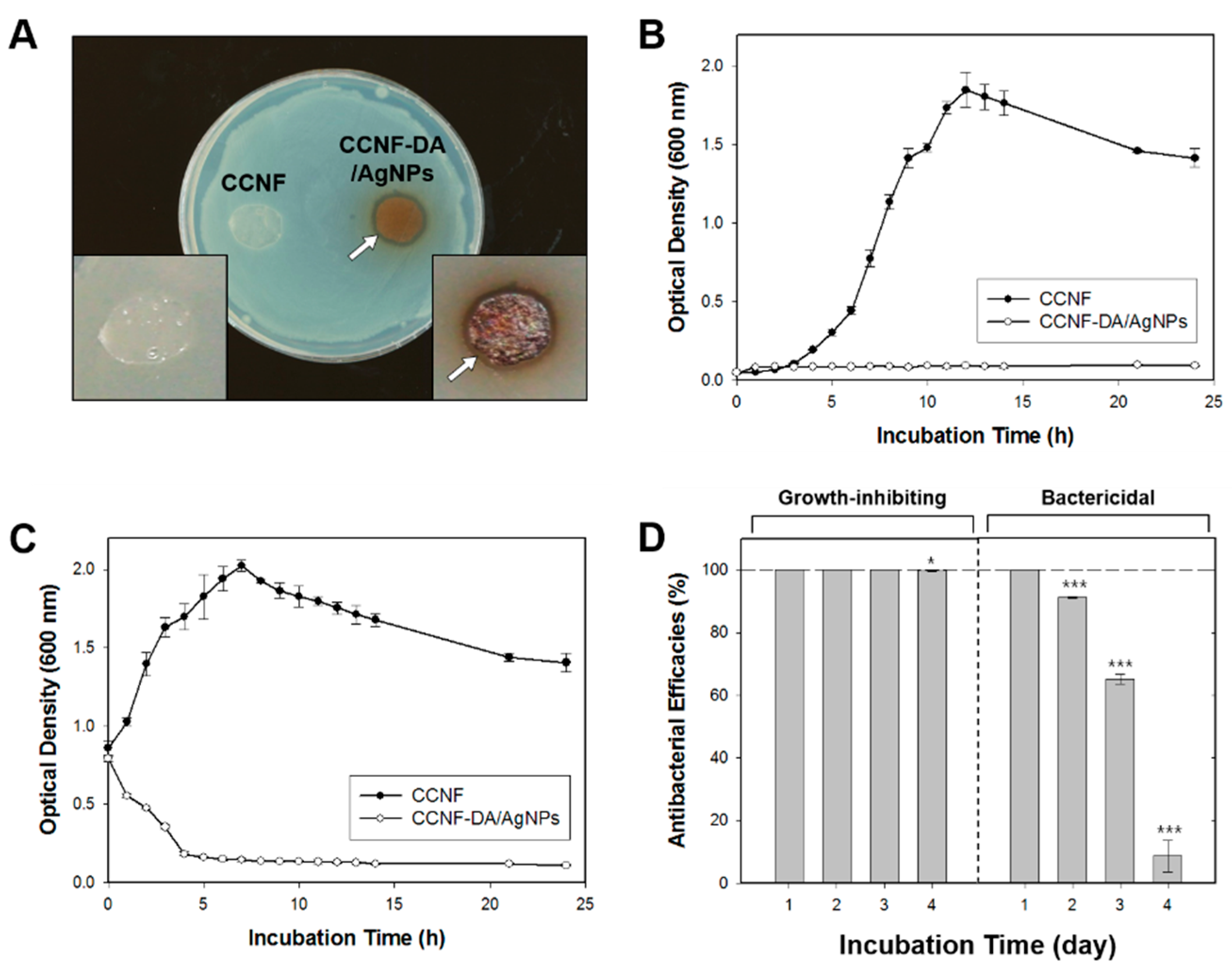
3.5. Antibacterial Test
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Eichhorn, S.; Davies, G. Modelling the crystalline deformation of native and regenerated cellulose. Cellulose 2006, 13, 291–307. [Google Scholar] [CrossRef]
- Tashiro, K.; Kobayashi, M. Theoretical evaluation of three-dimensional elastic constants of native and regenerated celluloses: Role of hydrogen bonds. Polymer 1991, 32, 1516–1526. [Google Scholar] [CrossRef]
- Diddens, I.; Murphy, B.; Krisch, M.; Muller, M. Anisotropic elastic properties of cellulose measured using inelastic X-ray scattering. Macromolecules 2008, 41, 9755–9759. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Nishiyama, Y.; Putaux, J.L.; Vignon, M.; Isogai, A. Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose. Biomacromolecules 2006, 7, 1687–1691. [Google Scholar] [CrossRef] [PubMed]
- Quellmalz, A.; Mihranyan, A. Citric acid cross-linked nanocellulose-based paper for size-exclusion nanofiltration. ACS Biomater. Sci. Eng. 2015, 1, 271–276. [Google Scholar] [CrossRef]
- Bodin, A.; Ahrenstedt, L.; Fink, H.; Brumer, H.; Risberg, B.; Gatenholm, P. Modification of nanocellulose with a xyloglucan–RGD conjugate enhances adhesion and proliferation of endothelial cells: Implications for tissue engineering. Biomacromolecules 2007, 8, 3697–3704. [Google Scholar] [CrossRef] [PubMed]
- Nystrom, G.; Mihranyan, A.; Razaq, A.; Lindström, T.; Nyholm, L.; Strømme, M. A nanocellulose polypyrrole composite based on microfibrillated cellulose from wood. J. Phys. Chem. B 2010, 114, 4178–4182. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Fullenkamp, D.E.; Rivera, J.G.; Gong, Y.K.; Lau, K.A.; He, L.; Varshney, R.; Messersmith, P.B. Mussel-inspired silver-releasing antibacterial hydrogels. Biomaterials 2012, 23, 3783–3791. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Duan, B.; Fang, Z.; Song, J.; Wang, C.; Messersmith, P.B.; Duan, H. Interfacial assembly of mussel-inspired Au@Ag@ polydopamine core-shell nanoparticles for recyclable nanocatalysts. Adv. Mater. 2014, 26, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-C.; Zhang, M.; Li, A.-J.; He, X.-W.; Yin, X.-B. 3,4-Dihydroxy-l-phenylalanine for preparation of gold nanoparticles and as electron transfer promoter in H2O2 biosensor. Electroanalysis 2011, 23, 2421–2428. [Google Scholar] [CrossRef]
- Tang, J.; Shi, Z.; Berry, R.M.; Tam, K.C. Mussel-inspired green metallization of silver nanoparticles on cellulose nanocrystals and their enhanced catalytic reduction of 4-nitrophenol in the presence of β-cyclodextrin. Ind. Eng. Chem. Res. 2015, 54, 3299–3308. [Google Scholar] [CrossRef]
- Cai, J.; Kimura, S.; Wada, M.; Kuga, S. Nanoporous cellulose as metal nanoparticles support. Biomacromolecules 2009, 10, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Ferraria, A.M.; Boufi, S.; Battaglini, N.; do Rego, A.M.B.; ReiVilar, M. Hybrid systems of silver nanoparticles generated on cellulose surfaces. Langmuir 2010, 26, 1996–2001. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Sacui, I.A.; Nieuwendaal, R.C.; Burnett, D.J.; Stranick, S.J.; Jorfi, M.; Weder, C.; Foster, E.J.; Olsson, R.T.; Gilman, J.W. Comparison of the properties of cellulose nanocrystals and cellulose nanofibrils isolated from bacteria, tunicate, and wood processed using acid, enzymatic, mechanical, and oxidative methods. ACS Appl. Mater. Interfaces 2014, 6, 6127–6138. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, E.; Pettersson, T.; Ankerfors, M.; Wågberg, L. Adhesive layer-by-layer films of carboxymethylated cellulose nanofibril-dopamine covalent bioconjugates inspired by marine mussel threads. ACS Nano 2012, 6, 4731–4739. [Google Scholar] [CrossRef] [PubMed]
- Arnow, L.E. Colorimetric determination of the components of 3,4-dihydroxyphenylalaninetyrosine mixtures. J. Biol. Chem. 1937, 118, 531–537. [Google Scholar]
- Li, X.; Lenhart, J.J.; Walker, H.W. Aggregation kinetics and dissolution of coated silver nanoparticles. Langmuir 2011, 28, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Black, K.C.; Liu, Z.; Messersmith, P.B. Catechol redox induced formation of metal core-polymer shell nanoparticles. Chem. Mater. 2011, 23, 1130–1135. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.K.; Seo, J.H.; Choi, B.-H.; Kim, B.J.; Shin, H.H.; Hwang, B.H.; Cha, H.J. Surface-independent antibacterial coating using silver nanoparticle-generating engineered mussel glue. ACS Appl. Mater. Interfaces 2014, 6, 20242–20253. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.X.; Kim, S.; Lee, D.; Hwang, D.S. Tunicate-mimetic nanofibrous hydrogel adhesive with improved wet adhesion. Acta Biomater. 2015, 20, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Emons, A.M.C.; Mulder, B.M. The making of the architecture of the plant cell wall: How cells exploit geometry. Proc. Natl. Acad. Sci. USA 1998, 95, 7215–7219. [Google Scholar] [CrossRef] [PubMed]
- Reis, D.; Vian, B. Helicoidal pattern in secondary cell walls and possible role of xylans in their construction. C. R. Biol. 2004, 327, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Uematsu, T.; Kimura, S.; Enomae, T.; Isogai, A. Self-aligned integration of native cellulose nanofibrils towards producing diverse bulk materials. Soft Matter 2011, 7, 8804–8809. [Google Scholar] [CrossRef]
- Woltman, S.J.; Jay, G.D.; Crawford, G.P. Liquid-crystal materials find a new order in biomedical applications. Nat. Mater. 2007, 6, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Naik, R.R.; Stringer, S.J.; Agarwal, G.; Jones, S.E.; Stone, M.O. Biomimetic synthesis and patterning of silver nanoparticles. Nat. Mater. 2002, 1, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, N.; Dougherty, D.A. Nanomechanics of cation–π interactions in aqueous solution. Trends Pharmacol. Sci. 2002, 23, 281–287. [Google Scholar]
- Lu, Q.; Oh, D.X.; Lee, Y.; Jho, Y.; Hwang, D.S.; Zeng, H. Nanomechanics of cation–π interactions in aqueous solution. Angew. Chem. Int. Ed. 2013, 52, 3944–3948. [Google Scholar] [CrossRef] [PubMed]
- Mohammad-Shiri, H.; Ghaemi, M.; Riahi, S.; Akbari-Sehat, A. Computational and electrochemical studies on the redox reaction of dopamine in aqueous solution. Int. J. Electrochem. Sci. 2011, 6, 317–336. [Google Scholar]
- Cui, W.; Li, M.; Liu, J.; Wang, B.; Zhang, C.; Jiang, L.; Cheng, Q. A Strong integrated strength and toughness artificial nacre based on dopamine cross-linked graphene oxide. ACS Nano 2014, 8, 9511–9517. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.S.; Harrington, M.J.; Lu, Q.; Masic, A.; Zeng, H.; Waite, J.H. Mussel foot protein-1 (mcfp-1) interaction with titania surfaces. J. Mater. Chem. 2012, 22, 15530–15533. [Google Scholar] [CrossRef] [PubMed]
- Bober, P.; Liu, J.; Mikkonen, K.S.; Ihalainen, P.; Pesonen, M.; Plumed-Ferrer, C.; von Wright, A.; Lindfors, T.; Xu, C.L.; Latonen, R.M. Biocomposites of nanofibrillated cellulose, polypyrrole, and silver nanoparticles with electroconductive and antimicrobial properties. Biomacromolecules 2014, 15, 3655–3663. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Gao, H.; Feng, J.; Ding, B.; Cao, X.; Kuga, S.; Wang, Y.; Zhang, L.; Cai, J. In situ synthesis of robust conductive cellulose/polypyrrole composite aerogels and their potential application in nerve regeneration. Angew. Chem. Int. Ed. 2014, 53, 5380–5384. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bian, L.; Zhou, P.; Tang, J.; Tang, W. Core-sheath structured bacterial cellulose/polypyrrole nanocomposites with excellent conductivity as supercapacitors. J. Mater. Chem. A 2013, 1, 578–584. [Google Scholar] [CrossRef]
- Kim, Y.; Zhu, J.; Yeom, B.; di Prima, M.; Su, X.; Kim, J.-G.; Yoo, S.J.; Uher, C.; Kotov, N.A. Stretchable nanoparticle conductors with self-organized conductive pathways. Nature 2013, 500, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Zhao, Y.; Hu, C.; Cheng, H.; Hu, Y.; Zhang, Z.; Shi, G.; Qu, L. All-graphene core-sheath microfibers for all-solid-state, stretchable fibriform supercapacitors and wearable electronic textiles. Adv. Mater. 2013, 25, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Su, Y.; Wang, D.W.; Li, F.; Du, J.H.; Cheng, H.M. Graphene-cellulose paper flexible supercapacitors. Adv. Energy Mater. 2011, 1, 917–922. [Google Scholar] [CrossRef]
- Mihardja, S.S.; Sievers, R.E.; Lee, R.J. The effect of polypyrrole on arteriogenesis in an acute rat infarct model. Biomaterials 2008, 29, 4205–4210. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Hwang, D.S.; Liu, Y.; Zeng, H. Molecular interactions of mussel protective coating protein, mcfp-1, from Mytilus californianus. Biomaterials 2012, 33, 1903–1911. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.-L.; Jo, Y.K.; Cha, M.; Cha, Y.J.; Yoon, D.K.; Sanandiya, N.D.; Prajatelistia, E.; Oh, D.X.; Hwang, D.S. Mussel-Inspired Anisotropic Nanocellulose and Silver Nanoparticle Composite with Improved Mechanical Properties, Electrical Conductivity and Antibacterial Activity. Polymers 2016, 8, 102. https://doi.org/10.3390/polym8030102
Nguyen H-L, Jo YK, Cha M, Cha YJ, Yoon DK, Sanandiya ND, Prajatelistia E, Oh DX, Hwang DS. Mussel-Inspired Anisotropic Nanocellulose and Silver Nanoparticle Composite with Improved Mechanical Properties, Electrical Conductivity and Antibacterial Activity. Polymers. 2016; 8(3):102. https://doi.org/10.3390/polym8030102
Chicago/Turabian StyleNguyen, Hoang-Linh, Yun Kee Jo, Minkyu Cha, Yun Jeong Cha, Dong Ki Yoon, Naresh D. Sanandiya, Ekavianty Prajatelistia, Dongyeop X. Oh, and Dong Soo Hwang. 2016. "Mussel-Inspired Anisotropic Nanocellulose and Silver Nanoparticle Composite with Improved Mechanical Properties, Electrical Conductivity and Antibacterial Activity" Polymers 8, no. 3: 102. https://doi.org/10.3390/polym8030102
APA StyleNguyen, H.-L., Jo, Y. K., Cha, M., Cha, Y. J., Yoon, D. K., Sanandiya, N. D., Prajatelistia, E., Oh, D. X., & Hwang, D. S. (2016). Mussel-Inspired Anisotropic Nanocellulose and Silver Nanoparticle Composite with Improved Mechanical Properties, Electrical Conductivity and Antibacterial Activity. Polymers, 8(3), 102. https://doi.org/10.3390/polym8030102