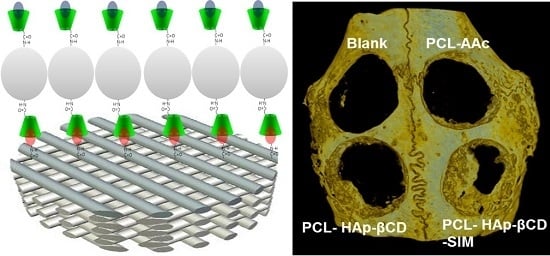
Development of Poly(ɛ-caprolactone) Scaffold Loaded with Simvastatin and Beta-Cyclodextrin Modified Hydroxyapatite Inclusion Complex for Bone Tissue Engineering
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Poly(ɛ-caprolactone) (PCL) Scaffold Preparation
2.3. Preparation of β-Cyclodextrin (βCD) Grafted Hydroxyapatite (HAp)
2.4. Surface Coating of PCL Scaffold with HAp and Simvastatin Loading
2.5. Characterization of βCD-Grafted HAp and PCL Scaffolds
2.6. Cell Culture
2.7. In Vivo Animal Study
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characterization of PCL Scaffolds
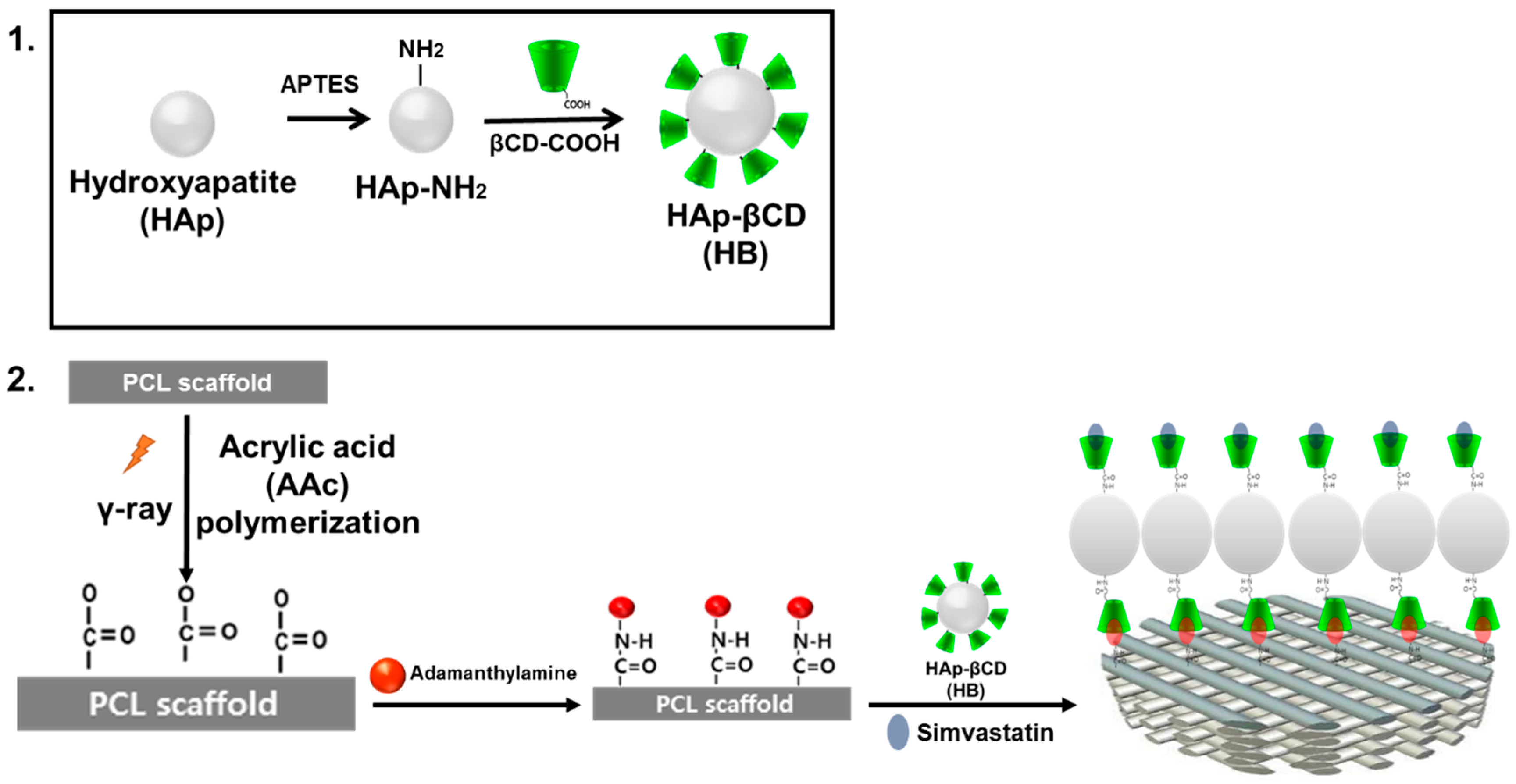
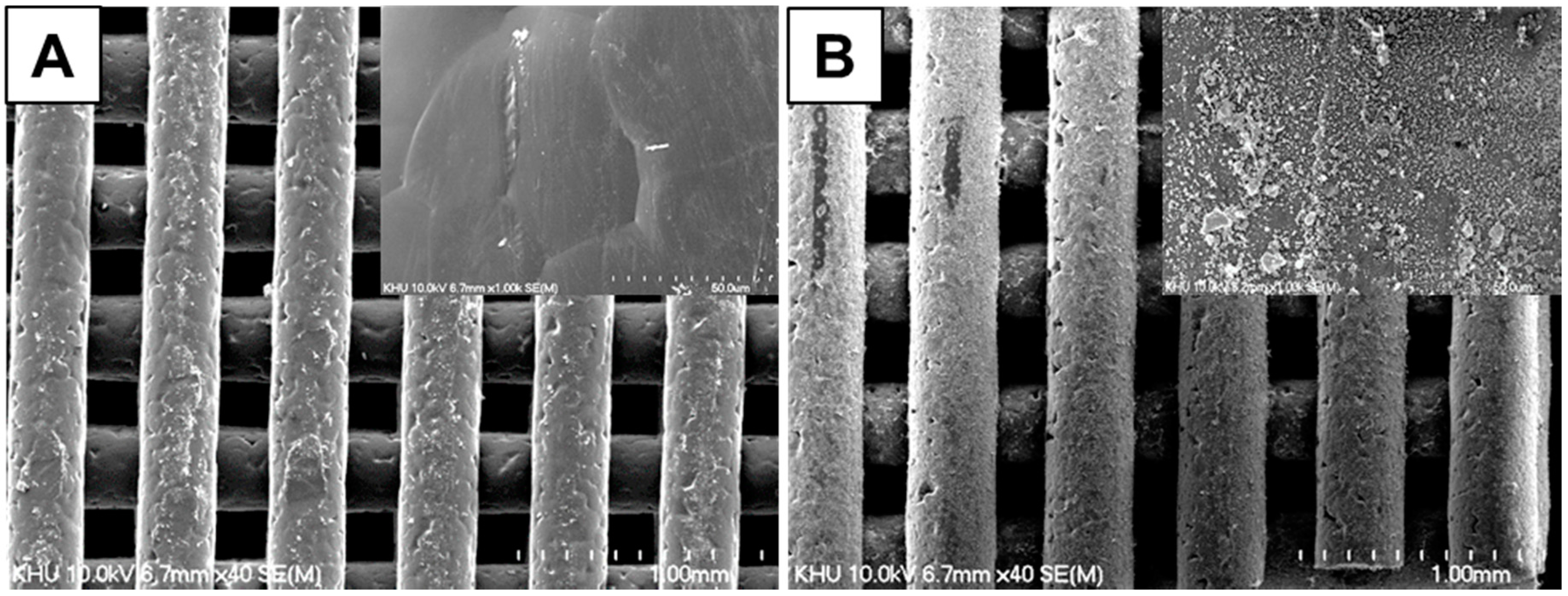
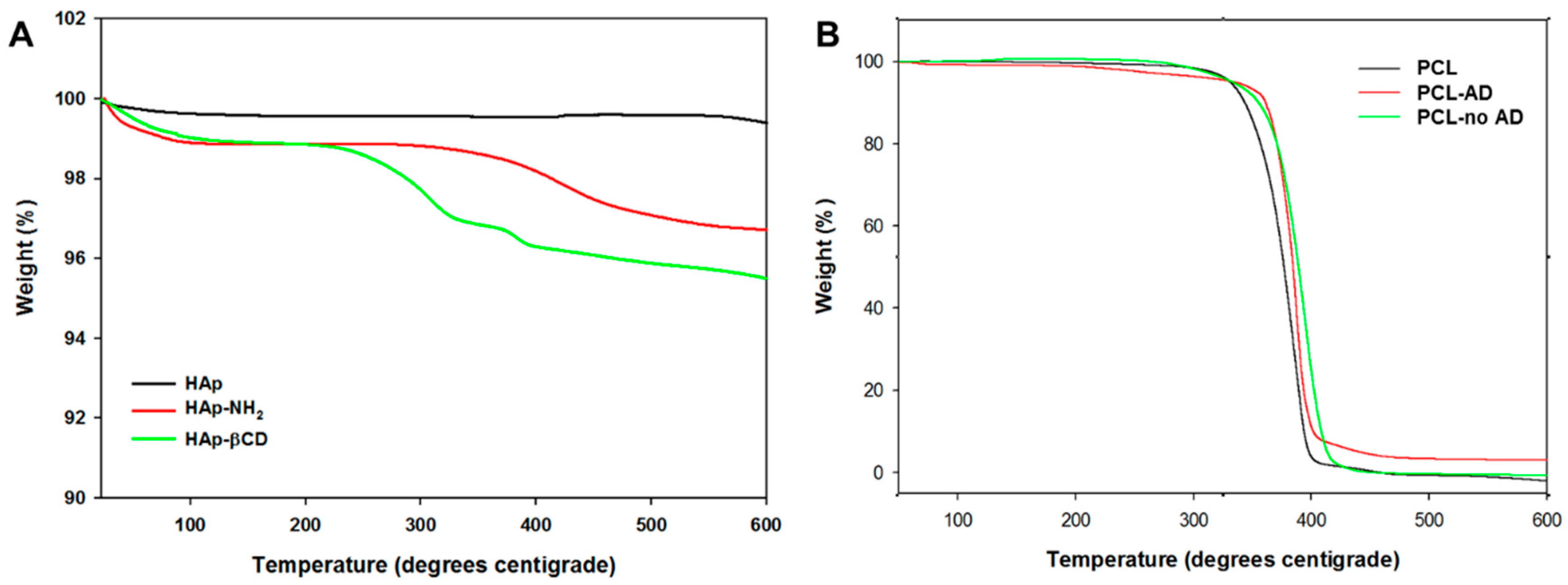
3.2. Characterization of βCD-Modified HAp and AD-Modified PCL Scaffold
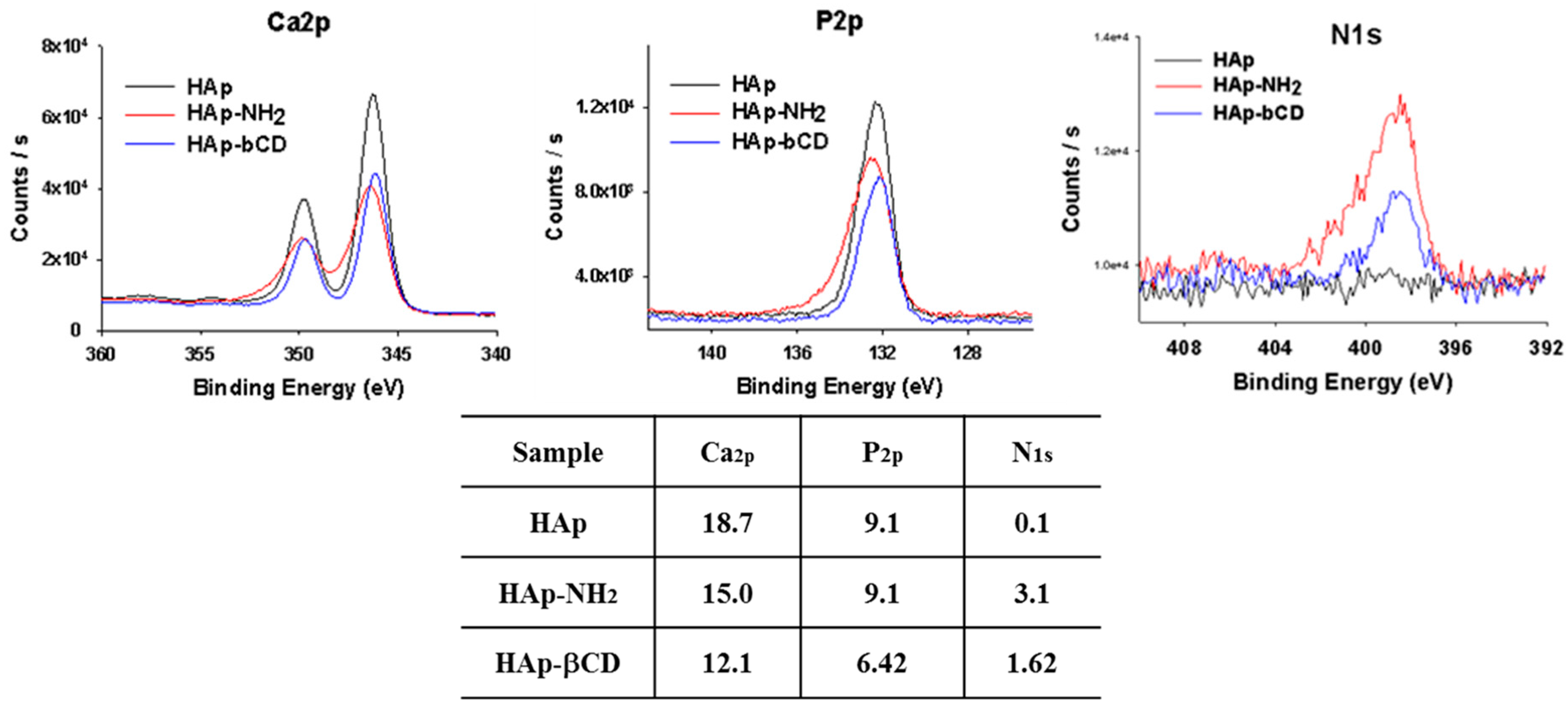
| Heading | Hap-NH2 | Hap-βCD |
|---|---|---|
| Amine contents (μmol/mg) | 21.9 ± 2.9 | 3.5 ± 0.4 |
3.3. In Vitro Cell Test of PCL Scaffolds
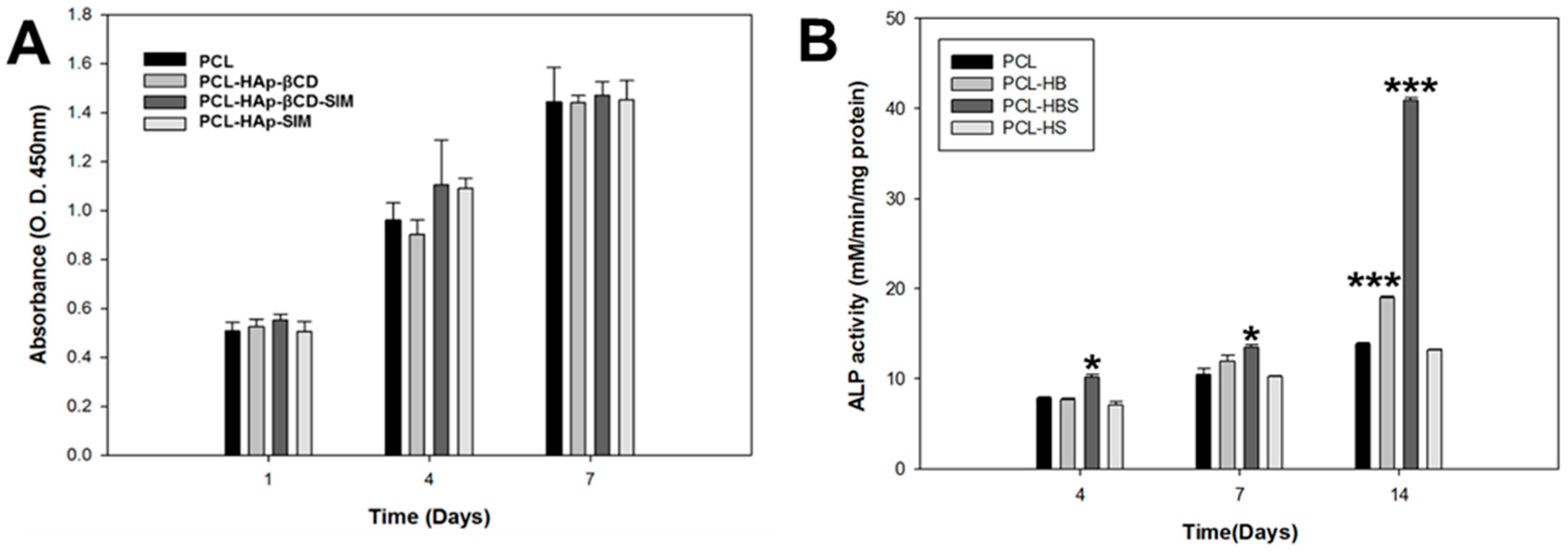
3.4. In vivo Animal Study
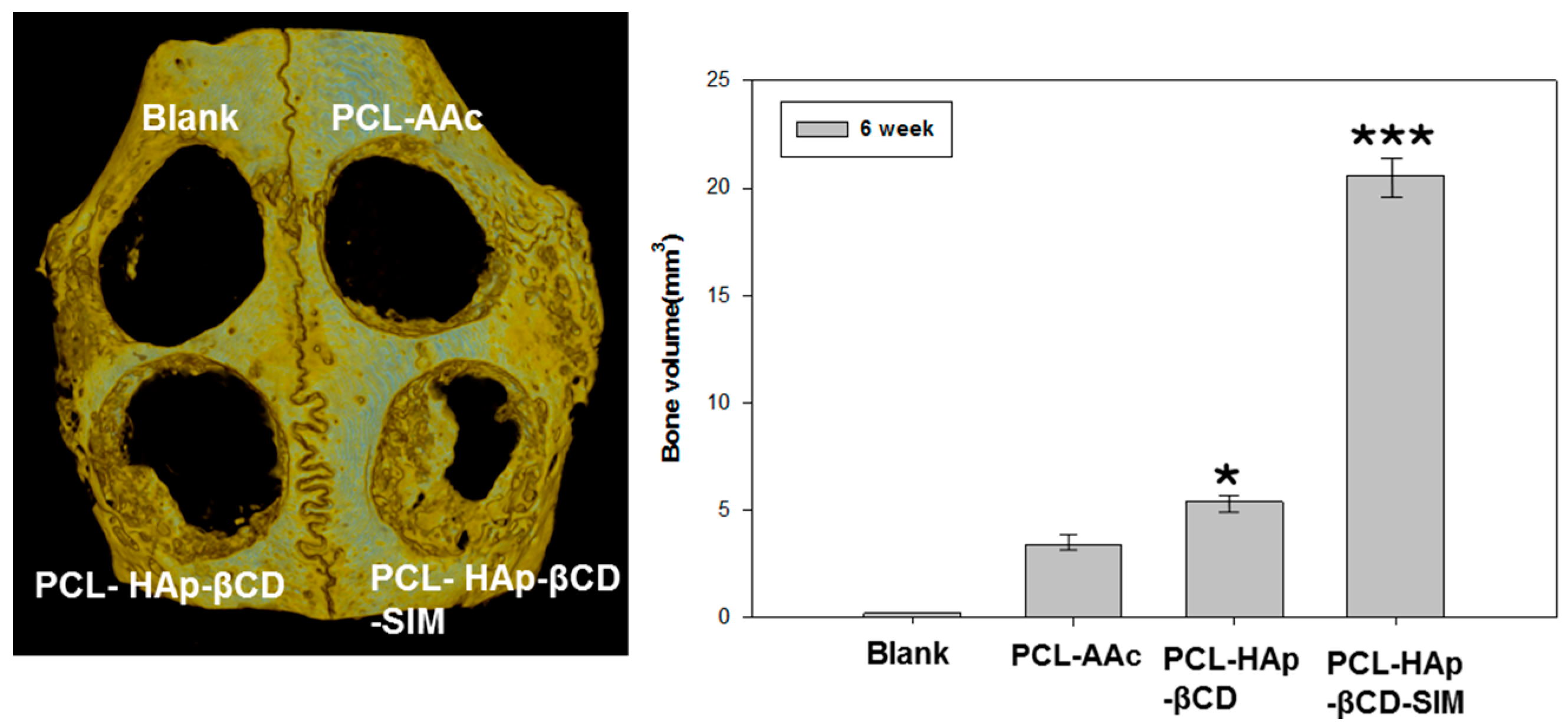
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.Y.; Zhou, L.H.; Zhang, Q.C.; Chen, Y.M.; Sun, W.; Xu, F.; Lu, T.J. Microfluidic hydrogels for tissue engineering. Biofabrication 2011, 3, 012001. [Google Scholar] [CrossRef] [PubMed]
- Griffith, L.G.; Naughton, G. Tissue engineering—Current challenges and expanding opportunities. Science 2002, 295, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Lohfeld, S.; McHugh, P. Laser sintering for the fabrication of tissue engineering scaffolds. In Computer-Aided Tissue Engineering; Liebschner, M.A.K., Ed.; Humana Press: New York, NY, USA, 2012; Volume 868, pp. 303–310. [Google Scholar]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.M.; Holmes, B.; Faucett, S.; Zhang, L.G. Three-dimensional printing of nanomaterial scaffolds for complex tissue regeneration. Tissue Eng. Part B Rev. 2015, 21, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Sachlos, E.; Czernuszka, J.T. Making tissue engineering scaffolds work. Review: The application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur. Cell Mater. 2003, 5, 29–39, discussion 39–40. [Google Scholar]
- Kwon, B.-J.; Kim, J.; Kim, Y.H.; Lee, M.H.; Baek, H.S.; Lee, D.H.; Kim, H.-L.; Seo, H.J.; Lee, M.H.; Kwon, S.-Y.; et al. Biological advantages of porous hydroxyapatite scaffold made by solid freeform fabrication for bone tissue regeneration. Artif. Organs 2013, 37, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.M.; Adewunmi, A.; Schek, R.M.; Flanagan, C.L.; Krebsbach, P.H.; Feinberg, S.E.; Hollister, S.J.; Das, S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 2005, 26, 4817–4827. [Google Scholar] [CrossRef] [PubMed]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Moutos, F.T.; Freed, L.E.; Guilak, F. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat. Mater. 2007, 6, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.P.; Chen, Y.Y.; Hsieh, M.F.; Lee, H.M. Solid freeform fabrication and in-vitro response of osteoblast cells of mPEG-PCL-mPEG bone scaffolds. Biomed. Microdevices 2013, 15, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Lei, D.; Li, S.; Huang, P.; Qi, Q.; Sun, Y.; Zhang, Y.; Wang, Z.; You, Z.; Ye, X.; et al. Hybrid small-diameter vascular grafts: Anti-expansion effect of electrospun poly ε-caprolactone on heparin-coated decellularized matrices. Biomaterials 2016, 76, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, J.; Kim, Y.; Kim, J.; Lee, J.; Shin, J.-W.; Kwon, I.; Kim, W. Fabrication of biomimetic PCL scaffold using rapid prototyping for bone tissue engineering. Macromol. Res. 2014, 22, 882–887. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, G.; Cai, Y.; Ji, H.; Zhou, G.; Zhao, X.; Tang, R.; Zhang, M. In vitro effects of nanophase hydroxyapatite particles on proliferation and osteogenic differentiation of bone marrow-derived mesenchymal stem cells. J. Biomed. Mater. Res. A 2009, 90, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Dinarvand, P.; Seyedjafari, E.; Shafiee, A.; Jandaghi, A.B.; Doostmohammadi, A.; Fathi, M.H.; Farhadian, S.; Soleimani, M. New approach to bone tissue engineering: Simultaneous application of hydroxyapatite and bioactive glass coated on a poly(l-lactic acid) scaffold. ACS Appl. Mater. Interfaces 2011, 3, 4518–4524. [Google Scholar] [CrossRef] [PubMed]
- Shor, L.; Güçeri, S.; Wen, X.; Gandhi, M.; Sun, W. Fabrication of three-dimensional polycaprolactone/hydroxyapatite tissue scaffolds and osteoblast-scaffold interactions in vitro. Biomaterials 2007, 28, 5291–5297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ma, P.X. Biomimetic polymer/apatite composite scaffolds for mineralized tissue engineering. Macromol. Biosci. 2004, 4, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhong, R.-Q.; Zhang, H.-Y.; Song, H.-B. A unique tetramer of 4:5 β-cyclodextrin-ferrocene in the solid state. Chem. Commun. 2005, 2211–2213. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, F.; Uekama, K. Cyclodextrin-based controlled drug release system. Adv. Drug Deliv. Rev. 1999, 36, 125–141. [Google Scholar] [CrossRef]
- Mundy, G.; Garrett, R.; Harris, S.; Chan, J.; Chen, D.; Rossini, G.; Boyce, B.; Zhao, M.; Gutierrez, G. Stimulation of bone formation in vitro and in rodents by statins. Science 1999, 286, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Sun, J.S.; Tsuang, Y.H.; Chen, M.H.; Weng, P.W.; Lin, F.H. Simvastatin promotes osteoblast viability and differentiation via Ras/Smad/Erk/BMP-2 signaling pathway. Nutr. Res. 2010, 30, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Lee, S.H.; Kim, W.D. Fabrication of porous polycaprolactone/hydroxyapatite (PCL/HA) blend scaffolds using a 3D plotting system for bone tissue engineering. Bioprocess Biosyst. Eng. 2010, 34, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Malaval, L.; Aubin, J.E. Global amplification polymerase chain reaction reveals novel transitional stages during osteoprogenitor differentiation. J. Cell Sci. 2003, 116, 1787–1796. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.C.; Sipe, J.B.; Hessle, L.; Dhanyamraju, R.; Atti, E.; Camacho, N.P.; Millan, J.L. Impaired calcification around matrix vesicles of growth plate and bone in alkaline phosphatase-deficient mice. Am. J. Pathol. 2004, 164, 841–847. [Google Scholar] [CrossRef]
- Liu, C.; Wu, Z.; Sun, H.-C. The effect of simvastatin on mRNA expression of transforming growth factor-β1, bone morphogenetic protein-2 and vascular endothelial growth factor in tooth extraction socket. Int. J. Oral Sci. 2009, 1, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Pagkalos, J.; Cha, J.M.; Kang, Y.; Heliotis, M.; Tsiridis, E.; Mantalaris, A. Simvastatin induces osteogenic differentiation of murine embryonic stem cells. J. Bone Miner. Res. 2010, 25, 2470–2478. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.B.; Kim, J.E.; Bae, M.S.; Park, S.A.; Balikov, D.A.; Sung, H.-j.; Jeon, H.B.; Park, H.K.; Um, S.H.; Lee, K.S.; et al. Development of Poly(ɛ-caprolactone) Scaffold Loaded with Simvastatin and Beta-Cyclodextrin Modified Hydroxyapatite Inclusion Complex for Bone Tissue Engineering. Polymers 2016, 8, 49. https://doi.org/10.3390/polym8020049
Lee JB, Kim JE, Bae MS, Park SA, Balikov DA, Sung H-j, Jeon HB, Park HK, Um SH, Lee KS, et al. Development of Poly(ɛ-caprolactone) Scaffold Loaded with Simvastatin and Beta-Cyclodextrin Modified Hydroxyapatite Inclusion Complex for Bone Tissue Engineering. Polymers. 2016; 8(2):49. https://doi.org/10.3390/polym8020049
Chicago/Turabian StyleLee, Jung Bok, Ji Eun Kim, Min Soo Bae, Su A Park, Daniel A. Balikov, Hak-joon Sung, Hoon Bong Jeon, Hun Kuk Park, Soong Ho Um, Kook Sun Lee, and et al. 2016. "Development of Poly(ɛ-caprolactone) Scaffold Loaded with Simvastatin and Beta-Cyclodextrin Modified Hydroxyapatite Inclusion Complex for Bone Tissue Engineering" Polymers 8, no. 2: 49. https://doi.org/10.3390/polym8020049
APA StyleLee, J. B., Kim, J. E., Bae, M. S., Park, S. A., Balikov, D. A., Sung, H.-j., Jeon, H. B., Park, H. K., Um, S. H., Lee, K. S., & Kwon, I. K. (2016). Development of Poly(ɛ-caprolactone) Scaffold Loaded with Simvastatin and Beta-Cyclodextrin Modified Hydroxyapatite Inclusion Complex for Bone Tissue Engineering. Polymers, 8(2), 49. https://doi.org/10.3390/polym8020049