Magnetically-Responsive Hydrogels for Modulation of Chondrogenic Commitment of Human Adipose-Derived Stem Cells
Abstract
:1. Introduction
2. Experimental Section
2.1. Development of MNPs-κC Hydrogels
2.2. Human Adipose Derived Stem Cells (hASCs): Isolation and Culture
2.3. Encapsulation of hASCs in MNPs-κC Hydrogels
2.4. Morphological Evaluation of MNPs-κC Hydrogels
2.5. Cell Viability and Metabolic Activity of hASCs in MNPs-κC Hydrogels
2.6. hASCs Proliferation in MNPs-κC Hydrogels
2.7. Chondrogenic Staining of hASCs Encapsulated in MNPs-κC Hydrogels
2.8. Gene Expression of hASCs Laden in MNPs-κC Hydrogels
| Target Gene | Primer Sequences (5′-3′) | Tm (°C) |
|---|---|---|
| Sox9 | TACGACTACACCGACCACCA | 58.4 |
| TTAGGATCATCTCGGCCATC | ||
| Collagen I | CATCTCCCCTTCGTTTTTGA | 55.3 |
| CCAAATCCGATGTTTCTGCT | ||
| Collagen II | GACAATCTGGCTCCCAAC | 56.4 |
| ACAGTCTTGCCCCACTTAC | ||
| GAPDH | ACAGTCAGCCGCATCTTCTT | 57.3 |
| ACGACCAAATCCGTTGACTC |
2.9. Statistical Analysis
3. Results
3.1. Morphological Evaluation of MNPs-κC Hydrogels

3.2. Influence of the MNPs Concentration on the Cell Morphology, Viability and Proliferation
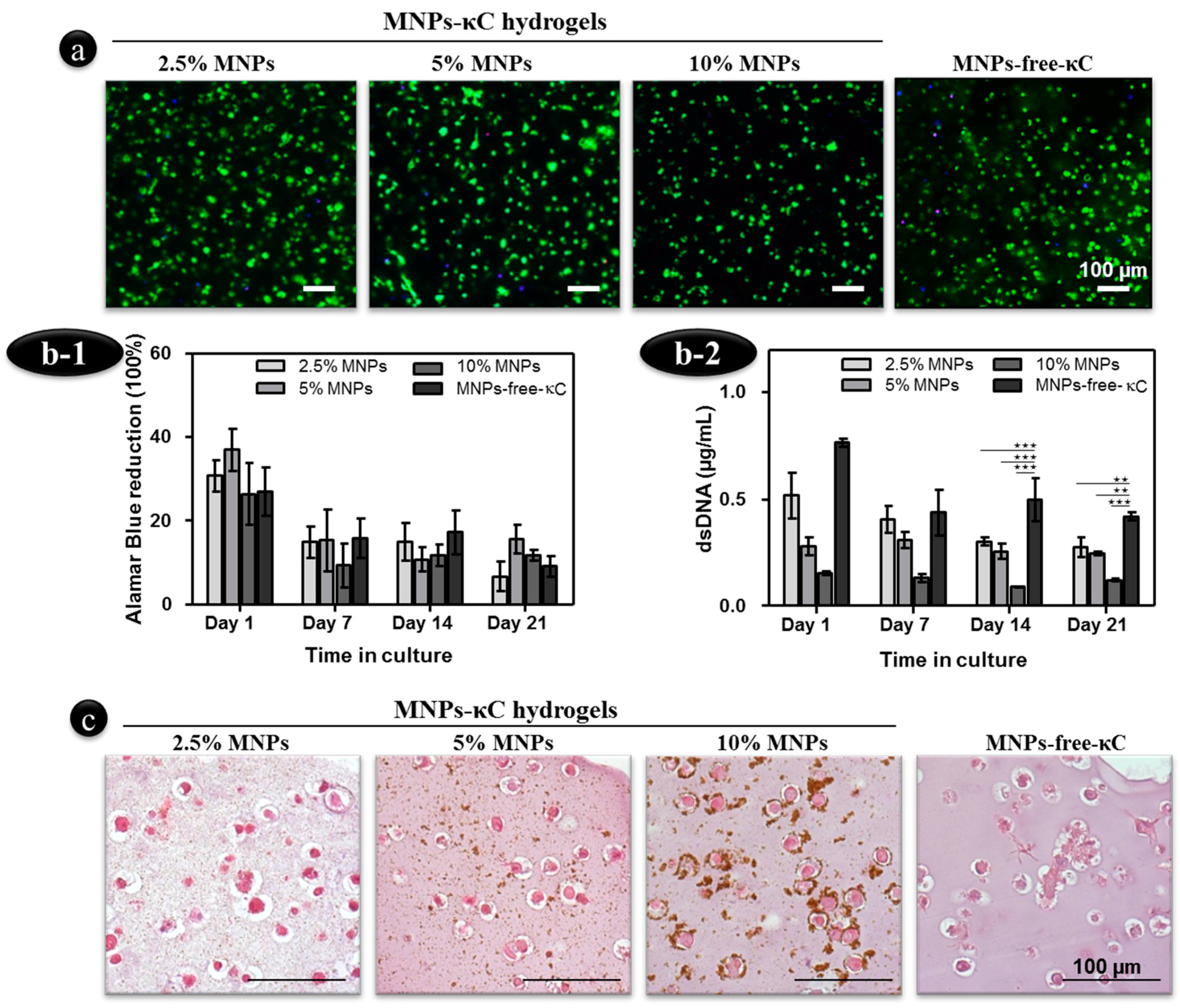
3.3. Reponse of hASCs Laden MNPs-κC Hydrogels under the Influence of an External Magnetic Stimulus

Assessment of hASCs Chondrogenic Commitment in MNPs-κC Hydrogels

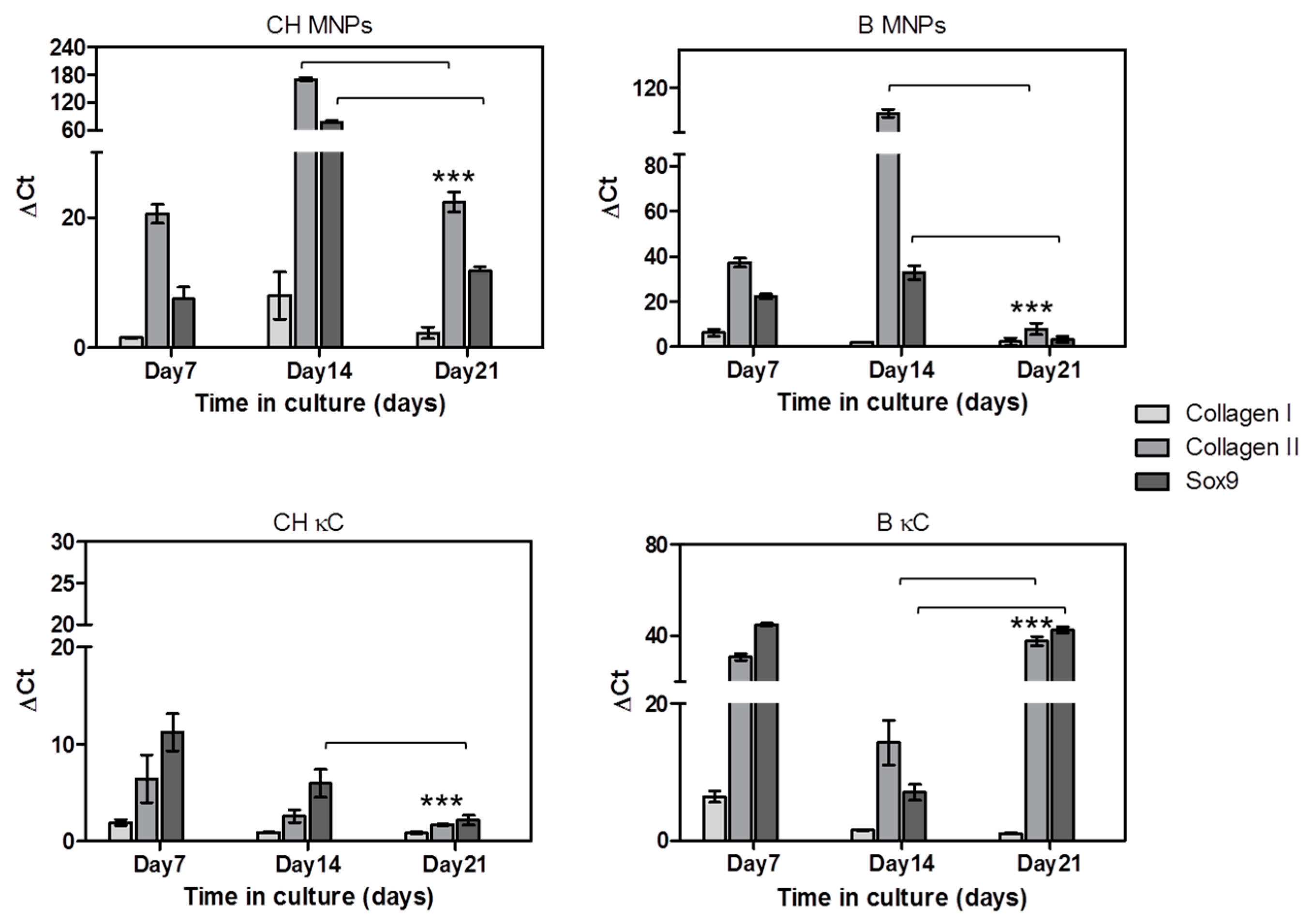
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shubayev, V.I.; Pisanic Ii, T.R.; Jin, S. Magnetic nanoparticles for theragnostics. Adv. Drug Deliv. Rev. 2009, 61, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.-H.; Salabas, E.L.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Estelrich, J.; Escribano, E.; Queralt, J.; Busquets, M.A. Iron oxide nanoparticles for magnetically-guided and magnetically-responsive drug delivery. Int. J. Mol. Sci. 2015, 16, 8070–8101. [Google Scholar] [CrossRef] [PubMed]
- Arruebo, M.; Fernández-Pacheco, R.; Ibarra, M.R.; Santamaría, J. Magnetic nanoparticles for drug delivery. Nano Today 2007, 2, 22–32. [Google Scholar] [CrossRef]
- Amirfazli, A. Nanomedicine: Magnetic nanoparticles hit the target. Nat. Nanotechnol. 2007, 2, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bock, N.; Riminucci, A.; Dionigi, C.; Russo, A.; Tampieri, A.; Landi, E.; Goranov, V.A.; Marcacci, M.; Dediu, V. A novel route in bone tissue engineering: Magnetic biomimetic scaffolds. Acta Biomater. 2010, 6, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. J. R. Soc. Interface 2011, 8, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Whatley, B.R.; Li, X.; Zhang, N.; Wen, X. Magnetic-directed patterning of cell spheroids. J. Biomed. Mater. Res. A 2013, 102, 1537–1547. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Webster, T.J. Magnetic nanoparticles: Biomedical applications and challenges. J. Mater. Chem. 2010, 20, 8760–8767. [Google Scholar] [CrossRef]
- Santo, V.E.; Rodrigues, M.T.; Reis, R.L.; Gomes, M.E. Contributions and future perspectives on the use of magnetic nanoparticles as diagnostic and therapeutic tools in the field of regenerative medicine. Expert Rev. Mol. Diagn. 2013, 13, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Kanczler, J.M.; Sura, H.S.; Magnay, J.; Green, D.; Oreffo, R.O.; Dobson, J.P.; El Haj, A.J. Controlled differentiation of human bone marrow stromal cells using magnetic nanoparticle technology. Tissue Eng. A 2010, 16, 3241–3250. [Google Scholar] [CrossRef] [PubMed]
- Fayol, D.; Frasca, G.; le Visage, C.; Gazeau, F.; Luciani, N.; Wilhelm, C. Use of magnetic forces to promote stem cell aggregation during differentiation, and cartilage tissue modeling. Adv. Mater. 2013, 25, 2611–2616. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.; Gonçalves, A.I.; Rodrigues, M.T.; Reis, R.L.; Gomes, M.E. The effect of magnetic stimulation on the osteogenic and chondrogenic differentiation of human stem cells derived from the adipose tissue (hASCs). J. Magn. Magn. Mater. 2015, 393, 526–536. [Google Scholar] [CrossRef]
- Sniadecki, N.J.; Anguelouch, A.; Yang, M.T.; Lamb, C.M.; Liu, Z.; Kirschner, S.B.; Liu, Y.; Reich, D.H.; Chen, C.S. Magnetic microposts as an approach to apply forces to living cells. Proc. Natl. Acad. Sci. USA 2007, 104, 14553–14558. [Google Scholar] [CrossRef] [PubMed]
- Tasoglu, S.; Yu, C.H.; Gungordu, H.I.; Guven, S.; Vural, T.; Demirci, U. Guided and magnetic self-assembly of tunable magnetoceptive gels. Nat. Commun. 2014. [Google Scholar] [CrossRef] [PubMed]
- Tampieri, A.; Landi, E.; Valentini, F.; Sandri, M.; D’Alessandro, T.; Dediu, V.; Marcacci, M. A conceptually new type of bio-hybrid scaffold for bone regeneration. Nanotechnology 2011, 22, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Popa, E.G.; Gomes, M.E.; Reis, R.L. Cell delivery systems using alginate-carrageenan hydrogel beads and fibers for regenerative medicine applications. Biomacromolecules 2011, 12, 3952–3961. [Google Scholar] [CrossRef] [PubMed]
- Mihaila, S.M.; Popa, E.G.; Reis, R.L.; Marques, A.P.; Gomes, M.E. Fabrication of endothelial cell-laden carrageenan microfibers for microvascularized bone tissue engineering applications. Biomacromolecules 2014, 15, 2849–2860. [Google Scholar] [CrossRef] [PubMed]
- Santo, V.E.; Frias, A.M.; Carida, M.; Cancedda, R.; Gomes, M.E.; Mano, J.F.; Reis, R.L. Carrageenan-based hydrogels for the controlled delivery of PDGF-BB in bone tissue engineering applications. Biomacromolecules 2009, 10, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Rocha, P.M.; Santo, V.E.; Gomes, M.E.; Reis, R.L.; Mano, J.F. Encapsulation of adipose-derived stem cells and transforming growth factor-β1 in carrageenan-based hydrogels for cartilage tissue engineering. J. Bioact. Compat. Polym. 2011, 26, 493–507. [Google Scholar] [CrossRef]
- Popa, E.; Reis, R.; Gomes, M. Chondrogenic phenotype of different cells encapsulated in κ-carrageenan hydrogels for cartilage regeneration strategies. Biotechnol. Appl. Biochem. 2012, 59, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Popa, E.G.; Rodrigues, M.T.; Coutinho, D.F.; Oliveira, M.B.; Mano, J.F.; Reis, R.L.; Gomes, M.E. Cryopreservation of cell laden natural origin hydrogels for cartilage regeneration strategies. Soft Matter 2013, 9, 875–885. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Liu, H.; Zhang, Z.; Jam, M.; Dudeja, P.K.; Michel, G.; Linhardt, R.J.; Tobacman, J.K. Carrageenan-induced innate immune response is modified by enzymes that hydrolyze distinct galactosidic bonds. J. Nutr. Biochem. 2010, 21, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Popa, E.G.; Reis, R.L.; Gomes, M.E. Seaweed polysaccharide-based hydrogels used for the regeneration of articular cartilage. Crit. Rev. Biotechnol. 2014, 35, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Salbach, J.; Rachner, T.D.; Rauner, M.; Hempel, U.; Anderegg, U.; Franz, S.; Simon, J.C.; Hofbauer, L.C. Regenerative potential of glycosaminoglycans for skin and bone. J. Mol. Med. 2012, 90, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Popa, E.G.; Carvalho, P.P.; Dias, A.F.; Santos, T.C.; Santo, V.E.; Marques, A.P.; Viegas, C.A.; Dias, I.R.; Gomes, M.E.; Reis, R.L. Evaluation of the in vitro and in vivo biocompatibility of carrageenan-based hydrogels. J. Biomed. Mater. Res. A 2014, 102, 4087–4097. [Google Scholar] [CrossRef] [PubMed]
- Daniel-da-Silva, A.L.; Trindade, T.; Goodfellow, B.J.; Costa, B.F.; Correia, R.N.; Gil, A.M. In situ synthesis of magnetite nanoparticles in carrageenan gels. Biomacromolecules 2007, 8, 2350–2357. [Google Scholar] [CrossRef] [PubMed]
- Mahdavinia, G.R.; Etemadi, H. In situ synthesis of magnetic CaraPVA IPN nanocomposite hydrogels and controlled drug release. Mater. Sci. Eng. C 2014, 45, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro, A.M.; Daniel-da-Silva, A.L.; Fateixa, S.; Trindade, T. κ-carrageenan hydrogel nanocomposites with release behavior mediated by morphological distinct au nanofillers. Carbohydr. Polym. 2012, 91, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Mahdavinia, G.R.; Etemadi, H.; Soleymani, F. Magnetic/pH-responsive beads based on caboxymethyl chitosan and κ-carrageenan and controlled drug release. Carbohydr. Polym. 2014, 128, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Mitsumata, T.; Honda, A.; Kanazawa, H.; Kawai, M. Magnetically tunable elasticity for magnetic hydrogels consisting of carrageenan and carbonyl iron particles. J. Phys. Chem. B 2012, 116, 12341–12348. [Google Scholar] [CrossRef] [PubMed]
- Santo, V.E.; Duarte, A.R.C.; Popa, E.G.; Gomes, M.E.; Mano, J.F.; Reis, R.L. Enhancement of osteogenic differentiation of human adipose derived stem cells by the controlled release of platelet lysates from hybrid scaffolds produced by supercritical fluid foaming. J. Control Release 2012, 162, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Santo, V.E.; Popa, E.G.; Mano, J.F.; Gomes, M.E.; Reis, R.L. Natural assembly of platelet lysate-loaded nanocarriers into enriched 3D hydrogels for cartilage regeneration. Acta Biomater. 2015, 19, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Popa, E.; Caridade, S.; Mano, J.F.; Reis, R.L.; Gomes, M.E. Chondrogenic potential of injectable κ-carrageenan hydrogel with encapsulated adipose stem cells for cartilage tissue-engineering applications. J. Tissue Eng. Regen. Med. 2013, 9, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Rada, T.; Reis, R.L.; Gomes, M.E. Distinct stem cells subpopulations isolated from human adipose tissue exhibit different chondrogenic and osteogenic differentiation potential. Stem Cell Rev. Rep. 2011, 7, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Rada, T.; Reis, R.L.; Gomes, M.E. Novel method for the isolation of adipose stem cells (ASCs). J. Tissue Eng. Regen. Med. 2009, 3, 158–159. [Google Scholar] [CrossRef] [PubMed]
- Daniel-da-Silva, A.L.; Loio, R.; Lopes-da-Silva, J.A.; Trindade, T.; Goodfellow, B.J.; Gil, A.M. Effects of magnetite nanoparticles on the thermorheological properties of carrageenan hydrogels. J. Colloid Interface Sci. 2008, 324, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Daniel-da-Silva, A.L.; Moreira, J.; Neto, R.; Estrada, A.C.; Gil, A.M.; Trindade, T. Impact of magnetic nanofillers in the swelling and release properties of κ-carrageenan hydrogel nanocomposites. Carbohydr. Polym. 2012, 87, 328–335. [Google Scholar] [CrossRef]
- Roeder, E.; Henrionnet, C.; Goebel, J.C.; Gambier, N.; Beuf, O.; Grenier, D.; Chen, B.; Vuissoz, P.A.; Gillet, P.; Pinzano, A. Dose-response of superparamagnetic iron oxide labeling on mesenchymal stem cells chondrogenic differentiation: A multi-scale in vitro study. PLoS ONE 2014. [Google Scholar] [CrossRef] [PubMed]
- Markides, H.; Rotherham, M.; El Haj, A.J. Biocompatibility and toxicity of magnetic nanoparticles in regenerative medicine. J. Nanomater. 2012. [Google Scholar] [CrossRef]
- Haudenschild, D.R.; McPherson, J.M.; Tubo, R.; Binette, F. Differential expression of multiple genes during articular chondrocyte redifferentiation. Anat. Rec. 2001, 263, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Son, B.; Kim, H.D.; Kim, M.; Kim, J.A.; Lee, J.; Shin, H.; Hwang, N.S.; Park, T.H. Physical stimuli-induced chondrogenic differentiation of mesenchymal stem cells using magnetic nanoparticles. Adv. Healthc. Mater. 2015, 4, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Fuhrer, R.; Hofmann, S.; Hild, N.; Vetsch, J.R.; Herrmann, I.K.; Grass, R.N.; Stark, W.J. Pressureless mechanical induction of stem cell differentiation is dose and frequency dependent. PLoS ONE 2013. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.D.; Brady, M.A.; St-Pierre, J.P.; Stevens, M.M.; Overby, D.R.; Ethier, C.R. Stimulation of chondrogenic differentiation of adult human bone marrow-derived stromal cells by a moderate-strength static magnetic field. Tissue Eng. A 2014, 20, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Lefebvre, V. Unraveling the transcriptional regulatory machinery in chondrogenesis. J. Bone Miner. Metab. 2011, 29, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Tsuchimochi, K.; Ijiri, K. The control of chondrogenesis. J. Cell. Biochem. 2006, 97, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Kasten, A.; Muller, P.; Bulnheim, U.; Groll, J.; Bruellhoff, K.; Beck, U.; Steinhoff, G.; Moller, M.; Rychly, J. Mechanical integrin stress and magnetic forces induce biological responses in mesenchymal stem cells which depend on environmental factors. J. Cell. Biochem. 2010, 111, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wu, C.A.; Rengarajan, V.; Finley, T.D.; Keles, H.O.; Sung, Y.; Li, B.; Gurkan, U.A.; Demirci, U. Three-dimensional magnetic assembly of microscale hydrogels. Adv. Mater. 2011, 23, 4254–4260. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, E.G.; Santo, V.E.; Rodrigues, M.T.; Gomes, M.E. Magnetically-Responsive Hydrogels for Modulation of Chondrogenic Commitment of Human Adipose-Derived Stem Cells. Polymers 2016, 8, 28. https://doi.org/10.3390/polym8020028
Popa EG, Santo VE, Rodrigues MT, Gomes ME. Magnetically-Responsive Hydrogels for Modulation of Chondrogenic Commitment of Human Adipose-Derived Stem Cells. Polymers. 2016; 8(2):28. https://doi.org/10.3390/polym8020028
Chicago/Turabian StylePopa, Elena G., Vítor E. Santo, Márcia T. Rodrigues, and Manuela E. Gomes. 2016. "Magnetically-Responsive Hydrogels for Modulation of Chondrogenic Commitment of Human Adipose-Derived Stem Cells" Polymers 8, no. 2: 28. https://doi.org/10.3390/polym8020028
APA StylePopa, E. G., Santo, V. E., Rodrigues, M. T., & Gomes, M. E. (2016). Magnetically-Responsive Hydrogels for Modulation of Chondrogenic Commitment of Human Adipose-Derived Stem Cells. Polymers, 8(2), 28. https://doi.org/10.3390/polym8020028







