Artificial Neural Network and Response Surface Methodology Modeling in Ionic Conductivity Predictions of Phthaloylchitosan-Based Gel Polymer Electrolyte
Abstract
:1. Introduction
2. Materials and Methods
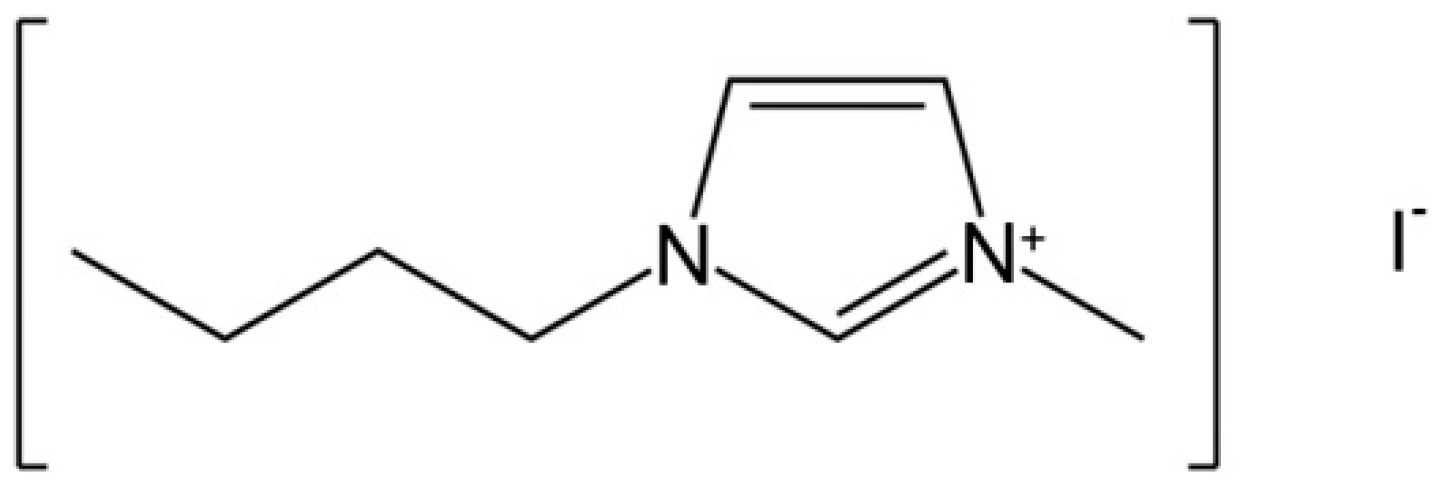
2.1. Sample Preparation
2.2. General Characterization
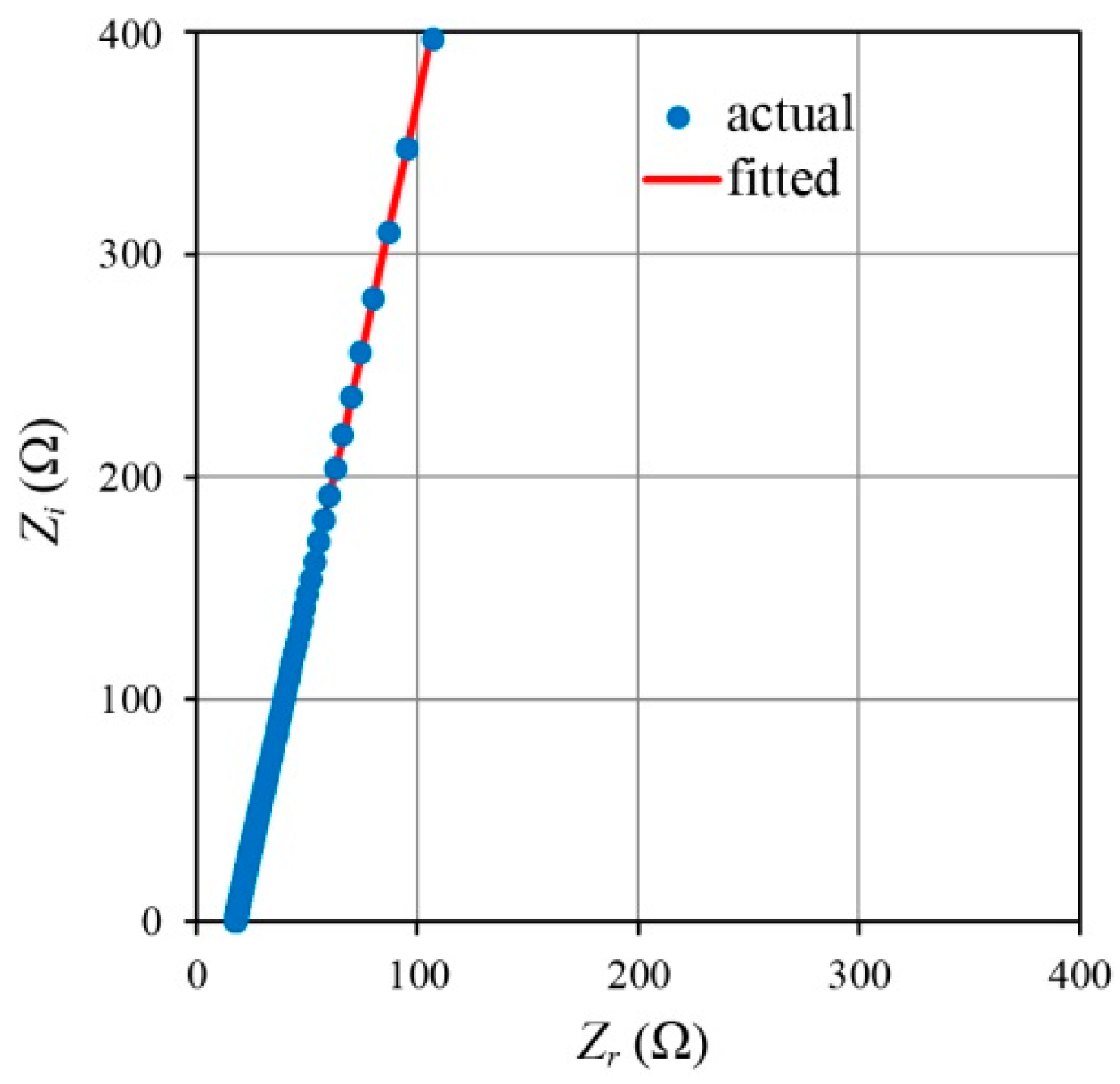
2.3. Electrochemical Impedance Spectroscopy (EIS)
3. Results and Discussion
3.1. FTIR Spectra of the PhCh–(LiI:CsI:BMII)

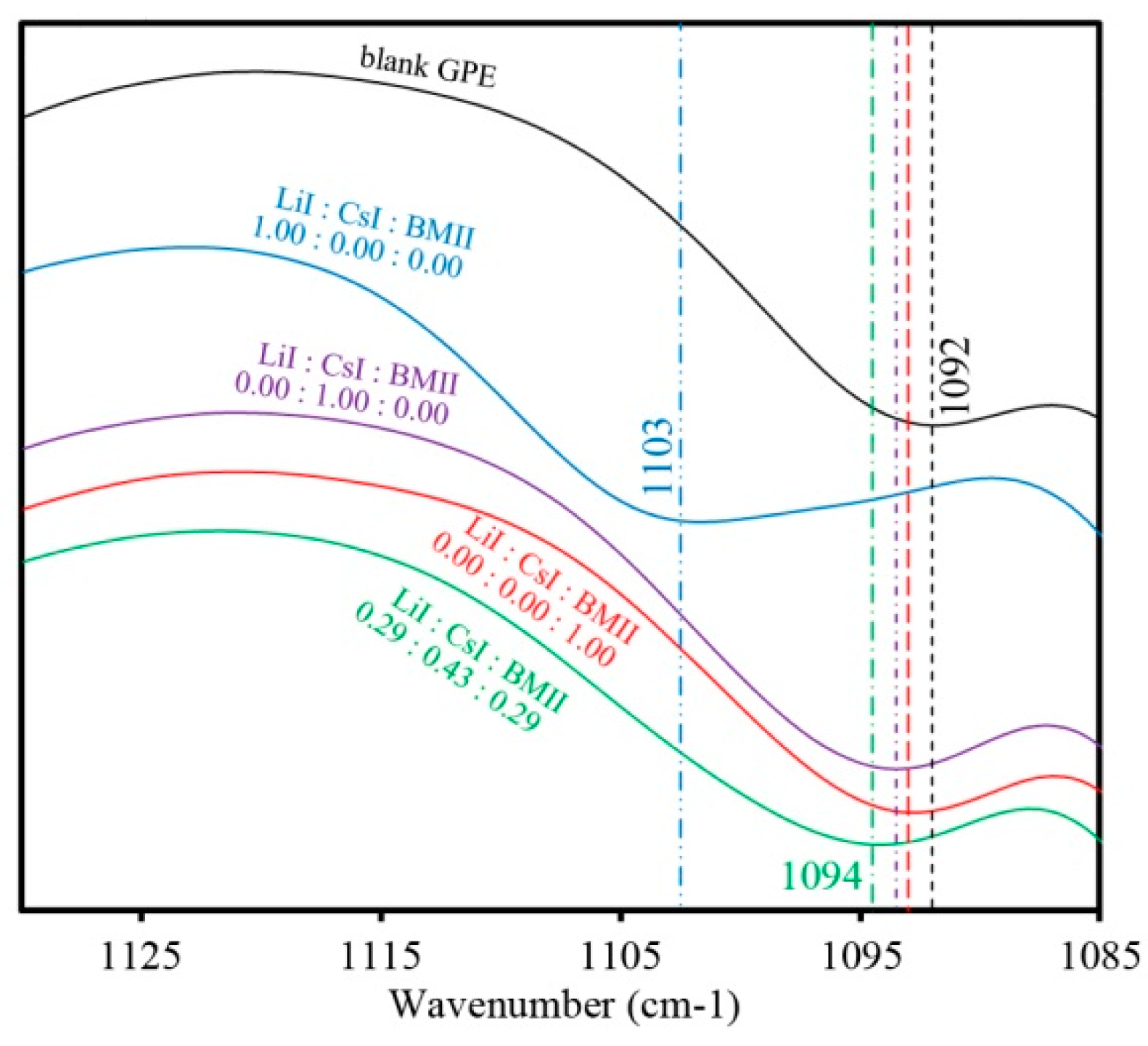
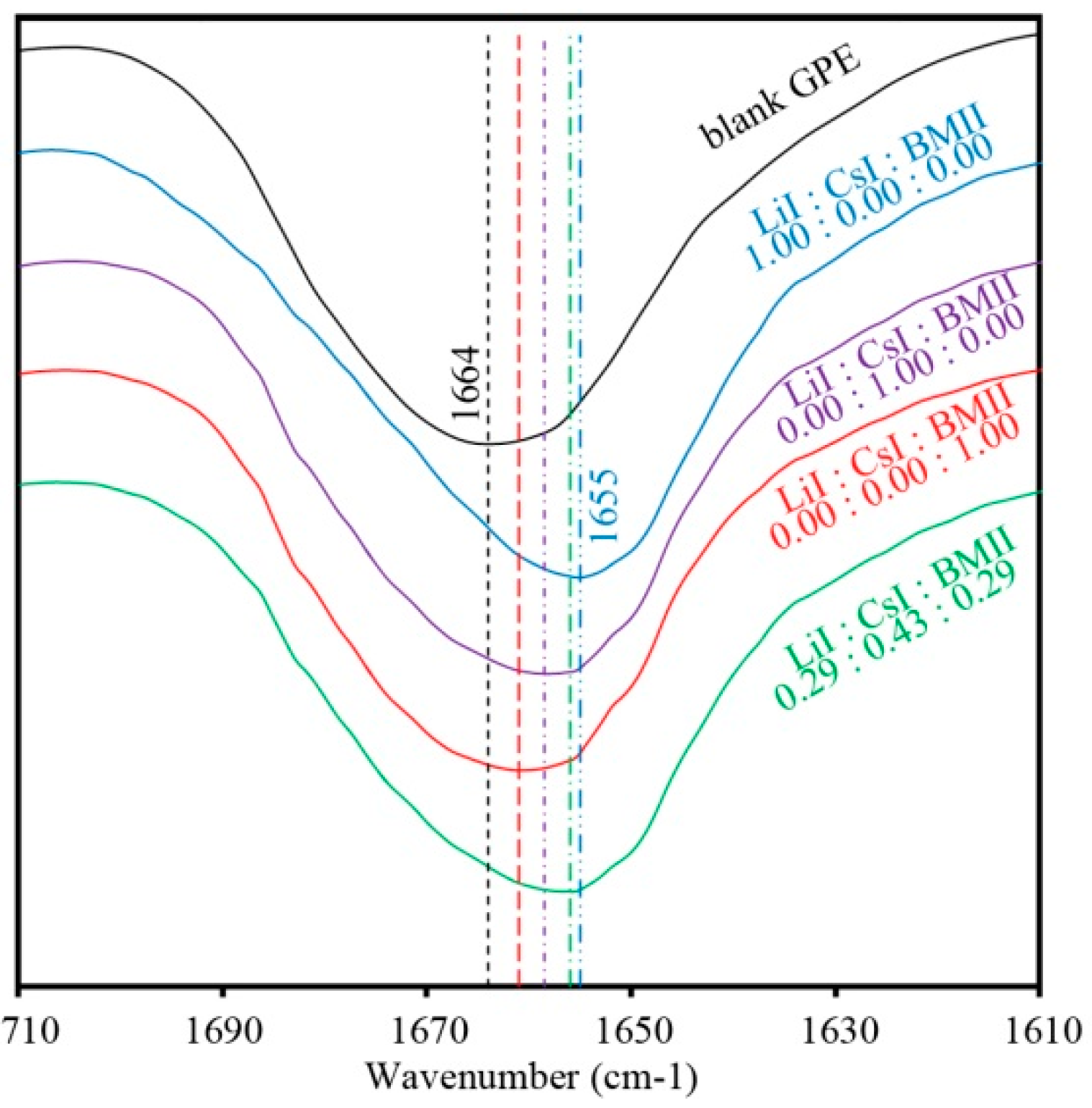
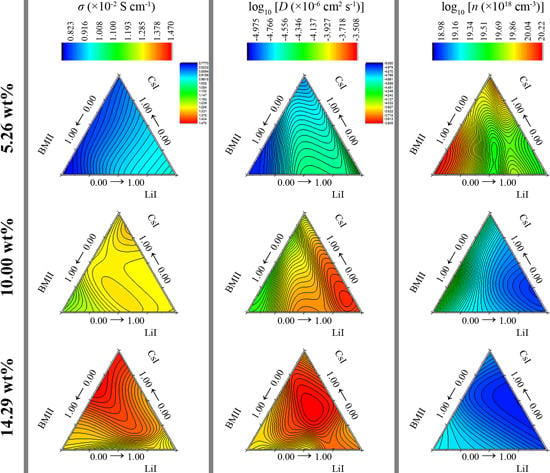
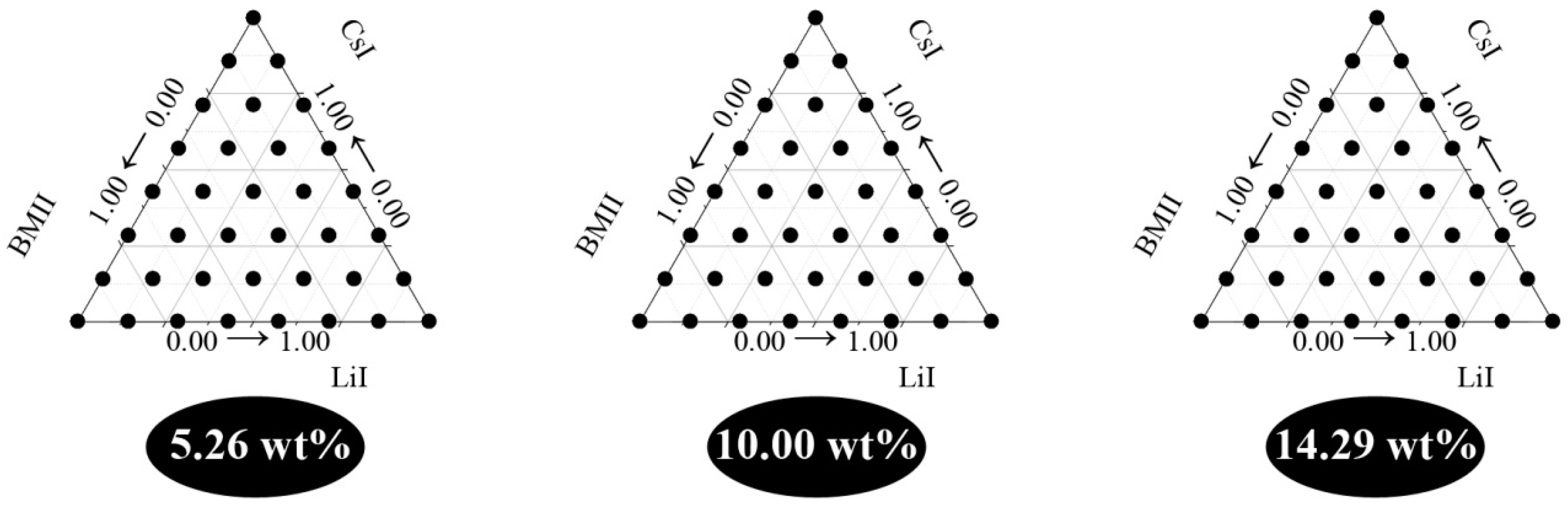
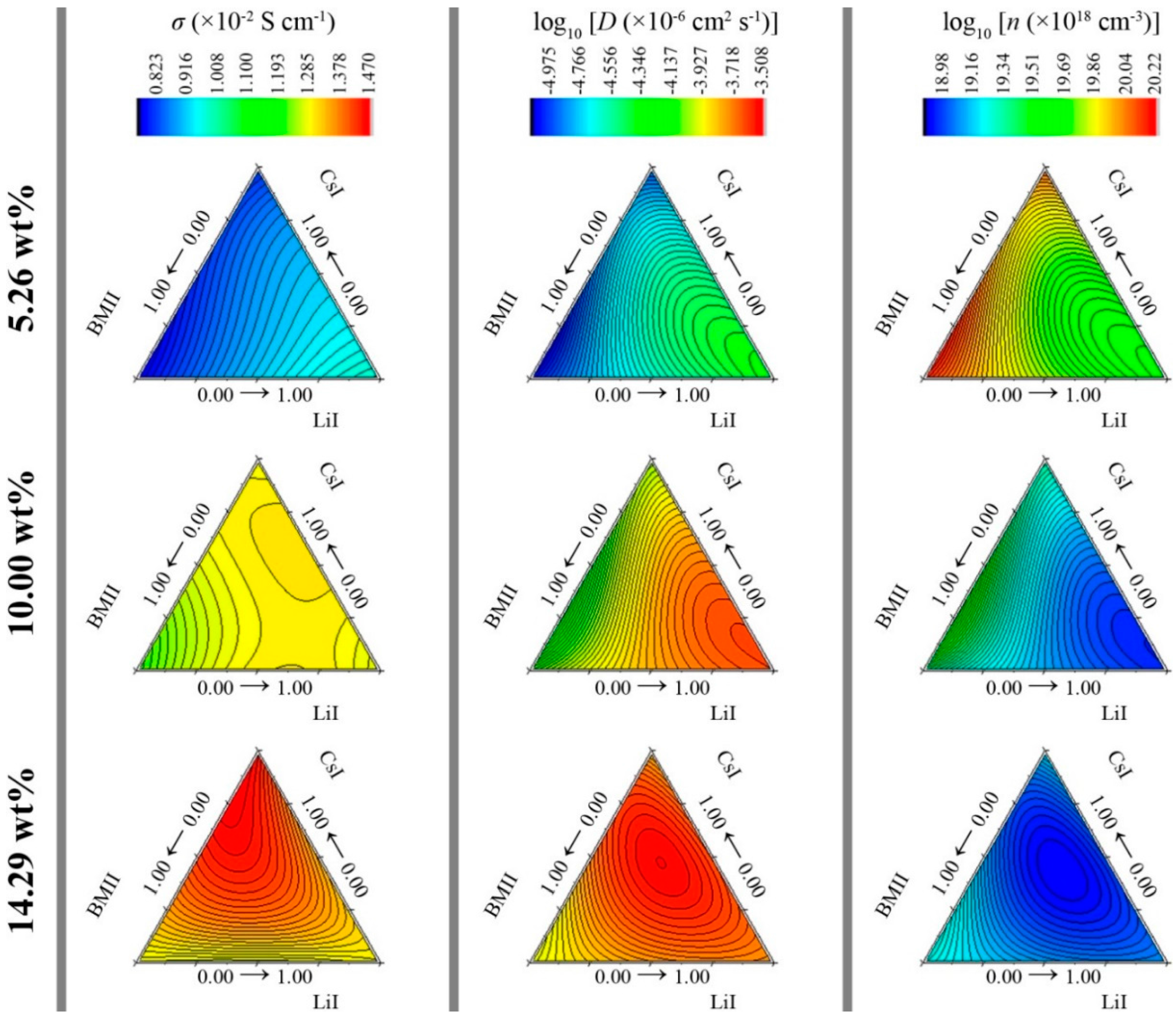
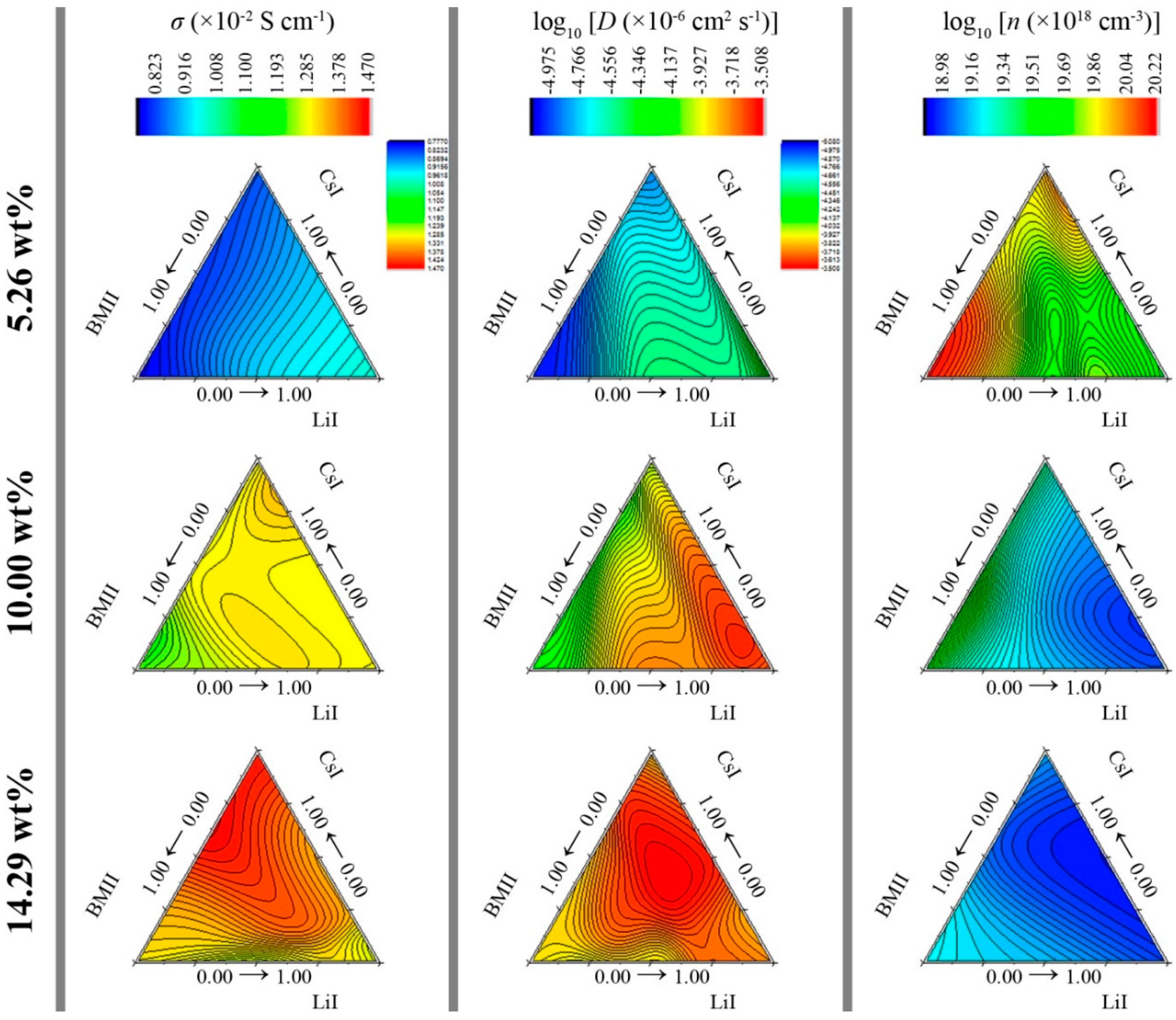
3.2. Surface Modeling
| σ (×10−2 S·cm−1) | D (×10−6 cm2·s−1) | n (×1018 cm−3) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LiI ratio | CsI ratio | BMII ratio | 5.26 wt % | 10.00 wt % | 14.29 wt % | 5.26 wt % | 10.00 wt % | 14.29 wt % | 5.26 wt % | 10.00 wt % | 14.29 wt % |
| 0.43 | 0.57 | 0.00 | 0.913 | 1.313 | 1.332 | 34.83 | 180.40 | 185.24 | 42.25 | 11.71 | 11.57 |
| 0.43 | 0.43 | 0.14 | 0.898 | 1.249 | 1.374 | 14.79 | 118.45 | 348.48 | 98.06 | 17.04 | 6.35 |
| 0.43 | 0.29 | 0.29 | 0.906 | 1.314 | 1.439 | 37.90 | 142.42 | 306.05 | 38.59 | 14.87 | 7.59 |
| 0.57 | 0.43 | 0.00 | 0.912 | 1.307 | 1.326 | 49.96 | 301.30 | 285.20 | 29.38 | 6.98 | 7.58 |
| 0.57 | 0.29 | 0.14 | 0.917 | 1.290 | 1.386 | 25.43 | 204.88 | 302.36 | 58.01 | 10.14 | 7.79 |
| 0.57 | 0.14 | 0.29 | 0.967 | 1.278 | 1.371 | 50.76 | 297.61 | 200.65 | 30.73 | 6.91 | 11.06 |
| 0.71 | 0.29 | 0.00 | 0.938 | 1.295 | 1.348 | 39.03 | 168.79 | 181.47 | 38.66 | 12.71 | 12.36 |
| 0.71 | 0.14 | 0.14 | 0.940 | 1.288 | 1.338 | 24.12 | 214.20 | 233.37 | 62.75 | 9.68 | 9.28 |
| 0.71 | 0.00 | 0.29 | 0.935 | 1.348 | 1.295 | 21.09 | 246.71 | 221.55 | 71.65 | 8.85 | 9.65 |
| 0.86 | 0.14 | 0.00 | 0.987 | 1.289 | 1.307 | 59.82 | 242.16 | 194.78 | 26.55 | 8.58 | 10.95 |
| 0.86 | 0.00 | 0.14 | 0.972 | 1.278 | 1.289 | 35.05 | 228.26 | 169.68 | 44.69 | 9.01 | 12.62 |
| 1.00 | 0.00 | 0.00 | 0.985 | 1.264 | 1.270 | 48.09 | 198.48 | 222.75 | 33.17 | 10.25 | 9.18 |
| 0.29 | 0.29 | 0.43 | 0.883 | 1.298 | 1.415 | 23.23 | 214.89 | 365.06 | 61.49 | 9.72 | 6.29 |
| 0.29 | 0.43 | 0.29 | 0.831 | 1.317 | 1.424 | 16.27 | 190.98 | 370.30 | 82.13 | 11.13 | 6.48 |
| 0.14 | 0.43 | 0.43 | 0.874 | 1.296 | 1.448 | 17.37 | 93.58 | 265.67 | 80.93 | 22.30 | 9.10 |
| 0.14 | 0.57 | 0.29 | 0.851 | 1.298 | 1.452 | 34.80 | 88.54 | 382.44 | 39.37 | 23.84 | 6.33 |
| 0.00 | 0.57 | 0.43 | 0.825 | 1.277 | 1.455 | 20.20 | 99.26 | 140.14 | 65.73 | 20.71 | 17.21 |
| 0.29 | 0.57 | 0.14 | 0.912 | 1.295 | 1.403 | 45.18 | 168.66 | 266.22 | 32.56 | 12.39 | 8.92 |
| 0.29 | 0.71 | 0.00 | 0.879 | 1.339 | 1.380 | 15.35 | 153.54 | 219.23 | 92.24 | 14.06 | 10.33 |
| 0.00 | 0.71 | 0.29 | 0.794 | 1.292 | 1.495 | 7.76 | 71.91 | 356.08 | 164.91 | 28.93 | 6.80 |
| 0.14 | 0.71 | 0.14 | 0.835 | 1.297 | 1.445 | 14.07 | 110.76 | 155.18 | 95.60 | 18.87 | 15.03 |
| 0.14 | 0.86 | 0.00 | 0.859 | 1.312 | 1.424 | 13.31 | 150.49 | 241.17 | 103.96 | 14.03 | 9.76 |
| 0.00 | 0.86 | 0.14 | 0.847 | 1.277 | 1.466 | 29.36 | 66.24 | 256.99 | 47.80 | 31.12 | 9.22 |
| 0.00 | 1.00 | 0.00 | 0.806 | 1.280 | 1.433 | 10.67 | 95.69 | 118.54 | 121.46 | 21.53 | 19.71 |
| 0.43 | 0.14 | 0.43 | 0.889 | 1.276 | 1.363 | 53.79 | 82.04 | 156.36 | 26.60 | 25.09 | 14.36 |
| 0.57 | 0.00 | 0.43 | 0.933 | 1.314 | 1.248 | 31.63 | 197.06 | 113.00 | 47.51 | 10.99 | 17.78 |
| 0.00 | 0.43 | 0.57 | 0.836 | 1.269 | 1.406 | 13.63 | 50.09 | 136.36 | 98.67 | 40.87 | 16.61 |
| 0.14 | 0.29 | 0.57 | 0.828 | 1.326 | 1.364 | 14.27 | 114.07 | 146.85 | 93.46 | 18.73 | 15.76 |
| 0.29 | 0.14 | 0.57 | 0.879 | 1.267 | 1.351 | 21.73 | 199.37 | 159.29 | 65.23 | 10.00 | 14.06 |
| 0.43 | 0.00 | 0.57 | 0.926 | 1.293 | 1.240 | 37.34 | 159.30 | 155.07 | 39.96 | 13.06 | 12.89 |
| 0.00 | 0.29 | 0.71 | 0.759 | 1.222 | 1.345 | 4.51 | 32.77 | 141.64 | 270.96 | 60.05 | 15.80 |
| 0.14 | 0.14 | 0.71 | 0.831 | 1.258 | 1.336 | 14.12 | 79.94 | 180.58 | 94.68 | 25.34 | 11.90 |
| 0.29 | 0.00 | 0.71 | 0.823 | 1.239 | 1.234 | 15.92 | 100.72 | 125.95 | 83.32 | 19.82 | 15.78 |
| 0.00 | 0.14 | 0.86 | 0.779 | 1.188 | 1.318 | 11.88 | 48.46 | 128.09 | 105.62 | 39.85 | 16.57 |
| 0.14 | 0.00 | 0.86 | 0.815 | 1.229 | 1.314 | 8.89 | 96.66 | 131.55 | 148.58 | 20.53 | 16.49 |
| 0.00 | 0.00 | 1.00 | 0.785 | 1.213 | 1.281 | 7.64 | 66.66 | 148.05 | 165.32 | 29.31 | 14.12 |
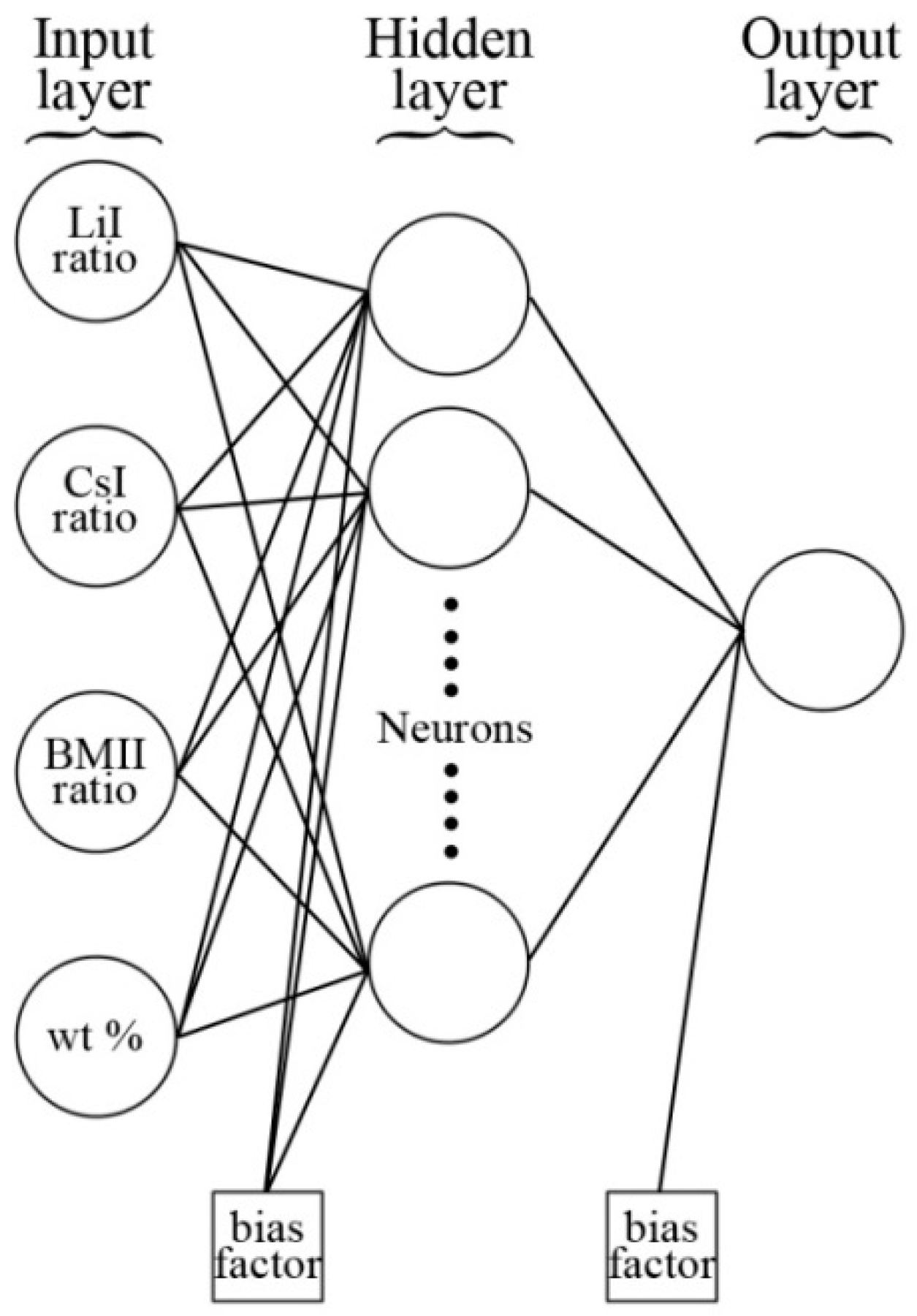
- is the normalized input variable;
- is the new minimum value of the normalized input variable;is the new maximum value of the normalized input variable;
- is the original input variable;
- is the minimum value of the original input variable;
- is the maximum value of the original input variable.
| Response | RSM R2 | RSM Adjusted R2 | S/N Ratio | 4-h-1 ANN R2 |
|---|---|---|---|---|
| Conductivity, σ(×10−2 S·cm−1) | 0.9919 | 0.9897 | 65.14 | 0.9936 |
| log10 Diffusion coefficient, log10 [D (×10−6 cm2·s−1)] | 0.9218 | 0.9091 | 28.07 | 0.9317 |
| log10 number density of charge carriers, log10 [n (×1018 cm−3)] | 0.8920 | 0.8743 | 23.91 | 0.8989 |
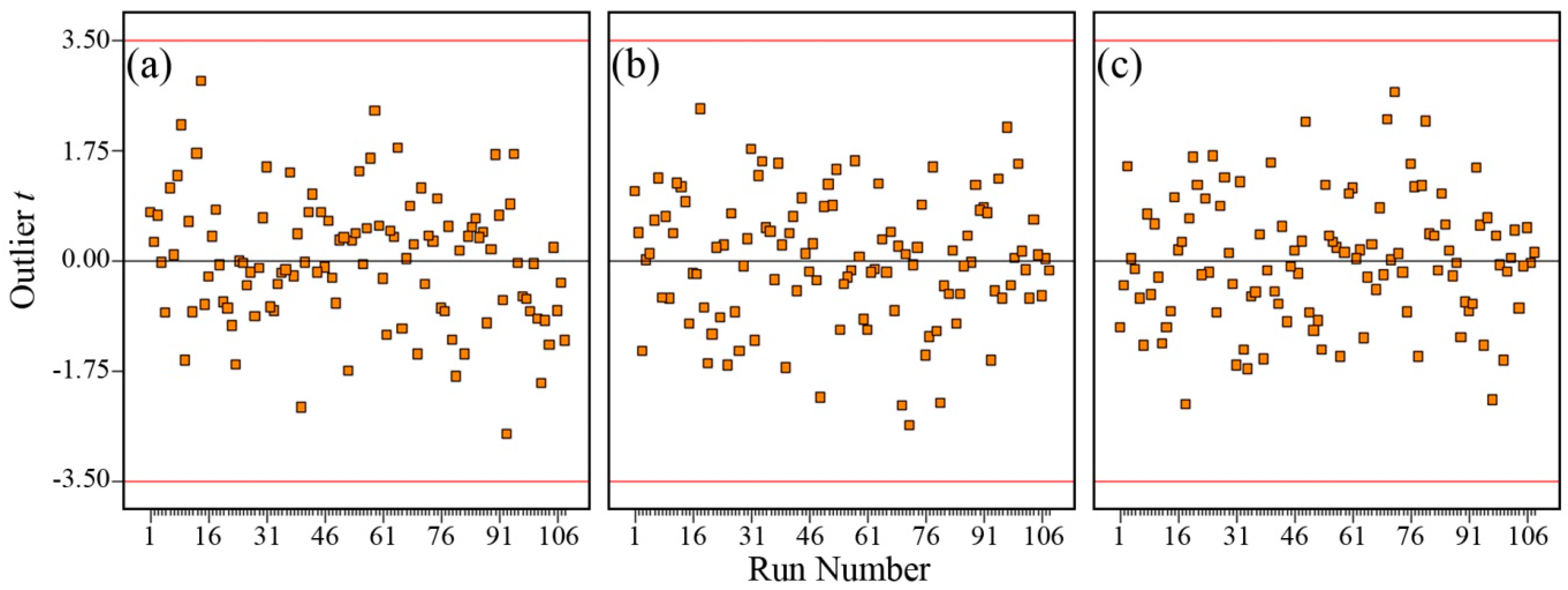

3.3. Determination of the Importance of Each Input Variable
| Variable | σ (×10−2 S·cm−1) | log10 [D (×10−6 cm2·s−1)] | log10 [n (×1018 cm−3)] | |||
|---|---|---|---|---|---|---|
| ANN PaD% | RSM PaD% | ANN PaD% | RSM PaD% | ANN PaD% | RSM PaD% | |
| x1, LiI | 28.40 | 33.73 | 18.04 | 25.35 | 31.01 | 31.83 |
| x2, CsI | 16.52 | 33.50 | 3.22 | 35.88 | 5.57 | 33.91 |
| x3, BMII | 19.68 | 30.91 | 4.81 | 37.94 | 7.04 | 34.24 |
| x4, wt % | 35.39 | 1.85 | 73.94 | 0.84 | 56.38 | 0.02 |

| Variable | σ(×10−2 S·cm−1) | log10 [D (×10−6 cm2·s−1)] | log10 [n (×1018 cm−3)] | |||
|---|---|---|---|---|---|---|
| ANN PaD2% | RSM PaD2% | ANN PaD2% | RSM PaD2% | ANN PaD2% | RSM PaD2% | |
| x1x2, LiI-CsI | 19.30 | 12.08 | 11.01 | 32.34 | 7.75 | 34.18 |
| x1x3, LiI-BMII | 35.78 | 13.71 | 19.78 | 27.04 | 7.49 | 27.28 |
| x2x3, CsI-BMII | 12.92 | 22.44 | 10.63 | 25.33 | 2.88 | 25.73 |
| x1x4, LiI-wt % | 14.60 | 10.15 | 32.25 | 3.71 | 60.62 | 3.23 |
| x2x4, CsI-wt % | 8.82 | 23.44 | 11.20 | 6.08 | 11.28 | 4.97 |
| x3x4, BMII-wt % | 8.58 | 18.18 | 15.14 | 5.50 | 9.98 | 4.61 |
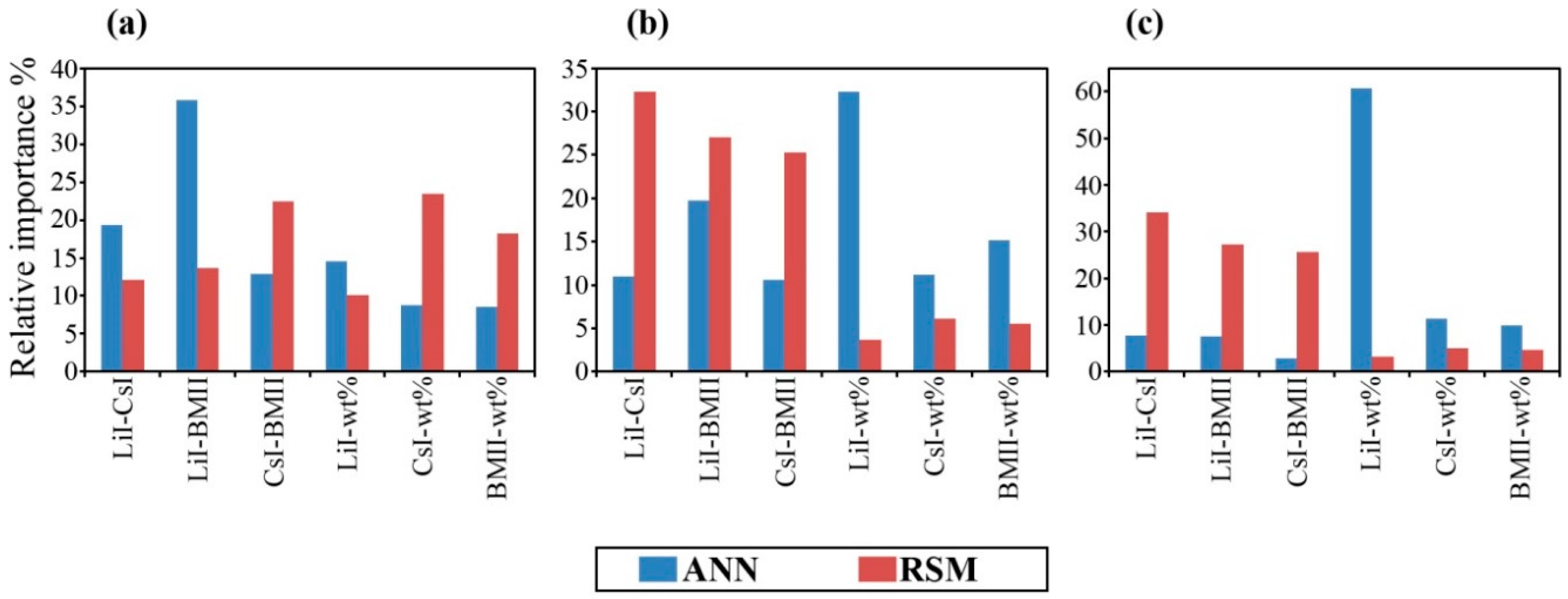
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Kim, E.; Xiong, Y.; Cheng, Y.; Wu, H.-C.; Liu, Y.; Morrow, B.; Ben-Yoav, H.; Ghodssi, R.; Rubloff, G.; Shen, J.; et al. Chitosan to connect biology to electronics: Fabricating the bio-device interface and communicating across this interface. Polymers 2014, 7, 1. [Google Scholar] [CrossRef]
- Varshney, P.; Gupta, S. Natural polymer-based electrolytes for electrochemical devices: A review. Ionics 2011, 17, 479–483. [Google Scholar] [CrossRef]
- Ye, Y.-S.; Rick, J.; Hwang, B.-J. Water soluble polymers as proton exchange membranes for fuel cells. Polymers 2012, 4, 913. [Google Scholar] [CrossRef]
- Hu, C.; Guo, J.; Wen, J. Hierarchical nanostructure CuO with peach kernel-like morphology as anode material for lithium-ion batteries. Ionics 2013, 19, 253–258. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Antony, R.; Thanikaikarasan, S.; Balakumar, S.; Shajan, X.S. Combined effect of nanochitosan and succinonitrile on structural, mechanical, thermal, and electrochemical properties of plasticized nanocomposite polymer electrolytes (PNCPE) for lithium batteries. Ionics 2013, 19, 747–755. [Google Scholar] [CrossRef]
- Pandiselvi, K.; Thambidurai, S. Chitosan-ZnO/polyaniline ternary nanocomposite for high-performance supercapacitor. Ionics 2014, 20, 551–561. [Google Scholar] [CrossRef]
- Mohamad, S.A.; Yahya, R.; Ibrahim, Z.A.; Arof, A.K. Photovoltaic activity in a ZNTE/PEO–chitosan blend electrolyte junction. Sol. Energy Mater. Sol. C 2007, 91, 1194–1198. [Google Scholar] [CrossRef]
- Buraidah, M.H.; Teo, L.P.; Yusuf, S.N.F.; Noor, M.M.; Kufian, M.Z.; Careem, M.A.; Majid, S.R.; Taha, R.M.; Arof, A.K. TiO2/chitosan-NH4I(+I2)-BMII-based dye-sensitized solar cells with anthocyanin dyes extracted from black rice and red cabbage. Int. J. Photoenergy 2011, 2011, 11. [Google Scholar] [CrossRef]
- Jin, E.M.; Park, K.-H.; Park, J.-Y.; Lee, J.-W.; Yim, S.-H.; Zhao, X.G.; Gu, H.-B.; Cho, S.-Y.; Fisher, J.G.; Kim, T.-Y. Preparation and characterization of chitosan binder-based electrode for dye-sensitized solar cells. Int. J. Photoenergy 2013, 2013, 7. [Google Scholar] [CrossRef]
- Xiang, Y.; Yang, M.; Guo, Z.; Cui, Z. Alternatively chitosan sulfate blending membrane as methanol-blocking polymer electrolyte membrane for direct methanol fuel cell. J. Membr. Sci. 2009, 337, 318–323. [Google Scholar] [CrossRef]
- Binsu, V.V.; Nagarale, R.K.; Shahi, V.K.; Ghosh, P.K. Studies on n-methylene phosphonic chitosan/poly(vinyl alcohol) composite proton-exchange membrane. React. Funct. Polym. 2006, 66, 1619–1629. [Google Scholar] [CrossRef]
- Seo, J.A.; Koh, J.H.; Roh, D.K.; Kim, J.H. Preparation and characterization of crosslinked proton conducting membranes based on chitosan and PSSA-MA copolymer. Solid State Ionics 2009, 180, 998–1002. [Google Scholar] [CrossRef]
- Winie, T.; Arof, A.K. Transport properties of hexanoyl chitosan-based gel electrolyte. Ionics 2006, 12, 149–152. [Google Scholar] [CrossRef]
- Aziz, N.A.; Majid, S.R.; Arof, A.K. Synthesis and characterizations of phthaloyl chitosan-based polymer electrolytes. J. Non-Cryst. Solids 2012, 358, 1581–1590. [Google Scholar] [CrossRef]
- Yusuf, S.N.F.; Aziz, M.F.; Hassan, H.C.; Bandara, T.M.W.J.; Mellander, B.-E.; Careem, M.A.; Arof, A.K. Phthaloylchitosan-based gel polymer electrolytes for efficient dye-sensitized solar cells. J. Chem. 2014, 2014, 8. [Google Scholar] [CrossRef]
- Dissanayake, M.A.K.L.; Thotawatthage, C.A.; Senadeera, G.K.R.; Bandara, T.M.W.J.; Jayasundera, W.J.M.J.S.R.; Mellander, B.E. Efficiency enhancement by mixed cation effect in dye-sensitized solar cells with pan based gel polymer electrolyte. J. Photoch. Photobiol. A 2012, 246, 29–35. [Google Scholar] [CrossRef]
- Morris, D. The lattice energies of the alkali halides. Acta Crystallogr. 1956, 9, 197–198. [Google Scholar] [CrossRef]
- Nakakoshi, M.; Shiro, M.; Fujimoto, T.; Machinami, T.; Seki, H.; Tashiro, M.; Nishikawa, K. Crystal structure of 1-butyl-3-methylimidazolium iodide. Chem. Lett. 2006, 35, 1400–1401. [Google Scholar] [CrossRef]
- Chávez-Ramírez, A.U.; Muñoz-Guerrero, R.; Durón-Torres, S.M.; Ferraro, M.; Brunaccini, G.; Sergi, F.; Antonucci, V.; Arriaga, L.G. High power fuel cell simulator based on artificial neural network. Int. J. Hydrogen Energy 2010, 35, 12125–12133. [Google Scholar] [CrossRef]
- Hirzin, R.S.F.N.; Azzahari, A.D.; Yahya, R.; Hassan, A. Optimizing the usability of unwanted latex yield by in situ depolymerization and functionalization. Ind. Crop. Prod. 2015, 74, 773–783. [Google Scholar] [CrossRef]
- Lv, X.-Y.; Cui, X.-R.; Long, Y.-F.; Su, J.; Wen, Y.-X. Optimization of titanium and vanadium co-doping in LiFePO4/C using response surface methodology. Ionics 2015, 21, 2447–2455. [Google Scholar] [CrossRef]
- Parthiban, T.; Ravi, R.; Kalaiselvi, N. Exploration of artificial neural network [ANN] to predict the electrochemical characteristics of lithium-ion cells. Electrochim. Acta 2007, 53, 1877–1882. [Google Scholar] [CrossRef]
- Lobato, J.; Cañizares, P.; Rodrigo, M.A.; Linares, J.J.; Piuleac, C.-G.; Curteanu, S. The neural networks based modeling of a polybenzimidazole-based polymer electrolyte membrane fuel cell: Effect of temperature. J. Power Sources 2009, 192, 190–194. [Google Scholar] [CrossRef]
- Ou, S.; Achenie, L.E.K. A hybrid neural network model for pem fuel cells. J. Power Sources 2005, 140, 319–330. [Google Scholar] [CrossRef]
- Lee, W.-Y.; Park, G.-G.; Yang, T.-H.; Yoon, Y.-G.; Kim, C.-S. Empirical modeling of polymer electrolyte membrane fuel cell performance using artificial neural networks. Int. J. Hydrogen Energy 2004, 29, 961–966. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, H.; Wang, L.; Liang, G.; Wang, L.; Shen, X. Artificial neural network-based QSPR study on absorption maxima of organic dyes for dye-sensitised solar cells. Mol. Simulat. 2011, 37, 1–10. [Google Scholar] [CrossRef]
- Lin, H.-C.; Su, C.-T.; Wang, C.-C.; Chang, B.-H.; Juang, R.-C. Parameter optimization of continuous sputtering process based on taguchi methods, neural networks, desirability function, and genetic algorithms. Expert Syst. Appl. 2012, 39, 12918–12925. [Google Scholar] [CrossRef]
- Torrecilla, J.S.; Rojo, E.; García, J.; Oliet, M.; Rodríguez, F. Determination of toluene, n-heptane, [emim][EtSO4], and [bmim][MeSO4] ionic liquids concentrations in quaternary mixtures by UV−Vis spectroscopy. Ind. Eng. Chem. Res. 2009, 48, 4998–5003. [Google Scholar] [CrossRef]
- Fatehi, M.-R.; Raeissi, S.; Mowla, D. Estimation of viscosity of binary mixtures of ionic liquids and solvents using an artificial neural network based on the structure groups of the ionic liquid. Fluid Phase Equilibr. 2014, 364, 88–94. [Google Scholar] [CrossRef]
- Nishimura, S.; Kohgo, O.; Kurita, K.; Kuzuhara, H. Chemospecific manipulations of a rigid polysaccharide: Syntheses of novel chitosan derivatives with excellent solubility in common organic solvents by regioselective chemical modifications. Macromolecules 1991, 24, 4745–4748. [Google Scholar] [CrossRef]
- Bandara, T.M.W.J.; Mellander, B.-E. Evaluation of mobility, diffusion coefficient and density of charge carriers in ionic liquids and novel electrolytes based on a new model for dielectric response. In Ionic Liquids: Theory, Properties, New Approaches; Kokorin, A., Ed.; InTech: Rijeka, Croatia, 2011; pp. 383–406. [Google Scholar]
- Linford, R.G. Solid State Ionics Devices; World Scientific: Singapore, 1988. [Google Scholar]
- Arof, A.K.; Amirudin, S.; Yusof, S.Z.; Noor, I.M. A method based on impedance spectroscopy to determine transport properties of polymer electrolytes. Phys. Chem. Chem. Phys. 2014, 16, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ma, X.; Wang, X.; Liang, X. Determination of the interaction using FTIR within the composite gel polymer electrolyte. J. Mol. Struct. 2013, 1031, 30–37. [Google Scholar] [CrossRef]
- Box, G.E.P.; Cox, D.R. An analysis of transformations. J. R. Stat. Soc. B Met. 1964, 26, 211–252. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments, 5th ed.; John Wiley & Sons: New York, NY, USA, 1997. [Google Scholar]
- Burnham, K.; Anderson, D.; Huyvaert, K. Aic model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behav. Ecol. Sociobiol. 2011, 65, 23–35. [Google Scholar] [CrossRef]
- Spiess, A.-N.; Neumeyer, N. An evaluation of R2 as an inadequate measure for nonlinear models in pharmacological and biochemical research: A monte carlo approach. BMC Pharmacol. 2010, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. Information theory and an extension of the maximum likelihood principle. In Selected Papers of Hirotugu Akaike; Parzen, E., Tanabe, K., Kitagawa, G., Eds.; Springer: New York, NY, USA, 1998; pp. 199–213. [Google Scholar]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Akaike, H. On the likelihood of a time series model. J. R. Stat. Soc. D Stat. 1978, 27, 217–235. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002. [Google Scholar]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 3rd ed.; John Wiley & Sons: New York, NY, USA, 2009. [Google Scholar]
- Olden, J.D.; Jackson, D.A. Illuminating the “black box”: A randomization approach for understanding variable contributions in artificial neural networks. Ecol. Model. 2002, 154, 135–150. [Google Scholar] [CrossRef]
- Paruelo, J.; Tomasel, F. Prediction of functional characteristics of ecosystems: A comparison of artificial neural networks and regression models. Ecol. Model. 1997, 98, 173–186. [Google Scholar] [CrossRef]
- Lek, S.; Guégan, J.F. Artificial neural networks as a tool in ecological modelling, an introduction. Ecol. Model. 1999, 120, 65–73. [Google Scholar] [CrossRef]
- Özesmi, S.L.; Özesmi, U. An artificial neural network approach to spatial habitat modelling with interspecific interaction. Ecol. Model. 1999, 116, 15–31. [Google Scholar] [CrossRef]
- Dimopoulos, I.; Chronopoulos, J.; Chronopoulou-Sereli, A.; Lek, S. Neural network models to study relationships between lead concentration in grasses and permanent urban descriptors in athens city (Greece). Ecol. Model. 1999, 120, 157–165. [Google Scholar] [CrossRef]
- Gevrey, M.; Dimopoulos, I.; Lek, S. Two-way interaction of input variables in the sensitivity analysis of neural network models. Ecol. Model. 2006, 195, 43–50. [Google Scholar] [CrossRef]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azzahari, A.D.; Yusuf, S.N.F.; Selvanathan, V.; Yahya, R. Artificial Neural Network and Response Surface Methodology Modeling in Ionic Conductivity Predictions of Phthaloylchitosan-Based Gel Polymer Electrolyte. Polymers 2016, 8, 22. https://doi.org/10.3390/polym8020022
Azzahari AD, Yusuf SNF, Selvanathan V, Yahya R. Artificial Neural Network and Response Surface Methodology Modeling in Ionic Conductivity Predictions of Phthaloylchitosan-Based Gel Polymer Electrolyte. Polymers. 2016; 8(2):22. https://doi.org/10.3390/polym8020022
Chicago/Turabian StyleAzzahari, Ahmad Danial, Siti Nor Farhana Yusuf, Vidhya Selvanathan, and Rosiyah Yahya. 2016. "Artificial Neural Network and Response Surface Methodology Modeling in Ionic Conductivity Predictions of Phthaloylchitosan-Based Gel Polymer Electrolyte" Polymers 8, no. 2: 22. https://doi.org/10.3390/polym8020022
APA StyleAzzahari, A. D., Yusuf, S. N. F., Selvanathan, V., & Yahya, R. (2016). Artificial Neural Network and Response Surface Methodology Modeling in Ionic Conductivity Predictions of Phthaloylchitosan-Based Gel Polymer Electrolyte. Polymers, 8(2), 22. https://doi.org/10.3390/polym8020022