Abstract
In recent years, there have been major advances and increasing amounts of research on the utilization of natural polymeric materials as drug delivery vehicles due to their biocompatibility and biodegradability. Seaweed polysaccharides are abundant resources and have been extensively studied for several biological, biomedical, and functional food applications. The exploration of seaweed polysaccharides for drug delivery applications is still in its infancy. Alginate, carrageenan, fucoidan, ulvan, and laminarin are polysaccharides commonly isolated from seaweed. These natural polymers can be converted into nanoparticles (NPs) by different types of methods, such as ionic gelation, emulsion, and polyelectrolyte complexing. Ionic gelation and polyelectrolyte complexing are commonly employed by adding cationic molecules to these anionic polymers to produce NPs of a desired shape, size, and charge. In the present review, we have discussed the preparation of seaweed polysaccharide-based NPs using different types of methods as well as their usage as carriers for the delivery of various therapeutic molecules (e.g., proteins, peptides, anti-cancer drugs, and antibiotics). Seaweed polysaccharide-based NPs exhibit suitable particle size, high drug encapsulation, and sustained drug release with high biocompatibility, thereby demonstrating their high potential for safe and efficient drug delivery.
1. Introduction
Seaweed is an important marine resource for human kind, and in particular, for the multi-billion dollar companies that have been operating based on seaweed-derived polysaccharides for approximately the last six decades [1,2,3,4]. The cell walls of seaweed are mainly composed of polysaccharides. These polysaccharides are generally small sugar units linked with glycosidic bonds. In recent years, significant research has been conducted on seaweed for the production of bioenergy and the development of food applications due to the abundance of this resource [5,6,7,8,9,10,11,12,13]. Applications of diverse seaweed polysaccharides (e.g., alginate, carrageenan, ulvan, and laminarin) in drug delivery, tissue engineering, and biosensor areas have been reported [14]. Recently, particular attention has been directed toward developing drug delivery systems using seaweed polysaccharides, which is an important field of biomedical research. Among the various synthetic and natural polymers that have been extensively studied for biomedical applications, particularly for drug delivery [15,16,17,18,19,20], natural seaweed polysaccharides that have been formulated into nanoparticles (NPs) for drug delivery systems (DDS) will be discussed in this review. Natural polysaccharides for DDS have main advantages in their biocompatibility and charge properties [21]. They are also inexpensive materials due to their abundance [22,23,24].
2. Polysaccharide-Based Nanoparticles for Drug Delivery
(C6H5O10)n is the general formula for typical polysaccharides. The number of units (n) can vary from 40 to 3000 [25]. Natural polysaccharides are commonly obtained from several resources, including algae, animals, plants, and microbes. Cellulose, chitin, chitosan, alginate, heparin, hyaluronic acid, chondroitin sulfate, pectin, pullulan, amylose, dextran, ulvan, carrageenan, and their derivatives have been widely studied for several biological and biomedical applications, including those in the fields of tissue engineering, wound management, drug delivery, and biosensors [26,27,28]. Furthermore, polysaccharides can be divided into two groups according to their charge. For example, chitosan is a positively charged (cationic) polysaccharide, whereas alginate, carrageenan, and fucoidan are negatively charged (anionic) polysaccharides [21]. Generally, polysaccharides are considered safe, biocompatible, stable, hydrophilic, and biodegradable, and they can be modified into different forms, such as chemically modified polysaccharides, hydrogels, scaffolds, fibers, and NPs. NPs have many advantages for drug delivery purposes compared with larger (micro-sized) particles because they easily penetrate into targeted areas [29,30,31,32,33,34,35,36,37,38,39].
Polysaccharide-based NPs can be obtained using different types of methods. In particular, the most widely studied methods are ionic linking, covalent cross-linking, self-assembly, and polyelectrolyte systems. Research on polysaccharide-based NPs (e.g., alginate, carrageenan, and fucoidan) for DDS has been increasing dramatically over the last decade (Figure 1) [21,40]. Polysaccharide-based NPs have advantages due to abundant availability and biocompatible properties, which make them important candidates for drug delivery system [41,42,43,44]. Posocco et al. (2015) [45] suggested that polysaccharide-based materials exhibit the following advantages:
- Their sources are abundant and they can be available in a well-characterized state.
- They can be modified to form different materials using chemical and enzymatic methods.
- They are biodegradable and biocompatible and exhibit low immunogenicity.
- They can be useful in stimuli-responsive DDS.
- They can be produced complexed and conjugated with proteins and bioactives.
- They can be modified as gels.
- They can give rise to interpenetrated polymeric networks.
- Ionic polysaccharides are mucoadhesive.
Based on these properties, polysaccharides can be useful as drug delivery carriers.
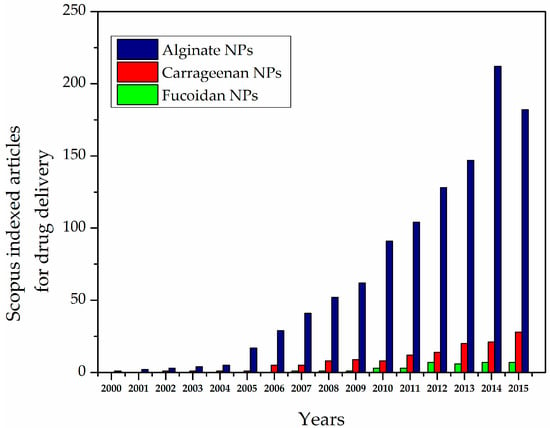
Figure 1.
Scopus-indexed articles for alginate-, carrageenan-, and fucoidan-based nanoparticles (NPs) for drug delivery.
3. Seaweed Polysaccharide-Based Nanoparticles for Drug Delivery
Seaweed can be classified as red, green, or blue. The cell walls of seaweed are often composed of polysaccharides. For approximately four decades, research has been conducted on the structures and applications of seaweed polysaccharides, especially on their functional food applications [46]. Polysaccharides including agar, alginate, fucoidan, carrageenan, and laminarin have been isolated from seaweed [6,25,47].
Seaweed polysaccharides have hydrophilic surface groups, such as hydroxyl, carboxyl, and sulfate groups, which interact with biological tissues easily [48]. Owing to these properties of seaweed polysaccharides, the usage of seaweed polysaccharides in DDS is increasing.
The main difference between the sulfated polysaccharides and other polysaccharides is surface charge. Most of the algae-derived polysaccharides are anionic in nature. Some seaweed-derived polysaccharides have anionic sulfate groups, which are not present in polysaccharides of terrestrial and animal origin [49]. These seaweed polysaccharide-based NPs avoid aggregation during blood circulation by reduced interaction with serum proteins.
4. Alginate
Alginate is a water soluble, anionic polymer, commonly produced from marine brown algae. It is mainly composed of α-l-guluronic acid (G) and β-d-mannuronic acid (M) residues linked by 1,4-glycosidic linkages (Figure 2A). It is nontoxic, biocompatible, biodegradable, and inexpensive, and thus it is extensively used for several biological, biomedical, and functional food applications [8,50,51]. Alginate NPs can be prepared by different types of methods, including ionic cross-linking, covalent cross-linking, self-assembly, complexation methods, and emulsion methods [39,52,53,54,55,56,57,58,59].
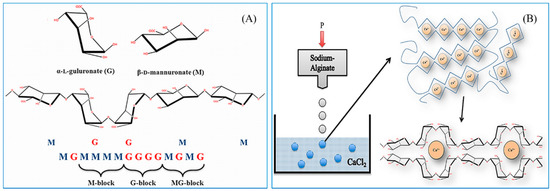
Figure 2.
(A) The structure of alginate; (B) The formulation of egg box-shaped NPs by an ionic gelation method. The figures were adopted with permission from [60].
4.1. Production of Alginate NPs
Considerable attention has been directed toward preparative methods to produce the desired properties of alginate NPs for effective drug delivery systems [61,62,63]. Different types of methods are explained here.
4.1.1. Ionic Cross-Linked Alginate NPs (Ionotropic Gelation)
The preparation of alginate NPs by ionic gelation is generally simple and mild. They can be produced by cross-linking alginate with various ions, such as Ca2+, Ba2+, and Al3+ [64]. Alginate NPs are commonly formed by the addition of calcium ions at a particular concentration; this is one of the highly explored methods [65]. Ionic cross-linked alginate NPs usually form egg box shapes, as illustrated in Figure 2B. However, sometimes this method tends to produce micro-sized particles rather than NPs. Therefore, process optimization is important to produce alginate NPs of a desired shape. The optimization can be performed by tailoring calcium ion concentration, alginate concentration, addition speed, pH, temperature, and stirring speed.
4.1.2. Preparation of Alginate NPs Using Emulsions
The size of alginate NPs prepared by emulsions is usually below 250 nm. This size is highly desirable for drug delivery applications due to enhanced cellular uptake. Machado et al. [66] developed calcium alginate NPs by a water-in-oil (W/O) emulsion. Tetraethylene glycol monododecyl ether, as a nonionic surfactant in decane, was mixed with alginate solution at different concentrations to form emulsions. Then, CaCl2 was added into the W/O nanoemulsions to form alginate NPs. Finally, alginate NPs were separated from the aqueous phase. The diameter of the developed NPs was approximately 200 nm [53,56,66,67,68].
4.1.3. Polyelectrolyte Complexation of Alginate NPs
The production of NPs with polyelectrolyte complex (PEC) systems has gained much attention due to its simple procedure for drug delivery applications. Generally, PECs can be formed by mixing oppositely charged polyelectrolytes and allowing them to interact electrostatically [69]. Aqueous polycationic solutions (chitosan or poly-l-lysine) were mixed with polyanionic alginate solutions at room temperature to immediately produce alginate-cationic polymeric NPs [70,71]. pH, temperature, and stirring speed may play major roles in controlling the size of these alginate NPs [72].
4.2. Alginate NPs in Drug Delivery Systems
Alginate NPs have been extensively studied for DDS due to their high encapsulation efficiency of highly effective drugs, proteins, and peptides. Alginate NPs usually do not agglomerate in organs while they deliver drugs or proteins [73]. Alginate NPs chemically modified with encapsulation materials may exhibit prolonged periods of material delivery. NP stability is an important parameter in DDS. Azevedo et al. [74] developed alginate-chitosan NPs with high stability. They were stored at 4 °C in solution for a period of five months. Their particle size and zeta potential were measured during that period of time. Particle size may change, and they may aggregate over time; this may due to the weak electrostatic interactions between alginate and chitosan. However, the addition of a stabilizer can overcome this type of issue. For example, the addition of vitamin B2 maintained the stability of alginate–chitosan NPs over a five-month period of time [74].
4.2.1. Alginate NPs in Protein and Peptide Delivery
Quality of life can be reduced significantly by health problems and common diseases. It was estimated that 9% of adults aged 18+ years and approximately 1.5 million deaths were directly caused by diabetes. The World Health Organization (WHO) predicts that by 2030, diabetes will be the 7th leading cause of death [75,76]. Insulin is one of the main treatments for diabetes, and the bioavailability of oral insulin is limited by the gastrointestinal tract. As a result, the targeted delivery of insulin is a main objective of NP-based insulin delivery. Polymers play an important role in insulin delivery [77]. Table 1 shows the usage of various alginate NPs for protein delivery, such as insulin delivery.

Table 1.
Alginate NPs for protein drug delivery.
| Serial number | Materials | Method | Particle size | Drug | References |
|---|---|---|---|---|---|
| 1 | Alginate–chitosan | Ionotropic and polyelectrolyte complex | 800 nm | Insulin | [69] |
| 2 | Alginate–chitosan | Ionotropic pre-gelation | 100–200 nm | Insulin | [77] |
| 3 | Alginate | W/O emulsion | 2604 nm | Insulin | [78] |
| 4 | Alginate–chitosan | Polyelectrolyte complex | 700 nm | Insulin | [79] |
| 5 | Alginate–chitosan | Gelification | 750 nm | Insulin | [80] |
| 6 | Alginate–chitosan–TPP | Ionic gelation | 260 to 525 nm | Insulin | [81] |
| 7 | Alginate–oligochitosan | W/O in microemulsion | 136 nm | BSA | [82] |
| 8 | Alginate NPs | Microemulsion | 350 nm | BSA | [83] |
| 9 | Alginate–chitosan | Gelification | 200 nm | BSA | [84] |
Reis et al. [78] developed alginate NPs using a W/O emulsion method and physical cross-linking with calcium ions; it was demonstrated that calcium ions play an important role in controlling particle size. The mass ratio of calcium ions to alginate was 7% (w/w). The encapsulation efficiency of insulin in the alginate NPs was more than 71%. The smaller particle size was achieved by adjusting the calcium and alginate solution concentrations; higher encapsulation efficiency and lower insulin release at pH 1.2 were also attained in this way [78]. At higher calcium ion concentrations, there are more calcium ions free to react with the M and G alginate monomers, forming more rigid alginate polymer chains and ultimately allowing sustainable insulin release from the alginate.
Sarmento et al. [69] prepared alginate NPs by ionotropic pre-gelation with CaCl2 followed by a PEC process with chitosan polysaccharides. The pH and mass ratio of the polymers and calcium ions play crucial roles influencing the NP formation. Approximately 800-nm particle sizes were produced by this method at pH 4.7 with a 6:1 mass ratio of alginate to chitosan. Fourier transform infrared spectroscopy results revealed the efficient encapsulation of insulin in the NPs [69]. In work by the same group, alginate NPs were formed by ionic gelation and used for insulin delivery [79]. In vivo results of alginate–chitosan NPs loaded with insulin were obtained from diabetic rats. Orally administered NPs lowered glucose levels by more than 40% at dosages of 50 and 100 IU/kg [80].
The size of the alginate–chitosan NPs was further decreased to less than 250 nm using the same ionotropic pre-gelation method by controlling the polymer mass ratio (Figure 3). The average size of the NPs obtained by this method was approximately 100–200 nm. The encapsulation efficiency of the insulin in the alginate-chitosan NPs was approximately 85%, and sustained release and nontoxicity were observed when the NPs were used as a peroral treatment [77] (Figure 3).
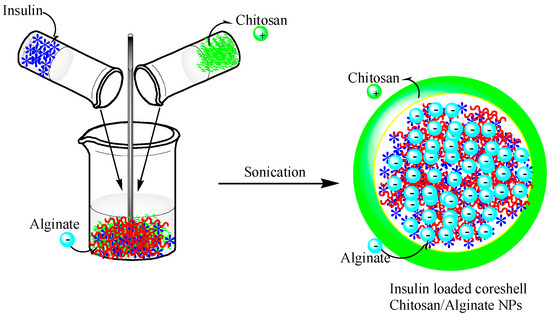
Figure 3.
A schematic showing the preparation of chitosan-alginate NPs incorporating insulin. This figure was adopted and redrawn from [77]. Copyright 2015, Elsevier.
Goycoolea et al. [81] developed chitosan–alginate NPs with pentasodium tripolyphosphate (TPP) using ionic gelation and PEC. The particle size was dependent on the molecular weight of alginate. The particle size increased from 260 to 525 nm with increased alginate molecular weight. Insulin was used as a model drug, and the encapsulation efficiency was found range from 41% to 52%. Insulin-loaded chitosan–alginate–TPP NPs showed efficient systemic absorption in rabbits [81].
Alginate-chitosan NPs have been used for the effective delivery of bovine serum albumin (BSA). Wang et al. [82] developed NPs based on low molecular weight alginate and chito–oligosaccharides using a microemulsion method. The size of the NPs was approximately 136 nm. The encapsulation efficiency reached approximately 88.4%. The developed NPs were nontoxic, biocompatible, and uniform in size, which suggested that they could be used as vehicles for other drugs [82]. Using the same microemulsion method, alginate NPs were developed using aqueous CaCl2, dioctyl sodium sulfosuccinate, and isopropyl myristate. The particle size of the alginate NPs was approximately 350 nm, as measured by DLS. The sustained release of BSA from the alginate NPs was observed. The loading efficiency of BSA was approximately 40% [83]. Li et al. [84] developed chitosan–alginate NPs for BSA delivery. The particle size of the NPs was approximately 200 nm. The release of BSA from the NPs was pH dependent [84].
4.2.2. Alginate NPs for Cancer Drug Delivery
Cancer has a major impact on society across the world. The number of new cancer cases will rise to 22 million within the next two decades [85]. Currently, surgery, chemotherapy, and radiation are the main therapies for cancer; however, it has been several years since chemotherapy has been used as the primary treatment for cancer because of the extent to which it can kill normal healthy cells. To overcome this issue, DDS with NPs have become alternative methods of targeting only cancer cell, increasing the availability of drugs to cancer cells and leaving normal cells unaffected [86]. Different types of NPs have been extensively studied for cancer drug delivery. Over the last five decades, liposome-, polymer-, dendrimer-, and protein-based NPs and inorganic NPs have been utilized as drug carriers to treat cancer [87]. NPs based on both synthetic polymers (e.g., poly(lactic-co-glycolic acid), polylactic acid, and polycaprolactone) and natural polymers (e.g., alginate, chitosan, carrageenan, and fucoidan) have been used as drug carriers to deliver several cancer drugs, such as doxorubicin and 5-fluorouracil (5-Fu) (Table 2).

Table 2.
Alginate NPs for cancer drug delivery.
| Serial number | Materials | Method | Particle size | Drug | References |
|---|---|---|---|---|---|
| 1 | Alginate | Gelification with CaCl2 and poly-l-lysine | 250–850 nm | Doxorubicin | [88] |
| 2 | Alginate | CaCl2 cross-linking | 214 ± 11 nm | Doxorubicin | [89] |
| 3 | Glycyrrhetinic acid–Alginate NPs | Chemical modification | 80 and 100 nm | Doxorubicin | [90] |
| 4 | Alginate NPs | Chemical modification | 241 nm | Doxorubicin | [91] |
| 5 | Aerosol OT-alginate NPs | Emulsification cross-linking method | 39 ± 7 nm | Doxorubicin and methylene blue | [92] |
| 6 | Alginate–CaCO3 NPs | Coprecipitation method | 100–400 nm | Doxorubicin and p53 | [93,94] |
| 7 | Chitosan–alginate NPs | Emulsion method | 200 nm | 5-Fluorouracil | [95] |
| 8 | Alginate–chitosan | Ionic gelation | 329–505 nm | 5-Fluorouracil | [96] |
| 9 | Alginate-chitosan-Pluronic F127 | Ionotropic pre gelation | 100 ± 20 nm | Curcumin | [97] |
| 10 | Alginate NPs | Oligonucleotide/Poly lysine | NA | Antisense oligonucleotide | [98] |
| 11 | Alginate–chitosan | Ionotropic gelation method | 230 to 627 nm | Gemcitabine | [99] |
| 12 | Bovine serum albumin and thiolated alginate | Coacervation | 350 to 500 nm | Tamoxifen | [100] |
Rajaonarivony et al. [88] developed alginate NPs with calcium ions and poly-l-lysine by a gelification method. The particle size of the alginate NPs was approximately 250–850 nm, and they were used for doxorubicin delivery. From this study, significant research has been performed to develop alginate NPs for various drug delivery purposes using a similar type of method [88]. Zhang et al. [89] developed alginate NPs with a CaCl2 cross-linking method. Alginate was modified with a liver targeting molecule (i.e., glycyrrhetinic acid) and chemically characterized. The doxorubicin-loaded glycyrrhetinic acid-alginate NPs exhibited a size of approximately 214 ± 11 nm. The drug could be released from the NPs for 20 days, and the treatment had the capacity to kill hepatocellular carcinoma cells effectively [89]. The same group examined the in vivo therapeutic efficacy of the developed NPs using a mouse liver tumor model. The chemical modification of the alginate NPs with glycyrrhetinic acid increased the biodistribution of doxorubicin. Doxorubicin reached 67.8 ± 4.9 μg/g in the liver after intravenous administration, which was significantly higher compared with the results of both non-glycyrrhetinic acid-modified NPs and the drug only [90]. By the continuous research on complexing NPs, glycyrrhetinic acid-modified alginate (GA–ALG) and doxorubicin-modified alginate (DOX–ALG) were prepared by self-assembly [91] (Figure 4). pH-Sensitive glycyrrhetinic acid–alginate/doxorubicin–alginate NPs (GA-ALG/DOX-ALG NPs) demonstrated efficient treatment of liver cancer. As shown in Figure 5A, DOX concentration in the liver of the GA-ALG/DOX-ALG NPs group reached 27.6 µg/g, which was higher than that of the DOX·HCl (8.1 µg/g). Further, DOX release from GA-ALG/DOX-ALG NPs showed pH-sensitivity; less than 10% of the drugs was released at pH 7.4 within 9 days while 58.7% of drug was released at pH 4.0 (Figure 5B). Confocal laser scanning microscopy images of HepG2 cells incubated with GA-ALG/DOX-ALG NPs and DOX-ALG NPs at the same DOX concentration (10 µg DOX/mL) showed that GA-ALG/DOX-ALG NPs were efficienty taken up by the cells (Figure 5C). H22 tumor tissue treated with GA-ALG/DOX-ALG NPs showed more effective inhibition of tumor growth compared with bare DOX and DOX-ALG NPs (Figure 5D).
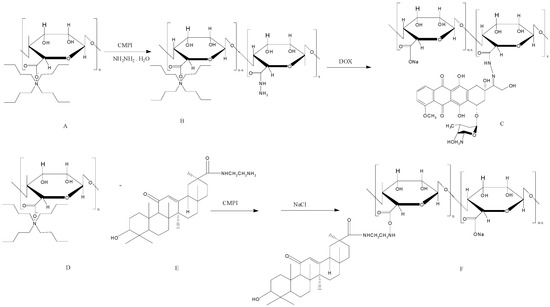
Figure 4.
The synthesis route of doxorubicin-modified alginate (DOX–ALG) (top) and glycyrrhetinic acid-modified alginate (GA–ALG) (bottom). The figures were adopted and redrawn from [91].
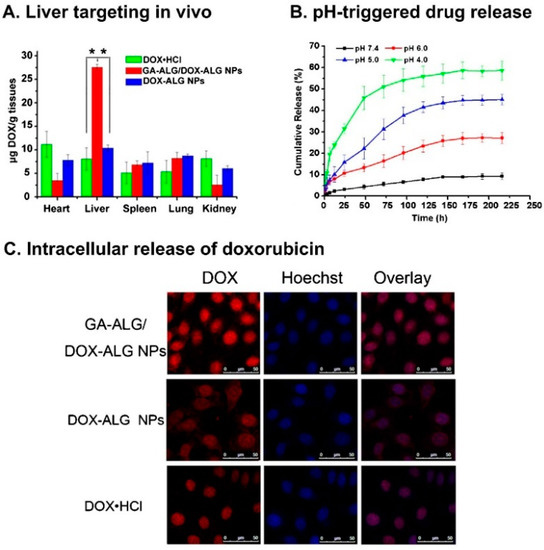
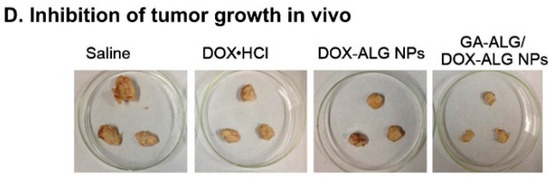
Figure 5.
(A) The results of an in vivo liver targeting study; (B) the release results at different pH levels; and (C) the cellular uptake of doxorubicin using glycyrrhetinic acid–alginate (GA–ALG)/doxorubicin–alginate (DOX–ALG) NP complexes and doxorubicin–alginate (DOX–ALG) NPs; (D) H22 tumor tissue slices from mice treated with saline, doxorubicin, doxorubicin-alginate (DOX–ALG) NPs, and glycyrrhetinic acid-alginate (GA–ALG)/doxorubicin-alginate (DOX–ALG) NP complexes. The figures were adopted and redrawn with permission from [91]. Copyright 2013, Elsevier.
Surfactant-polymer hybrid NPs using alginate and an anionic surfactant, aerosol-OT (AOT), were prepared for combined chemotherapy and photodynamic therapy. The NPs were able to deliver both doxorubicin and methylene blue. Increased nuclear and cellular accumulation of doxorubicin and methylene blue enhanced the production of reactive oxygen species that contributed to the superior toxicity [92].
Alginate–calcium carbonate–doxorubicin-p53 NPs were prepared by a co-precipitation technique. p53 is a tumor suppressor gene that plays a pivotal role in DNA repair, apoptosis, and cell cycle regulation. Zhao et al. [93,94] stated that, “inhibiting p53 mutations, the reintroduction of wild type (wt) p53 into tumor cells harboring p53 mutations, may also enhance the sensitivity of tumor cells to chemotherapeutic agents through the inhibition of the P-gp expression related to drug resistance. On the other hand, wt p53 protein is positive in response to a variety of stress signals including DNA damage caused by antitumor drugs”. Thus, the combination of p53 and doxorubicin may increase the efficacy of the cancer treatment. The developed particle size, approximately 100 to 400 nm, depended on the polymer content. The NPs showed a high drug encapsulation efficiency and completely inhibited the growth of the HeLa cells. These NPs were used for both gene and drug delivery purposes [93,94]. Xing et al. developed chitosan–alginate NPs by an emulsion method to incorporate 5-Fu. 5-Fu is a pyrimidine analog drug that has been used to treat cancer for several decades. The resulting particle size was found to be approximately 200 nm. A drug release of 50% was observed at 12 h in vitro [95]. Using the same 5-Fu drug, sodium alginate-chitosan NPs were prepared by an ionic gelation technique. The developed NPs showed a size ranging from approximately 329–505 nm. The encapsulation efficiency of 5-Fu mainly depended on the molar ratios of sodium alginate and chitosan (6%–26%) [96].
Recent studies have reported that curcumin has several biological activities, such as anti-inflammatory, antioxidant, and antimicrobial activity and the inhibition of different types of tumor cells. Das et al. [97] developed alginate–chitosan–pluronic F127 NPs for curcumin drug delivery. The encapsulation efficiency of the NPs was improved by the addition of pluronic F127. The size of the NPs was found to be approximately 100 nm [97]. Other studies using alginate NPs for cancer drug delivery have also been reported elsewhere [98,99,100].
4.2.3. Alginate NPs for Antibiotic and Antimicrobial Drug Delivery
Several antimicrobial drugs are available on the market to kill bacteria, viruses, and fungi [101]. Zahoor et al. [102] developed alginate NPs as antitubercular drug carriers. Isoniazid, rifampicin, and pyrazinamide were encapsulated by the alginate NPs. The encapsulation efficiency of these drugs was approximately 70%–90%. The size of the alginate NPs was approximately 235.5 nm with a polydispersity index of 0.439 [71,102,103] (Table 3).
Choonara et al. (2011) developed alginate NPs with an ionic cross-linking and reverse emulsion method [104]. Ghaffari et al. [105] developed alginate–chitosan NPs encapsulating ciprofloxacin with a particle size of approximately 520 ± 16 nm. The loading efficiency of ciprofloxacin was 88%. A sustained release of ciprofloxacin was observed over 45 h [105]. Bi-specific and biodegradable chitosan-alginate polyelectrolyte NPs were developed by Arora et al. [72] for amoxicillin delivery. The particle size of the developed NPs was 264 nm. By increasing the chitosan concentration in the polyelectrolyte system, the particle size was increased [72]. Chopra et al. [106] developed chitosan–alginate NPs for streptomycin delivery. The size of the developed NPs was 328 nm, and the encapsulation efficiency of the drug was 93.32% [106]. Other alginate-chitosan NPs encapsulating antimicrobial drugs have also been developed [107,108].

Table 3.
Alginate NPs for antibiotic drug delivery.
| Serial number | Materials | Method | Particle Size | Drug | References |
|---|---|---|---|---|---|
| 1 | Alginate NPs | Cation-induced gelification | NA | Rifampicin, isoniazid, pyrazinamide and ethambutol | [71] |
| 2 | Alginate–chitosan | Polyelectrolyte complex | 264–638 nm | Amoxicillin | [72] |
| 3 | Alginate NPs | Cation-induced gelification | 235.5 ± 0 nm | Rifampicin | [102] |
| 4 | Alginate NPs | Cation-induced gelification | 235.5 ± 0 nm | Isoniazid, rifampicin, pyrazinamide, and ethambutol | [103] |
| 5 | Alginate | Reverse emulsion | 240 ± 8.7 nm | Rifampicin and isoniazid | [104] |
| 6 | Calcium alginate | Polyelectrolyte complex | 520 nm | Ciprofloxacin | [105] |
| 7 | Alginate–chitosan | Ionotropic pre-gelation | 328 nm | Streptomycin | [106] |
| 8 | Alginate–chitosan–silica | Polyelectrolyte complex | NA | Piperacillin-tazobactam, cefepime, piperacillin, imipenem, gentamicin, ceftazidime | [107] |
| 9 | Alginate–chitosan | Gelification | 50–250 nm | Nisin | [108] |
4.2.4. Alginate NPs for Other Drug Delivery
Alginate NPs are excellent for encapsulating various drugs. Methylene blue, fluorescein sodium salt, nifedipine, gatifloxacin, rhodamine 6G, EGFR phosphorothioated 21-mer antisense 50, turmeric oil, epidermal growth factor, Bupivacaine, vitamin D3, 5-aminolevulinic acid, tuftsin, candida rugosa lipase, ibuprofen, ivermectin, enoxaparin, nitric oxide, benzoyl peroxide, and quinapyramine have all been encapsulated in alginate NPs for drug delivery [109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131] (Table 4).

Table 4.
Alginate NPs for other drug delivery.
| Serial number | Materials | Method | Particle size | Drug | References |
|---|---|---|---|---|---|
| 1 | Sodium alginate–chitosan | Ionic gelation, polyelectrolyte | 205 to 572 nm | Gatifloxacin | [70] |
| 2 | Sodium alginate: CaCl2-(poly-l-lysine or chitosan) | Ionic gelation | 544 ± 53 nm | Methylene blue | [109] |
| 3 | Silica/alginate | NA | 50–200 nm | [110] | |
| 4 | Alginate–chitosan | Ionotropic gelation | 600 nm | Fluorescein sodium salt | [111] |
| 5 | Alginate–chitosan | Polyelectrolyte | 20–50 nm | Nifedipine | [112] |
| 6 | OT-alginate hydrogel loaded with Fe3O4 | emulsification-cross-linking process | 25 and 50 nm | Rhodamine 6G | [113] |
| 7 | Alginate–chitosan | Precipitation method | 194 nm | EGFR Phosphorothioated 21-mer antisense 50 | [114] |
| 8 | Alginate–chitosan | Gelification | 522 ± 15 nm | Turmeric oil | [115] |
| 9 | Alginate–chitosan | NA | NA | Epidermal growth factor receptor | [116] |
| 10 | Alginate–chitosan | Polyelectrolyte | NA | Bupivacaine | [117] |
| 11 | Alginate–chitosan | NA | 600–650 nm | pAcGFP1-C1 plasmid | [118] |
| 12 | Hydrophobic alginate derivative | Chemical modification | 200–400 nm, | Vitamin D3 | [119] |
| 13 | Alginate folic acid chitosan | Ionic gelation | 115 nm | 5-aminolevulinic acid | [120] |
| 14 | Alginate NPs | Gelation method | 200 nm | Tuftsin | [121] |
| 15 | Superparamagnetic sodium alginate NPs | W/O emulsion method | 25–30 nm | Candida rugosa lipase | [122] |
| 16 | superparamagnetic alginate NPs | Coprecipitation | 200 nm | Ibuprofen | [123] |
| 17 | Thiolated chitosan alginate | NA | 265.7 ± 7.4 to 471.0 ± 6.4 nm | Ocular drug | [124] |
| 18 | Chitosan–alginate NPs | Coacervation | 155 nm | Ivermectin | [125] |
| 19 | Chitosan–alginate NPs | Ionic gelation | 213 nm | Enoxaparin | [126] |
| 20 | Chitosan–alginate NPs | NA | NA | Nitric oxide | [127] |
| 21 | Chitosan–alginate NPs | Polyelectrolyte complex | 50 nm | Benzoyl peroxide | [128] |
| 22 | Alginate beads | W/O emulsion | 200 to 1,000 nm | NA | [129] |
| 23 | Alginate | NA | NA | Pesticide | [130] |
| 24 | Sodium alginate NPs | Emulsion-cross-linking technology | 60 nm | Quinapyramine | [131] |
4.3. Alginate NP Patents
There are several patents regarding alginate-based NPs with different types of preparative methods. The methods of W/O emulsion and ionic cross-linking with calcium ions are patented [132]. Aerosol alginate NPs with doxorubicin, verapamil, and clonidine are also patented [133].
5. Carrageenan NPs
Carrageenan is an anionic, sulfated polysaccharide and is commonly isolated from red seaweed. It is mainly composed of d-galactose and 3,6-anhydro-d-galactose with glyosidic units. Carrageenan has been widely used for functional food applications and cancer treatments [134,135,136,137,138]. Recently, carrageenan has also been used for several biomedical applications [139,140,141,142,143], which were intensively reviewed by Li et al. [144]. The extraction procedure, structure, and subsequent product applications have also been discussed by Prajapati et al. (2014) in detail [22,145]. Three different types of carrageenan are available, depending on the extraction procedure: kappa (κ), iota (ι), and lamda (λ) carrageenan [146] (Figure 6).
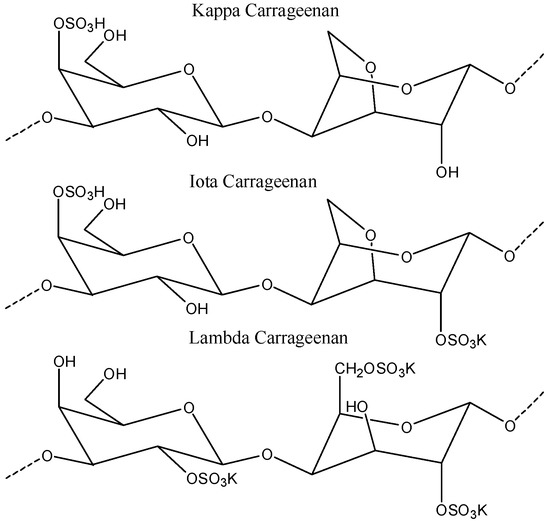
Figure 6.
The structure of κ carrageenan, ι carrageenan, and λ carrageenan. The figures were adopted and redrawn from [146].
5.1. Production of Carrageenan NPs
The negative surface charge of carrageenan can form a PEC with positively charged ion molecules. NPs formed by chitosan-carrageenan complexing have been studied for drug delivery purposes. These NPs can be prepared by the ionic gelation or polyelectrolyte complexing methods by mixing carrageenan with cationic polymers such as chitosan [147] (Figure 7).
Long-term NP stability is a major challenge of polysaccharide-based NPs used for DDS. Rodrigues et al. [148] reported chitosan-carrageenan NPs that were developed using a simple polyelectrolyte complexation method. The developed NPs were stored at 4 °C in an aqueous solution, and their size and zeta potential were measured. No statistically significant changes were observed in the size and zeta potential. This indicated that the stability of the NPs was not dependent on the mass ratio of polymers [148]. In work from the same group, the addition of TPP to the chitosan-carrageenan mixture was observed to increase the stability of the NPs for over 250 days [149], suggesting that TPP can act as an effective stabilizer.
Figure 7.
Structure of (A) chitosan; (B) carrageenan; and (C) tripolyphosphate (TPP). The figures were adopted and redrawn from [149].
5.2. Carrageenan NPs as Drug Delivery Vehicles
The most widely used method to prepare carrageenan NPs is the polyelectrolyte method, which is very simple and requires mild conditions. In recent years, particular attention has been directed toward carrageenan-chitosan NPs for the delivery of drug molecules (Table 5). A very mild, feasible, and convenient polyelectrolyte method for the production of carrageenan–chitosan NPs was investigated [150]. Bulger et al. [151] developed chitosan-carrageenan NPs by ionotropic gelation for the controlled release of recombinant human erythropoietin (rHu-EPO). The size of the developed NPs ranged from 200 to 1000 nm. The encapsulation efficiency of the rHu-EPO was approximately 47.97% ± 4.10%. In addition, approximately 50% of the encapsulated rHu-EPO was released over two weeks in a sustained manner [151]. It has been reported that the prepared NPs were nontoxic to L929 cells. Moreover, ovalbumin was used as a model protein, and the loading efficiency of the ovalbumin varied from 4% to 17% [152]. Cross-linked carrageenan nanogels were prepared using a microemulsion method. The size of the NPs was smaller than 100 nm [153]. Chitosan–carrageen–TPP NPs by ionic gelation were developed [149,154]. The size of the NPs was approximately 150–300 nm [149,154]. Other carrageenan-based NPs for DDS have also been reported [155,156,157].

Table 5.
Carrageenan NP production methods and delivery systems.
| Serial number | Materials | Method | Particle size | Drug | References |
|---|---|---|---|---|---|
| 1 | Chitosan–carrageenan NPs | Ionotropic gelation | 200 to 1000 nm | rHu-EPO | [151] |
| 2 | Chitosan/carrageenan | Ionic complexation | 350–650 nm | Ovalbumin | [152] |
| 3 | Cross-linked–carrageenan NPs | Reverse microemulsion | 100 nm | Methylene blue | [153] |
| 4 | Chitosan/carrageenan/TPP | Ionic gelation | 150–300 nm | BSA | [149,154] |
| 5 | Carrageenan/protamine | Self-assembled | 100–150 nm | NA | [155] |
| 6 | Carboxymethyl chitosan and carrageenan | NA | NA | Riboflavin | [156] |
| 7 | Carrageenan hydrogel | Gelation | NA | Methylene blue | [157] |
6. Fucoidan NPs
Fucoidan is an anionic, sulfated polysaccharide found in brown seaweed (e.g., Laminaria japonica, Macrocystis pyrifera, Fucus vesiculosus, and Ascophyllum nodosum). It is mainly composed of α-(1-3)-linked fucose units or repeating disaccharide units of α-(1-3)- and α-(1-4)-linked fucose residues with O-2 branches (Figure 8). It has excellent bioactivity, including antivirus, antitumor, antithrombotic, anticoagulant, anti-inflammatory, and antioxidant activity [158,159,160,161]. Research on fucoidan for biomedical applications is still at the early stage of determining its exact function [162,163,164,165]. Some studies have been conducted regarding fucoidan-based NPs for the delivery of curcumin, doxorubicin, and growth factors.
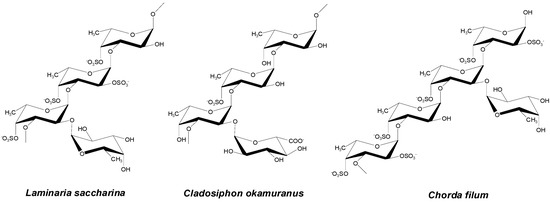
Figure 8.
Structures of fucoidan. The figure was adopted with permission from [158].
6.1. Production of Fucoidan NPs
Chitosan/fucoidan-based NPs were synthesized using different types of methods, such as self-assembly, coacervation, polyelectrolyte complexing, ionic cross-linking, chemical modification, and emulsion (Table 6). Pinheiro et al. (2014) developed chitosan-fucoidan NPs using self-assembly for the delivery of bioactive compounds [166]. Lee and Lim et al. (2014) discussed the formation of chitosan-fucoidan NPs in two papers in detail [167,168]. The size of the developed chitosan–fucoidan NPs ranged from approximately 365–900 nm. A 1:1 ratio of chitosan to fucoidan was the optimum condition to produce NPs with a small size, high yield, and good stability. They also found that pH 5 was optimum to produce the polyelectrolyte NPs [167,168]. Kimura et al. [169] developed fucoidan-based NPs and assessed their activity against osteosarcoma. The experimental results suggested that the fucoidan NPs were more effective than native fucoidan [169]. Fucoidan nanogels with a particle size of approximately 123 nm were produced and used for cancer research [170]. Stable chitosan–fucoidan NPs encapsulating basic fibroblast growth factor (bFGF) were developed for nerve tissue engineering [171]. The particles were able to protect bFGF from degradation by enzymes. The particles were stable for a period of eight days. O-carboxymethyl chitosan/fucoidan NPs were prepared by ionic crosslinking and used for curcumin delivery [172] (Figure 9). The synthesized curcumin-loaded chitosan/fucoidan NPs dramatically increased the cellular uptake of curcumin. Fucolidan NPs by coacervation process and anionic emulsion polymerization were also developed [173,174].
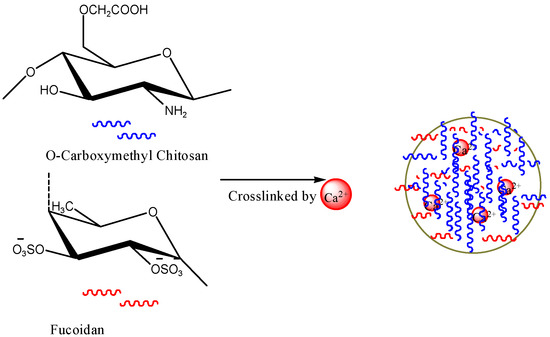
Figure 9.
The formation of fucoidan NPs. The figures were adopted and redrawn from [172].

Table 6.
The production of fucoidan NPs.
| Serial number | Materials | Method | Particle size | References |
|---|---|---|---|---|
| 1 | Chitosan-fucoidan NPs | Self-assembled | 365–900 nm | [167,168] |
| 2 | Fucoidan lipid NPs | Chemical modification | 100 nm | [169] |
| 3 | Fucoidan nanogels | Graft with hexadecylamine | 123 nm | [170] |
| 4 | Chitosan-fucoidan | Coacervation process | 154 and 453 nm | [173] |
| 5 | Fucoidan-coated poly(isobutylcyanoacrylate) NPs | Anionic emulsion polymerization | 193 ± 4 nm to 399 ± 0.7 nm | [174] |
6.2. Fucoidan NPs for Growth Factor Delivery
A diverse set of fucoidan NPs for the delivery of growth factors has been reported (Table 7). Huang et al. developed chitosan fucoidan-based NPs as vehicles for stromal cell-derived factor-1 (SDF-1) [175]. Chitosan–TPP–fucoidan NPs were developed using ionic gelation and PEC methods. The encapsulation efficiency of the chitosan-TPP-fucoidan NPs with SDF-1 was 60%–68%. The developed NPs showed a spherical diameter of approximately 173–403 nm. The amount of released SDF-1 from the chitosan-TPP-fucoidan NPs ranged from 17 to 23 ng/mL [175]. In work from the same group, chitosan-fucoidan NPs were produced by a PEC process and used for nerve tissue engineering. The size of the NPs was approximately 200 nm. The developed chitosan-fucoidan NPs were nontoxic to PC12 cells at a concentration of 125 ng/mL. Fucoidan-chitosan NPs were also prepared by a PEC processs with sonication [176]. BSA-loaded fucoidan-chitosan NPs showed a sustained release of BSA.

Table 7.
Fucoidan NPs for growth factor delivery.
| Serial number | Materials | Method | Size | Drug | References |
|---|---|---|---|---|---|
| 1 | Chitosan–fucoidan NPs | Polyelectrolyte complexing | 200 nm | bFGF | [171] |
| 2 | Chitosan–TPP–fucoidan | Ionic gelation and polyelectrolyte complexing | 173–403 nm | SDF-1 | [175] |
| 3 | Fucoidan–chitosan NPs | Polyelectrolyte complexing | 860 nm | BSA | [176] |
6.3. Fucoidan NPs for Cancer Drug Delivery
A number of studies have reported that fucoidan itself has the capability of eliminating cancer cells by inducing apoptosis [177,178,179,180,181,182,183,184]. Therefore, various fucoidan-based NPs encapsulating anticancer drugs have been intensively developed in the pursuit of efficient cancer therapies (Table 8). Huang et al. (2011) developed chitosan-fucoidan NPs by ionic gelation for curcumin delivery [185]. Curcumin can be used as a natural anticancer drug, but its application has been hindered due to low bioavailability[186]. To improve bioavailability, curcumin-loaded NPs have been attempted [187,188,189]. The encapsulation efficiency of curcumin in chitosan-fucoidan NPs was higher than 85%. The release of curcumin increases with increasing pH; while the release of curcumin from the chitosan-fucoidan NPs was inhibited at pH 1.2, its release was increased at pH 6.0 and 7.0 [185]. In work from the same group, fucoidan NPs were developed using o-carboxymethyl chitosan for curcumin delivery. Ionic cross-linking has been used to produce these NPs. The encapsulation efficiency increased significantly to 92.8%. Curcumin was efficiently released from the chitosan-fucoidan NPs in a pH-dependent manner. While the release of curcumin was effective at pH 7.4, the release of curcumin was minimal at pH 2.5 [172,190]. Fucoidan NPs encapsulating DOX were also developed for cancer therapy [191]. The drug encapsulation efficiency was found to be 71.1% and 3.6%. The particle size was approximately 140 nm [191]. In HCT-8 cells (MDR model cells) exposed to DOX-loaded AcFu NPs, a time-dependent cellular internalization of the drugs was observed. Over 99% of the total DOX load was internalized by the HCT-8 cells after 2 h, whereas 1.99% and 1.79% of a fucoidan–DOX mixture and free DOX were internalized, respectively (Figure 10A–D). Only the DOX-loaded AcFu NPs could be clearly identified in confocal images (Figure 10E). In HCT-116 cells (non-MDR cells), the cellular uptake of free DOX was similar to that of the AcFu nanoparticle-encapsulated DOX (Figure 10F). However, these researchers mentioned that the mechanism behind this result was unclear mechanism (Figure 10 and Table 8).

Table 8.
Fucoidan NPs for cancer drug delivery.
| Serial number | Materials | Method | Particle size | Drug | References |
|---|---|---|---|---|---|
| 1 | Chitosan–fucoidan NPs | Self-assembled | Approximately 100 nm | PLL | [166] |
| 2 | O-carboxymethyl chitosan/fucoidan | Ionic cross-linking | 270 nm | Curcumin | [172] |
| 3 | Chitosan–fucoidan | Ionic gelation | 173 nm | Curcumin | [185] |
| 4 | Fucoidan NPs | Self-assembly | 140 nm | Doxorubicin | [191] |
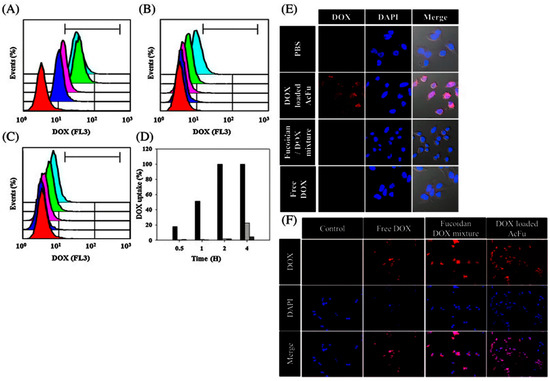
Figure 10.
The time-dependent cellular uptake efficiency of doxorubicin was estimated by FACS analysis. Flow cytometry analysis of cells treated with (A) doxorubicin-loaded acetylated fucoidan NPs (AcFu NP); (B) natural fucoidan–doxorubicin mixtures; and (C) free doxorubicin. The colors in these graphs indicate the time after sample treatment: red—control; blue—30 min; pink—1 h; green—2 h; and sky blue—4 h. The uptake efficiencies at each time point are indicated by the bar graph in (D); (Black: doxorubicin-loaded AcFu NPs; gray: natural fucoidan–doxorubicin mixture; dark gray: free doxorubicin.); (E) Confocal images of doxorubicin uptake 4 h after treatment; (F) Confocal images of doxorubicin uptake in HCT-116 cells 4 h after sample treatment. The figures were adopted with permission from [191]. Copyright 2013, Elsevier.
7. Future Research in Seaweed Polysaccharide NPs
Ionic gelation and PEC methods provide excellent opportunities to produce large amounts of natural polymer-based NPs. However, there are several factors to be considered for developing natural polymer-based NPs, including the molecular weight of the polymers, addition time, pH, stirring speed, and temperature. To date, few in vitro, in vivo studies, and particle formation studies have been performed using alginate, carrageenan, and fucoidan NPs for drug delivery. There is a need for more in vivo research on carrageenan NPs and fucoidan NPs for further commercialization and use in clinical settings [192].
7.1. Active Targeting Molecules
Proper NP charge, size, and shape can improve drug delivery efficacy. In addition to those factors, engineering NPs with targeting moieties can significantly enhance drug delivery efficacy through the high accumulation of drugs in the targeted disease areas. In recent years, various targeting moieties, including peptides, small molecules, and polysaccharides themselves, have been incorporated into polysaccharide-based NPs to obtain targeted delivery. Somatostatin receptors, A54 hepatocarcinoma binding peptide, RGD peptide, and small molecules (e.g., glycyrrhetinic acid and vitamin E succinate) have also been used as targeting moieties [40]. Polysaccharides such as chitosan have also been known to have a capacity to promote drug absorption in the small intestine due to mucoadhesion [40,193,194,195,196,197].
7.2. Other Seaweed Polysaccharides
Future research can be focused on the formation of NPs from other seaweed polysaccharide-based biomaterials, such as ulvan and laminarin. Different seaweed polysaccharides have their own merits and applications. Ulvan is an anionic polysaccharide and thus easily forms NPs with cationic polymers such as chitosan, which indicates its potential as a biocompatible drug delivery carrier [198,199,200,201].
The seaweed polysaccharide NP preparations in this review were mainly based on combinations of chitosan and polyanions (e.g., alginate, carrageenan and fucoidan). The main reason to combine the chitosan and polyanions is to produce stable polymeric NPs, which can be achieved by the opposite charge interactions of chitosan and alginate. Developed NPs have been shown to protect the encapsulated materials and release drugs sustainably and effectively. Further advantages of the chitosan-polyanionic system include nontoxicity, biocompatibility and biodegradability [202].
8. Conclusions
In this review, we have discussed the production of various NPs using seaweed-based polysaccharides and their applications in drug delivery. The formation of seaweed polysaccharide-based NPs can easily be achieved by means of ionic gelation and PEC; these materials have the capacity to hold drug molecules and release them in specific locations. We believe that these methods will be increasingly utilized for the production of polysaccharide-based NPs in the future. Seaweed polysaccharide-based NPs have shown promising results in delivering proteins, peptides, anti-cancer drugs, and other drugs with increased bioavailability and sustained release properties. In particular, alginate-based NPs have extensively been studied for the delivery of anti-cancer drugs. In the last three decades, several studies have been conducted on seaweed polysaccharides both in vitro and in vivo; these studies have demonstrated the high stability and biocompatibility as well as sustained drug release achievable by these systems, which will support their future use in clinical settings. The introduction of targeting moieties to polysaccharide-based NPs will improve their therapeutic efficacy while also reducing undesired side effects.
Acknowledgments
This work was supported by the Post-Doctor Research Program (2015) through Incheon National University (INU), Incheon, Republic of Korea.
Author Contributions
Jayachandran Venkatesan and Sukumaran Anil developed the concept for the review and wrote the manuscript. Se-Kwon Kim and Min Suk Shim wrote and edited the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Smit, A. Medicinal and pharmaceutical uses of seaweed natural products: A review. J. Appl. Phycol. 2004, 16, 245–262. [Google Scholar] [CrossRef]
- Renn, D. Biotechnology and the red seaweed polysaccharide industry: Status, needs and prospects. Trends Biotechnol. 1997, 15, 9–14. [Google Scholar] [CrossRef]
- Araki, C. Seaweed polysaccharides. In Carbohydrate Chemistry of Substances of Biological Interests; Pergamon Press: London, UK, 1959; pp. 15–30. [Google Scholar]
- Mori, R. Seaweed polysaccharides. Adv. Carbohydr. Chem. 1953, 8, 315. [Google Scholar] [PubMed]
- Vera, J.; Castro, J.; Gonzalez, A.; Moenne, A. Seaweed polysaccharides and derived oligosaccharides stimulate defense responses and protection against pathogens in plants. Mar. Drugs 2011, 9, 2514–2525. [Google Scholar] [CrossRef] [PubMed]
- Rioux, L.E.; Turgeon, S.L.; Beaulieu, M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007, 69, 530–537. [Google Scholar] [CrossRef]
- Kraan, S. Algal Polysaccharides, Novel Applications and Outlook; INTECH Open Access Publisher: Rijeka, Croatia, 2012. [Google Scholar]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.-H.; Kim, S.-K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.J. Bioenergy from brown seaweeds, 2000. Available online: http://hdl.handle.net/11250/245591 (accessed on 02 January 2016).
- Kim, S.-K. Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; John Wiley & Sons: New York City, NY, USA, 2011. [Google Scholar]
- Panchanathan, M.; Jayachandran, V.; Se-Kwon, K. Marine algae: An important source of bioenergy production. In Marine Bioenergy; CRC Press: Boca Raton, FL, USA, 2015; pp. 45–70. [Google Scholar]
- Panchanathan, M.; Se-Kwon, K. Introduction to marine bioenergy. In Marine Bioenergy; CRC Press: Boca Raton, FL, USA, 2015; pp. 3–12. [Google Scholar]
- Wi, S.G.; Kim, H.J.; Mahadevan, S.A.; Yang, D.-J.; Bae, H.-J. The potential value of the seaweed Ceylon moss (Gelidium amansii) as an alternative bioenergy resource. Bioresour. Technol. 2009, 100, 6658–6660. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Lowe, B.; Anil, S.; Manivasagan, P.; Al Kheraif, A.A.; Kang, K.H.; Kim, S.K. Seaweed polysaccharides and their potential biomedical applications. Starch-Stärke 2015, 67, 381–390. [Google Scholar] [CrossRef]
- Sell, S.A.; Wolfe, P.S.; Garg, K.; McCool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The use of natural polymers in tissue engineering: A focus on electrospun extracellular matrix analogues. Polymers 2010, 2, 522–553. [Google Scholar] [CrossRef]
- Pillai, O.; Panchagnula, R. Polymers in drug delivery. Curr. Opin. Chem. Biol. 2001, 5, 447–451. [Google Scholar] [CrossRef]
- Tiwari, P.; Panthari, P.; Katare, D.P.; Kharkwal, H. Natural polymers in drug delivery. World J. Pharm. Pharm. Sci. 2014, 3, 1395–1409. [Google Scholar]
- Hubbell, J.A. Biomaterials in tissue engineering. Nat. Biotechnol. 1995, 13, 565–576. [Google Scholar] [CrossRef]
- Gerard, M.; Chaubey, A.; Malhotra, B. Application of conducting polymers to biosensors. Biosens. Bioelectron. 2002, 17, 345–359. [Google Scholar] [CrossRef]
- Cascone, M.G.; Sim, B.; Sandra, D. Blends of synthetic and natural polymers as drug delivery systems for growth hormone. Biomaterials 1995, 16, 569–574. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, V.D.; Maheriya, P.M.; Jani, G.K.; Solanki, H.K. Carrageenan: A natural seaweed polysaccharide and its applications. Carbohydr. Polym. 2014, 105, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.P.; Geckeler, K.E. Polymer nanoparticles: Preparation techniques and size-control parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Sudhir, S.C.; Dennis, H.R.; Sinjan, D. Nanoparticles prepared using natural and synthetic polymers. In Nanoparticulate Drug Delivery Systems; CRC Press: Boca Raton, FL, USA, 2007; pp. 51–60. [Google Scholar]
- Venugopal, V. Marine Polysaccharides: Food Applications; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.; Tamura, H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Skaugrud, Ø.; Hagen, A.; Borgersen, B.; Dornish, M. Biomedical and pharmaceutical applications of alginate and chitosan. Biotechnol. Genet. Eng. Rev. 1999, 16, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro-and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef] [PubMed]
- De Campos, A.M.; Diebold, Y.; Carvalho, E.L.; Sánchez, A.; Alonso, M.J. Chitosan nanoparticles as new ocular drug delivery systems: In vitro stability, in vivo fate, and cellular toxicity. Pharm. Res. 2004, 21, 803–810. [Google Scholar] [CrossRef] [PubMed]
- De Campos, A.M.; Sánchez, A.; Alonso, M.A.J. Chitosan nanoparticles: A new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. Int. J. Pharm. 2001, 224, 159–168. [Google Scholar] [CrossRef]
- Li, L.; Chen, D.; Zhang, Y.; Deng, Z.; Ren, X.; Meng, X.; Tang, F.; Ren, J.; Zhang, L. Magnetic and fluorescent multifunctional chitosan nanoparticles as a smart drug delivery system. Nanotechnology 2007, 18, 405102. [Google Scholar] [CrossRef]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Chitosan nanoparticles: A promising system in novel drug delivery. Chem. Pharm. Bull. 2010, 58, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, D.; Ninan, N.; Jayakumar, R.; Nair, S.V.; Menon, D. O-carboxymethyl chitosan nanoparticles for controlled release of non-steroidal anti-inflammatory drugs. Adv. Sci. Eng. Med. 2014, 6, 522–530. [Google Scholar] [CrossRef]
- Prabaharan, M. Chitosan-based nanoparticles for tumor-targeted drug delivery. Int. J. Biol. Macromol. 2015, 72, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Saranya, N.; Moorthi, A.; Saravanan, S.; Devi, M.P.; Selvamurugan, N. Chitosan and its derivatives for gene delivery. Int. J. Biol. Macromol. 2011, 48, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Tiyaboonchai, W. Chitosan nanoparticles: A promising system for drug delivery. Naresuan Univ. J. Sci. Technol. 2013, 11, 51–66. [Google Scholar]
- Zhang, H.; Oh, M.; Allen, C.; Kumacheva, E. Monodisperse chitosan nanoparticles for mucosal drug delivery. Biomacromolecules 2004, 5, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Paques, J.P.; van der Linden, E.; van Rijn, C.J.; Sagis, L.M. Preparation methods of alginate nanoparticles. Adv. Colloid Interface Sci. 2014, 209, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wardwell, P.R.; Bader, R.A. Polysaccharide-based micelles for drug delivery. Pharmaceutics 2013, 5, 329–352. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Fernández, A.; Pérez, E.; Teijón, J.; Benito, M.; Blanco, M. Polysaccharide-Based Nanoparticles for Controlled Release Formulations; INTECH Open Access Publisher: Rijeka, Croatia, 2012. [Google Scholar]
- Namazi, H.; Heydari, A.; Fathi, F. Nanoparticles Based on Modified Polysaccharides; INTECH Open Access Publisher: Rijeka, Croatia, 2012. [Google Scholar]
- Kulterer, M.R.; Reichel, V.E.; Kargl, R.; Köstler, S.; Sarbova, V.; Heinze, T.; Stana-Kleinschek, K.; Ribitsch, V. Functional polysaccharide composite nanoparticles from cellulose acetate and potential applications. Adv. Funct. Mater. 2012, 22, 1749–1758. [Google Scholar] [CrossRef]
- Saravanakumar, G.; Jo, D.-G.; H Park, J. Polysaccharide-based nanoparticles: A versatile platform for drug delivery and biomedical imaging. Curr. Med. Chem. 2012, 19, 3212–3229. [Google Scholar] [CrossRef] [PubMed]
- Posocco, B.; Dreussi, E.; de Santa, J.; Toffoli, G.; Abrami, M.; Musiani, F.; Grassi, M.; Farra, R.; Tonon, F.; Grassi, G. Polysaccharides for the delivery of antitumor drugs. Materials 2015, 8, 2569–2615. [Google Scholar] [CrossRef]
- Percival, E. The polysaccharides of green, red and brown seaweeds: Their basic structure, biosynthesis and function. Br. Phycol. J. 1979, 14, 103–117. [Google Scholar] [CrossRef]
- Barros, F.C.N.; da Silva, D.C.; Sombra, V.G.; Maciel, J.S.; Feitosa, J.P.A.; Freitas, A.L.P.; de Paula, R.C.M. Structural characterization of polysaccharide obtained from red seaweed Gracilaria caudata (J Agardh). Carbohydr. Polym. 2013, 92, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, S.; Kusaykin, M.; Trincone, A.; Tatiana, Z. Are multifunctional marine polysaccharides a myth or reality? Front. Chem. 2015, 3, 39. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.H.; Alves, A.; Popa, E.G.; Reys, L.L.; Gomes, M.E.; Sousa, R.A.; Silva, S.S.; Mano, J.F.; Reis, R.L. Marine algae sulfated polysaccharides for tissue engineering and drug delivery approaches. Biomatter 2012, 2, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A. Alginate particles as platform for drug delivery by the oral route: State-of-the-art. ISRN Pharm. 2014. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.P.; Neufeld, R.J.; Vilela, S.; Ribeiro, A.J.; Veiga, F. Review and current status of emulsion/dispersion technology using an internal gelation process for the design of alginate particles. J. Microencapsul. 2006, 23, 245–257. [Google Scholar] [CrossRef] [PubMed]
- You, J.-O. Efficient Gene Delivery with Calcium-Alginate Nanoparticles; ProQuest: Ann Arbor, MI, USA, 2006. [Google Scholar]
- Ciofani, G.; Raffa, V.; Obata, Y.; Menciassi, A.; Dario, P.; Takeoka, S. Magnetic driven alginate nanoparticles for targeted drug delivery. Curr. Nanosci. 2008, 4, 212–218. [Google Scholar] [CrossRef]
- Vauthier, C.; Bouchemal, K. Methods for the preparation and manufacture of polymeric nanoparticles. Pharm. Res. 2009, 26, 1025–1058. [Google Scholar] [CrossRef] [PubMed]
- Duceppe, N.; Tabrizian, M. Advances in using chitosan-based nanoparticles for in vitro and in vivo drug and gene delivery. Expert Opin. Drug Deliv. 2010, 7, 1191–1207. [Google Scholar] [CrossRef] [PubMed]
- Lertsutthiwong, P.; Rojsitthisak, P. Chitosan-alginate nanocapsules for encapsulation of turmeric oil. Die Pharm. An Int. J. Pharm. Sci. 2011, 66, 911–915. [Google Scholar]
- Sun, J.; Tan, H. Alginate-based biomaterials for regenerative medicine applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef]
- Paredes Juárez, G.A.; Spasojevic, M.; Faas, M.; de Vos, P. Immunological and technical considerations in application of alginate-based microencapsulation systems. Front. Bioeng. Biotechnol. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, M.; Zheng, M.; Guo, Q.; Wang, Y.; Wang, H.; Xie, X.; Huang, F.; Gong, R. Folate mediated self-assembled phytosterol-alginate nanoparticles for targeted intracellular anticancer drug delivery. Colloids Surf. B 2015, 129, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Bulmer, C.; Margaritis, A. Characterization of novel composite alginate chitosan-carrageenan nanoparticles for encapsulation of BSA as a model drug delivery system. Curr. Drug Deliv. 2015, 12, 351–357. [Google Scholar] [CrossRef]
- Katuwavila, K.; Perera, A.; Karunaratne, V.; Karunaratne, D. Determination of in vitro release kinetics of doxorubicin from chitosan and chitosan-alginate nanoparticles. In Proceedings of the Peradeniya University International Research Sessions (iPURSE), Peradeniya, Sri Lanka, 4–5 July 2014; Volume 18.
- Racoviţă, S.; Vasiliu, S.; Popa, M.; Luca, C. Polysaccharides based on micro-and nanoparticles obtained by ionic gelation and their applications as drug delivery systems. Rev. Roum. Chim. 2009, 54, 709–718. [Google Scholar]
- Hudson, D.; Margaritis, A. Biopolymer nanoparticle production for controlled release of biopharmaceuticals. Crit. Rev. Biotechnol. 2014, 34, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.H.E.; Lundberg, D.; Ribeiro, A.J.; Veiga, F.J.; Lindman, B.; Miguel, M.G.; Olsson, U. Preparation of calcium alginate nanoparticles using water-in-oil (w/o) nanoemulsions. Langmuir 2012, 28, 4131–4141. [Google Scholar] [CrossRef] [PubMed]
- You, J.O.; Peng, C.A. Calcium-Alginate Nanoparticles Formed by Reverse Microemulsion as Gene Carriers, Macromolecular Symposia; Wiley Online Library: New York, NY, USA, 2005; pp. 147–153. [Google Scholar]
- Yi, Y.-M.; Yang, T.-Y.; Pan, W.-M. Preparation and distribution of 5-fluorouracil 125I sodium alginate-bovine serum albumin nanoparticles. World J. Gastroenterol. 1999, 5, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, B.; Ferreira, D.; Veiga, F.; Ribeiro, A. Characterization of insulin-loaded alginate nanoparticles produced by ionotropic pre-gelation through DSC and FTIR studies. Carbohydr. Polym. 2006, 66, 1–7. [Google Scholar] [CrossRef]
- Motwani, S.K.; Chopra, S.; Talegaonkar, S.; Kohli, K.; Ahmad, F.J.; Khar, R.K. Chitosan–sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: Formulation, optimisation and in vitro characterisation. Eur. J. Pharm. Biopharm. 2008, 68, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Pandey, R.; Sharma, S.; Khuller, G. Alginate nanoparticles as antituberculosis drug carriers: Formulation development, pharmacokinetics and therapeutic potential. Indian J. Chest Dis. Allied Sci. 2006, 48, 171. [Google Scholar] [PubMed]
- Arora, S.; Gupta, S.; Narang, R.K.; Budhiraja, R.D. Amoxicillin loaded chitosan–alginate polyelectrolyte complex nanoparticles as mucopenetrating delivery system for H. Pylori. Sci. Pharm. 2011, 79, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Sarei, F.; Dounighi, N.M.; Zolfagharian, H.; Khaki, P.; Bidhendi, S.M. Alginate nanoparticles as a promising adjuvant and vaccine delivery system. Indian J. Pharm. Sci. 2013, 75, 442–449. [Google Scholar] [PubMed]
- Azevedo, M.A.; Bourbon, A.I.; Vicente, A.A.; Cerqueira, M.A. Alginate/chitosan nanoparticles for encapsulation and controlled release of vitamin B2. Int. J. Biol. Macromol. 2014, 71, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.; Sabik, J.F.; Ainkaran, P.; Blackstone, E.H. Coronary artery bypass grafting in diabetics: A growing healthcare cost crisis. J. Thorac Cardiovasc. Surg. 2015, 150, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Diabetes. Available online: http://www.who.int/mediacentre/factsheets/fs312/en/ (accessed on 2 January 2016).
- Mukhopadhyay, P.; Chakraborty, S.; Bhattacharya, S.; Mishra, R.; Kundu, P. pH-sensitive chitosan/alginate core-shell nanoparticles for efficient and safe oral insulin delivery. Int. J. Biol. Macromol. 2015, 72, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.P.; Neufeld, R.; Ribeiro, A.J.; Veiga, F. Design of insulin-loaded alginate nanoparticles: Influence of the calcium ion on polymer gel matrix properties. Chem. Ind. Chem. Eng. Q. 2006, 12, 47–52. [Google Scholar] [CrossRef]
- Sarmento, B.; Ribeiro, A.; Veiga, F.; Ferreira, D.; Neufeld, R. Insulin-loaded nanoparticles are prepared by alginate ionotropic pre-gelation followed by chitosan polyelectrolyte complexation. J. Nanosci. Nanotechnol. 2007, 7, 2833–2841. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, B.; Ribeiro, A.; Veiga, F.; Sampaio, P.; Neufeld, R.; Ferreira, D. Alginate/chitosan nanoparticles are effective for oral insulin delivery. Pharm. Res. 2007, 24, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- Goycoolea, F.M.; Lollo, G.; Remunán-López, C.; Quaglia, F.; Alonso, M.J. Chitosan-alginate blended nanoparticles as carriers for the transmucosal delivery of macromolecules. Biomacromolecules 2009, 10, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; He, N. Preparation, characterization and applications of low-molecular-weight alginate–oligochitosan nanocapsules. Nanoscale 2010, 2, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Nesamony, J.; Singh, P.R.; Nada, S.E.; Shah, Z.A.; Kolling, W.M. Calcium alginate nanoparticles synthesized through a novel interfacial cross-linking method as a potential protein drug delivery system. J. Pharm. Sci. 2012, 101, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Shi, X.W.; Du, Y.M.; Tang, Y.F. Quaternized chitosan/alginate nanoparticles for protein delivery. J. Biomed. Mater. Res. 2007, 83, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Cancer Statistics, National Cancer Institute. Available online: http://www.cancer.gov/about-cancer/what-is-cancer/statistics (accessed on 2 January 2016).
- Bhunchu, S.; Rojsitthisak, P. Biopolymeric alginate-chitosan nanoparticles as drug delivery carriers for cancer therapy. Die Pharm. An Int. J. Pharm. Sci. 2014, 69, 563–570. [Google Scholar]
- Alexis, F.; Pridgen, E.M.; Langer, R.; Farokhzad, O.C. Nanoparticle technologies for cancer therapy. In Drug Delivery; Springer: New York, NY, USA, 2010; pp. 55–86. [Google Scholar]
- Rajaonarivony, M.; Vauthier, C.; Couarraze, G.; Puisieux, F.; Couvreur, P. Development of a new drug carrier made from alginate. J. Pharm. Sci. 1993, 82, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, W.; Wang, C.; Tian, Q.; Huang, W.; Yuan, Z.; Chen, X. Cytotoxicity of liver targeted drug-loaded alginate nanoparticles. Sci. China Ser. B 2009, 52, 1382–1387. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, W.; Liu, T.; Wu, Y.; Guo, H.; Wang, P.; Tian, Q.; Wang, Y.; Yuan, Z. Doxorubicin-loaded glycyrrhetinic acid-modified alginate nanoparticles for liver tumor chemotherapy. Biomaterials 2012, 33, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lai, Q.; Wang, W.; Wu, Y.; Zhang, C.; Liu, Y.; Yuan, Z. Functional alginate nanoparticles for efficient intracellular release of doxorubicin and hepatoma carcinoma cell targeting therapy. Int. J. Pharm. 2013, 451, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Khdair, A.; Handa, H.; Mao, G.; Panyam, J. Nanoparticle-mediated combination chemotherapy and photodynamic therapy overcomes tumor drug resistance in vitro. Eur. J. Pharm. Biopharm. 2009, 71, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liu, C.-J.; Zhuo, R.-X.; Cheng, S.-X. Alginate/CaCO3 hybrid nanoparticles for efficient codelivery of antitumor gene and drug. Mol. Pharm. 2012, 9, 2887–2893. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhuo, R.-X.; Cheng, S.-X. Alginate modified nanostructured calcium carbonate with enhanced delivery efficiency for gene and drug delivery. Mol. Biosyst. 2012, 8, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Deng, L.; Dong, A. Chitosan/alginate nanoparticles stabilized by poloxamer for the controlled release of 5-fluorouracil. J. Appl. Polym. Sci. 2010, 117, 2354–2359. [Google Scholar] [CrossRef]
- Nagarwal, R.C.; Kumar, R.; Pandit, J. Chitosan coated sodium alginate–chitosan nanoparticles loaded with 5-FU for ocular delivery: In vitro characterization and in vivo study in rabbit eye. Eur. J. Pharm. Sci. 2012, 47, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Kasoju, N.; Bora, U. Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Aynie, I.; Vauthier, C.; Chacun, H.; Fattal, E.; Couvreur, P. Spongelike alginate nanoparticles as a new potential system for the delivery of antisense oligonucleotides. Antisense Nucleic Acid Drug Dev. 1999, 9, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Karar, P. Optimization of process variables for the preparation of chitosan-alginate nanoparticles. Int. J Pharm Pharm. Sci. 2011, 3, 78–80. [Google Scholar]
- Martinez, A.; Benito-Miguel, M.; Iglesias, I.; Teijon, J.; Blanco, M. Tamoxifen-loaded thiolated alginate-albumin nanoparticles as antitumoral drug delivery systems. J. Biomed. Mater. Res. 2012, 100, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pornpattananangkul, D.; Hu, C.-M.; Huang, C.-M. Development of nanoparticles for antimicrobial drug delivery. Curr. Med. Chem. 2010, 17, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, A.; Sharma, S.; Khuller, G. Inhalable alginate nanoparticles as antitubercular drug carriers against experimental tuberculosis. Int. J. Antimicrob. Agents 2005, 26, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Pandey, R.; Sharma, S.; Khuller, G.K. Pharmacokinetic and pharmacodynamic behaviour of antitubercular drugs encapsulated in alginate nanoparticles at two doses. Int. J. Antimicrob. Agents 2006, 27, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Choonara, Y.E.; Pillay, V.; Ndesendo, V.M.; du Toit, L.C.; Kumar, P.; Khan, R.A.; Murphy, C.S.; Jarvis, D.-L. Polymeric emulsion and crosslink-mediated synthesis of super-stable nanoparticles as sustained-release anti-tuberculosis drug carriers. Colloids Surf. B 2011, 87, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, S.; Varshosaz, J.; Haririan, I.; Khoshayand, M.R.; Azarmi, S.; Gazori, T. Ciprofloxacin loaded alginate/chitosan and solid lipid nanoparticles, preparation, and characterization. J. Dispers. Sci. Technol. 2012, 33, 685–689. [Google Scholar] [CrossRef]
- Chopra, M.; Kaur, P.; Bernela, M.; Thakur, R. Synthesis and optimization of streptomycin loaded chitosan-alginate nanoparticles. Int. J. Sci. Technol. Res. 2012, 1, 31–34. [Google Scholar]
- Balaure, P.C.; Andronescu, E.; Grumezescu, A.M.; Ficai, A.; Huang, K.-S.; Yang, C.-H.; Chifiriuc, C.M.; Lin, Y.-S. Fabrication, characterization and in vitro profile based interaction with eukaryotic and prokaryotic cells of alginate–chitosan–silica biocomposite. Int. J. Pharm. 2013, 441, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Zohri, M.; Alavidjeh, M.S.; Haririan, I.; Ardestani, M.S.; Ebrahimi, S.E.S.; Sani, H.T.; Sadjadi, S.K. A comparative study between the antibacterial effect of nisin and nisin-loaded chitosan/alginate nanoparticles on the growth of staphylococcus aureus in raw and pasteurized milk samples. Probiotics Antimicrob. Proteins 2010, 2, 258–266. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Robinson, D. Polymer relationships during preparation of chitosan–alginate and poly-l-lysine–alginate nanospheres. J. Control. Release 2003, 89, 101–112. [Google Scholar] [CrossRef]
- Boissiere, M.; Allouche, J.; Chanéac, C.; Brayner, R.; Devoisselle, J.-M.; Livage, J.; Coradin, T. Potentialities of silica/alginate nanoparticles as hybrid magnetic carriers. Int. J. Pharm. 2007, 344, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Ciofani, G.; Raffa, V.; Menciassi, A.; Dario, P. Alginate and chitosan particles as drug delivery system for cell therapy. Biomed. Microdevices 2008, 10, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Dai, Y.-N.; Zhang, J.-P.; Wang, A.-Q.; Wei, Q. Chitosan-alginate nanoparticles as a novel drug delivery system for nifedipine. Int. J. Biomed. Sci. 2008, 4, 221. [Google Scholar] [PubMed]
- Sudakar, C.; Dixit, A.; Regmi, R.; Naik, R.; Lawes, G.; Naik, V.M.; Vaishnava, P.P.; Toti, U.; Panyam, J. FeO incorporated AOT-alginate nanoparticles for drug delivery. IEEE Trans. Magn. 2008, 44, 2800–2803. [Google Scholar] [CrossRef]
- Gazori, T.; Khoshayand, M.R.; Azizi, E.; Yazdizade, P.; Nomani, A.; Haririan, I. Evaluation of alginate/chitosan nanoparticles as antisense delivery vector: Formulation, optimization and in vitro characterization. Carbohydr. Polym. 2009, 77, 599–606. [Google Scholar] [CrossRef]
- Lertsutthiwong, P.; Rojsitthisak, P.; Nimmannit, U. Preparation of turmeric oil-loaded chitosan-alginate biopolymeric nanocapsules. Mat. Sci. Eng. C 2009, 29, 856–860. [Google Scholar] [CrossRef]
- Azizi, E.; Namazi, A.; Haririan, I.; Fouladdel, S.; Khoshayand, M.R.; Shotorbani, P.Y.; Nomani, A.; Gazori, T. Release profile and stability evaluation of optimized chitosan/alginate nanoparticles as EGFR antisense vector. Int. J. Nanomed. 2010, 5, 455. [Google Scholar]
- Grillo, R.; de Melo, N.F.; de Araújo, D.R.; de Paula, E.; Rosa, A.H.; Fraceto, L.F. Polymeric alginate nanoparticles containing the local anesthetic bupivacaine. J. Drug Target. 2010, 18, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Chang, S.M.; Tsai, K.C.; Chen, W.S.; Lin, F.H.; Shieh, M.J. Effect of chitosan-alginate nanoparticles and ultrasound on the efficiency of gene transfection of human cancer cells. J. Gene Med. 2010, 12, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, C.-G.; Huang, Z.-H.; Xue, F.-F. Preparation and characterization of nanoparticles based on hydrophobic alginate derivative as carriers for sustained release of vitamin D3. J. Agric. Food. Chem. 2011, 59, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-J.; Lin, F.-H.; Tsai, H.-M.; Lin, C.-F.; Chin, H.-C.; Wong, J.-M.; Shieh, M.-J. Alginate-folic acid-modified chitosan nanoparticles for photodynamic detection of intestinal neoplasms. Biomaterials 2011, 32, 2174–2182. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Amiji, M. Tuftsin-modified alginate nanoparticles as a noncondensing macrophage-targeted DNA delivery system. Biomacromolecules 2012, 13, 1074–1085. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, X.; Li, Y.; Cui, Y.; Zhu, H.; Zhu, W. Preparation of superparamagnetic sodium alginate nanoparticles for covalent immobilization of candida rugosa lipase. J. Nanopart. Res. 2012, 14, 1–7. [Google Scholar] [CrossRef]
- Lu, C.; Liu, P. Effect of chitosan multilayers encapsulation on controlled release performance of drug-loaded superparamagnetic alginate nanoparticles. J. Mater. Sci. Mater. Med. 2012, 23, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Su, M.; Tang, S.; Wang, L.; Liang, X.; Meng, F.; Hong, Y.; Xu, Z. Synthesis of thiolated chitosan and preparation nanoparticles with sodium alginate for ocular drug delivery. Mol. Vis. 2012, 18, 1973–1982. [Google Scholar] [PubMed]
- Ali, M.; Afzal, M.; Verma, M.; Misra-Bhattacharya, S.; Ahmad, F.J.; Dinda, A.K. Improved antifilarial activity of ivermectin in chitosan–alginate nanoparticles against human lymphatic filarial parasite, brugia malayi. Parasitol. Res. 2013, 112, 2933–2943. [Google Scholar] [CrossRef] [PubMed]
- Bagre, A.P.; Jain, K.; Jain, N.K. Alginate coated chitosan core shell nanoparticles for oral delivery of enoxaparin: In vitro and in vivo assessment. Int. J. Pharm. 2013, 456, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Marcato, P.D.; Adami, L.F.; de Melo Barbosa, R.; Melo, P.; Ferreira, I.R.; de Paula, L.; Duran, N.; Seabra, A.B. Development of a sustained-release system for nitric oxide delivery using alginate/chitosan nanoparticles. Curr. Nanosci. 2013, 9, 1–7. [Google Scholar]
- Friedman, A.J.; Phan, J.; Schairer, D.O.; Champer, J.; Qin, M.; Pirouz, A.; Blecher-Paz, K.; Oren, A.; Liu, P.T.; Modlin, R.L. Antimicrobial and anti-inflammatory activity of chitosan–alginate nanoparticles: A targeted therapy for cutaneous pathogens. J. Investig. Dermatol. 2013, 133, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Paques, J.P.; van der Linden, E.; van Rijn, C.J.; Sagis, L.M. Alginate submicron beads prepared through w/o emulsification and gelation with Cacl2 nanoparticles. Food Hydrocoll. 2013, 31, 428–434. [Google Scholar] [CrossRef]
- Kumar, S.; Bhanjana, G.; Sharma, A.; Sidhu, M.; Dilbaghi, N. Synthesis, characterization and on field evaluation of pesticide loaded sodium alginate nanoparticles. Carbohydr. Polym. 2014, 101, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Manuja, A.; Kumar, S.; Dilbaghi, N.; Bhanjana, G.; Chopra, M.; Kaur, H.; Kumar, R.; Manuja, B.K.; Singh, S.K.; Yadav, S.C. Quinapyramine sulfate-loaded sodium alginate nanoparticles show enhanced trypanocidal activity. Nanomedicine 2014, 9, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Yang, S. Composition and Method for Preparing Alginate Nanocapsules. U.S. Patents 8,449,919 B2, 28 May 2013. [Google Scholar]
- Panyam, J.; Chavanpatil, M.D. Polymer-Surfactant Nanoparticles for Sustained Release of Compounds. U.S. Patent 20,110,020,457 A1, 27 January 2011. [Google Scholar]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef] [PubMed]
- Charalampopoulos, D.; Wang, R.; Pandiella, S.; Webb, C. Application of cereals and cereal components in functional foods: A review. Int. J. Food Microbiol. 2002, 79, 131–141. [Google Scholar] [CrossRef]
- Luo, M.; Shao, B.; Nie, W.; Wei, X.-W.; Li, Y.-L.; Wang, B.-L.; He, Z.-Y.; Liang, X.; Ye, T.-H.; Wei, Y.-Q. Antitumor and adjuvant activity of λ-carrageenan by stimulating immune response in cancer immunotherapy. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Murad, H.; Ghannam, A.; Al-Ktaifani, M.; Abbas, A.; Hawat, M. Algal sulfated carrageenan inhibits proliferation of MDA-MB-231 cells via apoptosis regulatory genes. Mol. Med. Rep. 2015, 11, 2153–2158. [Google Scholar] [CrossRef] [PubMed]
- Şen, M.; Avcı, E.N. Radiation synthesis of poly (n-vinyl-2-pyrrolidone)–κ-carrageenan hydrogels and their use in wound dressing applications. I. Preliminary laboratory tests. J. Biomed. Mater. Res. 2005, 74, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural–origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev. 2007, 59, 207–233. [Google Scholar] [CrossRef] [PubMed]
- Santo, V.E.; Frias, A.M.; Carida, M.; Cancedda, R.; Gomes, M.E.; Mano, J.F.; Reis, R.L. Carrageenan-based hydrogels for the controlled delivery of PDGF-BB in bone tissue engineering applications. Biomacromolecules 2009, 10, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Rocha, P.M.; Santo, V.E.; Gomes, M.E.; Reis, R.L.; Mano, J.F. Encapsulation of adipose-derived stem cells and transforming growth factor-β1 in carrageenan-based hydrogels for cartilage tissue engineering. J. Bioact. Compatible Polym. 2011, 26, 493–507. [Google Scholar] [CrossRef]
- Mihaila, S.M.; Gaharwar, A.K.; Reis, R.L.; Marques, A.P.; Gomes, M.E.; Khademhosseini, A. Photocrosslinkable κ-carrageenan hydrogels for tissue engineering applications. Adv. Healthc. Mater. 2013, 2, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ni, R.; Shao, Y.; Mao, S. Carrageenan and its applications in drug delivery. Carbohydr. Polym. 2014, 103, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Melo-Silveira, R.F.; Almeida-Lima, J.; Rocha, H.A.O. Application of marine polysaccharides in nanotechnology. In Marine Medicinal Glycomics; Nova Science Publishers, Inc.: New York, NY, USA, 2013; pp. 95–114. [Google Scholar]
- Kadajji, V.G.; Betageri, G.V. Water soluble polymers for pharmaceutical applications. Polymers 2011, 3, 1972–2009. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.; Cardoso, L.; da Costa, A.M.R.; Grenha, A. Biocompatibility and stability of polysaccharide polyelectrolyte complexes aimed at respiratory delivery. Materials 2015, 8, 5647–5670. [Google Scholar] [CrossRef]
- Rodrigues, S.; Costa, A.M.R.D.; Grenha, A. Chitosan/carrageenan nanoparticles: Effect of cross-linking with tripolyphosphate and charge ratios. Carbohydr. Polym. 2012, 89, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.C.; Bourbon, A.I.; Quintas, M.A.C.; Coimbra, M.A.; Vicente, A.A. Κ-carrageenan/chitosan nanolayered coating for controlled release of a model bioactive compound. Innov. Food Sci. Emerg. Technol. 2012, 16, 227–232. [Google Scholar] [CrossRef]
- Bulmer, C.; Margaritis, A.; Xenocostas, A. Encapsulation and controlled release of recombinant human erythropoietin from chitosan-carrageenan nanoparticles. Curr. Drug Del. 2012, 9, 527–537. [Google Scholar] [CrossRef]
- Grenha, A.; Gomes, M.E.; Rodrigues, M.; Santo, V.E.; Mano, J.F.; Neves, N.M.; Reis, R.L. Development of new chitosan/carrageenan nanoparticles for drug delivery applications. J. Biomed. Mater. Res. A 2010, 92, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Daniel-da-Silva, A.L.; Ferreira, L.; Gil, A.M.; Trindade, T. Synthesis and swelling behavior of temperature responsive κ-carrageenan nanogels. J. Colloid Interface Sci. 2011, 355, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.; Cordeiro, C.; Seijo, B.; Remuñán-López, C.; Grenha, A. Hybrid nanosystems based on natural polymers as protein carriers for respiratory delivery: Stability and toxicological evaluation. Carbohydr. Polym. 2015, 123, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Dul, M.; Paluch, K.J.; Kelly, H.; Healy, A.M.; Sasse, A.; Tajber, L. Self-assembled carrageenan/protamine polyelectrolyte nanoplexes-investigation of critical parameters governing their formation and characteristics. Carbohydr. Polym. 2015, 123, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Mahdavinia, G.R.; Etemadi, H.; Soleymani, F. Magnetic/pH-responsive beads based on caboxymethyl chitosan and κ-carrageenan and controlled drug release. Carbohydr. Polym. 2015, 128, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Daniel-Da-Silva, A.L.; Moreira, J.; Neto, R.; Estrada, A.C.; Gil, A.M.; Trindade, T. Impact of magnetic nanofillers in the swelling and release properties of κ-carrageenan hydrogel nanocomposites. Carbohydr. Polym. 2012, 87, 328–335. [Google Scholar] [CrossRef]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.A.; Grenha, A. Polysaccharide nanoparticles for protein and peptide delivery: Exploring less-known materials. Adv. Protein Chem. Struct. Biol. 2015, 98, 223–261. [Google Scholar] [PubMed]
- Murakami, K.; Aoki, H.; Nakamura, S.; Nakamura, S.-i.; Takikawa, M.; Hanzawa, M.; Kishimoto, S.; Hattori, H.; Tanaka, Y.; Kiyosawa, T. Hydrogel blends of chitin/chitosan, fucoidan and alginate as healing-impaired wound dressings. Biomaterials 2010, 31, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Sezer, A.D.; Cevher, E. Fucoidan: A versatile biopolymer for biomedical applications. In Active Implants and Scaffolds for Tissue Regeneration; Springer: New York, NY, USA, 2011; pp. 377–406. [Google Scholar]
- Lee, H.M.; Kim, J.-K.; Cho, T.-S. Applications of ophthalmic biomaterials embedded with fucoidan. J. Ind. Eng. Chem. 2012, 18, 1197–1201. [Google Scholar] [CrossRef]
- Murakami, K.; Ishihara, M.; Aoki, H.; Nakamura, S.; Nakamura, S.I.; Yanagibayashi, S.; Takikawa, M.; Kishimoto, S.; Yokoe, H.; Kiyosawa, T. Enhanced healing of mitomycin c-treated healing-impaired wounds in rats with hydrosheets composed of chitin/chitosan, fucoidan, and alginate as wound dressings. Wound Repair Regen. 2010, 18, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.C.; Bourbon, A.I.; Cerqueira, M.A.; Maricato, É.; Nunes, C.; Coimbra, M.A.; Vicente, A.A. Chitosan/fucoidan multilayer nanocapsules as a vehicle for controlled release of bioactive compounds. Carbohydr. Polym. 2014, 115, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Lim, K.H. Polyelectrolyte complexes of chitosan self-assembled with fucoidan: An optimum condition to prepare their nanoparticles and their characteristics. Korean J. Chem. Eng. 2014, 31, 664–675. [Google Scholar] [CrossRef]
- Lee, E.J.; Lim, K.H. Formation of chitosan-fucoidan nanoparticles and their electrostatic interactions: Quantitative analysis. J. Biosci. Bioeng. 2015, 121, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Kimura, R.; Rokkaku, T.; Takeda, S.; Senba, M.; Mori, N. Cytotoxic effects of fucoidan nanoparticles against osteosarcoma. Mar. Drugs 2013, 11, 4267–4278. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Santos, N.; Almeida-Lima, J.; Vidal, A.A.J.; Gomes, D.L.; Oliveira, R.M.; Pedrosa, S.S.; Pereira, P.; Gama, F.M.; Rocha, H.A.O. Antiproliferative activity of fucan nanogel. Mar. Drugs 2012, 10, 2002–2022. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Yang, Y.T. Effect of basic fibroblast growth factor released from chitosan-fucoidan nanoparticles on neurite extension. J. Tissue Eng. Regen. Med. 2013. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Kuo, T.H. O-carboxymethyl chitosan/fucoidan nanoparticles increase cellular curcumin uptake. Food Hydrocoll. 2015, 53, 261–269. [Google Scholar] [CrossRef]
- Da Silva, L.C.; Garcia, T.; Mori, M.; Sandri, G.; Bonferoni, M.C.; Finotelli, P.V.; Cinelli, L.P.; Caramella, C.; Cabral, L.M. Preparation and characterization of polysaccharide-based nanoparticles with anticoagulant activity. Int. J. Nanomed. 2012, 7, 2975–2986. [Google Scholar] [CrossRef] [PubMed]
- Lira, M.C.B.; Santos-Magalhães, N.S.; Nicolas, V.; Marsaud, V.; Silva, M.P.C.; Ponchel, G.; Vauthier, C. Cytotoxicity and cellular uptake of newly synthesized fucoidan-coated nanoparticles. Eur. J. Pharm. Biopharm. 2011, 79, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Liu, T.J. Mobilization of mesenchymal stem cells by stromal cell-derived factor-1 released from chitosan/tripolyphosphate/fucoidan nanoparticles. Acta Biomater. 2012, 8, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yao, W.; Wang, S.; Geng, D.; Zheng, Q.; Chen, A. Preparation and characterization of fucoidan-chitosan nanospheres by the sonification method. J. Nanosci. Nanotechnol. 2014, 14, 3844–3849. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, K.; Manivasagan, P.; Venkatesan, J.; Kim, S.-K. Brown seaweed fucoidan: Biological activity and apoptosis, growth signaling mechanism in cancer. Int. J. Biol. Macromol. 2013, 60, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Park, S.Y.; Lee, J.-Y.; Park, J.H. Fucoidan present in brown algae induces apoptosis of human colon cancer cells. BMC Gastroenterol. 2010, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Delma, C.R.; Srinivasan, G.P.; Raviprakash, N.; Manna, S.K.; Somasundaram, S.T.; Aravindan, N. Fucoidan from turbinaria conoides attenuates pancreatic cancer progession by regulating p53-NFκB crosstalk. Cancer Res. 2015, 75, 5508–5508. [Google Scholar] [CrossRef]
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Dickinson, J.L. Fucoidan and cancer: A multifunctional molecule with anti-tumor potential. Mar. Drugs 2015, 13, 2327–2346. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, S.; Kim, S.-K. Fucoidan, a sulfated polysaccharides from brown algae as therapeutic target for cancer. In Handbook of Anticancer Drugs from Marine Origin; Springer: New York, NY, USA, 2015; pp. 145–164. [Google Scholar]
- Park, H.Y.; Kim, G.-Y.; Moon, S.-K.; Kim, W.J.; Yoo, Y.H.; Choi, Y.H. Fucoidan inhibits the proliferation of human urinary bladder cancer T24 cells by blocking cell cycle progression and inducing apoptosis. Molecules 2014, 19, 5981–5998. [Google Scholar] [CrossRef] [PubMed]
- Lowenthal, R.M.; Fitton, J.H. Are seaweed-derived fucoidans possible future anti-cancer agents? J. Appl. Phycol. 2015, 27, 2075–2077. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, Y.; Zhang, Y.; Zhang, D. Fucoidan induces cancer cell apoptosis by modulating the endoplasmic reticulum stress cascades. PLoS ONE 2014, 9, e108157. [Google Scholar]
- Huang, Y.C.; Lam, U.I. Chitosan/fucoidan ph sensitive nanoparticles for oral delivery system. J. Chin. Chem. Soc. 2011, 58, 779–785. [Google Scholar] [CrossRef]
- Fu, Y.; Gao, X.; Wan, Y.; Zhang, J.; Kong, D.; Wang, Z.; Zhao, Y. Dendritic nanoconjugate containing optimum folic acid for targeted intracellular curcumin delivery. RSC Adv. 2014, 4, 46020–46023. [Google Scholar] [CrossRef]
- Singh, R.; Lillard, J.W. Targeted delivery of curcumin to improve therapeutic outcome in breast cancer. Cancer Res. 2014, 74, 4476. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Rao, W.; Zhang, W.; Poventud-Fuentes, I.; Wang, Y.; Lei, Y.; Agarwal, P.; Weekes, B.; Li, C.; Lu, X.; Yu, J. Thermally responsive nanoparticle-encapsulated curcumin and its combination with mild hyperthermia for enhanced cancer cell destruction. Acta Biomater. 2014, 10, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Sreeranganathan, M.; Chennazhi, K.P.; Lakshmanan, V.-K.; Jayakumar, R. In vitro combinatorial anticancer effects of 5-fluorouracil and curcumin loaded n, O-carboxymethyl chitosan nanoparticles toward colon cancer and in vivo pharmacokinetic studies. Eur. J. Pharm. Biopharm. 2014, 88, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Jeong, D.; Na, K. Doxorubicin loading fucoidan acetate nanoparticles for immune and chemotherapy in cancer treatment. Carbohydr. Polym. 2013, 94, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S. Therapies from fucoidan: An update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef] [PubMed]
- Kanapathipillai, M.; Brock, A.; Ingber, D.E. Nanoparticle targeting of anti-cancer drugs that alter intracellular signaling or influence the tumor microenvironment. Adv. Drug Deliv. Rev. 2014, 79, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Lale, S.V.; Aravind, A.; Kumar, D.S.; Koul, V. As1411 aptamer and folic acid functionalized pH-responsive ATRP fabricated pPEGMA–PCL–pPEGMA polymeric nanoparticles for targeted drug delivery in cancer therapy. Biomacromolecules 2014, 15, 1737–1752. [Google Scholar] [CrossRef] [PubMed]
- Thambi, T.; Deepagan, V.; Yoon, H.Y.; Han, H.S.; Kim, S.-H.; Son, S.; Jo, D.-G.; Ahn, C.-H.; Suh, Y.D.; Kim, K. Hypoxia-responsive polymeric nanoparticles for tumor-targeted drug delivery. Biomaterials 2014, 35, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ho, W.; Zhang, X.; Bertrand, N.; Farokhzad, O. Cancer nanomedicine: From targeted delivery to combination therapy. Trends Mol. Med. 2015, 21, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Sheng, J. The antihyperlipidemic mechanism of high sulfate content ulvan in rats. Mar. Drugs 2015, 13, 3407–3421. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Samal, S.K.; Bartoli, C.; Morelli, A.; Smet, P.F.; Dubruel, P.; Chiellini, F. Biofunctionalization of ulvan scaffolds for bone tissue engineering. ACS Appl. Mater. Interfaces 2014, 6, 3211–3218. [Google Scholar] [CrossRef] [PubMed]
- Efthimiadou, E.K.; Metaxa, A.-F.; Kordas, G. Modified polysaccharides for drug delivery. Polysacch. Bioact. Biotechnol. 2015, 1805–1835. [Google Scholar]
- Graiff, A.; Ruth, W.; Kragl, U.; Karsten, U. Chemical characterization and quantification of the brown algal storage compound laminarin—A new methodological approach. J. Appl. Phycol. 2015. [Google Scholar] [CrossRef]
- Douglas, K.L.; Tabrizian, M. Effect of experimental parameters on the formation of alginate–chitosan nanoparticles and evaluation of their potential application as DNA carrier. J. Biomater. Sci. Polym. Ed. 2005, 16, 43–56. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).