Cationic Nanocylinders Promote Angiogenic Activities of Endothelial Cells
Abstract
:1. Introduction
2. Experimental Section
2.1. Fabrication of PLLA Nanocylinders (NCs)
2.2. Characterization of PLLA NCs
2.3. Cell Culture
2.4. HUVEC Migration Assay
2.5. Permeability Test
2.6. In Vitro Tubulogenesis
2.7. Quantitative Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR)
2.8. Statistical Analysis
3. Results and Discussion
3.1. NC Characterization

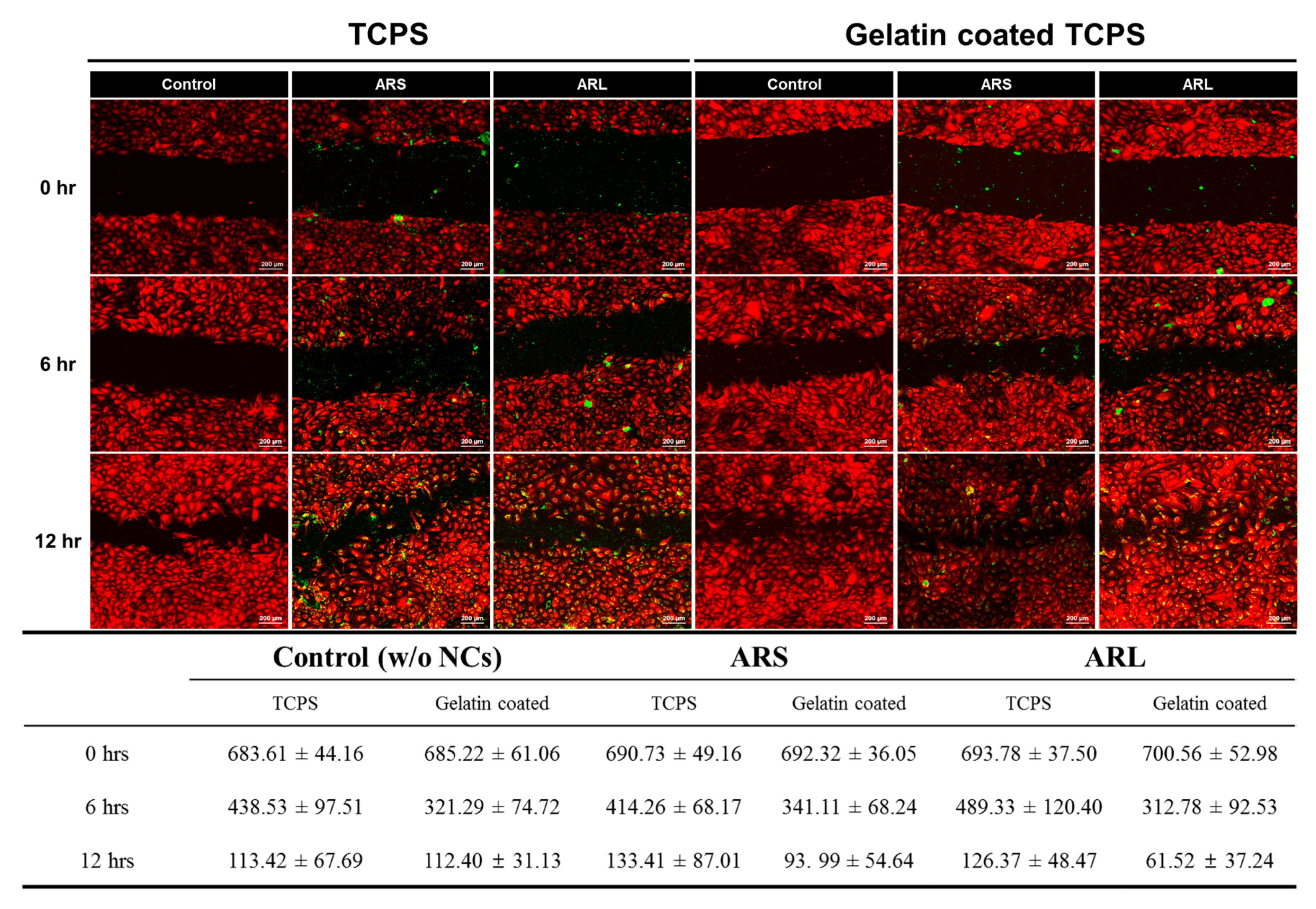
3.2. Cell Migration Study
3.3. Permeability Test
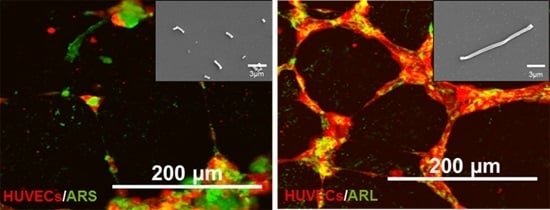
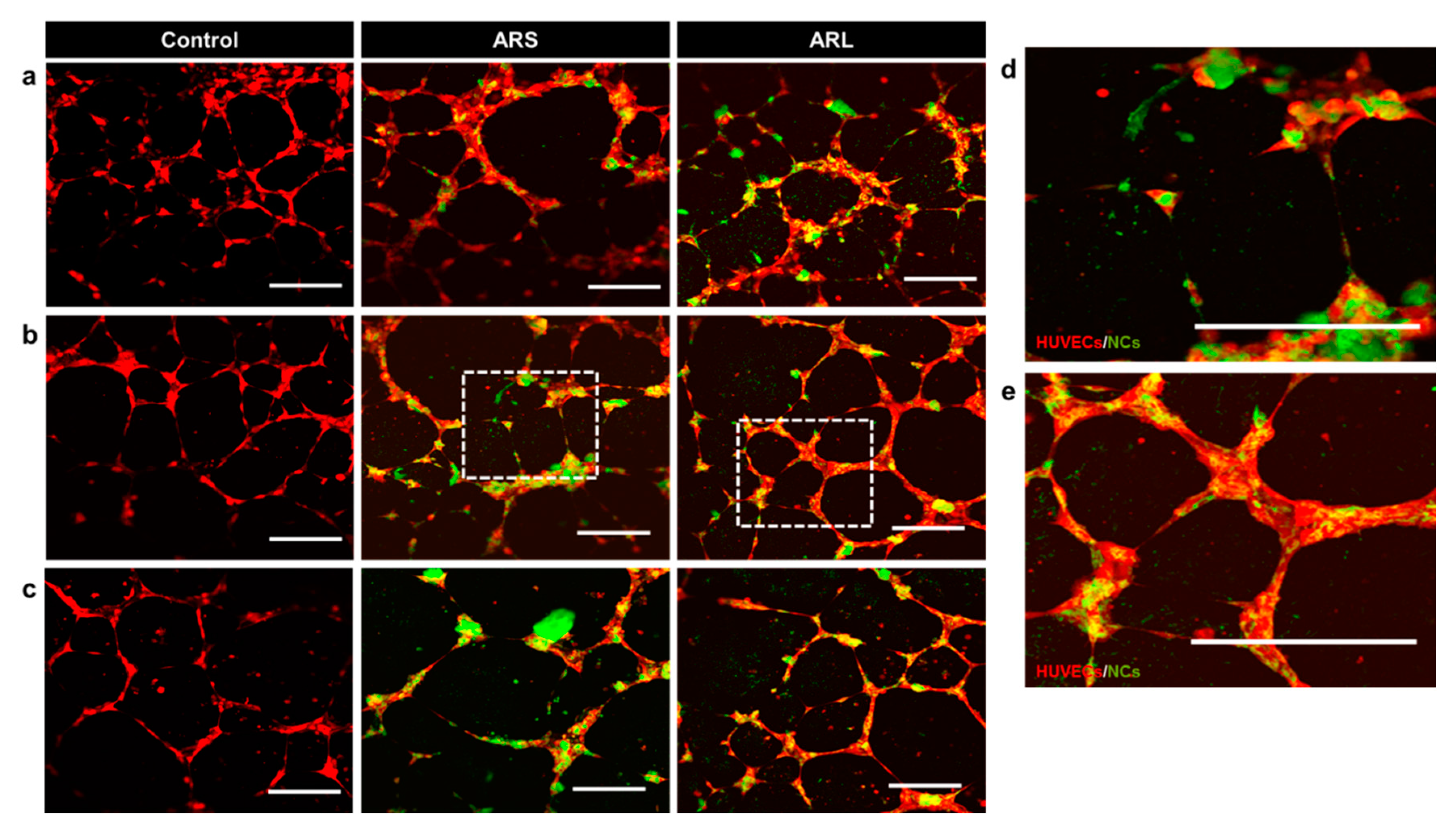
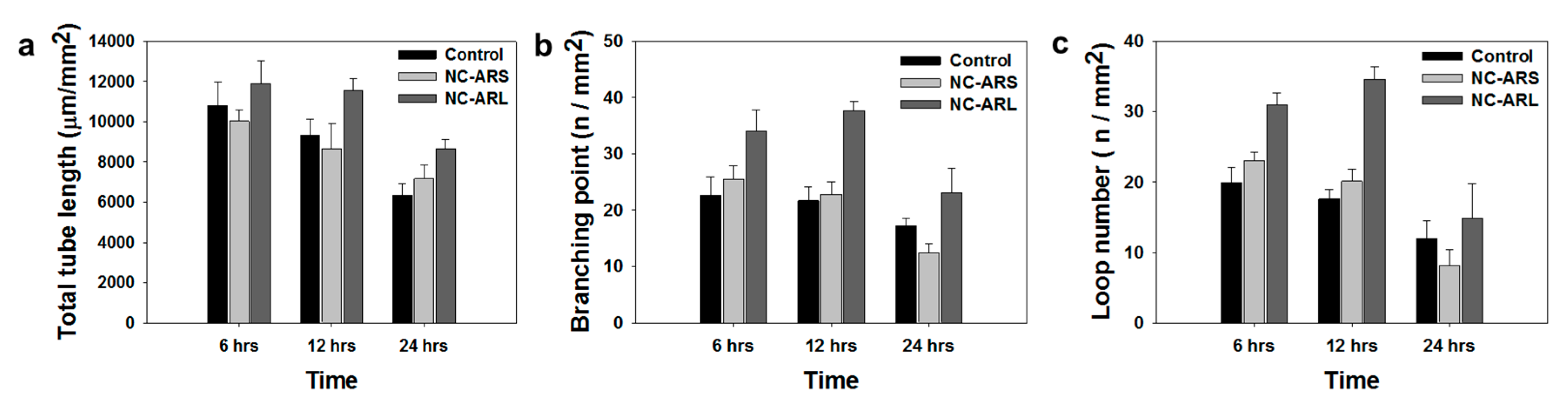
3.4. Tubulogenesis In Vitro
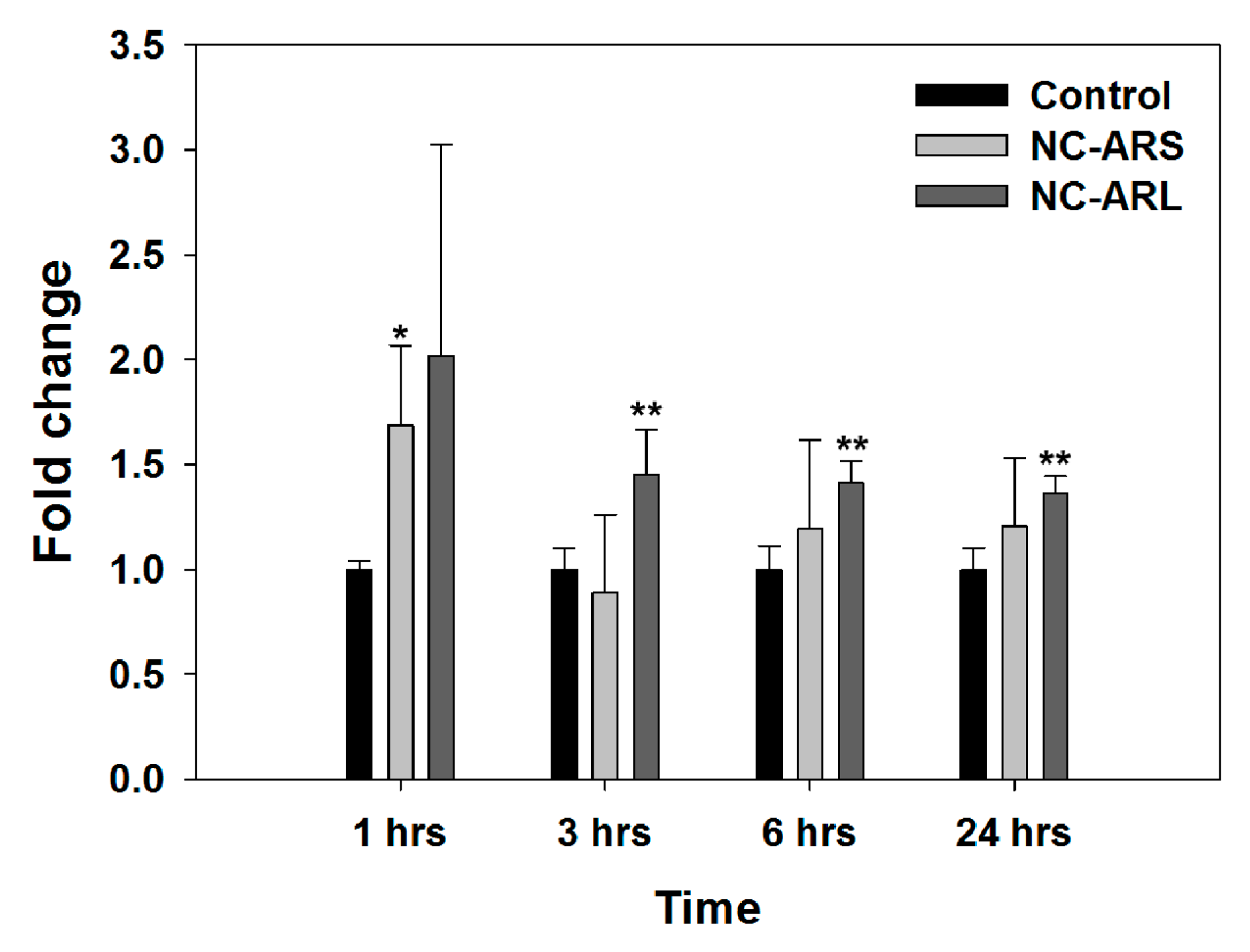
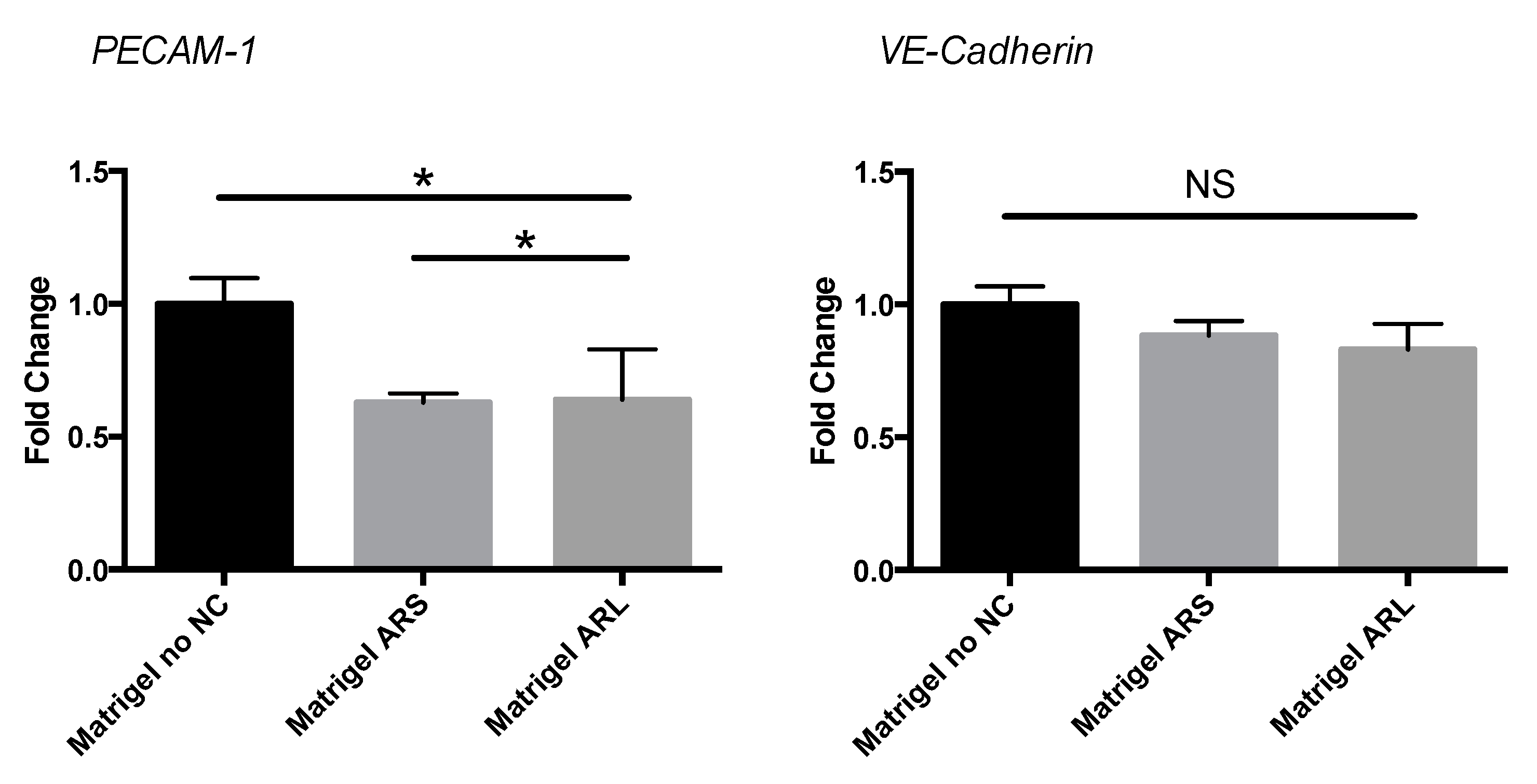
3.5. Gene Expression
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Boire, T.C.; Bronikowski, C.; Zachman, A.L.; Crowder, S.W.; Sung, H.J. Decoupling polymer properties to elucidate mechanisms governing cell behavior. Tissue Eng. B Rev. 2012, 18, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Lei, D.; Li, S.; Huang, P.; Qi, Q.; Sun, Y.; Zhang, Y.; Wang, Z.; You, Z.; Ye, X.; et al. Hybrid small-diameter vascular grafts: Anti-expansion effect of electrospun poly ε-caprolactone on heparin-coated decellularized matrices. Biomaterials 2016, 76, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Griffith, L.G.; Naughton, G. Tissue engineering—Current challenges and expanding opportunities. Science 2002, 295, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Khademhosseini, A. Modular tissue engineering: Engineering biological tissues from the bottom up. Soft Matter 2009, 5, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Utoh, R.; Ohashi, K.; Kikuchi, T.; Okano, T. Fabrication of functional 3D hepatic tissues with polarized hepatocytes by stacking endothelial cell sheets in vitro. J. Tissue Eng. Regen. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Owaki, T.; Shimizu, T.; Yamato, M.; Okano, T. Cell sheet engineering for regenerative medicine: Current challenges and strategies. Biotechnol. J. 2014, 9, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Hsiao, A.Y.; Takeuchi, S. Point-, line-, and plane-shaped cellular constructs for 3D tissue assembly. Adv. Drug Deliv. Rev. 2015, 95, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Jamilpour, N.; Wang, F.-Y.; Wong, P.K. Geometric control of capillary architecture via cell-matrix mechanical interactions. Biomaterials 2014, 35, 3273–3280. [Google Scholar] [CrossRef] [PubMed]
- Wallez, Y.; Huber, P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim. Biophys. Acta 2008, 1778, 794–809. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Gaspar, D.; Sorushanova, A.; Milcovich, G.; Spanoudes, K.; Mullen, A.M.; O’Brien, T.; Pandit, A.; Zeugolis, D.I. Scaffold and scaffold-free self-assembled systems in regenerative medicine. Biotechnol. Bioeng. 2015. [Google Scholar] [CrossRef] [PubMed]
- Rioja, A.Y.; Annamalai, R.T.; Paris, S.; Putnam, A.J.; Stegemann, J.P. Endothelial sprouting and network formation in collagen- and fibrin-based modular microbeads. Acta Biomater. 2016, 29, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.-C.; Park, A.Y.; Guan, J.-L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Setyawati, M.I.; Tay, C.Y.; Chia, S.L.; Goh, S.L.; Fang, W.; Neo, M.J.; Chong, H.C.; Tan, S.M.; Loo, S.C.J.; Ng, K.W.; et al. Titanium dioxide nanomaterials cause endothelial cell leakiness by disrupting the homophilic interaction of VE-cadherin. Nat. Commun. 2013, 4, 1673. [Google Scholar] [CrossRef] [PubMed]
- Arnaoutova, I.; Kleinman, H.K. In vitro angiogenesis: Endothelial cell tube formation on gelled basement membrane extract. Nat. Protoc. 2010, 5, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Willems, E.; Leyns, L.; Vandesompele, J. Standardization of real-time PCR gene expression data from independent biological replicates. Anal. Biochem. 2010, 379, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gao, C.; Liu, X.; He, T.; Shen, J. Immobilization of biomacromolecules onto aminolyzed poly(l-lactic acid) toward acceleration of endothelium regeneration. Tissue Eng. 2004, 10, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.G.; Park, T.G. Biodegradable polymer nanocylinders fabricated by transverse fragmentation of electrospun nanofibers through aminolysis. Macromol. Rapid Commun. 2008, 29, 1231–1236. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, C.; Liu, X.; Shen, J. Surface modification of polycaprolactone membrane via aminolysis and biomacromolecule immobilization for promoting cytocompatibility of human endothelial cells. Biomacromolecules 2002, 3, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Gao, C.; Xie, Y.; Gong, Y.; Shen, J. Collagen-coated polylactide microspheres as chondrocyte microcarriers. Biomaterials 2005, 26, 6305–6313. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-J.; Danielson, K.G.; Alexander, P.G.; Tuan, R.S. Biological response of chondrocytes cultured in three-dimensional nanofibrous poly(ε-caprolactone) scaffolds. J. Biomed. Mater. Res. A 2003, 67A, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Croll, T.I.; O’Connor, A.J.; Stevens, G.W.; Cooper-White, J.J. Controllable surface modification of poly(lactic-co-glycolic acid) (PLGA) by hydrolysis or aminolysis I: Physical, chemical, and theoretical aspects. Biomacromolecules 2004, 5, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Leong, M.F.; Ong, W.F.; Chan-Park, M.B.; Chian, K.S. Esophageal epithelium regeneration on fibronectin grafted poly(l-lactide-co-caprolactone) (PLLC) nanofiber scaffold. Biomaterials 2007, 28, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Jeong, S.I.; Bae, M.S.; Heo, D.N.; Heo, J.S.; Hwang, Y.-S.; Lee, H.-W.; Kwon, I.K. Poly(l-lactic acid) nanocylinders as nanofibrous structures for macroporous gelatin scaffolds. J. Nanosci. Nanotechnol. 2011, 11, 6371–6376. [Google Scholar] [CrossRef] [PubMed]
- Todaro, G.J.; Lazar, G.K.; Green, H. The initiation of cell division in a contact-inhibited mammalian cell line. J. Cell. Physiol. 1965, 66, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Sacharidou, A.; Koh, W.; Stratman, A.N.; Mayo, A.M.; Fisher, K.E.; Davis, G.E. Endothelial lumen signaling complexes control 3D matrix-specific tubulogenesis through interdependent Cdc42- and MT1-MMP-mediated events. Blood 2010, 115, 5259–5269. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.B.; Balikov, D.A.; Yang, J.W.; Kim, K.S.; Park, H.K.; Kim, J.K.; Kwon, I.K.; Bellan, L.M.; Sung, H.-J. Cationic Nanocylinders Promote Angiogenic Activities of Endothelial Cells. Polymers 2016, 8, 15. https://doi.org/10.3390/polym8010015
Lee JB, Balikov DA, Yang JW, Kim KS, Park HK, Kim JK, Kwon IK, Bellan LM, Sung H-J. Cationic Nanocylinders Promote Angiogenic Activities of Endothelial Cells. Polymers. 2016; 8(1):15. https://doi.org/10.3390/polym8010015
Chicago/Turabian StyleLee, Jung Bok, Daniel A. Balikov, Jae Won Yang, Ki Seok Kim, Hun Kuk Park, Jeong Koo Kim, Il Keun Kwon, Leon M. Bellan, and Hak-Joon Sung. 2016. "Cationic Nanocylinders Promote Angiogenic Activities of Endothelial Cells" Polymers 8, no. 1: 15. https://doi.org/10.3390/polym8010015
APA StyleLee, J. B., Balikov, D. A., Yang, J. W., Kim, K. S., Park, H. K., Kim, J. K., Kwon, I. K., Bellan, L. M., & Sung, H.-J. (2016). Cationic Nanocylinders Promote Angiogenic Activities of Endothelial Cells. Polymers, 8(1), 15. https://doi.org/10.3390/polym8010015