Melt Free-Radical Grafting of Maleic Anhydride onto Biodegradable Poly(lactic acid) by Using Styrene as A Comonomer
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Sample Preparation
2.3. Purification
| Samples Code | Formulation | Grafting Temperature °C | |||
|---|---|---|---|---|---|
| PLA | MA | St | DCP | ||
| 1 | 100 | 0 | 0 | 0 | 180 |
| 2 | 100 | 4.50 | 0.00 | 0.50 | 180 |
| 3 | 100 | 4.50 | 2.25 | 0.50 | 180 |
| 4 | 100 | 4.50 | 4.50 | 0.50 | 180 |
| 5 | 100 | 4.50 | 9.00 | 0.50 | 180 |
| 6 | 100 | 4.50 | 18.00 | 0.50 | 180 |
| 7 | 100 | 4.50 | 9.00 | 0.50 | 165 |
| 8 | 100 | 4.50 | 9.00 | 0.50 | 170 |
| 9 | 100 | 4.50 | 9.00 | 0.50 | 190 |
| 10 | 100 | 1.50 | 3.00 | 0.17 | 180 |
| 11 | 100 | 3.00 | 6.00 | 0.33 | 180 |
| 12 | 100 | 6.00 | 12.00 | 0.67 | 180 |
2.4. Characterizations
2.4.1. Fourier Transform Infrared Spectroscopy (FT-IR)
2.4.2. Titration
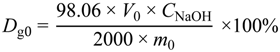
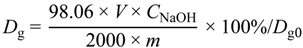
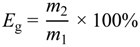
2.4.3. Thermogravimetric Analysis (TGA)
2.4.4. Molar Mass Characterization
2.4.5. Differential Scanning Calorimetry (DSC)
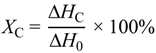
3. Results and Discussion
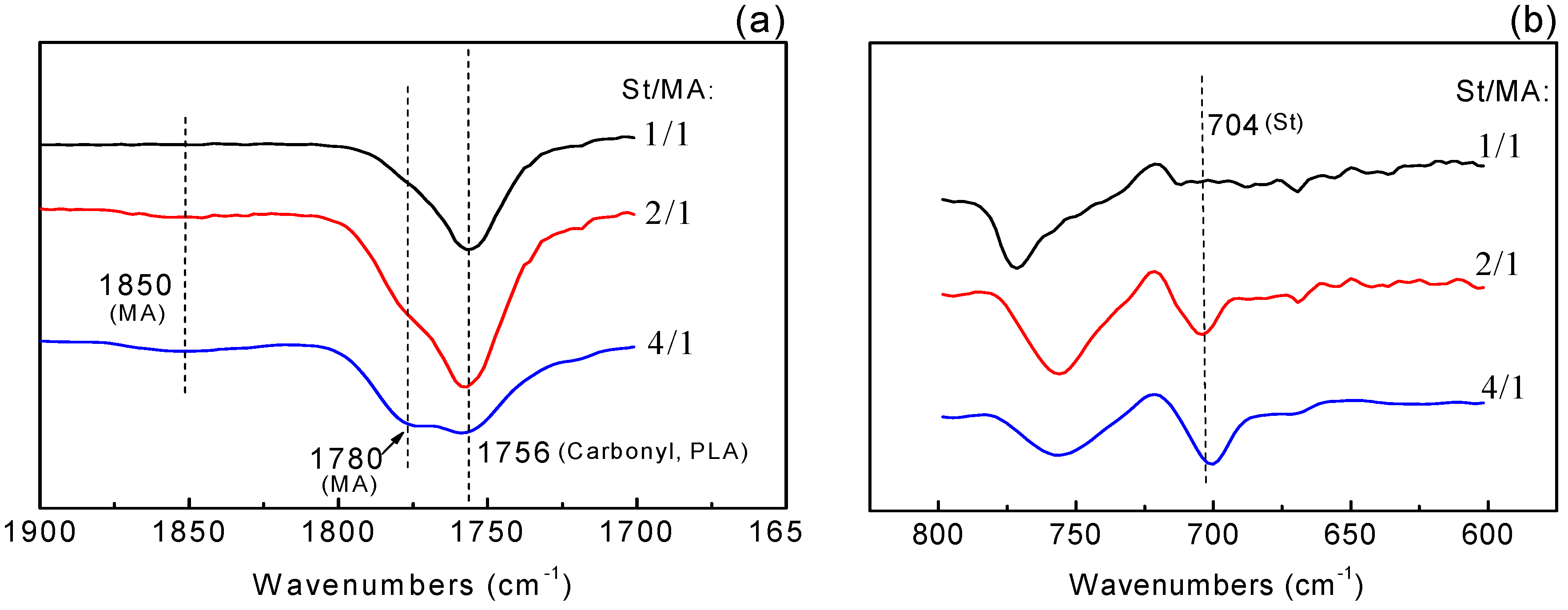
3.1. Analysis on PLA Grafting MA System
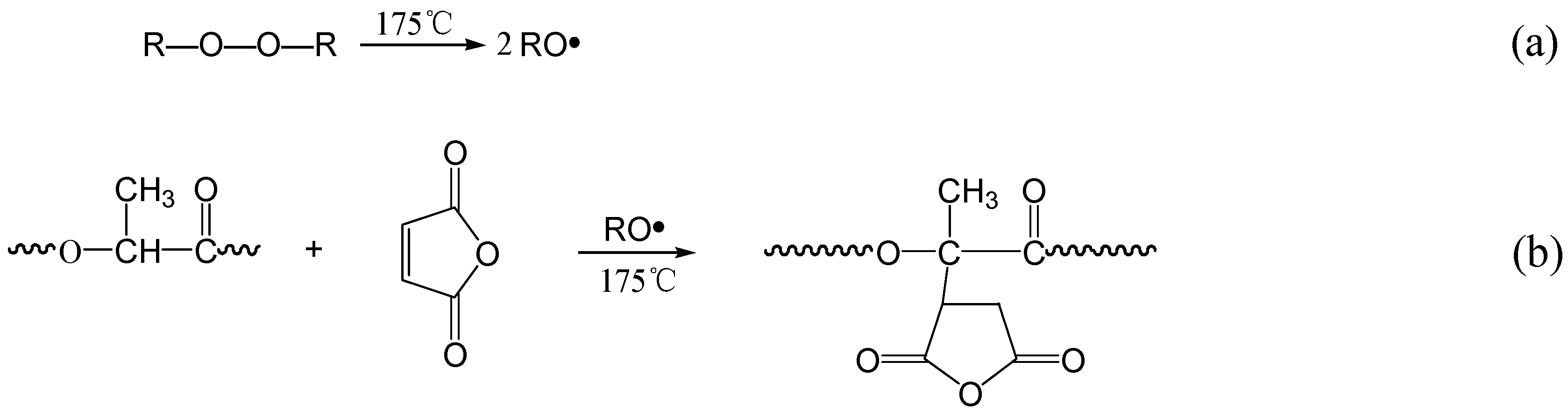
3.2. Grafting Degree and Grafting Efficiency of MA on PLA Chains
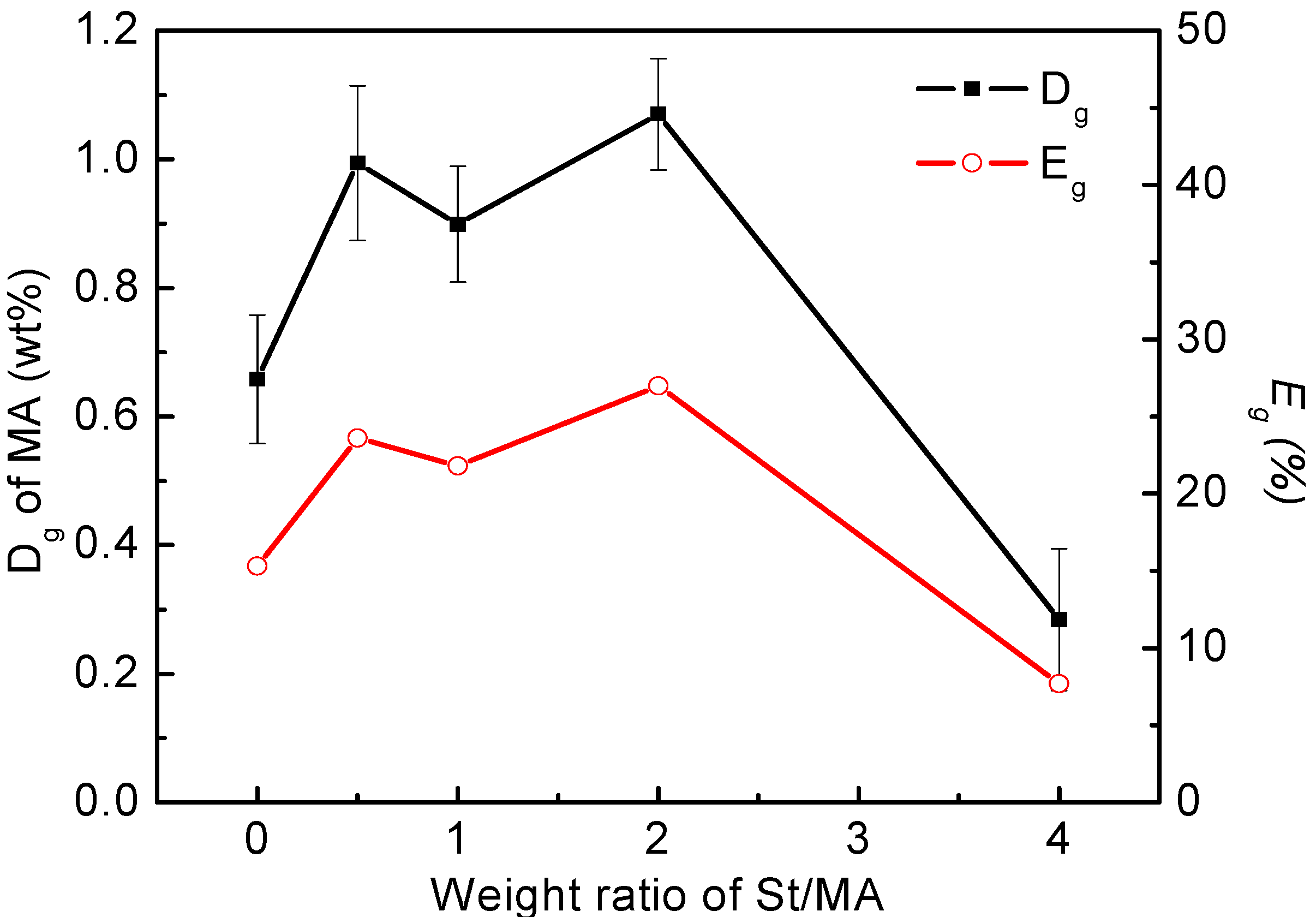
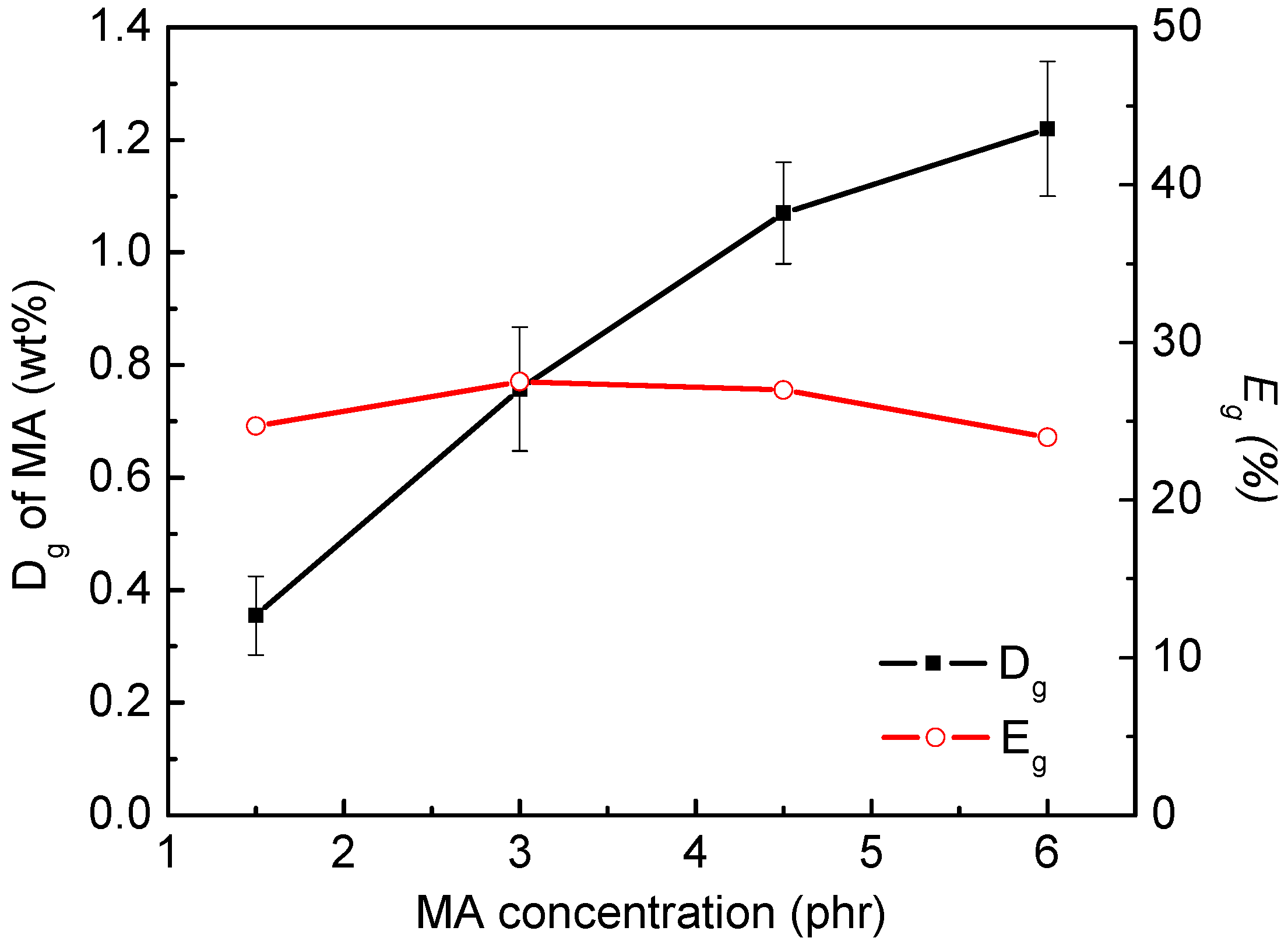
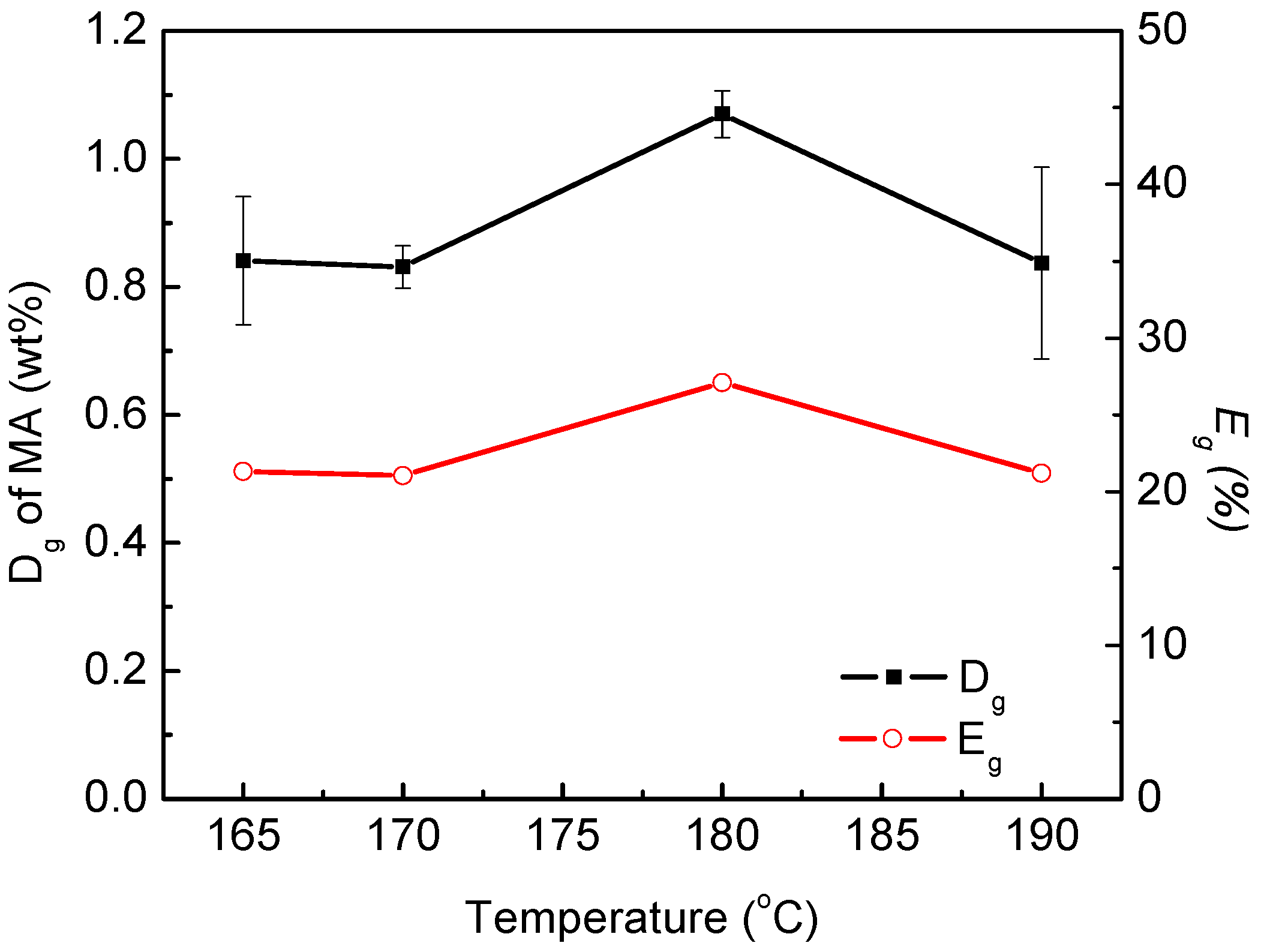
3.3. Thermogravimetric Analysis of Maleated PLA
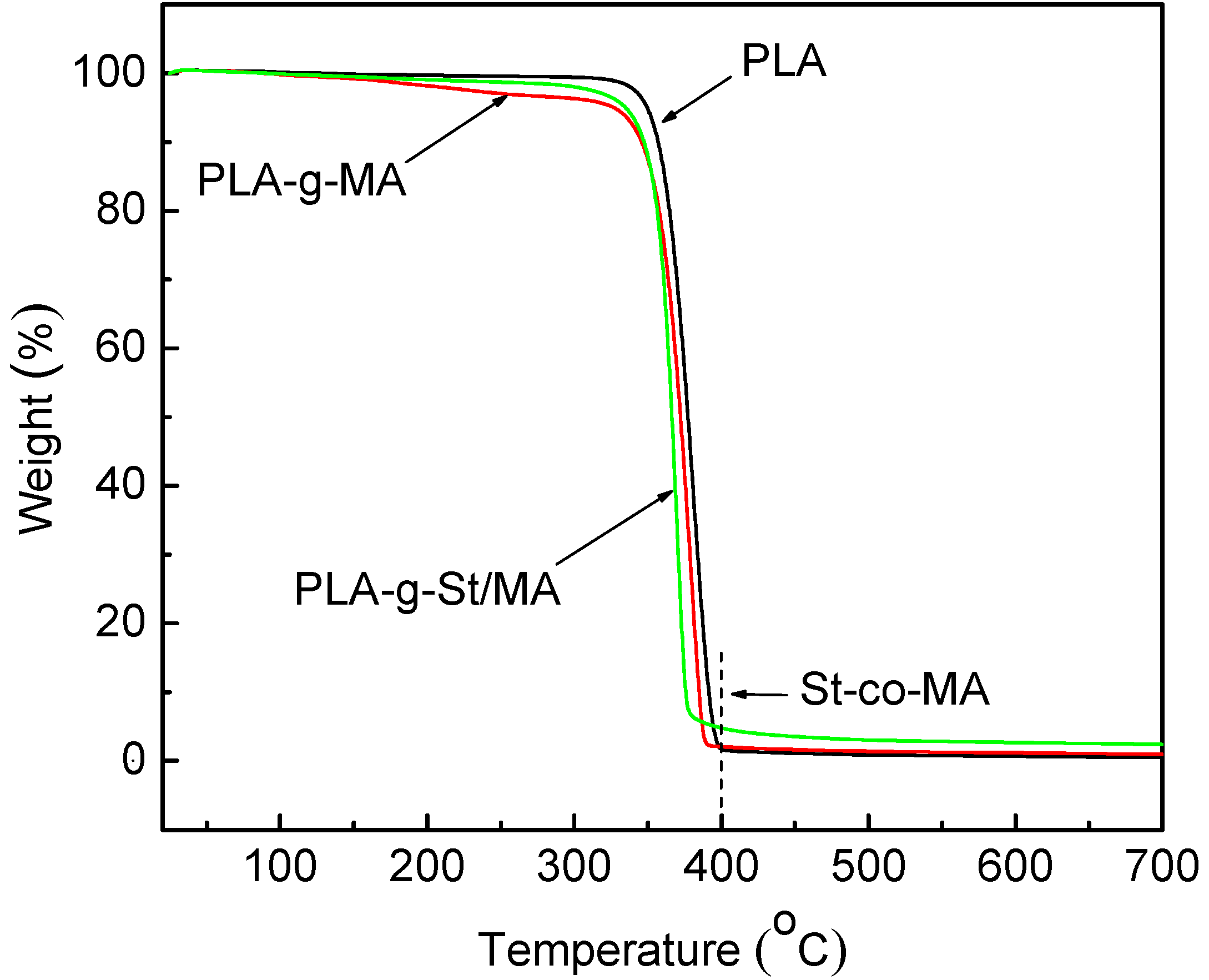
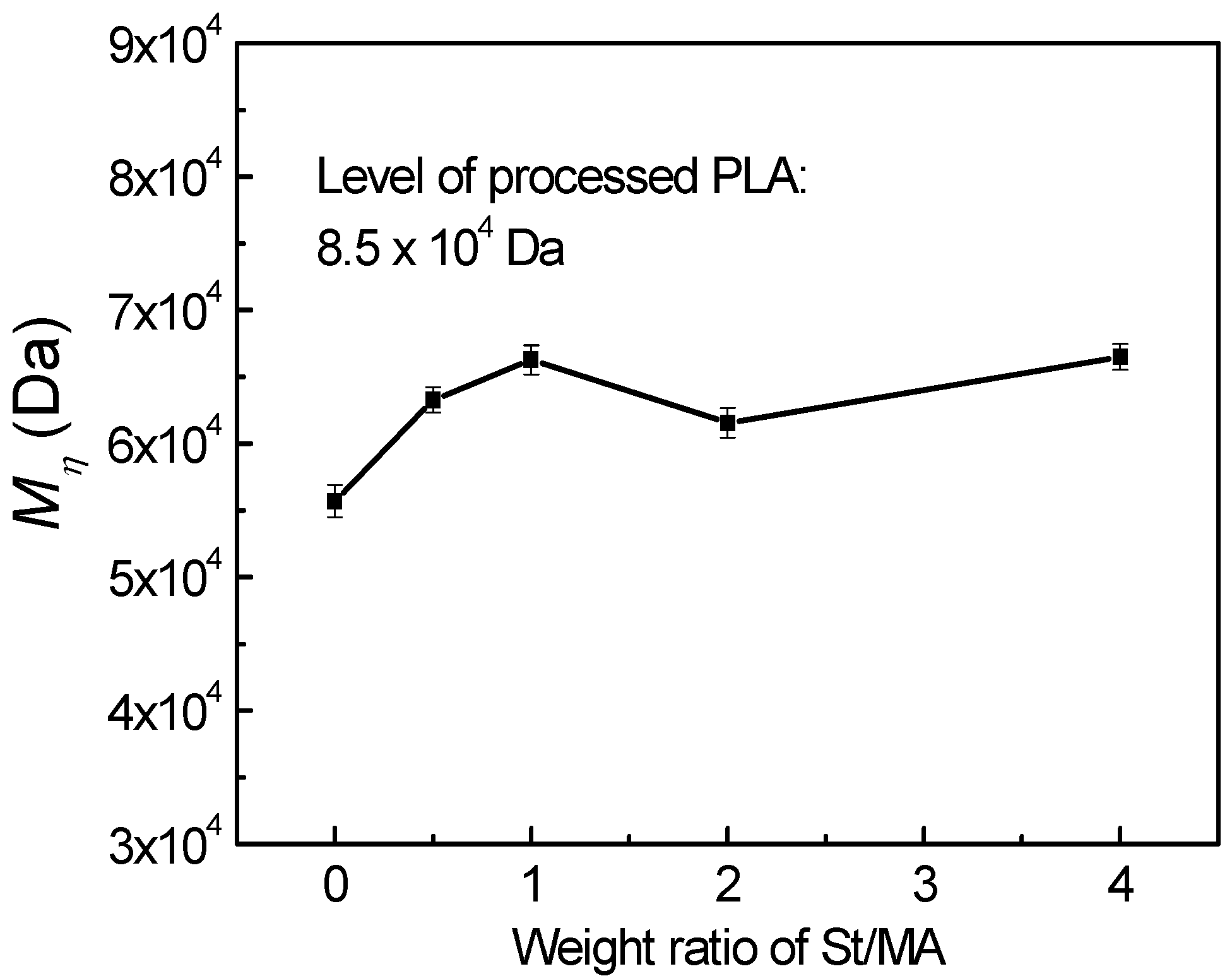
3.4. Influence of Grafting on the Molecular Weight of PLA
3.5. Crystallization and Melting Behavior of PLA and Maleated PLA
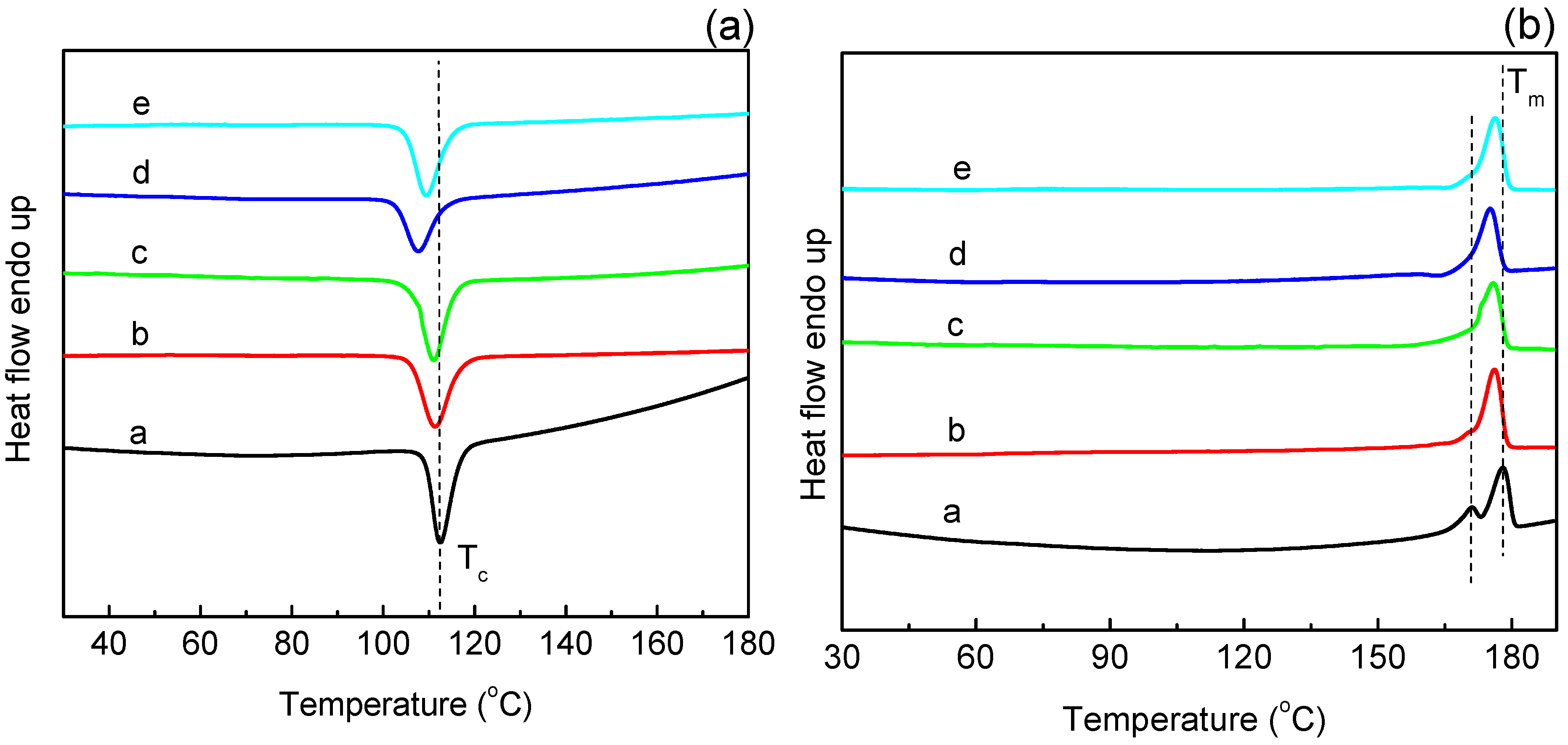
| Samples | St/MA 1 (wt/wt) | Crystallization Process | Melt Process | |||
|---|---|---|---|---|---|---|
| Tonset °C | Tc °C | Xc % | Tm1 °C | |||
| PLA 2 | - | 117 | 113 | 30 | 178 | |
| Maleated PLA | 0/1 | 117 | 111 | 32 | 176 | |
| 1/1 | 116 | 111 | 31 | 175 | ||
| 2/1 | 112 | 107 | 26 | 175 | ||
| 4/1 | 114 | 109 | 28 | 176 | ||
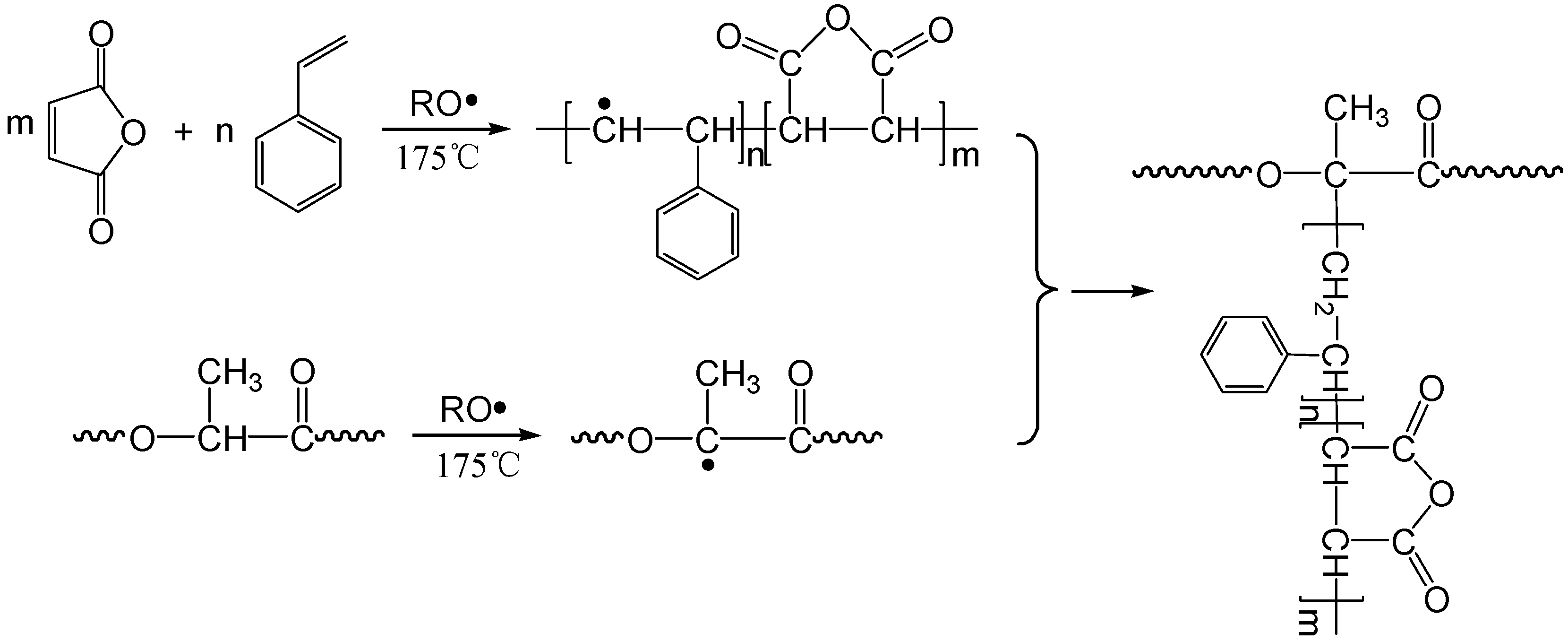
3.6. Grafting Mechanism of MA onto PLA in the Presence of Styrene

4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Reddy, M.M.; Vivekanandhan, S.; Misra, M.; Bhatia, S.K.; Mohanty, A.K. Biobased plastics and bionanocomposites: Current status and future opportunities. Prog. Polym. Sci. 2013, 38, 1653–1689. [Google Scholar] [CrossRef]
- Raquez, J.M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nano-composites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Martin, O.; Averous, L. Poly(lactic acid): Plasticization and properties of biodegradable multiphase systems. Polymer 2001, 42, 6209–6219. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Zhang, J.F.; Sun, X. Mechanical properties of poly(lactic acid)/starch composites compatibilized by maleic anhydride. Biomacromolecules 2004, 5, 1446–1451. [Google Scholar] [CrossRef]
- Chen, L.; Qiu, X.; Xie, Z.; Hong, Z.; Sun, J.; Chen, X.; Jing, X. Poly(l-lactide)/starch blends compatibilized with poly(l-lactide)-g-starch copolymer. Carbohydr. Polym. 2006, 65, 75–80. [Google Scholar] [CrossRef]
- Schwach, E.; Six, J.L.; Avérous, L. Biodegradable blends based on starch and poly (lactic acid): Comparison of different strategies and estimate of compatibilization. J. Polym. Environ. 2008, 16, 286–297. [Google Scholar] [CrossRef]
- Zare, A.; Morshed, M.; Bagheri, R.; Karimi, K. Effect of various parameters on the chemical grafting of amide monomers to poly (lactic acid). Fibers Polym. 2013, 14, 1783–1793. [Google Scholar] [CrossRef]
- He, J.; Ding, L.; Deng, J.; Yang, W. Oil absorbent beads containing β-cyclodextrin moieties: Preparation via suspension polymerization and high oil absorbency. Polym. Adv. Technol. 2012, 23, 810–816. [Google Scholar] [CrossRef]
- Peng, C.; Chen, H.; Wang, J.; Chen, Z.; Ni, M.; Chen, Y.; Yuan, T. Controlled degradation of polylactic acid grafting N-vinyl pyrrolidone induced by gamma ray radiation. J. Appl. Polym. Sci. 2013, 130, 704–709. [Google Scholar] [CrossRef]
- Choi, K.M.; Choi, M.C.; Han, D.H.; Park, T.S.; Ha, C.S. Plasticization of poly(lactic acid)(PLA) through chemical grafting of poly(ethylene glycol) (PEG) via in situ reactive blending. Eur. Polym. J. 2013, 49, 2356–2364. [Google Scholar] [CrossRef]
- Li, J.; Kong, M.; Cheng, X.J.; Dang, Q.F.; Zhou, X.; Wei, Y.N.; Chen, X.G. Preparation of biocompatible chitosan grafted poly(lactic acid) nanoparticles. Inter. J. Biol. Macromol. 2012, 51, 221–227. [Google Scholar] [CrossRef]
- Li, X.T.; Sun, J.; Chen, S.; Chen, G.Q. In vitro investigation of maleated poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) for its biocompatibility to mouse fibroblast L929 and human microvascular endothelial cells. J. Biomed. Mater. Res. Part A 2008, 87, 832–842. [Google Scholar]
- Detyothin, S.; Selke, S.E.; Narayan, R.; Rubino, M.; Auras, R. Reactive functionalization of poly(lactic acid), PLA: Effects of the reactive modifier, initiator and processing conditions on the final grafted maleic anhydride content and molecular weight of PLA. Polym. Degrad. Stab. 2013, 98, 2697–2708. [Google Scholar] [CrossRef]
- Wu, C.S. Renewable resource-based composites of recycled natural fibers and maleated polylactide bioplastic: Characterization and biodegradability. Polym. Degrad. Stab. 2009, 94, 1076–1084. [Google Scholar] [CrossRef]
- Zhou, C.; Shi, Q.; Guo, W.; Terrell, L.; Qureshi, A.T.; Hayes, D.J.; Wu, Q. Electrospun bio-nanocomposite scaffolds for bone tissue engineering by cellulose nanocrystals reinforcing maleic anhydride grafted PLA. ACS Appl. Mater. Inter. 2013, 5, 3847–3854. [Google Scholar]
- Hong, J.; Kim, D.S. Preparation and physical properties of polylactide/cellulose nanowhisker/nanoclay composites. Polym. Compos. 2013, 34, 293–298. [Google Scholar] [CrossRef]
- Zhu, R.; Liu, H.; Zhang, J. Compatibilizing effects of maleated poly(lactic acid) (PLA) on properties of PLA/Soy protein composites. Industr. Engin. Chem. Res. 2012, 51, 7786–7792. [Google Scholar] [CrossRef]
- Cartier, H.; Hu, G.H. Styrene-assisted melt free radical grafting of glycidyl methacrylate onto polypropylene. J. Polym. Sci. Part A: Polym. Chem. 1998, 36, 1053–1063. [Google Scholar] [CrossRef]
- Signori, F.; Badalassi, M.; Bronco, S.; Ciardelli, F. Radical functionalization of poly(butylene succinate-co-adipate): Effect of cinnamic co-agents on maleic anhydride grafting. Polymer 2011, 52, 4656–4663. [Google Scholar]
- Chen, X.; Wu, H.; Yu, J.; Guo, S.; Luo, Z. Maleic anhydride/styrene melt grafting and crosslinking onto ethylene-octene copolymer. Polym. Eng. Sci. 2008, 48, 2289–2296. [Google Scholar] [CrossRef]
- Hu, G.H.; Cartier, H. Styrene-assisted melt free radical grafting of glycidyl methacrylate onto an ethylene and propylene rubber. J. Appl. Polym. Sci. 1999, 71, 125–133. [Google Scholar] [CrossRef]
- Li, Y.; Xie, X.M.; Guo, B.H. Study on styrene-assisted melt free-radical grafting of maleic anhydride onto polypropylene. Polymer 2001, 42, 3419–3425. [Google Scholar]
- Ma, P.; Cai, X.; Zhang, Y.; Wang, S.; Dong, W.; Chen, M.; Lemstra, P.J. In-situ compatibilization of poly(lactic acid) and poly (butylene adipate-co-terephthalate) blends by using dicumyl peroxide as a free-radical initiator. Polym. Degrad. Stab. 2014, 102, 145–151. [Google Scholar] [CrossRef]
- Witte, P.V.D.; Dijkstra, P.J.; Berg, J.V.D.; Feijen, J. Phase behavior of polylactides in solvent-nonsolvent mixtures. J. Polym. Sci. Part B: Polym. Phys. 1996, 34, 2553–2568. [Google Scholar] [CrossRef]
- Södergård, A.; Stolt, M. Properties of lactic acid based polymers and their correlation with composition. Prog. Polym. Sci. 2002, 27, 1123–1163. [Google Scholar] [CrossRef]
- Machado, A.V.; Covas, J.A.; Van Duin, M. Effect of polyolefin structrure on maleic anhydride grafting. Polymer 2001, 42, 3649–3655. [Google Scholar] [CrossRef]
- Machado, A.V.; Van Duin, M.; Covas, J.A. Monitoring polyolefin modification along the axis of a twin-screw extruder: II. Maleic anhydride grafting. J. Polym. Sci. part A: Polym. Chem. 2000, 38, 3919–3932. [Google Scholar] [CrossRef]
- Piemonte, V.; Gironi, F. Kinetics of hydrolytic degradation of PLA. J. Polym. Environ. 2013, 21, 313–318. [Google Scholar] [CrossRef]
- Hong, S.G.; Lin, Y.C.; Lin, C.H. Improvement of the thermal stability of polyhydroxybutyrates by grafting with maleic anhydride by different methods: differential scanning calorimetry, thermogravimetric analysis, and gel permeation chromatography. J. Appl. Polym. Sci. 2008, 110, 2718–2726. [Google Scholar] [CrossRef]
- Ma, P.M.; Wang, R.Y.; Wang, S.F.; Zhang, Y.; Zhang, Y.X.; Hristova, D. Effects of fumed silica on the crystallization behavior and thermal properties of poly-(hydroxybutyrate-co-hydroxyvalerate). J. Appl. Polym. Sci. 2008, 108, 1770–1777. [Google Scholar] [CrossRef]
- Abt, T.; Sánchez-Soto, M.; Martínez de Ilarduya, A. Toughening of in situ polymerized cyclic butylene terephthalate by chain extension with a bifunctional epoxy resin. Eur. Polym. J. 2012, 48, 163–171. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ma, P.; Jiang, L.; Ye, T.; Dong, W.; Chen, M. Melt Free-Radical Grafting of Maleic Anhydride onto Biodegradable Poly(lactic acid) by Using Styrene as A Comonomer. Polymers 2014, 6, 1528-1543. https://doi.org/10.3390/polym6051528
Ma P, Jiang L, Ye T, Dong W, Chen M. Melt Free-Radical Grafting of Maleic Anhydride onto Biodegradable Poly(lactic acid) by Using Styrene as A Comonomer. Polymers. 2014; 6(5):1528-1543. https://doi.org/10.3390/polym6051528
Chicago/Turabian StyleMa, Piming, Long Jiang, Tao Ye, Weifu Dong, and Mingqing Chen. 2014. "Melt Free-Radical Grafting of Maleic Anhydride onto Biodegradable Poly(lactic acid) by Using Styrene as A Comonomer" Polymers 6, no. 5: 1528-1543. https://doi.org/10.3390/polym6051528
APA StyleMa, P., Jiang, L., Ye, T., Dong, W., & Chen, M. (2014). Melt Free-Radical Grafting of Maleic Anhydride onto Biodegradable Poly(lactic acid) by Using Styrene as A Comonomer. Polymers, 6(5), 1528-1543. https://doi.org/10.3390/polym6051528




