Effects of Aging on Sucrose-Based Poly(ester-urethane)s: Thermal, Ultraviolet, and Hydrolytic Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sucrose-Based Polyurethane Synthesis
2.3. Thermal Aging
2.4. UV Exposure
2.5. Hydrolytic Degradation
2.6. Characterization Methods
2.6.1. Fourier Transform Infrared (FT-IR) Spectroscopy
2.6.2. Thermogravimetric Analysis (TGA)
2.6.3. Differential Scanning Calorimetry (DSC)
2.6.4. Mechanical Analysis
2.6.5. Contact Angle and Surface Free Energy
2.6.6. Atomic Force Microscopy
2.6.7. Scanning Electron Microscopy
3. Results and Discussion
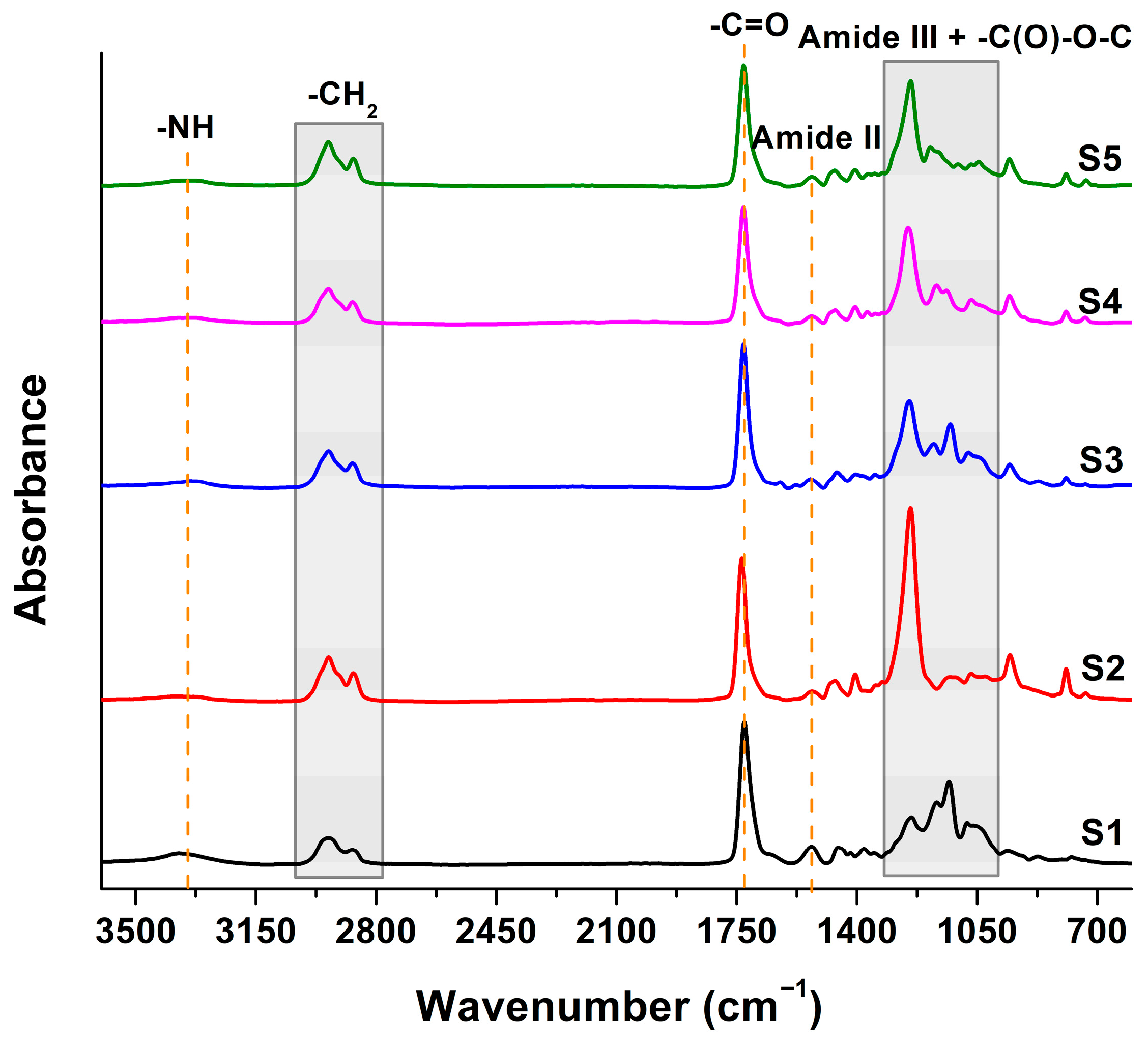
3.1. Chemical and Structure Changes Measured by FT-IR Spectroscopy
3.1.1. Structural Changes After Thermal Aging
3.1.2. Structural Changes After UV Irradiation
3.2. Thermogravimetric Analysis
3.3. DSC Study
Effects of Thermal and UV Aging on Crystallization and Melting Behavior
3.4. Mechanical Properties
Mechanical Parameters After Thermal and UV Aging
3.5. Contact Angle and Surface Free Energy
Wettability After Thermal and UV Treatment
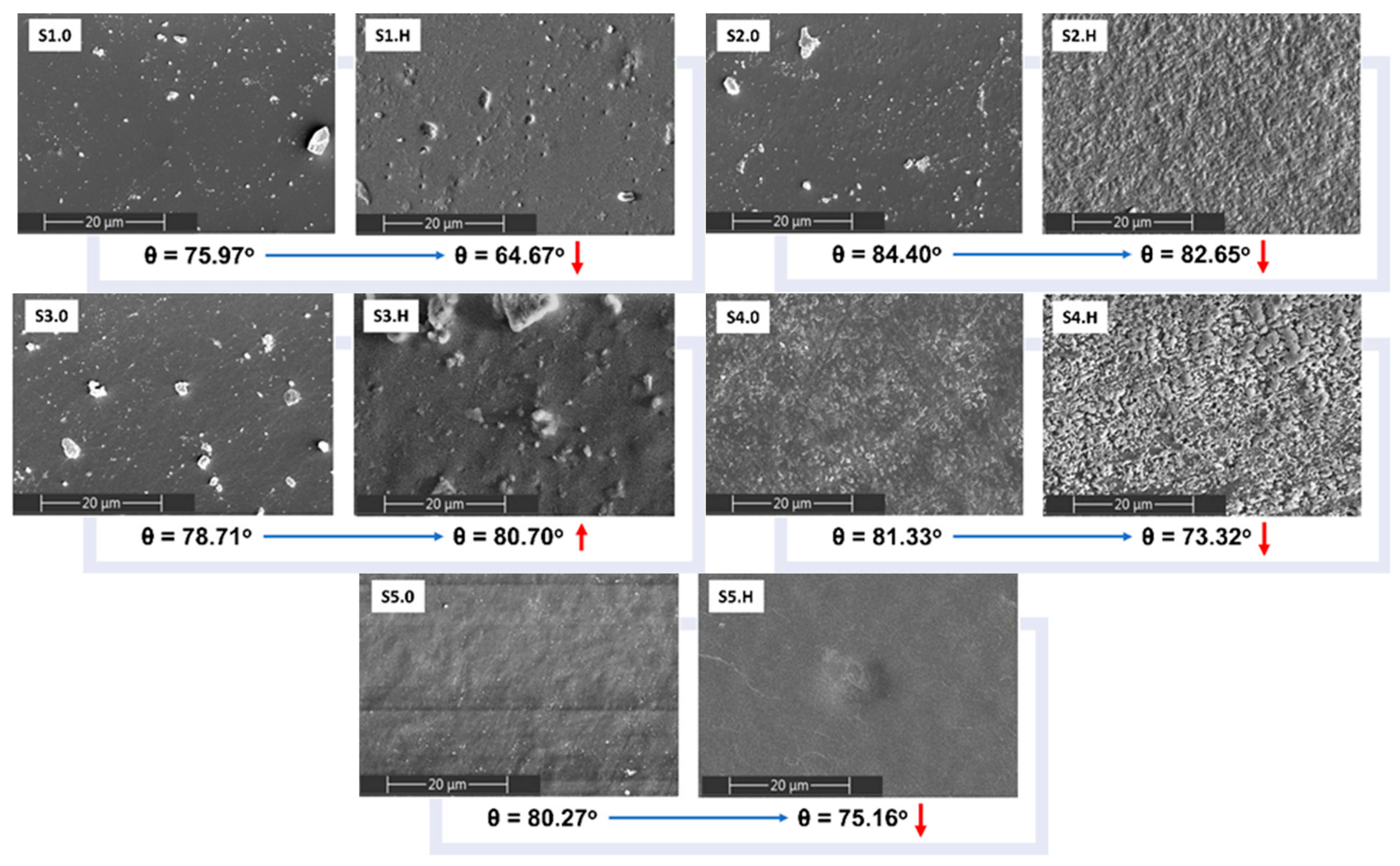
3.6. Surface Morphology Changes Induced by Thermal and UV Aging
3.7. Hydrolytic Degradation
3.7.1. Structural Modification During Hydrolysis
3.7.2. Surface Analysis by SEM and the Contact Angle Technique
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, C.K.; Lee, J.H.; Kim, I.S.; Kim, S.H. Castor oil-based polyols with gradually increasing functionalities for biopolyurethane synthesis. J. Appl. Polym. Sci. 2020, 137, 48304. [Google Scholar] [CrossRef]
- Du, H.; Zhao, Y.; Li, Q.; Wang, J.; Kang, M.; Wang, X. Synthesis and Characterization of Waterborne Polyurethane Adhesive from MDI and HDI. J. Appl. Polym. Sci. 2008, 110, 1396–1402. [Google Scholar] [CrossRef]
- Wang, C.; Wu, Y.; Li, Y.; Shao, Q.; Yan, X.; Han, C.; Wang, Z.; Liu, Z.; Guo, Z. Flame-retardant rigid polyurethane foam with a phosphorus-nitrogen single intumescent flame retardant. Polym. Adv. Technol. 2018, 29, 668–676. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, S.H.; Kim, S.H. Fabrication of bio-based polyurethane nanofibers incorporated with a triclosan/cyclodextrin complex for antibacterial applications. RSC Adv. 2020, 10, 3450–3458. [Google Scholar] [CrossRef]
- Wu, S.; Ma, S.; Zhang, Q.; Yang, C. A comprehensive review of polyurethane: Properties, applications and future perspectives. Polymer 2025, 327, 128361. [Google Scholar] [CrossRef]
- Plastics the Fast Facts 2025 Global and European Plastics Production and Economic Indicators. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2025/ (accessed on 24 November 2025).
- Polyurethane Market Size & Share Analysis—Growth Trends and Forecast (2025–2030). Available online: https://www.mordorintelligence.com/industry-reports/polyurethane-market (accessed on 24 November 2025).
- Lenges, C.; Behabtu, N.; Mok, J.; Sendijarevic, I.; Sendijarevic, A. Engineered polysaccharide alpha-1,3 glucan as isocyanate-reactive component in viscoelastic polyurethane foams. J. Appl. Polym. Sci. 2020, 138, e49979. [Google Scholar] [CrossRef]
- Bayan, R.; Karak, N. Bio-derived aliphatic hyperbranched polyurethane nanocomposites with inherent self healing tendency and surface hydrophobicity: Towards creating high performance smart materials. Compos. Part A Appl. Sci. Manuf. 2018, 110, 142–153. [Google Scholar] [CrossRef]
- Cherng, J.Y.; Hou, T.Y.; Shih, M.F.; Talsma, H.; Hennink, W.E. Polyurethane-based drug delivery systems. Int. J. Pharm. 2013, 450, 145–162. [Google Scholar] [CrossRef]
- Tan, S.; Abraham, T.; Ference, D.; MacOsko, C.W. Rigid polyurethane foams from a soybean oil-based Polyol. Polymer 2011, 52, 2840–2846. [Google Scholar] [CrossRef]
- de Luca Bossa, F.; Verdolotti, L.; Russo, V.; Campaner, P.; Minigher, A.; Lama, G.C.; Boggioni, L.; Tesser, R.; Lavorgna, M. Upgrading sustainable polyurethane foam based on greener polyols: Succinic-based polyol and Mannich-based polyol. Materials 2020, 13, 3170. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Daver, F.; Murdoch, B.J.; Ball, A.S.; Ivanova, E.P.; Adhikari, B. Biodegradation of novel bioplastics made of starch, polyhydroxyurethanes and cellulose nanocrystals in soil environment. Sci. Total Environ. 2022, 815, 152684. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-Y.; Wang, Y.-R.; Teng, D.-Y.; Xue, Y.-F.; Jiang, G.-C. Synthesis of Biomass Polyurethane and Its Properties. J. Polym. Mater. 2025, 42, 359–377. [Google Scholar] [CrossRef]
- Oprea, S.; Potolinca, V.O. Synthesis and characterization of novel linear and cross-linked polyurethane urea elastomers with 2,3-diaminopyridine in the main chain. High Perform. Polym. 2013, 25, 147–155. [Google Scholar] [CrossRef]
- Mülhaupt, R. Green Polymer Chemistry and Bio-based Plastics Dreams and Reality. Macromol. Chem. Phys. 2013, 214, 159–174. [Google Scholar] [CrossRef]
- Williams, C.K.; Hillmyer, M.A. Polymers from Renewable Resources: A Perspective for a Special Issue of Polymer Reviews. Polym. Rev. 2008, 48, 1–10. [Google Scholar] [CrossRef]
- Biswas, A.; Kim, S.; Gómez, A.; Buttrum, M.; Boddu, V.; Cheng, H.N. Microwave-Assisted Synthesis of Sucrose Polyurethanes and Their Semi-interpenetrating Polymer Networks with Polycaprolactone and Soybean Oil. Ind. Eng. Chem. Res. 2018, 57, 3227–3234. [Google Scholar] [CrossRef]
- Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Oleic and undecylenic acids as renewable feedstocks in the synthesis of polyols and polyurethanes. Polymers 2010, 2, 440–453. [Google Scholar] [CrossRef]
- Tenorio-Alfonso, A.; Sánchez, M.C.; Franco, J.M. A Review of the Sustainable Approaches in the Production of Bio-based Polyurethanes and Their Applications in the Adhesive Field. J. Polym. Environ. 2020, 28, 749–774. [Google Scholar] [CrossRef]
- Malani, R.S.; Malshe, V.C.; Thorat, B.N. Polyols and polyurethanes from renewable sources: Past, present, and future—Part 2: Plant-derived materials. J. Coat. Technol. Res. 2022, 19, 361–375. [Google Scholar] [CrossRef]
- Galbis, J.A.; de García-Martín, M.G.; de Paz, M.V.; Galbis, E. Bio-Based Polyurethanes from Carbohydrate Monomers. In Aspects of Polyurethanes; Yılmaz, F.S., Ed.; IntechOpen: London, UK, 2017; ISBN 978-953-51-3546-3. [Google Scholar]
- Mo, Y.; Huang, X.; Hu, C. Recent Advances in the Preparation and Application of Bio-Based Polyurethanes. Polymers 2024, 16, 2155. [Google Scholar] [CrossRef]
- Jayalath, P.; Ananthakrishnan, K.; Jeong, S.; Shibu, R.P.; Zhang, M.; Kumar, D.; Yoo, C.G.; Shamshina, J.L.; Therasme, O. Bio-Based Polyurethane Materials: Technical, Environmental, and Economic Insights. Processes 2025, 13, 1591. [Google Scholar] [CrossRef]
- Savelyev, Y.; Markovskaya, L.; Savelyeva, O.; Akhranovich, E.; Parkhomenko, N.; Travinskaya, T. Degradable polyurethane foams based on disaccharides. J. Appl. Polym. Sci. 2015, 132, 42131. [Google Scholar] [CrossRef]
- Lu, M.Y.; Surányi, A.; Viskolcz, B.; Fiser, B. Molecular design of sugar-based polyurethanes. Croat. Chem. Acta 2018, 91, 299–307. [Google Scholar] [CrossRef]
- Solanki, A.; Mehta, J.; Thakore, S. Structure—Property relationships and biocompatibility of carbohydrate crosslinked polyurethanes. Carbohydr. Polym. 2014, 110, 338–344. [Google Scholar] [CrossRef]
- Kizuka, K.; Inoue, S. Synthesis and Properties of Polyurethane Elastomers Containing Sucrose as a Cross-Linker. Open J. Org. Polym. Mater. 2015, 5, 103–112. [Google Scholar] [CrossRef]
- Kordován, M.Á.; Hegedus, C.; Czifrák, K.; Lakatos, C.; Kálmán-Szabó, I.; Daróczi, L.; Zsuga, M.; Kéki, S. Novel Polyurethane Scaffolds Containing Sucrose Crosslinker for Dental Application. Int. J. Mol. Sci. 2022, 23, 7904. [Google Scholar] [CrossRef]
- Nagy, L.; Nagy, M.; Vadkerti, B.; Daróczi, L.; Deák, G.; Zsuga, M.; Kéki, S. Designed Polyurethanes for Potential Biomedical and Pharmaceutical Applications: Novel Synthetic Strategy for Preparing Sucrose Containing Biocompatible and Biodegradable Polyurethane Networks. Polymers 2019, 11, 825. [Google Scholar] [CrossRef]
- Lakatos, C.; Kordován, M.Á.; Czifrák, K.; Nagy, L.; Vadkerti, B.; Daróczi, L.; Zsuga, M.; Keki, S. Synthesis of sucrose-HDI cooligomers: New polyols for novel polyurethane networks. Int. J. Mol. Sci. 2022, 23, 1444. [Google Scholar] [CrossRef]
- Xi, X.; Wu, Z.; Pizzi, A.; Gerardin, C.; Lei, H.; Zhang, B.; Du, G. Non—Isocyanate polyurethane adhesive from sucrose used for particleboard. Wood Sci. Technol. 2019, 53, 393–405. [Google Scholar] [CrossRef]
- Kloss, J.; de Souza, F.S.M.; da Silva, E.R.; Dionısio, J.A.; Akcelrud, L.; Zawadzki, S.F. Polyurethanes Elastomers Based on Poly(e-caprolactone) Diol: Biodegradation Evaluation. Macromol. Symp. 2006, 245–246, 651–656. [Google Scholar] [CrossRef]
- Boyer, A.; Lingome, C.E.; Condassamy, O.; Schappacher, M.; Moebs-Sanchez, S.; Queneau, Y.; Gadenne, B.; Alfose, C.; Henri, C. Glycolipids as a source of polyols for the design of original linear and cross-linked polyurethanes. Polym. Chem. 2013, 4, 296–306. [Google Scholar] [CrossRef]
- Ates, B.; Koytepe, S.; Karaaslan, M.G.; Balcioglu, S.; Gulgen, S. Biodegradable non-aromatic adhesive polyurethanes based on disaccharides for medical applications. Int. J. Adhes. Adhes. 2014, 49, 90–96. [Google Scholar] [CrossRef]
- Zhong, H.; Zhang, Q.; Lei, H.; Zhou, X.; Zhang, J.; Du, G.; Pizzi, A.; Xi, X. Preparation and Modification of Sucrose-Based Non-Isocyanate Polyurethane Adhesives for Plywood Bonding. Molecules 2025, 30, 1541. [Google Scholar] [CrossRef] [PubMed]
- Zong, J.; Chang, Z.Y.; Huang, D.; Li, S.; Wang, X.; Chen, S.; Li, J.; Zang, X. A Sucrose-Derived Copper Oxide/Polyurethane Foam for Efficient and Sustainable Solar-Driven Seawater Desalination. ACS Appl. Polym. Mater. 2025, 7, 15408–15419. [Google Scholar] [CrossRef]
- Pantelic, B.; Siaperas, R.; Budin, C.; de Boer, T.; Topakas, E.; Nikodinovic-Runic, J. Proteomic examination of polyester-polyurethane degradation by Streptomyces sp. PU10: Diverting polyurethane intermediates to secondary metabolite production. Microb. Biotechnol. 2024, 17, e14445. [Google Scholar] [CrossRef]
- Di Bisceglie, F.; Quartinello, F.; Vielnascher, R.; Guebitz, G.M.; Pellis, A. Cutinase-Catalyzed Polyester-Polyurethane Degradation: Elucidation of the Hydrolysis Mechanism. Polymers 2022, 14, 411. [Google Scholar] [CrossRef]
- Narayan, R.; Chattopadhyay, D.K.; Sreedhar, B.; Raju, K.V.S.N.; Mallikarjuna, N.N.; Aminabhavi, T.M. Degradation Profiles of Polyester—Urethane (Hydroxylated Polyester/Diphenylmethane Diisocyanate) and Polyester—Melamine (Hydroxylated Polyester/Hexamethoxymethylmelamine) Coatings: An Accelerated Weathering Study. J. Appl. Polym. Sci. 2005, 97, 1069–1081. [Google Scholar] [CrossRef]
- Makki, H.; Adema, K.N.S.; Hendrix, M.M.R.M.; Peters, E.A.J.F.; Laven, J.; Van Der Ven, L.G.J.; Van Benthem, R.A.T.M.; De With, G. Weathering of a polyester-urethane clearcoat: Lateral inhomogeneities. Polym. Degrad. Stab. 2015, 122, 180–186. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, L.; Qu, L.; Zhang, R.; Qin, Z.; Zhang, H.; Wei, J.; Xu, J.; Hou, Z. Cross-linked poly(ester urethane)/starch composite films with high starch content as sustainable food-packaging materials: Influence of cross-link density. Int. J. Biol. Macromol. 2024, 256, 128441. [Google Scholar] [CrossRef]
- Wuttisarn, R.; Niyomsin, S.; Pangon, A.; Veranitisagul, C.; Laobuthee, A.; Chirachanchai, S. α-amino acid-derived poly(ester-amide-urethane): A potential bio-based elastomers with tunable mechanics and methanolysis-driven degradability. Polym. Degrad. Stab. 2026, 244, 111786. [Google Scholar] [CrossRef]
- Kar, A.; Ahmad, M.; Mandal, M.; Karak, N. Elastomeric biodegradable poly(ester amide urethane) as a tough and robust material. Prog. Org. Coatings 2023, 182, 107684. [Google Scholar] [CrossRef]
- Oprea, S.; Potolinca, O. Synthesis of cross-linked polyurethane elastomers with fluorescein linkages. J. Mater. Sci. 2009, 44, 4181–4187. [Google Scholar] [CrossRef]
- Gradinaru, L.M.; Vlad, S.; Spiridon, I.; Petrescu, M. Durability of polyurethane membranes in artificial weathering environment. Polym. Test. 2019, 80, 106144. [Google Scholar] [CrossRef]
- Brzoska, J.; Smorawska, J.; Głowinska, E.; Datta, J. A green route for high-performance bio-based polyurethanes synthesized from modified bio-based isocyanates. Ind. Crop. Prod. 2024, 222, 119542. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.; Yang, W.; Zhang, Y.; Wu, W.; Luo, Y. Preparation and properties of block copolymerization thermoset polyurethane elastomers prepared in situ. Polymer 2024, 290, 126586. [Google Scholar] [CrossRef]
- Maaskant, E.; Aarsen, C.V.; van Es, D.S. Accelerated weathering of furanoate polyesters: Effect of molecular weight, crystallinity, and time. J. Appl. Polym. Sci. 2023, 140, e54062. [Google Scholar] [CrossRef]
- Tian, S.; Luo, Y.; Chen, J.; He, H.; Chen, Y.; Ling, Z. A Comprehensive Study on The Accelerated Weathering Properties of Polypropylene—Wood Composites with Non-Metallic Materials of Waste-Printed Circuit Board Powders Shenghui. Materials 2019, 12, 876. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, M.; Ding, Y.; Zhou, Y.; Dan, Y. Aging induced ductile-brittle-ductile transition in bisphenol A polycarbonate. J. Polym. Res. 2018, 25, 39. [Google Scholar] [CrossRef]
- Grasset, C.; Groeneveld, M.; Tranvik, L.J.; Robertson, L.P.; Hawkes, J.A. Hydrophilic Species Are the Most Biodegradable Components of Freshwater Dissolved Organic Matter. Environ. Sci. Technol. 2023, 57, 13463–13472. [Google Scholar] [CrossRef]
- Young, T. An Essay on the Cohesion of Fluids. Philos. Trans. R. Soc. London 1805, 95, 65–87. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- FOWKES, F.M. Dispersion Force Contributions to Surface and Interfacial Tensions, Contact Angles, and Heats of Immersion. In Contact Angle, Wettability, and Adhesion; Fowkes, F.M., Ed.; American Chemical Society: Washington, DC, USA, 1964; pp. 99–111. [Google Scholar]
- Hejda, F.; Solar, P.; Kousal, J. Surface Free Energy Determination by Contact Angle Measurements—A Comparison of Various Approaches. In Proceedings of the WDS’10 Proceedings of Contributed Papers, Part III, Prague, Czech Republic, 1–4 June 2010; Volumes 25–30, pp. 25–30. [Google Scholar]
- Król, P.; Król, B. Surface free energy of polyurethane coatings with improved hydrophobicity. Colloid Polym. Sci. 2012, 290, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, M.R.; Calvo, O.; Fenollar, O.; Garcia, D.; Balart, R. Characterization of the surface changes and the aging effects of low-pressure nitrogen plasma treatment in a polyurethane film. Polym. Test. 2008, 27, 75–83. [Google Scholar] [CrossRef]
- Yang, D.; Brett, J.K.; Celina, M.C. Hydrolysis of poly(ester urethane): In-depth mechanistic pathways through FTIR 2D-COS spectroscopy. Polym. Degrad. Stab. 2025, 231, 111094. [Google Scholar] [CrossRef]
- Salazar, M.R.; Pack, R.T. Degradation of a poly(ester urethane) elastomer. II. Kinetic modeling of the hydrolysis of a poly(butylene adipate). J. Polym. Sci. Part B Polym. Phys. 2002, 40, 192–200. [Google Scholar] [CrossRef]
- Papp, V.; Vadkerti, B.; Banyai, I.; Keki, S.; Keri, M. Structure and Swelling Properties of Biodegradable Cross-Linked Polyurethanes by Means of Nuclear Magnetic Resonance. J. Phys. Chem. B 2025, 129, 5607–5620. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.; Bryant, P.; Kurusingal, V.; Colwell, J.M.; Laycock, B. Degradation and stabilization of polyurethane elastomers. Prog. Polym. Sci. 2019, 90, 211–268. [Google Scholar] [CrossRef]
- Pretsch, T.; Jakob, I.; Muller, W. Hydrolytic degradation and functional stability of a segmented shape memory poly(ester urethane). Polym. Degrad. Stab. 2009, 94, 61–73. [Google Scholar] [CrossRef]
- Pegoretti, A.; Penati, A. Recycled poly(ethylene terephthalate) and its short glass fibres composites: Effects of hygrothermal aging on the thermo-mechanical behaviour. Polymer 2004, 45, 7995–8004. [Google Scholar] [CrossRef]
- Han, J.; Chen, B.; Ye, L.; Zhang, A.Y.; Zhang, J.; Feng, Z.G. Synthesis and characterization of biodegradable polyurethane based on poly(ε-caprolactone) and L-lysine ethyl ester diisocyanate. Front. Mater. Sci. China 2009, 3, 25–32. [Google Scholar] [CrossRef]
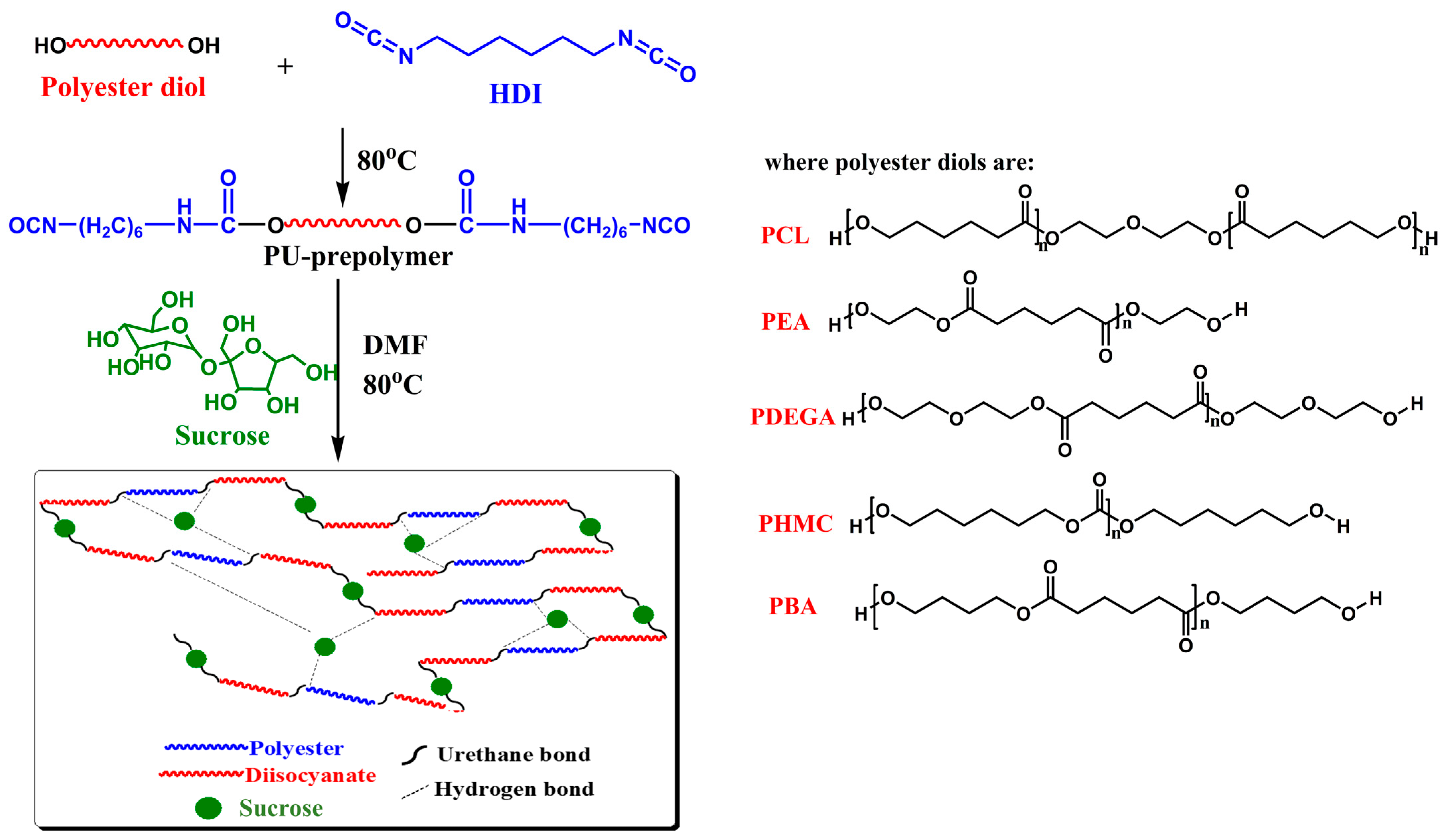













| Sample | Polyester Diol | Polyester Diol/HDI Molar Ratio | Polyester Diol/Sucrose Molar Ratio |
|---|---|---|---|
| S1 | PEA | 1/2 | 5/1 |
| S2 | PHMC | 1/2 | 5/1 |
| S3 | PDEGA | 1/2 | 5/1 |
| S4 | PBA | 1/2 | 5/1 |
| S5 | PCL | 1/2 | 5/1 |
| Sample | Degradation Stages/Mass Loss (°C/%) | T5% (a) (°C) | Tmax (b) (°C) | Char Residue (%) | ||
|---|---|---|---|---|---|---|
| I | II | III | ||||
| S1 | 280-395/25 | 395-470/69 | 470-520/2 | 320 | 441 | 3.35 |
| S2 | 280-345/13 | 345-425/83 | 425-520/3 | 315 | 384 | 0.47 |
| S3 | 280-345/8 | 345-480/89 | 480-520/1 | 321 | 447 | 1.44 |
| S4 | 280-330/6 | 330-465/88 | 465-520/5 | 317 | 431 | 0.96 |
| S5 | 280-345/7 | 345-470/88 | 470-520/4 | 328 | 430 | 0.74 |
| Sample | S1 | S2 | S3 | S4 | S5 | |
|---|---|---|---|---|---|---|
| Tg (°C) | untreated | −38 | −42 | −38 | −43 | −52 |
| −40 °C (d) | −38 | −41 | −39 | −46 | −53 | |
| 80 °C (e) | −37 | −45 | −44 | −44 | −53 | |
| UV (f) | −37 | −40 | −43 | −44 | −49 | |
| ∆Cp (J g−1 C−1) (a) | untreated | 0.529 | 0.456 | 0.667 | 0.212 | 0.268 |
| −40 °C (d) | 0.531 | 0.426 | 0.589 | 0.203 | 0.244 | |
| 80 °C (e) | 0.180 | 0.623 | 0.619 | 0.288 | 0.214 | |
| UV (f) | 0.538 | 0.488 | 0.709 | 0.150 | 0.236 | |
| Tm (°C) (b) | untreated | - | - | - | 50 | 41 |
| −40 °C (d) | - | - | - | 44 | 42 | |
| 80 °C (e) | - | - | - | 44 | 41 | |
| UV (f) | - | - | - | 43 | 47 | |
| ∆Hm (J g−1) (c) | untreated | - | - | - | 43.834 | 36.275 |
| −40 °C (d) | - | - | - | 43.951 | 35.146 | |
| 80 °C (e) | - | - | - | 59.742 | 36.850 | |
| UV (f) | - | - | - | 31.468 | 35.878 | |
| Sample | Retention Rates After Thermal and UV Aging (%) | |||||
|---|---|---|---|---|---|---|
| −40 °C | 80 °C | UV | ||||
| Rσ (a) | RE (b) | Rσ (a) | RE (b) | Rσ (a) | RE (b) | |
| S1 | 81.94 | 85.74 | 67.59 | 83.29 | 46.76 | 66.86 |
| S2 | 109.33 | 95.97 | 66.32 | 86.05 | 50.26 | 81.75 |
| S3 | 93.15 | 102.26 | 39.73 | 82.41 | 21.92 | 58.36 |
| S4 | 115.85 | 8.83 | 96.33 | 7.35 | 68.67 | 4.33 |
| S5 | 93.07 | 92.00 | 85.62 | 98.39 | 87.75 | 47.59 |
| Sample | S1 | S2 | S3 | S4 | S5 | |
|---|---|---|---|---|---|---|
| Sq (nm) | untreated | 17.40 | 137.90 | 80.04 | 29.71 | 27.51 |
| −40 °C (a) | 34.48 | 207.99 | 149.28 | 53.28 | 71.82 | |
| 80 °C (b) | 50.27 | 149.62 | 45.80 | 249.91 | 45.18 | |
| UV (c) | 34.32 | 160.47 | 7.07 | 63.74 | 162.51 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Potolinca, V.O.; Varganici, C.-D.; Doroftei, F.; Oprea, S. Effects of Aging on Sucrose-Based Poly(ester-urethane)s: Thermal, Ultraviolet, and Hydrolytic Stability. Polymers 2026, 18, 88. https://doi.org/10.3390/polym18010088
Potolinca VO, Varganici C-D, Doroftei F, Oprea S. Effects of Aging on Sucrose-Based Poly(ester-urethane)s: Thermal, Ultraviolet, and Hydrolytic Stability. Polymers. 2026; 18(1):88. https://doi.org/10.3390/polym18010088
Chicago/Turabian StylePotolinca, Violeta Otilia, Cristian-Dragos Varganici, Florica Doroftei, and Stefan Oprea. 2026. "Effects of Aging on Sucrose-Based Poly(ester-urethane)s: Thermal, Ultraviolet, and Hydrolytic Stability" Polymers 18, no. 1: 88. https://doi.org/10.3390/polym18010088
APA StylePotolinca, V. O., Varganici, C.-D., Doroftei, F., & Oprea, S. (2026). Effects of Aging on Sucrose-Based Poly(ester-urethane)s: Thermal, Ultraviolet, and Hydrolytic Stability. Polymers, 18(1), 88. https://doi.org/10.3390/polym18010088







