A Straightforward Methodology for the Quantification of Long Chain Branches in Polyethylene by 13C NMR Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
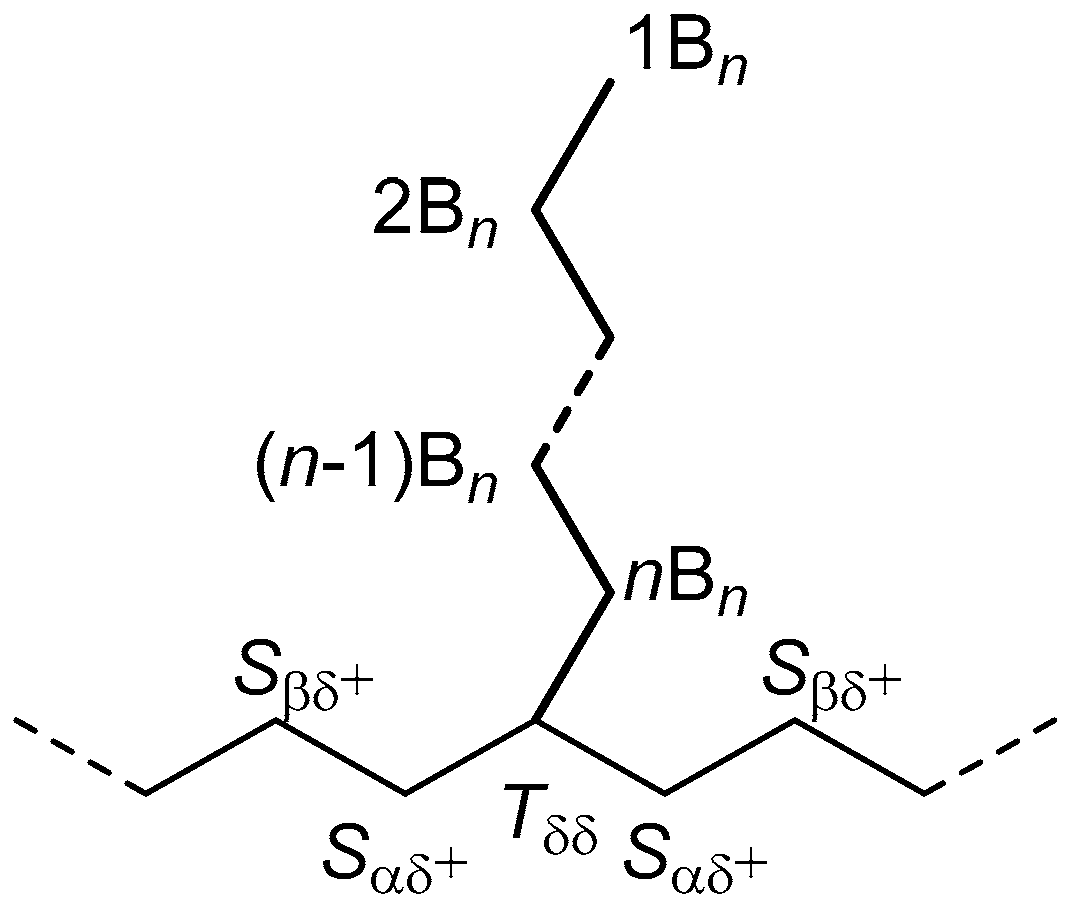
3.1. Detection of LCB in the Absence of Overlapping SCB
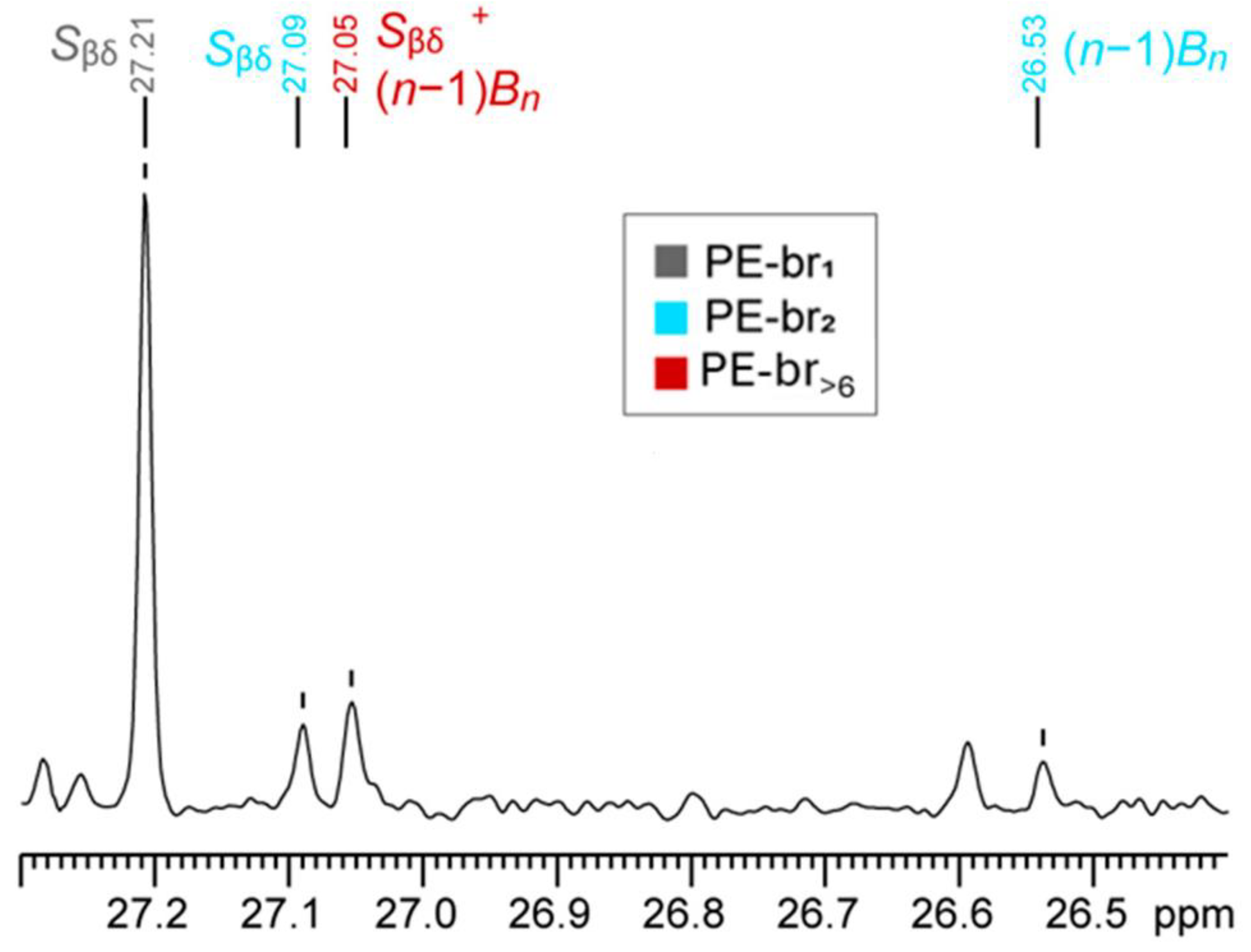
3.2. Detection of LCB in the Presence of Overlapping SCB
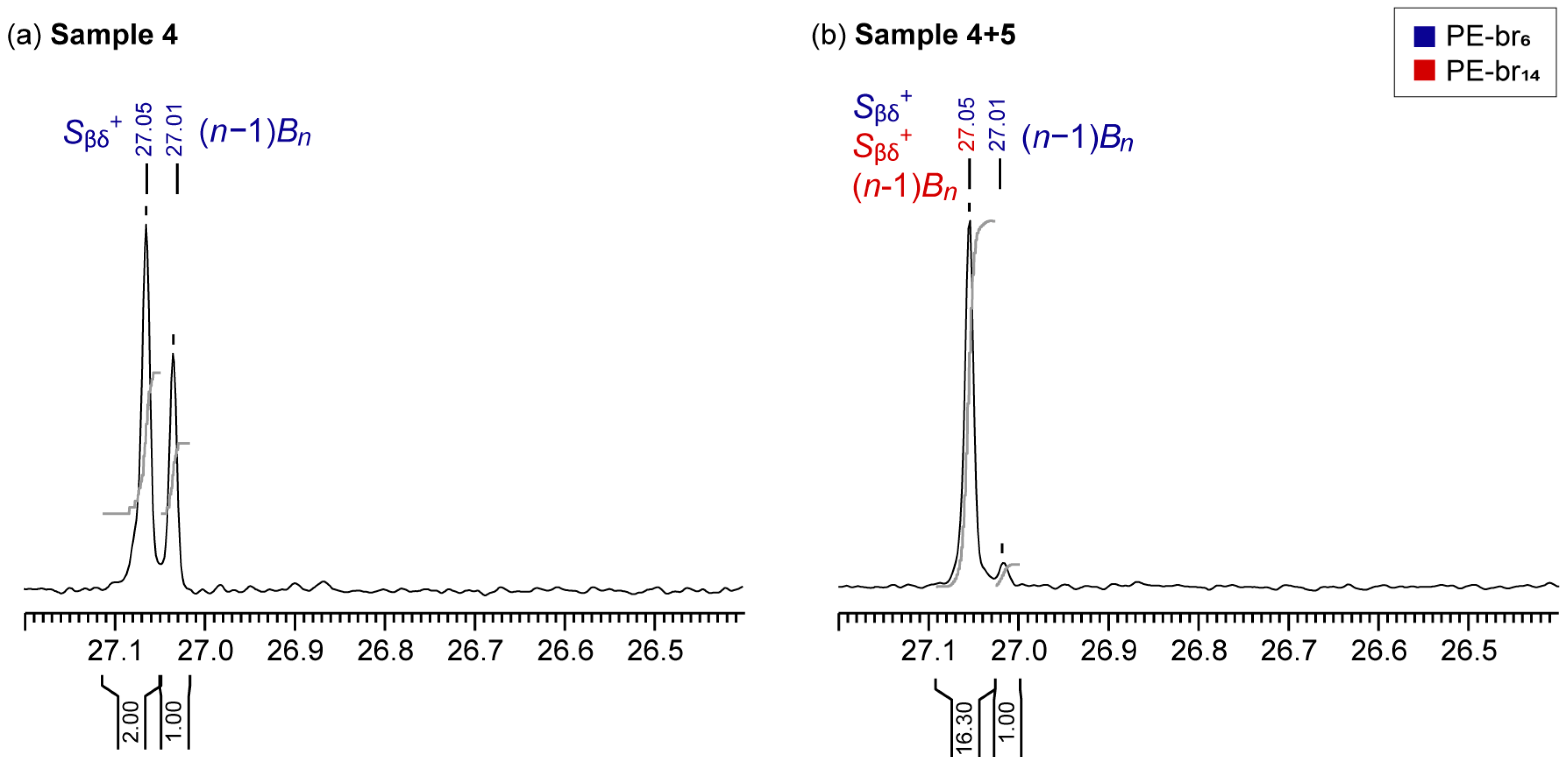
3.3. Analysis of a Representative Industrial Sample
4. Conclusions
- If only one Cβ signal at 27.05 ppm is present, the polymer contains only LCB
- If two Cβ signals at 27.05 and 27.01 ppm are present and exhibit a 2:1 integral ratio, the polymer contains only hexyl-type SCB
- If two Cβ signals at 27.05 and 27.01 ppm are present and exhibit an integral ratio higher than 2:1, the polymer contains both hexyl-type SCB and LCB, and the contribution of the latter can be estimated using Equation (1) (or Equation (2)).
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PE | Polyethylene |
| LDPE | Low-density polyethylene |
| LLDPE | Linear low-density polyethylene |
| HDPE | High-density polyethylene |
| LCB | Long chain branches |
| SCB | Short chain branches |
References
- Sauter, D.; Taoufik, M.; Boisson, C. Polyolefins, a Success Story. Polymers 2017, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Stürzel, M.; Mihan, S.; Mülhaupt, R. From Multisite Polymerization Catalysis to Sustainable Materials and All-Polyolefin Composites. Chem. Rev. 2016, 116, 1398–1433. [Google Scholar] [CrossRef] [PubMed]
- Busico, V.; Cipullo, R. Microstructure of Polypropylene. Prog. Polym. Sci. 2001, 26, 443–533. [Google Scholar] [CrossRef]
- Zanchin, G.; Leone, G. Polyolefin Thermoplastic Elastomers from Polymerization Catalysis: Advantages, Pitfalls and Future Challenges. Prog. Polym. Sci. 2021, 113, 101342. [Google Scholar] [CrossRef]
- Hees, T.; Zhong, F.; Stürzel, M.; Mülhaupt, R. Tailoring Hydrocarbon Polymers and All-Hydrocarbon Composites for Circular Economy. Macromol. Rapid Commun. 2019, 40, 1800608. [Google Scholar] [CrossRef]
- Klosin, J.; Fontaine, P.P.; Figueroa, R. Development of Group IV Molecular Catalysts for High Temperature Ethylene-α-Olefin Copolymerization Reactions. Acc. Chem. Res. 2015, 48, 2004–2016. [Google Scholar] [CrossRef]
- Simpson, G.A.; Vaughan, D.M. Ethylene Polymers, LLDPE. In Encyclopedia of Polymer Science and Technology, 4th ed.; Mark, H.F., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; Volume 41. [Google Scholar]
- Burfield, D.R. Correlation between Crystallinity and Ethylene Content in LLDPE and Related Ethylene Copolymers. Demonstration of the Applicability of a Simple Empirical Relationship. Macromolecules 1987, 20, 3020–3023. [Google Scholar] [CrossRef]
- Alizadeh, A.; Richardson, L.; Xu, J.; McCartney, S.; Marand, H.; Cheung, Y.W.; Chum, S. Influence of Structural and Topological Constraints on the Crystallization and Melting Behavior of Polymers. 1. Ethylene/1-Octene Copolymers. Macromolecules 1999, 32, 6221–6235. [Google Scholar] [CrossRef]
- Bensason, S.; Minick, J.; Moet, A.; Chum, S.; Hiltner, A.; Baer, E. Classification of Homogeneous Ethylene-Octene Copolymers Based on Comonomer Content. J. Polym. Sci. Part B Polym. Phys. 1996, 34, 1301–1315. [Google Scholar] [CrossRef]
- Piel, C.; Starck, P.; Seppälä, J.V.; Kaminsky, W. Thermal and Mechanical Analysis of Metallocene-Catalyzed Ethene-α-Olefin Copolymers: The Influence of the Length and Number of the Crystallizing Side Chains. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 1600–1612. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Lu, Z.; Sun, C.-C. Roles of Branch Content and Branch Length in Copolyethylene Crystallization: Molecular Dynamics Simulations. Macromolecules 2002, 35, 106–111. [Google Scholar] [CrossRef]
- van Doremaele, G.; van Duin, M.; Valla, M.; Berthoud, A. On the Development of Titanium Κ1-Amidinate Complexes, Commercialized as Keltan ACETM Technology, Enabling the Production of an Unprecedented Large Variety of EPDM Polymer Structures. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 2877–2891. [Google Scholar] [CrossRef]
- Budzelaar, P.H.M. Mechanisms of Branch Formation in Metal-Catalyzed Ethene Polymerization. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 221–241. [Google Scholar] [CrossRef]
- Usami, T.; Takayama, S. Fine-Branching Structure in High-Pressure, Low-Density Polyethylenes by 50.10-MHz Carbon-13 NMR Analysis. Macromolecules 1984, 17, 1756–1761. [Google Scholar] [CrossRef]
- Zhou, Z.; Pesek, S.; Klosin, J.; Rosen, M.S.; Mukhopadhyay, S.; Cong, R.; Baugh, D.; Winniford, B.; Brown, H.; Xu, K. Long Chain Branching Detection and Quantification in LDPE with Special Solvents, Polarization Transfer Techniques, and Inverse Gated 13C NMR Spectroscopy. Macromolecules 2018, 51, 8443–8454. [Google Scholar] [CrossRef]
- Liu, P.; Liu, W.; Wang, W.J.; Li, B.G.; Zhu, S. A Comprehensive Review on Controlled Synthesis of Long-Chain Branched Polyolefins: Part 3, Characterization of Long-Chain Branched Polymers. Macromol. React. Eng. 2017, 11, 1600012. [Google Scholar] [CrossRef]
- Ye, Z.; AlObaidi, F.; Zhu, S.; Subramanian, R. Long-Chain Branching and Rheological Properties of Ethylene-1-Hexene Copolymers Synthesized from Ethylene Stock by Concurrent Tandem Catalysis. Macromol. Chem. Phys. 2005, 206, 2096–2105. [Google Scholar] [CrossRef]
- Graessley, W.W. Effect of Long Branches on the Temperature Dependence of Viscoelastic Properties in Polymer Melts. Macromolecules 1982, 15, 1164–1167. [Google Scholar] [CrossRef]
- Shroff, R.N.; Mavridis, H. Assessment of NMR and Rheology for the Characterization of LCB in Essentially Linear Polyethylenes. Macromolecules 2001, 34, 7362–7367. [Google Scholar] [CrossRef]
- Janzen, J.; Colby, R.H. Diagnosing Long-Chain Branching in Polyethylenes. J. Mol. Struct. 1999, 485–486, 569–583. [Google Scholar] [CrossRef]
- Piel, C.; Stadler, F.J.; Kaschta, J.; Rulhoff, S.; Münstedt, H.; Kaminsky, W. Structure-Property Relationships of Linear and Long-Chain Branched Metallocene High-Density Polyethylenes Characterized by Shear Rheology and SEC-MALLS. Macromol. Chem. Phys. 2006, 207, 26–38. [Google Scholar] [CrossRef]
- Pathaweeisariyakul, T.; Narkchamnan, K.; Thitisuk, B.; Yau, W. Methods of Long Chain Branching Detection in PE by Triple-Detector Gel Permeation Chromatography. J. Appl. Polym. Sci. 2015, 132, 42222. [Google Scholar] [CrossRef]
- Bryant, W.M.D.; Voter, R.C. The Molecular Structure of Polyethylene. II. Determination of Short Chain Branching. J. Am. Chem. Soc. 1953, 75, 6113–6118. [Google Scholar] [CrossRef]
- Zhou, Z.; Duan, X.; Yu, Y.; Ma, L.; Moreno, A.; Xia, Y.; Chen, L.; Ye, S.; Cong, R. Recent Advances and Applications of NMR Techniques in Plastic Characterizations. Anal. Chem. 2025, 97, 5847–5865. [Google Scholar] [CrossRef] [PubMed]
- Randall, J.C. A review of high resolution liquid 13carbon nuclear magnetic resonance characterizations of ethylene-based polymers. J. Macromol. Sci. Part C 1989, 29, 201–317. [Google Scholar] [CrossRef]
- Spiess, H.W. 50th Anniversary Perspective: The Importance of NMR Spectroscopy to Macromolecular Science. Macromolecules 2017, 50, 1761–1777. [Google Scholar] [CrossRef]
- Zhou, Z.; Kümmerle, R.; Stevens, J.C.; Redwine, D.; He, Y.; Qiu, X.; Cong, R.; Klosin, J.; Montañez, N.; Roof, G. 13C NMR of Polyolefins with a New High Temperature 10mm Cryoprobe. J. Magn. Reson. 2009, 200, 328–333. [Google Scholar] [CrossRef]
- Zhou, Z.; Kuemmerle, R.; Rau, N.; Eldred, D.; Moreno, A.; Czarniecki, B.; Qiu, X.; Cong, R.; Gies, A.P.; Fan, L.; et al. Polyolefin Analyses with a 10 Mm Multinuclear NMR Cryoprobe. Anal. Chem. 2020, 92, 15596–15603. [Google Scholar] [CrossRef]
- Vittoria, A.; Mingione, A.; Abbate, R.A.; Cipullo, R.; Busico, V. High Throughput Experimentation Protocol for Quantitative Measurements of Regioselectivity in Ziegler-Natta Polypropylene Catalysis. Ind. Eng. Chem. Res. 2019, 58, 14729–14735. [Google Scholar] [CrossRef]
- Antinucci, G.; Vittoria, A.; Cipullo, R.; Busico, V. Regioirregular Monomeric Units in Ziegler–Natta Polypropylene: A Sensitive Probe of the Catalytic Sites. Macromolecules 2020, 53, 3789–3795. [Google Scholar] [CrossRef]
- Zhou, Z.; Anklin, C.; Kuemmerle, R.; Cong, R.; Qiu, X.; Decesare, J.; Kapur, M.B.; Patel, R. Very Sensitive 13C NMR Method for the Detection and Quantification of Long-Chain Branches in Ethylene-Hexene Linear Low-Density Polyethylene. Macromolecules 2021, 54, 5985–5990. [Google Scholar] [CrossRef]
- Zhou, Z.; Paradkar, R.; Cong, R.; Qiu, X.; Fan, L.; Kuemmerle, R.; Moreno, A.; Czarniecki, B. Analyses of Short Chain Branches in Polyolefins with Improved 1H NMR Spectroscopy. Anal. Chem. 2020, 92, 8350–8355. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Lee, Y.; Kwak, S.; Park, H.; Kim, B.; Kim, S.; Lee, K.H.; Cho, H.S.; Hwang, K.Y. Analysis of Chain Branch of Polyolefins by a New Proton NMR Approach. Anal. Chem. 2016, 88, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Kümmerle, R.; Qiu, X.; Redwine, D.; Cong, R.; Taha, A.; Baugh, D.; Winniford, B. A New Decoupling Method for Accurate Quantification of Polyethylene Copolymer Composition and Triad Sequence Distribution with 13C NMR. J. Magn. Reson. 2007, 187, 225–233. [Google Scholar] [CrossRef]
- Baugh, D.; Redwine, O.D.; Taha, A.; Reichek, K.; Potter, J. Using Solvents to Improve the Chemical Shift Differences between Short-Chain Branch Methines and Long-Chain Branch Methines in Polyethylene Copolymers. Polyolefin Charact. 2007, 257, 158–161. [Google Scholar]
- Zhou, Z.; Baugh, D.; Fontaine, P.P.; He, Y.; Shi, Z.; Mukhopadhyay, S.; Cong, R.; Winniford, B.; Miller, M. Long-Chain Branch Measurement in Substantially Linear Ethylene Polymers by 13C NMR with Halogenated Naphthalenes as Solvents. Macromolecules 2017, 50, 7959–7966. [Google Scholar] [CrossRef]
- Zhou, Z.; Anklin, C.; Cong, R.; Qiu, X.; Kuemmerle, R. Long-Chain Branch Detection and Quantification in Ethylene-Hexene LLDPE with 13C NMR. Macromolecules 2021, 54, 757–762. [Google Scholar] [CrossRef]
- Cong, R.; Parrott, A.; Hollis, C.; Cheatham, M.; Hill, T.; Bailey, K.; Zhou, Z.; Bautista, J.; Balding, P.; Fan, J. Quantification of Chemical Composition Distribution of Polyolefin Materials for Improved Accuracy and Speed. Macromolecules 2023, 56, 1492–1502. [Google Scholar] [CrossRef]
- Góra, M.; Tranchida, D.; Albrecht, A.; Müller, A.J.; Cavallo, D. Fast Successive Self-Nucleation and Annealing (SSA) Thermal Fractionation Protocol for the Characterization of Polyolefin Blends from Mechanical Recycling. J. Polym. Sci. 2022, 60, 3366–3378. [Google Scholar] [CrossRef]
- Long, Y.-Y.; Lv, J.; Li, B.-X. Fast Quantitative Analysis of High Density Polyethylene Using Adiabatic-Refocused INEPT Spectroscopy. Polymer 2024, 310, 127476. [Google Scholar] [CrossRef]
- Giampaolo, F.; Cipullo, R.; Cuomo, S.; Piccialli, F.; Busico, V. Automated Ultra-Fast 13C NMR Analysis of Polyolefin Materials. Anal. Chem. 2025, 97, 2503–2510. [Google Scholar] [CrossRef]
- Antinucci, G.; Pucciarelli, A.; Vittoria, A.; Zaccaria, F.; Urciuoli, G.; Ehm, C.; Cannavacciuolo, F.D.; Cipullo, R.; Busico, V. Fast Analytics of High-Impact Polypropylene (HIPP). ACS Appl. Polym. Mater. 2023, 5, 3894–3897. [Google Scholar] [CrossRef]
- Galland, G.B.; Quijada, R.; Rojas, R.; Bazan, G.; Komon, Z.J.A. NMR Study of Branched Polyethylenes Obtained with Combined Fe and Zr Catalysts. Macromolecules 2002, 35, 339–345. [Google Scholar] [CrossRef]
- Hou, L.; Fan, G.; Guo, M.; Hsieh, E.; Qiao, J. An improved method for distinguishing branches longer than six carbons (B6+) in polyethylene by solution 13C NMR. Polymer 2012, 53, 4329–4332. [Google Scholar] [CrossRef]
- Beigzadeh, D.; Soares, J.B.P.; Duever, T.A. Effect of Cocatalyst on the Chain Microstructure of Polyethylene Made with CGC-Ti/MAO/B(C6F5)3. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 3055–3061. [Google Scholar] [CrossRef]
- Wang, W.J.; Yan, D.; Zhu, S.; Hamielec, A.E. Kinetics of Long Chain Branching in Continuous Solution Polymerization of Ethylene Using Constrained Geometry Metallocene. Macromolecules 1998, 31, 8677–8683. [Google Scholar] [CrossRef]
- Kolodka, E.; Wang, W.; Charpentier, P.A.; Zhu, S.; Hamielec, A.E. Long-Chain Branching in Slurry Polymerization of Ethylene with Zirconocene Dichloride/Modified Methylaluminoxane. Polymer 2000, 41, 3985–3991. [Google Scholar] [CrossRef]
- Randall, J.C.; Zoepfl, F.J.; Silverman, J. A 13C NMR Study of Radiation-Induced Long-Chain Branching in Polyethylene. Die Makromol. Chem. Rapid Commun. 1983, 4, 149–157. [Google Scholar] [CrossRef]
- Ehm, C.; Vittoria, A.; Goryunov, G.P.; Izmer, V.V.; Kononovich, D.S.; Samsonov, O.V.; Budzelaar, P.H.M.; Voskoboynikov, A.Z.; Busico, V.; Uborsky, D.V.; et al. On the Limits of Tuning Comonomer Affinity of ‘Spaleck-Type’ Ansa-Zirconocenes in Ethene/1-Hexene Copolymerization: A High-Throughput Experimentation/QSAR Approach. Dalton Trans. 2020, 49, 10162–10172. [Google Scholar] [CrossRef]
- Urciuoli, G.; Zaccaria, F.; Zuccaccia, C.; Cipullo, R.; Budzelaar, P.H.M.; Tensi, L.; Vittoria, A.; Ehm, C.; Macchioni, A.; Busico, V. Al-Alkyl Borate Salt Cocatalysts for Olefin Polymerization: Exploration of N-Donor Ligand Variations. Inorg. Chem. Front. 2024, 11, 7872–7885. [Google Scholar] [CrossRef]
- Uborsky, D.V.; Mladentsev, D.Y.; Guzeev, B.A.; Borisov, I.S.; Vittoria, A.; Ehm, C.; Cipullo, R.; Hendriksen, C.; Friederichs, N.; Busico, V.; et al. C 1-Symmetric Si-Bridged (2-Indenyl)(1-Indenyl) Ansa -Metallocenes as Efficient Ethene/1-Hexene Copolymerization Catalysts. Dalton Trans. 2020, 49, 3015–3025. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Rinaldi, P.L.; McIntosh, L.H.; Quirk, R.P. Poly(Ethylene-Co-1-Octene) Characterization by High-Temperature Multidimensional NMR at 750 MHz. Macromolecules 2001, 34, 4757–4767. [Google Scholar] [CrossRef]
- Liu, W.; Ray, D.G.; Rinaldi, P.L. Resolution of Signals from Long-Chain Branching in Polyethylene by 13C NMR at 188.6 MHz. Macromolecules 1999, 32, 3817–3819. [Google Scholar] [CrossRef]
- Randall, J.C. Characterization of Long-Chain Branching in Polyethylenes Using High-Field Carbon-13 NMR. ACS Symp. Ser. 1980, 142, 93–118. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaccaria, F.; Pucciarelli, A.; Cipullo, R.; Busico, V. A Straightforward Methodology for the Quantification of Long Chain Branches in Polyethylene by 13C NMR Spectroscopy. Polymers 2025, 17, 1274. https://doi.org/10.3390/polym17091274
Zaccaria F, Pucciarelli A, Cipullo R, Busico V. A Straightforward Methodology for the Quantification of Long Chain Branches in Polyethylene by 13C NMR Spectroscopy. Polymers. 2025; 17(9):1274. https://doi.org/10.3390/polym17091274
Chicago/Turabian StyleZaccaria, Francesco, Andrea Pucciarelli, Roberta Cipullo, and Vincenzo Busico. 2025. "A Straightforward Methodology for the Quantification of Long Chain Branches in Polyethylene by 13C NMR Spectroscopy" Polymers 17, no. 9: 1274. https://doi.org/10.3390/polym17091274
APA StyleZaccaria, F., Pucciarelli, A., Cipullo, R., & Busico, V. (2025). A Straightforward Methodology for the Quantification of Long Chain Branches in Polyethylene by 13C NMR Spectroscopy. Polymers, 17(9), 1274. https://doi.org/10.3390/polym17091274








