Unveiling Thermal Degradation and Fire Behavior of 110 kV Ultra-High-Voltage Flame-Retardant Cable Sheath After Thermal Aging
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Thermal Aging
2.3. Characterization
2.3.1. LOI Test
2.3.2. UL-94 Test
2.3.3. Contact Angle Test
2.3.4. Cone Calorimeter Test
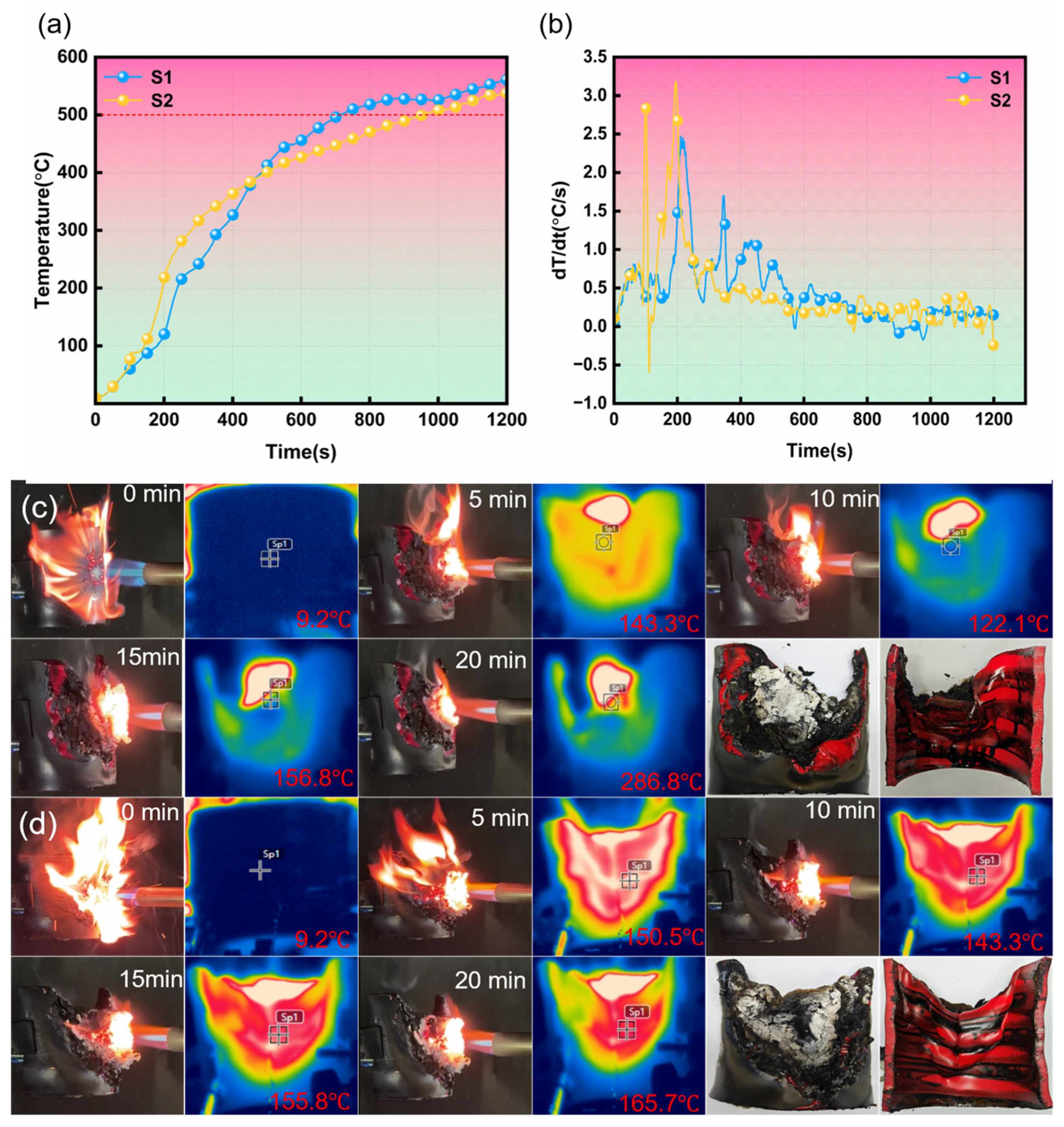
2.3.5. Muffle Furnace Test
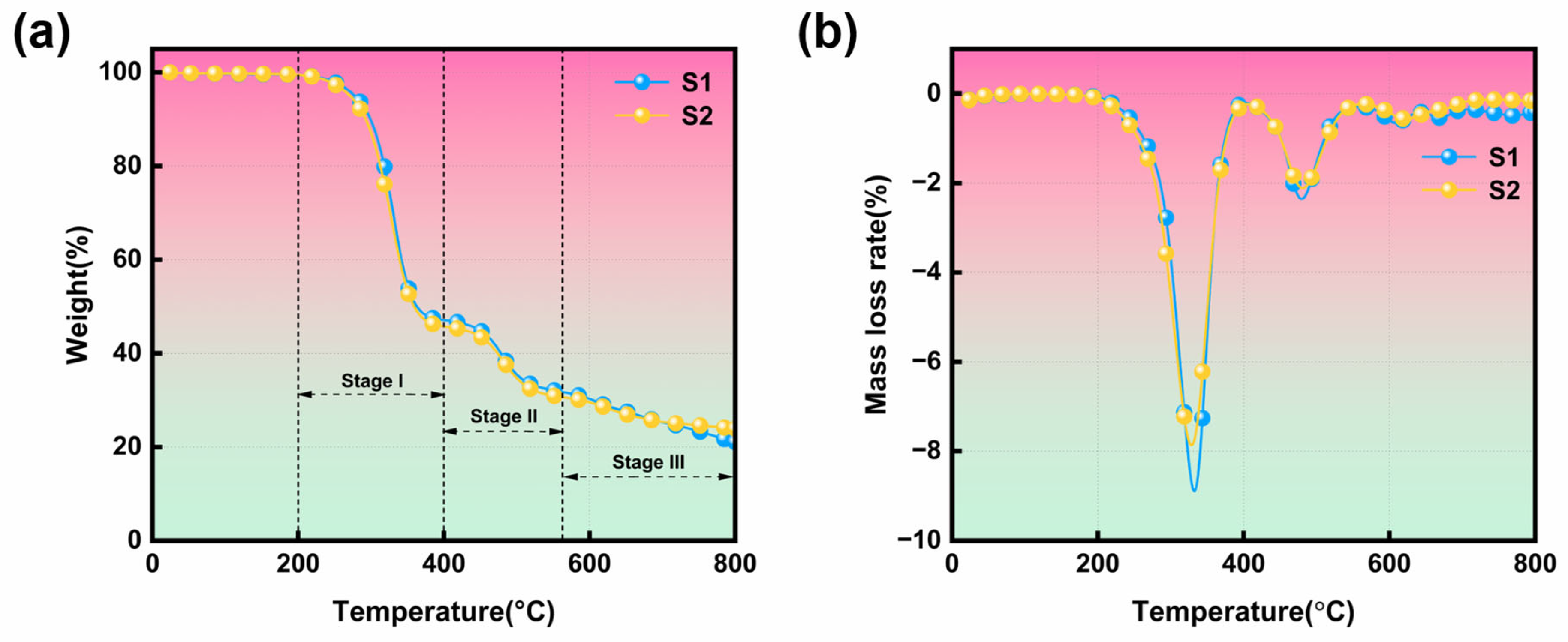
2.3.6. TGA Test
2.3.7. Open Flame Test
2.3.8. FTIR Test
2.3.9. SEM Test
3. Results and Discussion
3.1. Morphological Analysis and Hydrophilicity Analysis
3.2. LOI and UL-94 Tests
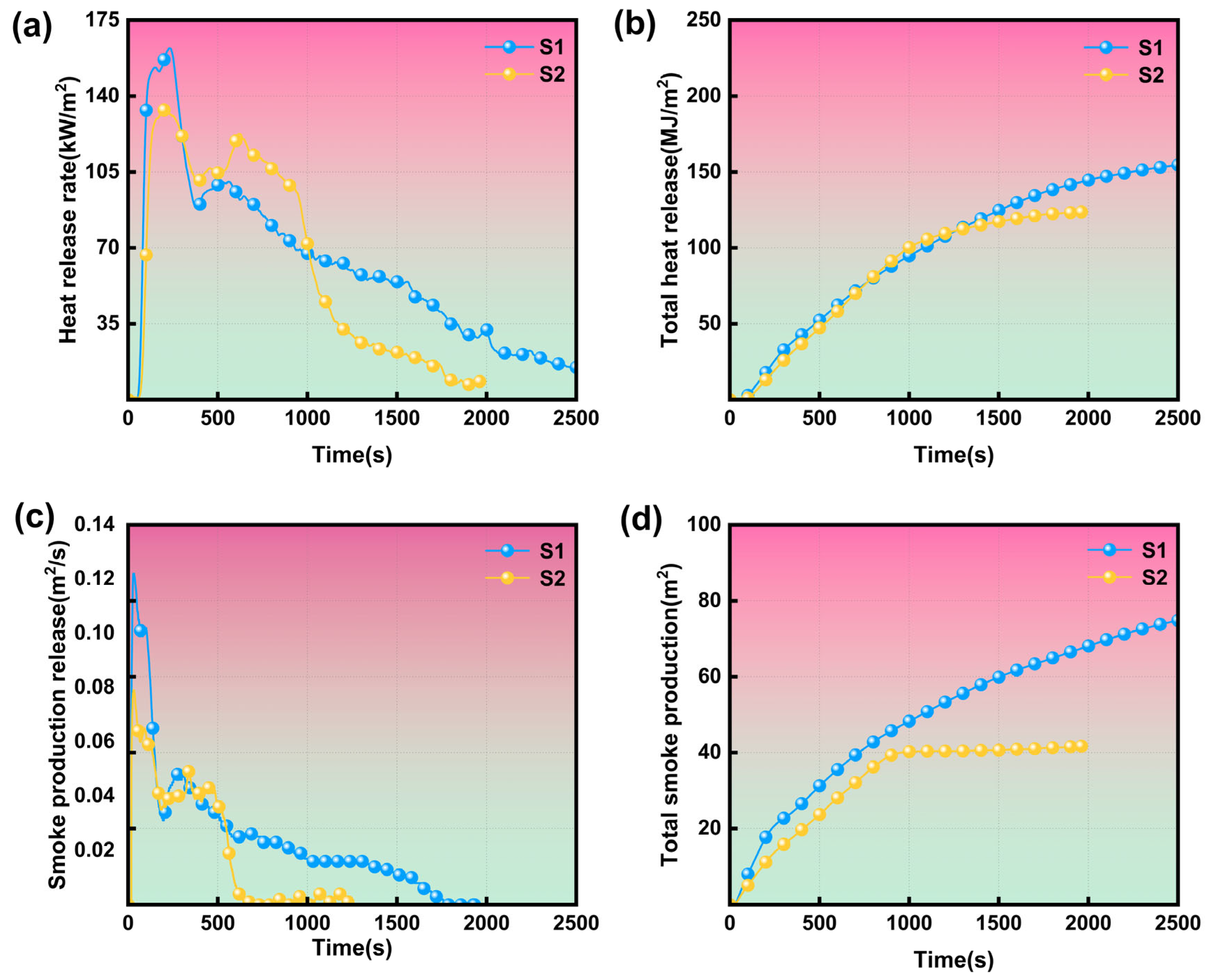
3.3. Forced Flaming Combustion Test
3.4. Fire Resistance
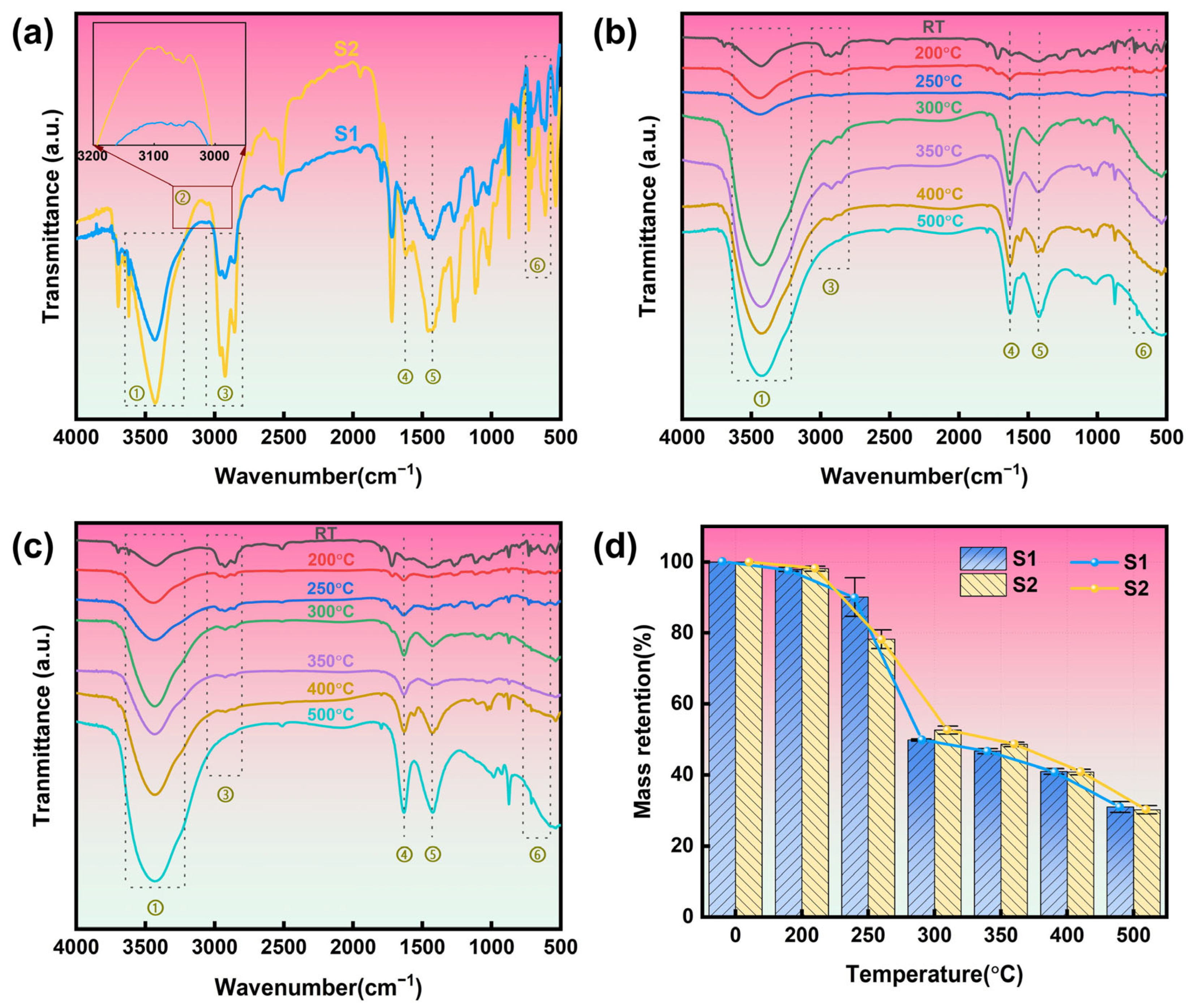
3.5. Thermal Stability Analysis
3.6. Aging Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, W.J.; Liu, X.J.; Yuan, L.J.; Xiao, H.; Lu, J.M. Science, Regulating the structure of crosslinked polyethylene and its application in ultra-high voltage cables. Polym. Eng. 2024, 64, 496–505. [Google Scholar] [CrossRef]
- Ahmad, S.; Rizvi, Z.H.; Wuttke, F. Unveiling soil thermal behavior under ultra-high voltage power cable operations. Sci. Rep. 2025, 15, 7315. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.R.; Zhao, M.; Yu, G.; Kang, N.; Zhang, J.; Guo, Y.; Lu, S. Dynamic risk assessment framework for fire of power critical infrastructure: The case study of UHV converter transformer. Qual. Reliab. Eng. Int. 2024, 41, 71–97. [Google Scholar] [CrossRef]
- Yan, L.; Wang, W.; Xu, Z. Impact of thermal aging temperatures on the combustion behavior and mechanical properties of flame-retardant electrical cables. J. Therm. Anal. Calorim. 2024, 149, 9045–9056. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, N.; Lin, J.; Lu, S.; Liew, K.M. On the pyrolysis characteristic parameters of four flame-retardant classes of PVC sheathless cable insulation materials. J. Anal. Appl. Pyrolysis 2023, 170, 105901. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, H.; Peng, L.; Zheng, Z.; Zeng, W.; Bi, K.; Cheng, C.; Chow, W. Burning behavior of cable tray located on a wall with different cable arrangements. Fire Mater. 2018, 43, 64–73. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, B.; Yang, Y.; Shi, Y.; Liu, C. Study on combustion characteristics and smoke components of aviation cable under low pressure. J. Therm. Anal. Calorim. 2024, 149, 8215–8224. [Google Scholar] [CrossRef]
- Meinier, R.; Sonnier, R.; Zavaleta, P.; Suard, S.; Ferry, L. Fire behavior of halogen-free flame retardant electrical cables with the cone calorimeter. J. Hazard. Mater. 2018, 342, 306–316. [Google Scholar] [CrossRef]
- Quennehen, P.; Royaud, I.; Seytre, G.; Gain, O.; Rain, P.; Espilit, T.; François, S. Determination of the aging mechanism of single core cables with PVC insulation. Polym. Degrad. Stab. 2015, 119, 96–104. [Google Scholar] [CrossRef]
- Liu, H.; Wang, W.; Yan, L.; Xu, Z. Flammability degradation behavior and ageing mechanism of flame-retardant cable sheath under different ageing conditions. Polym. Degrad. Stab. 2024, 230, 111019. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H.; Zhang, J.; Yang, S.; Zhang, Z.; Zhao, W. Thermal degradation of flame-retarded high-voltage cable sheath and insulation via TG-FTIR. J. Anal. Appl. Pyrolysis 2018, 134, 167–175. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, T.; Ning, X.; Liu, Y.; Wang, J. Thermal degradation kinetics study of polyvinyl chloride (PVC) sheath for new and aged cables. Waste Manag. 2019, 99, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.-B.; Chen, X.; Dai, C.; Zhang, M.; Paramane, A.; Zheng, L.; Tanaka, Y. Effect of thermal ageing on physico-chemical and electrical properties of EHVDC XLPE cable insulation. IEEE Trans. Dielectr. Electr. Insul. 2021, 28, 1012–1019. [Google Scholar] [CrossRef]
- Boukezzi, L.; Boubakeur, A. Prediction of mechanical properties of XLPE cable insulation under thermal aging: Neural network approach. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 2125–2134. [Google Scholar] [CrossRef]
- Arunjothi, R.; Raja, G.; Meena, K. Characteristics of Power Cable Sheathing Materials with Thermal ageing. In Proceedings of the 2021 IEEE International Conference on the Properties and Applications of Dielectric Materials (ICPADM), Johor Bahru, Malaysia, 12–14 July 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 398–401. [Google Scholar]
- Anandakumaran, K.; Stonkus, D.J. Determination of PVC cable insulation degradation. J. Vinyl Technol. 2004, 14, 24–28. [Google Scholar] [CrossRef]
- Henrist, C.; Rulmont, A.; Cloots, R.; Gilbert, B.; Bernard, A.; Beyer, G. Toward the understanding of the thermal degradation of commercially available fire-resistant cable. Mater. Lett. 2000, 46, 160–168. [Google Scholar] [CrossRef]
- Tian, R.; Li, K.; Lin, Y.; Lu, C.; Duan, X. Characterization Techniques of Polymer Aging: From Beginning to End. Chem. Rev. 2023, 123, 3007–3088. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Wang, S.; Fu, M.; Tian, F.; Chen, X.; Zhang, Y. Comprehensive assessment of the thermal aging effects on fire risks of PVC cable. J. Therm. Anal. Calorim. 2024, 149, 3785–3793. [Google Scholar] [CrossRef]
- Vahabi, H.; Sonnier, R.; Ferry, L. Effects of ageing on the fire behaviour of flame-retarded polymers: A review. Polym. Int. 2015, 64, 313–328. [Google Scholar] [CrossRef]
- Emanuelsson, V.; Simonson, M.; Gevert, T. The effect of accelerated ageing of building wires. Fire Mater. 2007, 31, 311–326. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, R.; Ning, X.; Xie, T.; Wang, J. Thermal degradation properties of LDPE insulation for new and aged fine wires. J. Therm. Anal. Calorim. 2019, 137, 461–471. [Google Scholar] [CrossRef]
- Kim, M.H.; Seo, H.J.; Lee, S.K.; Lee, M.C. Influence of Thermal Aging on the Combustion Characteristics of Cables in Nuclear Power Plants. Energies 2021, 14, 2003. [Google Scholar] [CrossRef]
- Nowlen, S. The Impact of Thermal Aging on the Flammability of Electric Cables; US Nuclear Regulatory Commission (NRC): Washington, DC, USA, 1991. [Google Scholar]
- Zhang, J.; Guo, Y.; Shao, W.; Xiao, F. Benign design of intumescent fire protection coatings for steel structures containing biomass humic acid as carbon source. Constr. Build. Mater. 2023, 409, 134001. [Google Scholar] [CrossRef]
- Ito, M.; Nagai, K. Analysis of degradation mechanism of plasticized PVC under artificial aging conditions. Polym. Degrad. Stab. 2007, 92, 260–270. [Google Scholar] [CrossRef]
- Pagacz, J.; Chrzanowski, M.; Krucińska, I.; Pielichowski, K. Thermal aging and accelerated weathering of PVC/MMT nanocomposites: Structural and morphological studies. J. Appl. Polym. Sci. 2015, 132, 42090. [Google Scholar] [CrossRef]
- Liu, J.; Lv, Y.; Luo, Z.; Wang, H.; Wei, Z. Molecular chain model construction, thermo-stability, and thermo-oxidative degradation mechanism of poly (vinyl chloride). RSC Adv. 2016, 6, 31898–31905. [Google Scholar] [CrossRef]
- Liu, H.; Wang, W.; Yan, L.; Xu, Z. Fire-Resistant Degradation Mechanism of Polyvinyl Chloride Cable Sheaths Exposed to Different Accelerated Ageing Conditions. Thermochim. Acta 2025, 748, 179983. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, C.; Du, C.; Xu, K.; Li, Y.; Xu, M.; Bourbigot, S.; Fontaine, G.; Li, B.; Liu, L. High-strength, thermal-insulating, fire-safe bio-based organic lightweight aerogel based on 3D network construction of natural tubular fibers. Compos. B Eng. 2023, 261, 110809. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, R.; Wang, X.; He, J.; Wang, J. Pyrolysis and Combustion of Polyvinyl Chloride (PVC) Sheath for New and Aged Cables via Thermogravimetric Analysis-Fourier Transform Infrared (TG-FTIR) and Calorimeter. Materials 2018, 11, 1997. [Google Scholar] [CrossRef]
- Czogała, J.; Pankalla, E.; Turczyn, R. Recent attempts in the design of efficient PVC plasticizers with reduced migration. Materials 2021, 14, 844. [Google Scholar] [CrossRef]
- Xiao, F.; Fontaine, G.; Li, K.; Bourbigot, S. Combination effect of zinc borate in intumescent polybutylene succinate systems: Study of the carbonaceous structures using solid-state NMR. Polym. Degrad. Stab. 2022, 206, 110192. [Google Scholar] [CrossRef]
- Xiao, F.; Fontaine, G.; Bourbigot, S. Improvement of flame retardancy and antidripping properties of intumescent polybutylene succinate combining piperazine pyrophosphate and zinc borate. ACS Appl. Polym. Mater. 2022, 4, 1911–1921. [Google Scholar] [CrossRef]
- Martinka, J.; Rantuch, P.; Sulová, J.; Martinka, F. Assessing the fire risk of electrical cables using a cone calorimeter. J. Therm. Anal. Calorim. 2019, 135, 3069–3083. [Google Scholar] [CrossRef]
- Xiao, F.; Fontaine, G.; Bourbigot, S. A highly efficient intumescent polybutylene succinate: Flame retardancy and mechanistic aspects. Polym. Degrad. Stab. 2022, 196, 109830. [Google Scholar] [CrossRef]
- Park, J.W.; Lim, O.K.; You, W.J. Analysis on the fire growth rate index considering of scale factor, volume fraction, and ignition heat source for polyethylene foam pipe insulation. Energies 2020, 13, 3644. [Google Scholar] [CrossRef]
- Chen, D.-J.; Zhang, Y.-L.; He, J.-Y.; Li, X.-M. Making polycarbonate flame retardant: Flame retardant selection and calorimetric analyses. Polym. Test. 2023, 117, 107876. [Google Scholar] [CrossRef]
- Kerekes, Z.; Restás, Á.; Lublóy, É. The effects causing the burning of plastic coatings of fire-resistant cables and its consequences. J. Therm. Anal. Calorim. 2020, 139, 775–787. [Google Scholar] [CrossRef]
- Deng, J.; Yu, K.-L.; Wang, C.-P.; Chen, L.-J.; Lyu, H.-F.; Shu, C.-M. Pyrolysis mechanism of different thermal aging of PVC insulation copper cable for home decoration. Thermochim. Acta 2023, 729, 179611. [Google Scholar] [CrossRef]
- Beneš, M.; Milanov, N.; Matuschek, G.; Kettrup, A.; Plaček, V.; Balek, V. Thermal degradation of PVC cable insulation studied by simultaneous TG-FTIR and TG-EGA methods. J. Therm. Anal. Calorim. 2004, 78, 621–630. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C.; Antonie, S.R.; Chiriac, M.; Precup, M.; Yang, J.; Roy, C. Study of the natural ageing of PVC insulation for electrical cables. Polym. Degrad. Stab. 2000, 67, 209–221. [Google Scholar] [CrossRef]
- Ramesh, S.; Leen, K.H.; Kumutha, K.; Arof, A.K. FTIR studies of PVC/PMMA blend based polymer electrolytes. Spectrochim. Acta A 2007, 66, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Beltran, M.; Marcilla, A. Fourier transform infrared spectroscopy applied to the study of PVC decomposition. Eur. Polym. J. 1997, 33, 1135–1142. [Google Scholar] [CrossRef]

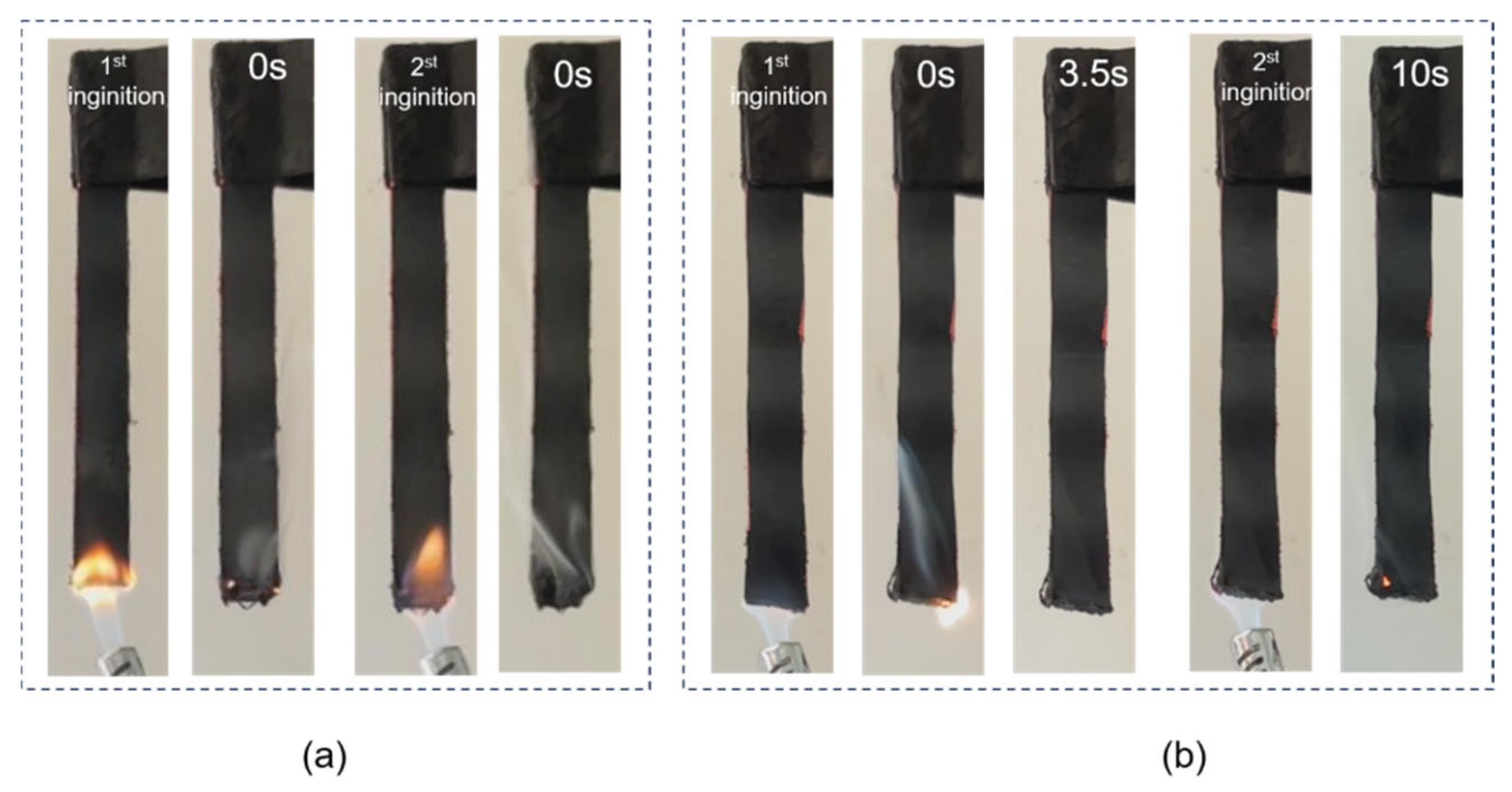

| Sample | t1 [s] | t2 [s] | LOI [%] | Dripping | Rating |
|---|---|---|---|---|---|
| S1 | 0 | 0 | 28.5 | No | V-0 |
| S2 | 3.5 | 0 | 28.3 | No | V-0 |
| Sample | TTI [s] | pHRR1 [kW/m2] | pHRR2 [kW/m2] | THR [MJ/m2] | PSPR1 [m2/s] | PSPR2 [m2/s] | TSP [m2] | FIGRA [kW/(m2 s)] | MARHE [kW/m2] |
|---|---|---|---|---|---|---|---|---|---|
| S1 | 6 | 162.1 | 100.5 | 163.6 | 0.12 | 0.05 | 76.7 | 1.33 | 0.11 |
| S2 | 8 | 133.7 | 122.6 | 123.7 | 0.08 | 0.05 | 41.7 | 0.92 | 0.10 |
| Sample | T5% [°C] | Tpeak1 [°C] | DTGpeak1 [%min−1] | Tpeak2 [°C] | DTGpeak2 [%min−1] | Char Residue [%] |
|---|---|---|---|---|---|---|
| S1 | 277.5 | 332.5 | 8.87 | 479.6 | 2.4 | 21.0 |
| S2 | 271.5 | 329.2 | 7.86 | 481.8 | 2.1 | 23.9 |
| Number | Wavenumber (cm−1) | Functional Group |
|---|---|---|
| ① | 3220–3640 | O-H stretching vibration |
| ② | 3040–3110 | C=C-H |
| ③ | 2800–3060 | CH2 |
| ④ | 1620 | C=C |
| ⑤ | 1425 | CH2 |
| ⑥ | 570–740 | C-Cl |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; He, W.; Huo, X.; Lu, X.; Li, K.; Xiao, F. Unveiling Thermal Degradation and Fire Behavior of 110 kV Ultra-High-Voltage Flame-Retardant Cable Sheath After Thermal Aging. Polymers 2025, 17, 1273. https://doi.org/10.3390/polym17091273
Jiang Y, He W, Huo X, Lu X, Li K, Xiao F. Unveiling Thermal Degradation and Fire Behavior of 110 kV Ultra-High-Voltage Flame-Retardant Cable Sheath After Thermal Aging. Polymers. 2025; 17(9):1273. https://doi.org/10.3390/polym17091273
Chicago/Turabian StyleJiang, Yaqiang, Wei He, Xinke Huo, Xuelian Lu, Kaiyuan Li, and Fei Xiao. 2025. "Unveiling Thermal Degradation and Fire Behavior of 110 kV Ultra-High-Voltage Flame-Retardant Cable Sheath After Thermal Aging" Polymers 17, no. 9: 1273. https://doi.org/10.3390/polym17091273
APA StyleJiang, Y., He, W., Huo, X., Lu, X., Li, K., & Xiao, F. (2025). Unveiling Thermal Degradation and Fire Behavior of 110 kV Ultra-High-Voltage Flame-Retardant Cable Sheath After Thermal Aging. Polymers, 17(9), 1273. https://doi.org/10.3390/polym17091273








