Mechanical, Antibacterial, and Physico-Chemical Properties of Three Different Polymer-Based Direct Restorative Materials: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparations
2.2. Compression Strength & Fracture Mode
2.3. Porosity
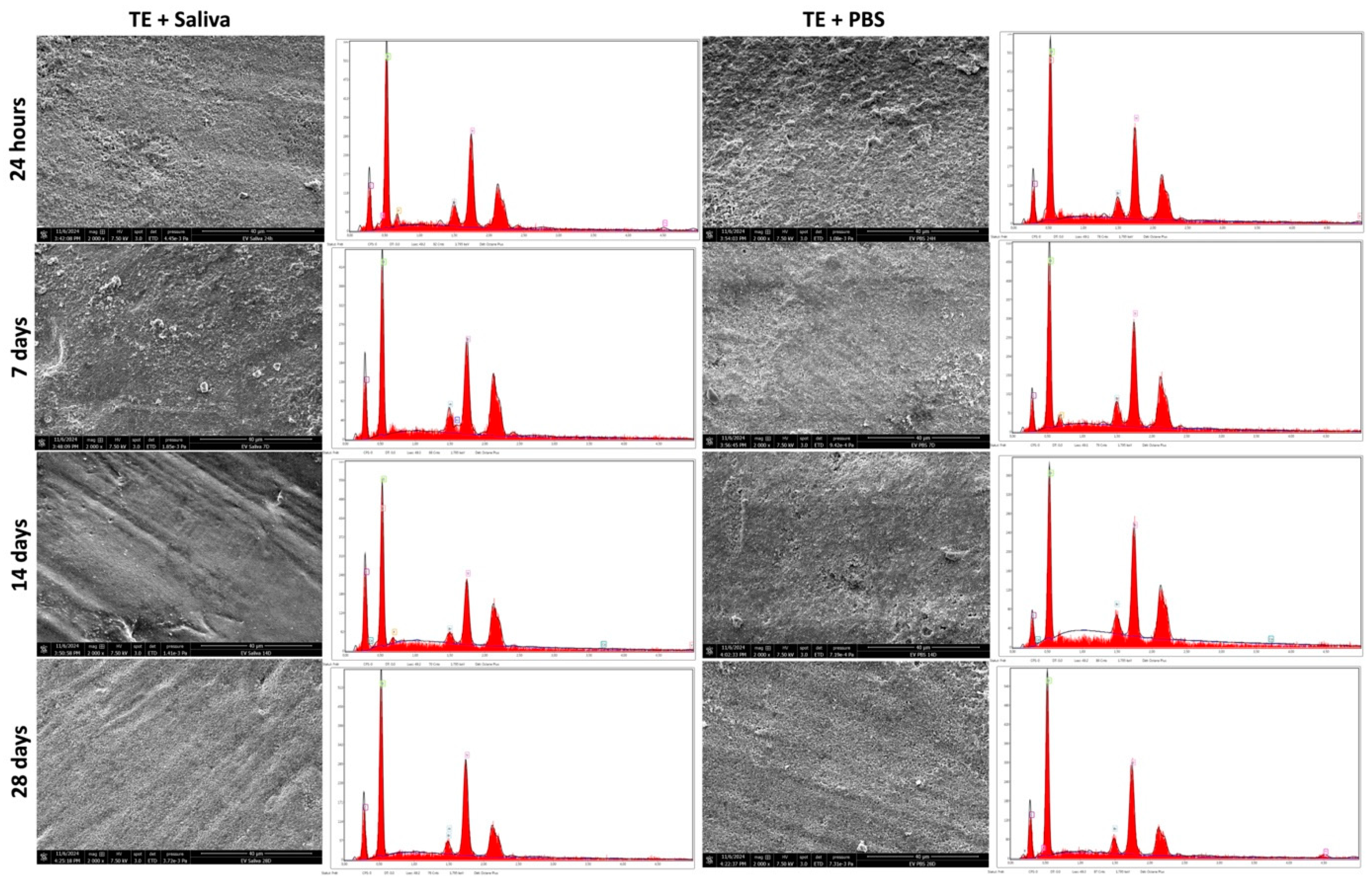
2.4. Morphological Changes After PBS/Saliva Immersion
2.5. Wettability
2.6. Antibacterial Activity
2.7. Statistical Analysis
3. Results
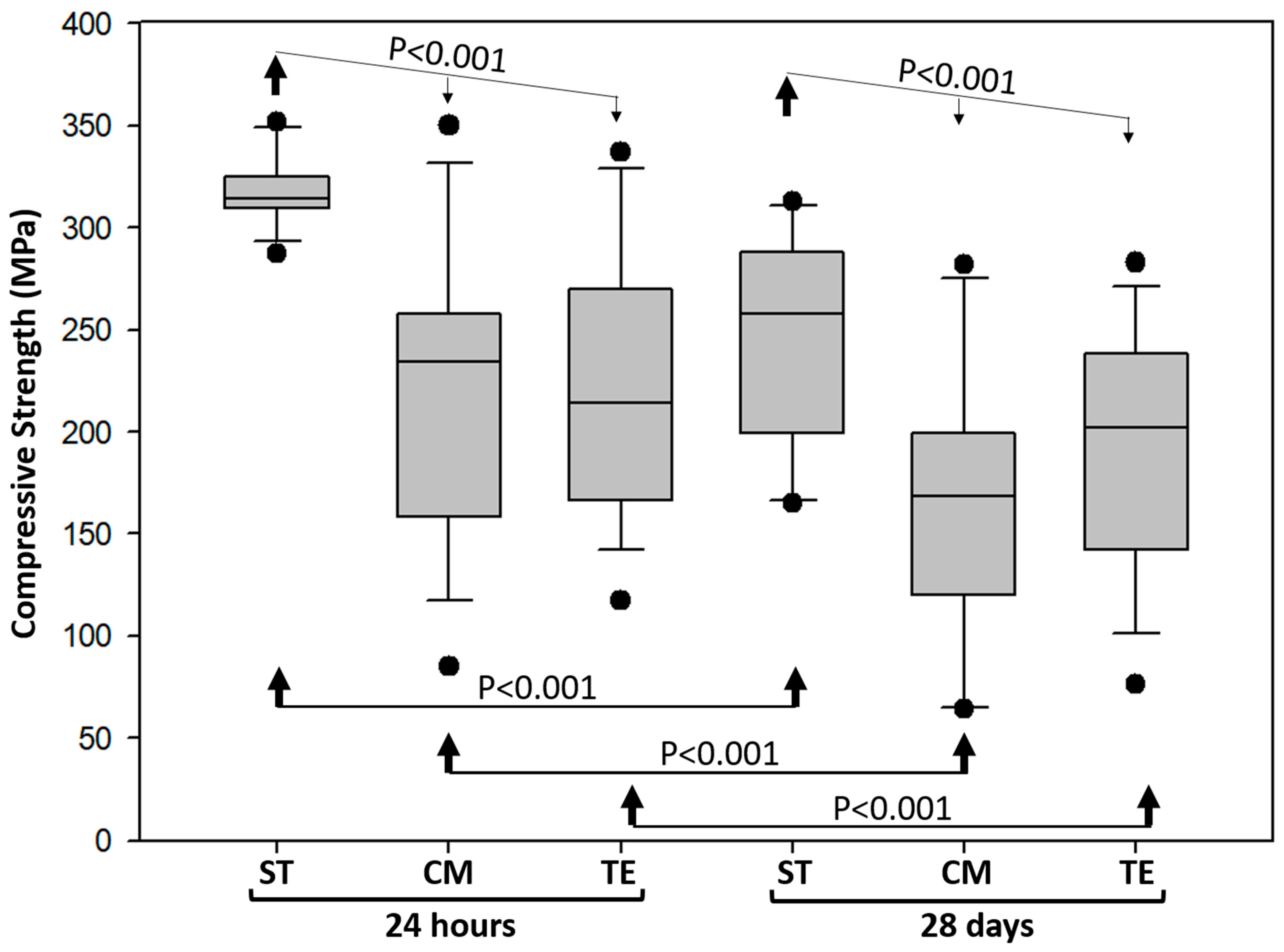
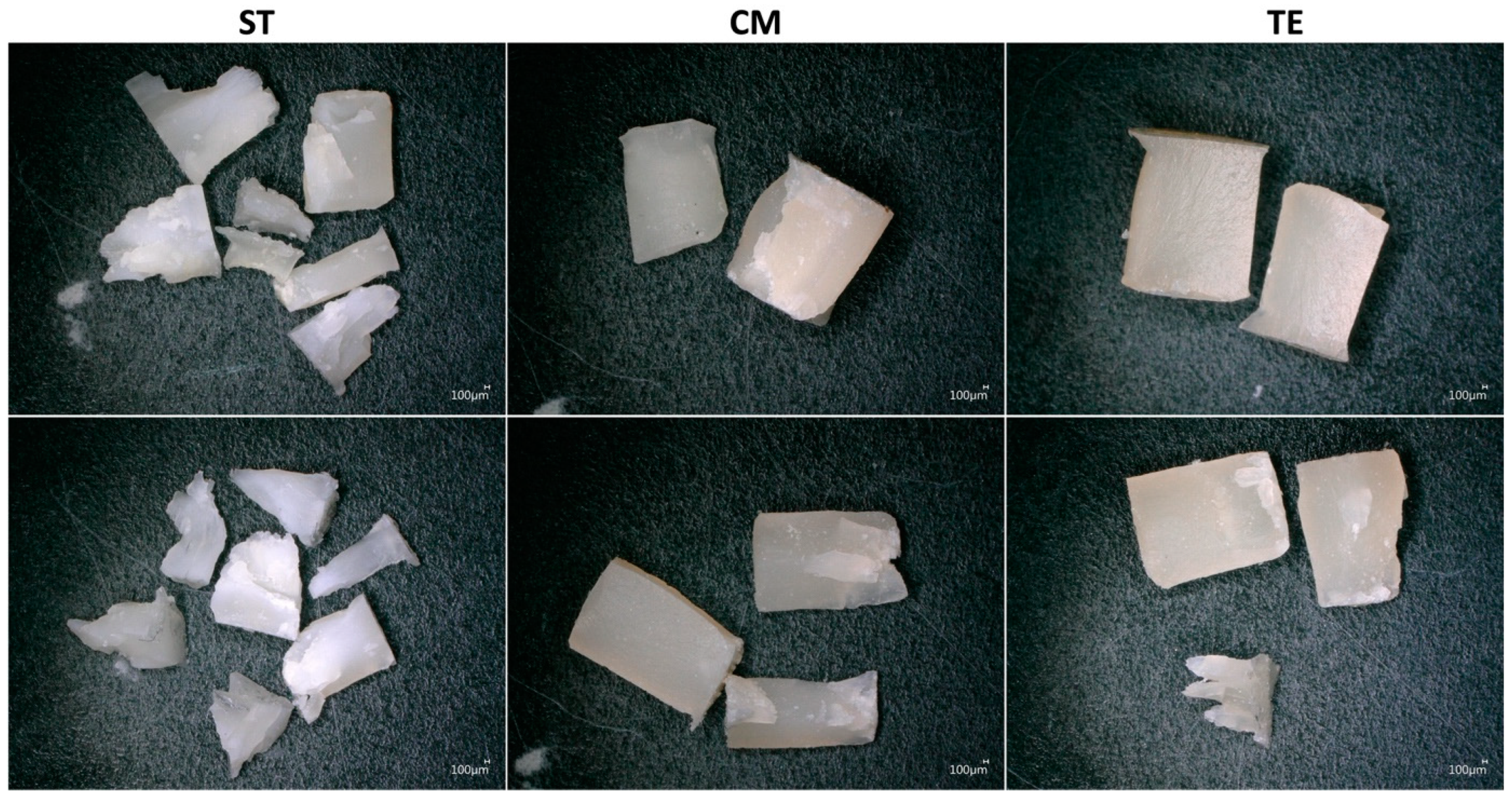
3.1. Compressive Strength and Fracture Mode
3.2. Porosity
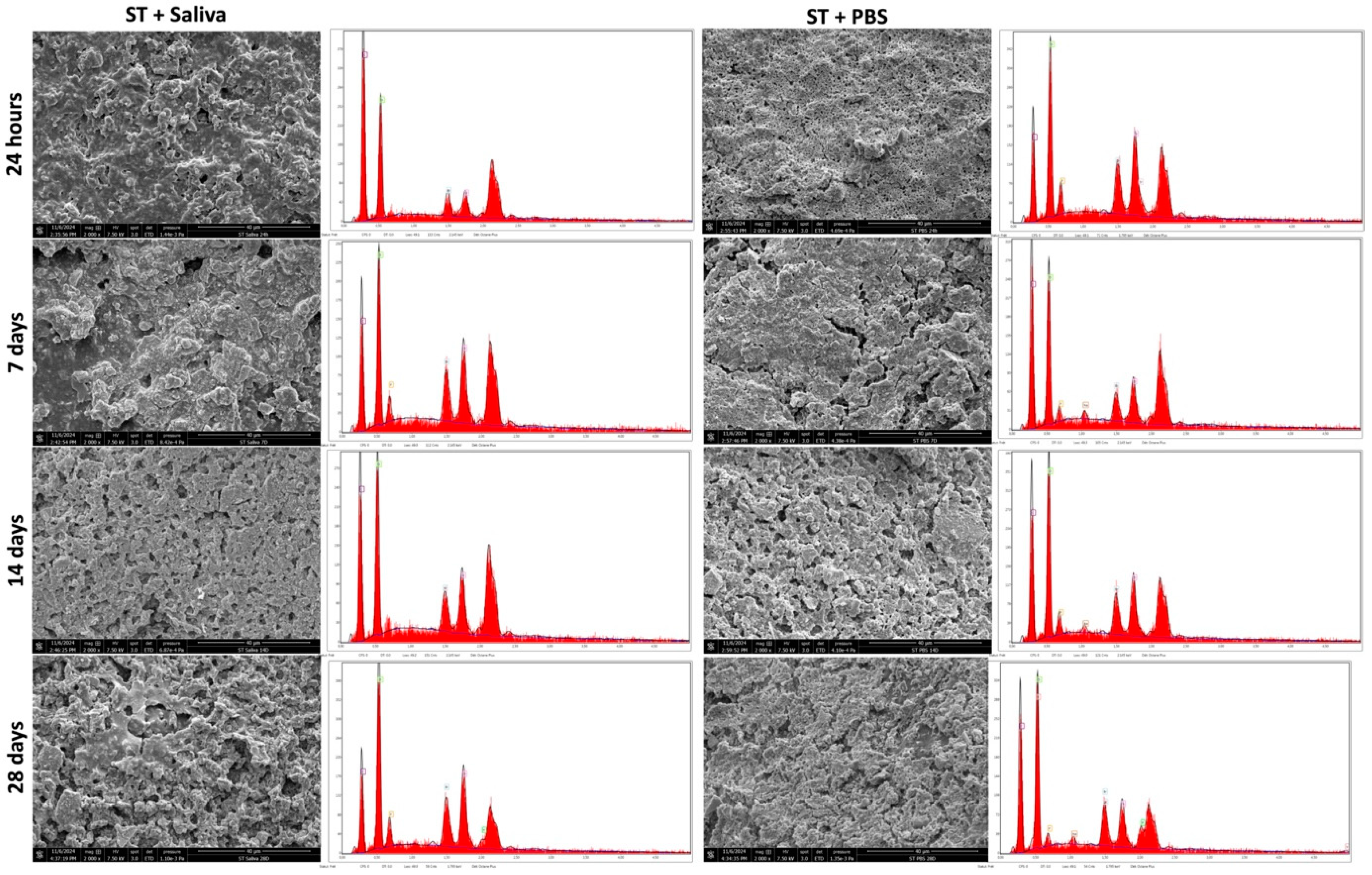
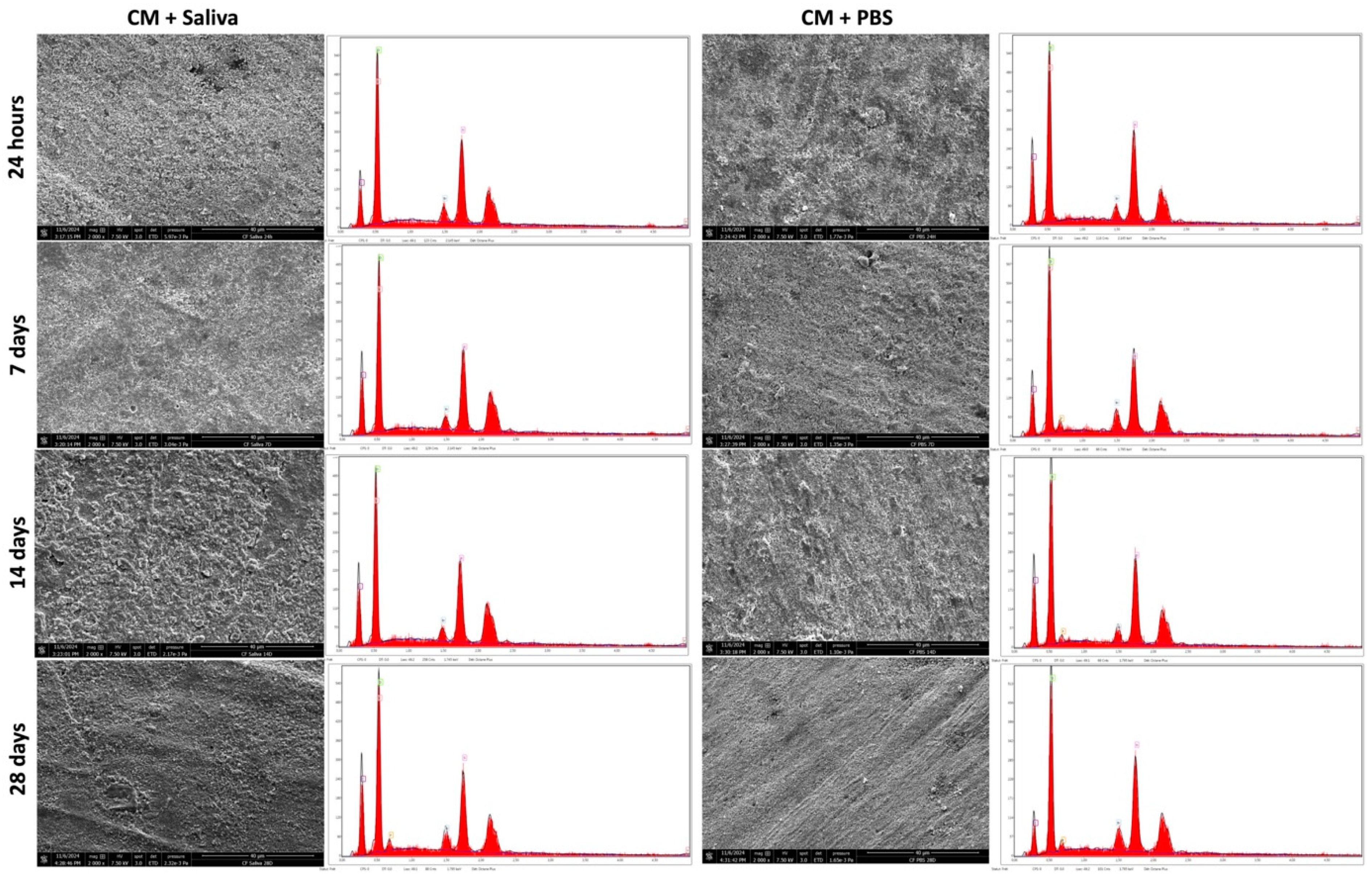
3.3. SEM & EDX Analysis
3.4. Wettability
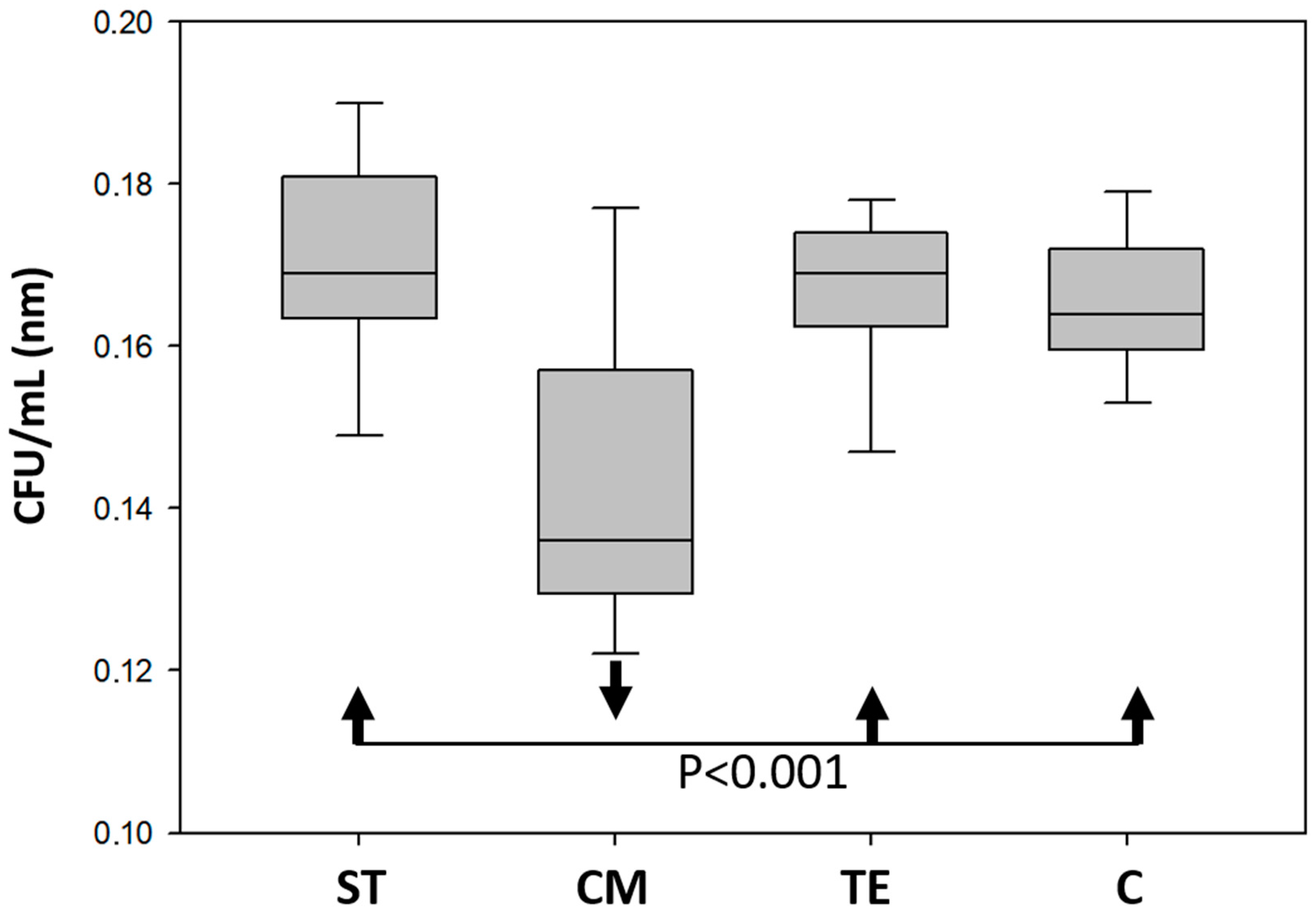
3.5. Antibacterial Activity
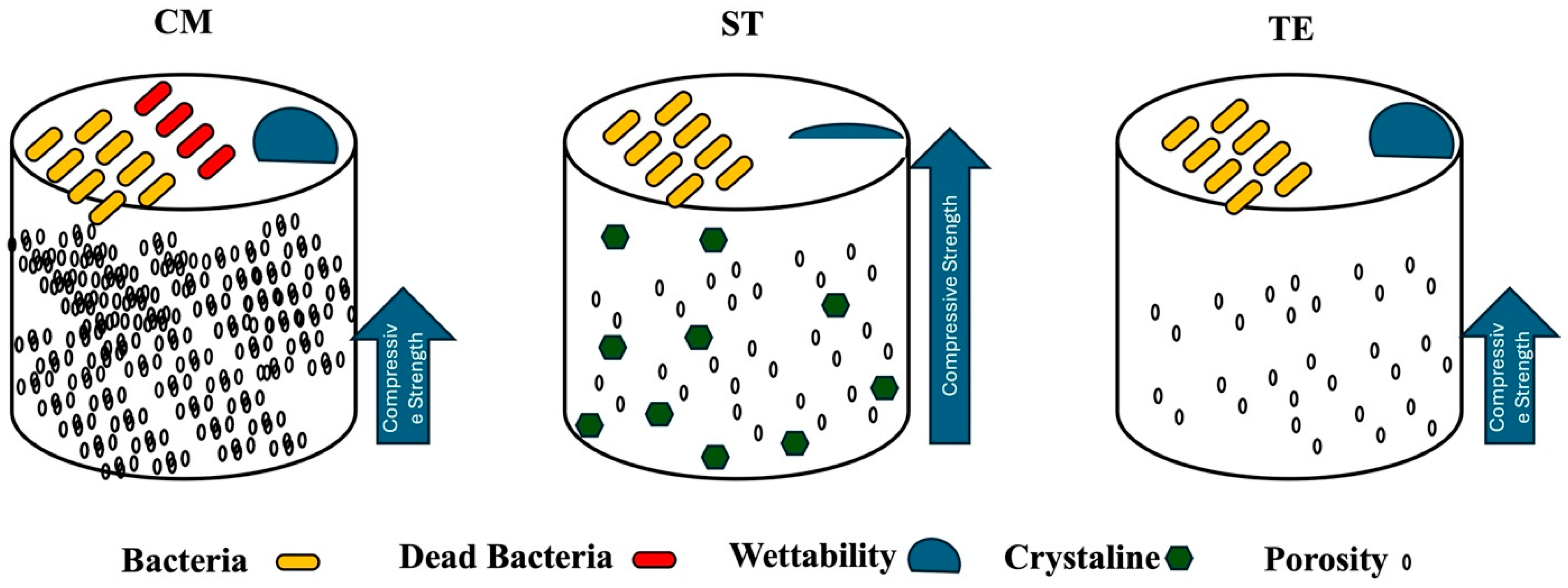
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conrads, G.; About, I. Pathophysiology of Dental Caries. Monogr. Oral Sci. 2018, 27, 1–10. [Google Scholar] [PubMed]
- Sun, R.; Xu, X.; Dong, Y.; Li, J.; Guan, W.; Huang, Y.; Li, S.; Wang, Y.; Li, J. Global and Regional Trends in Prevalence of Untreated Caries in Permanent Teeth: Age-Period-Cohort Analysis from 1990 to 2019 and Projections until 2049. J. Dent. 2024, 147, 105122. [Google Scholar] [CrossRef] [PubMed]
- Bernabe, E.; Marcenes, W.; Hernandez, C.R.; Bailey, J.; Abreu, L.G.; Alipour, V.; Amini, S.; Arabloo, J.; Arefi, Z.; Arora, A.; et al. Global, Regional, and National Levels and Trends in Burden of Oral Conditions from 1990 to 2017: A Systematic Analysis for the Global Burden of Disease 2017 Study. J. Dent. Res. 2020, 99, 362–373. [Google Scholar]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Dorri, M.; Martinez-Zapata, M.J.; Walsh, T.; Marinho, V.C.; Sheiham, A.; Zaror, C. Atraumatic Restorative Treatment versus Conventional Restorative Treatment for Managing Dental Caries. Cochrane Database Syst. Rev. 2017, 12, CD008072. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Resin Composite—State of the Art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef]
- Moraschini, V.; Fai, C.K.; Alto, R.M.; Dos Santos, G.O. Amalgam and Resin Composite Longevity of Posterior Restorations: A Systematic Review and Meta-Analysis. J. Dent. 2015, 43, 1043–1050. [Google Scholar] [CrossRef]
- German, M.J. Developments in Resin-Based Composites. Br. Dent. J. 2022, 232, 638–643. [Google Scholar] [CrossRef]
- Da Rosa Rodolpho, P.A.; Cenci, M.S.; Donassollo, T.A.; Loguércio, A.D.; Demarco, F.F. A Clinical Evaluation of Posterior Composite Restorations: 17-Year Findings. J. Dent. 2006, 34, 427–435. [Google Scholar] [CrossRef]
- PA Rodolfo, B.; Collares, K.; Correa, M.B.; Demarco, F.F.; Opdam, N.J.; Cenci, M.S.; Moraes, R.R. Clinical performance of posterior resin composite restorations after up to 33 years. Dent. Mater. 2022, 38, 680–688. [Google Scholar]
- Saini, R.S.; Vyas, R.; Vaddamanu, S.K.; Quadri, S.A.; Mosaddad, S.A.; Heboyan, A. Efficacy of different adhesive systems in bonding direct resin composite restorations: A systematic review and meta-analysis. Evid. Based Dent. 2025, 1–9. [Google Scholar] [CrossRef]
- Meereis, C.T.W.; Münchow, E.A.; de Oliveira da Rosa, W.L.; da Silva, A.F.; Piva, E. Polymerization Shrinkage Stress of Resin-Based Dental Materials: A Systematic Review and Meta-Analyses of Composition Strategies. J. Mech. Behav. Biomed. Mater. 2018, 82, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.J.; Faria-E-Silva, A.L.; Rodrigues, M.P.; Vilela, A.B.F.; Pfeifer, C.S.; Tantbirojn, D.; Versluis, A. Polymerization Shrinkage Stress of Composite Resins and Resin Cements—What Do We Need to Know? Braz. Oral Res. 2017, 31, 62. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.M.; de Almeida Neves, A.; Lukomska-Szymanska, M.; Farrar, P.; Cascales, Á.F.; Sauro, S. Bonding Performance and Interfacial Adaptation of Modern Bulk-Fill Restorative Composites after Aging in Artificial Saliva: An In Vitro Study. Clin. Oral Investig. 2024, 28, 132. [Google Scholar] [CrossRef] [PubMed]
- Faria, E.S.A.L.; Pfeifer, C.S. Development of Dual-Cured Resin Cements with Long Working Time, High Conversion in Absence of Light, and Reduced Polymerization Stress. Dent. Mater. 2020, 36, 293–301. [Google Scholar] [CrossRef]
- Dimitriadi, M.; Petropoulou, A.; Zinelis, S.; Eliades, G. Degree of Conversion of Dual-Cured Composite Luting Agents: The Effect of Transition Metal-Based Touch-Cure Activators. J. Dent. 2024, 147, 105147. [Google Scholar] [CrossRef]
- Thadathil Varghese, J.; Raju, R.; Farrar, P.; Prentice, L.; Prusty, B.G. Comparative Analysis of Self-Cure and Dual-Cure Dental Composites on Their Physico-Mechanical Behaviour. Aust. Dent. J. 2023, 69, 124–138. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y. Depth-Dependence of Degree of Conversion and Microhardness for Dual-Cure and Light-Cure Composites. Oper. Dent. 2020, 45, 396–406. [Google Scholar] [CrossRef]
- Elsharawy, R.; Elawsya, M.; AbdAllah, A.; El Embaby, A. Polymerization Efficiency of Different Bulk-Fill Resin Composites Cured by Monowave and Polywave Light-Curing Units: A Comparative Study. Quintessence Int. 2024, 55, 264–272. [Google Scholar]
- Guarneri, J.A.G.; Maucoski, C.; Ghaffari, S.; MacNeil, B.D.; Price, R.B.; Arrais, C.A.G. Ability of a Novel Primer to Enhance the Polymerization of a Self-Cured Resin Composite. Dent. Mater. 2025, 41, 42–50. [Google Scholar] [CrossRef]
- Yahyazadehfar, M.; Ivancik, J.; Majd, H.; An, B.; Zhang, D.; Arola, D. On the Mechanics of Fatigue and Fracture in Teeth. Appl. Mech. Rev. 2014, 66, 030803. [Google Scholar] [CrossRef]
- Alrahlah, A.; Silikas, N.; Watts, D.C. Post-Cure Depth of Cure of Bulk Fill Dental Resin Composites. Dent. Mater. 2014, 30, 149–154. [Google Scholar] [CrossRef] [PubMed]
- MacAulay, M.; Tam, L.E.; Santerre, J.P.; Finer, Y. In Vivo Biodegradation of BisGMA- and Urethane-Modified BisGMA-Based Resin Composite Materials. JDR Clin. Transl. Res. 2017, 2, 397–405. [Google Scholar] [CrossRef]
- Kharouf, N.; Sauro, S.; Hardan, L.; Jmal, H.; Bachagha, G.; Macaluso, V.; Addiego, F.; Inchingolo, F.; Haikel, Y.; Mancino, D. Compressive Strength and Porosity Evaluation of Innovative Bidirectional Spiral Winding Fiber Reinforced Composites. J. Clin. Med. 2022, 11, 6754. [Google Scholar] [CrossRef] [PubMed]
- Černý, M.; Petruš, J.; Chamradová, I. The Influence of Porosity on Mechanical Properties of PUR-Based Composites: Experimentally Derived Mathematical Approach. Polymers 2023, 15, 1960. [Google Scholar] [CrossRef] [PubMed]
- Szczesio-Wlodarczyk, A.; Sokolowski, J.; Kleczewska, J.; Bociong, K. Ageing of Dental Composites Based on Methacrylate Resins—A Critical Review of the Causes and Method of Assessment. Polymers 2020, 12, 882. [Google Scholar] [CrossRef]
- Rodríguez, H.A.; Kriven, W.M.; Casanova, H. Development of Mechanical Properties in Dental Resin Composite: Effect of Filler Size and Filler Aggregation State. Mater. Sci. Eng. C 2019, 101, 274–282. [Google Scholar] [CrossRef]
- Al-Zain, A.O.; Baeesa, L.; Jassoma, E.; Alghilan, M.A.; Hariri, M.; Ismail, E.H.; Münchow, E.A. Assessment of Internal Porosities for Different Placement Techniques of Bulk-Fill Resin-Based Composites: A Micro-Computed Tomography Study. Clin. Oral Investig. 2023, 27, 7489–7499. [Google Scholar] [CrossRef]
- Aminoroaya, A.; Neisiany, R.E.; Khorasani, S.N.; Panahi, P.; Das, O.; Madry, H.; Ramakrishna, S. A Review of Dental Composites: Challenges, Chemistry Aspects, Filler Influences, and Future Insights. Compos. Part B Eng. 2021, 216, 108852. [Google Scholar] [CrossRef]
- Buelvas, D.D.A.; Besegato, J.F.; Vicentin, B.L.S.; Jussiani, E.I.; Hoeppner, M.G.; Andrello, A.C.; Di Mauro, E. Impact of Light-Cure Protocols on the Porosity and Shrinkage of Commercial Bulk Fill Dental Resin Composites with Different Flowability. J. Polym. Res. 2020, 27, 292. [Google Scholar] [CrossRef]
- Pires, P.M.; Neves, A.D.A.; Makeeva, I.M.; Schwendicke, F.; Faus-Matoses, V.; Yoshihara, K.; Sauro, S. Contemporary Restorative Ion-Releasing Materials: Current Status, Interfacial Properties, and Operative Approaches. Br. Dent. J. 2020, 229, 450–458. [Google Scholar] [CrossRef] [PubMed]
- De Araújo-Neto, V.G.; Sebold, M.; de Castro, E.F.; Feitosa, V.P.; Giannini, M. Evaluation of Physico-Mechanical Properties and Filler Particles Characterization of Conventional, Bulk-Fill, and Bioactive Resin-Based Composites. J. Mech. Behav. Biomed. Mater. 2021, 115, 104288. [Google Scholar] [CrossRef]
- Wiegand, A.; Buchalla, W.; Attin, T. Review on Fluoride-Releasing Restorative Materials—Fluoride Release and Uptake Characteristics, Antibacterial Activity, and Influence on Caries Formation. Dent. Mater. 2007, 23, 343–362. [Google Scholar] [CrossRef]
- Randolph, L.D.; Palin, W.M.; Leloup, G.; Leprince, J.G. Filler Characteristics of Modern Dental Resin Composites and Their Influence on Physico-Mechanical Properties. Dent. Mater. 2016, 32, 1586–1599. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.F.; Pires, P.M.; Alambiaga-Caravaca, A.M.; Spagnuolo, G.; Hibbitts, A.; Sauro, S. Remineralisation of Mineral-Deficient Dentine Induced by Experimental Ion-Releasing Materials in Combination with a Biomimetic Dual-Analogue Primer. J. Dent. 2025, 152, 105468. [Google Scholar] [CrossRef] [PubMed]
- Scougall Vilchis, R.J.; Hotta, Y.; Yamamoto, K. Examination of Six Orthodontic Adhesives with Electron Microscopy, Hardness Tester, and Energy Dispersive X-Ray Microanalyzer. Angle Orthod. 2008, 78, 655–661. [Google Scholar] [CrossRef]
- Scougall Vilchis, R.J.; Hotta, Y.; Hotta, M.; Idono, T.; Yamamoto, K. Examination of Composite Resins with Electron Microscopy, Microhardness Tester, and Energy Dispersive X-Ray Microanalyzer. Dent. Mater. J. 2009, 28, 102–112. [Google Scholar] [CrossRef]
- Di Francescantonio, M.; Pacheco, R.R.; Aguiar, T.R.; Boaro, L.C.C.; Braga, R.R.; Martins, A.L.; Giannini, M. Evaluation of Composition and Morphology of Filler Particles in Low-Shrinkage and Conventional Composite Resins Carried Out by Means of SEM and EDX. J. Clin. Dent. Res. 2016, 13, 49–58. [Google Scholar] [CrossRef]
- Butera, A.; Pascadopoli, M.; Gallo, S.; Lelli, M.; Tarterini, F.; Giglia, F.; Scribante, A. SEM/EDS Evaluation of the Mineral Deposition on a Polymeric Composite Resin of a Toothpaste Containing Biomimetic Zn-Carbonate Hydroxyapatite (microRepair®) in Oral Environment: A Randomized Clinical Trial. Polymers 2021, 13, 2740. [Google Scholar] [CrossRef]
- Komalsingsakul, A.; Klaophimai, A.; Srisatjaluk, R.L.; Senawongse, P. Effect of the Surface Roughness of Composite Resins on the Water Contact Angle and Biofilm Formation. Mahidol Dent. J. 2019, 39, 75–84. [Google Scholar]
- Tulumbaci, F.; Korkut, E.; Ozer, H.; Özcan, M. Evaluation of Wettability Characteristics and Adhesion of Resin Composite to Photo-Polymerized Pulp-Capping Materials with and without Bioactive Glass. Braz. Dent. Sci. 2019, 22, 335–343. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, N.; Weir, M.D.; Reynolds, M.A.; Bai, Y.; Xu, H.H. Bioactive Dental Composites and Bonding Agents Having Remineralizing and Antibacterial Characteristics. Dent. Clin. 2017, 61, 669–687. [Google Scholar] [CrossRef] [PubMed]
- Mai, S.; Zhang, Q.; Liao, M.; Ma, X.; Zhong, Y. Recent Advances in Direct Adhesive Restoration Resin-Based Dental Materials with Remineralizing Agents. Front. Dent. Med. 2022, 3, 868651. [Google Scholar] [CrossRef]
- Tang, Y.; Lei, L.; Yang, D.; Zheng, J.; Zeng, Q.; Xiao, H.; Zhou, Z. Calcium Release-Mediated Adsorption and Lubrication of Salivary Proteins on Resin-Based Dental Composites. J. Mech. Behav. Biomed. Mater. 2022, 135, 105437. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Matin, K.; Nikaido, T.; Foxton, R.M.; Tagami, J. Effect of Surface Characteristics on Adherence of S. mutans Biofilms to Indirect Resin Composites. Dent. Mater. J. 2007, 26, 915–923. [Google Scholar] [CrossRef]
- Ono, M.; Nikaido, T.; Ikeda, M.; Imai, S.; Hanada, N.; Tagami, J.; Matin, K. Surface Properties of Resin Composite Materials Relative to Biofilm Formation. Dent. Mater. J. 2007, 26, 613–622. [Google Scholar] [CrossRef]
- D’Ercole, S.; De Angelis, F.; Biferi, V.; Noviello, C.; Tripodi, D.; Di Lodovico, S.; D’Arcangelo, C. Antibacterial and Antibiofilm Properties of Three Resin-Based Dental Composites Against Streptococcus mutans. Materials 2022, 15, 1891. [Google Scholar] [CrossRef]
- Dureja, A.; Acharya, S.R.; Kini, S.; Mayya, A.; Hedge, V. Biocompatibility and Performance of Dental Composite Restorations: A Narrative Review on Free Monomer Release, Concerns and Solutions. Eng. Proc. 2023, 59, 160. [Google Scholar]


| Material | Chemical Composition | Manufacturer’ Instructions | Lot |
|---|---|---|---|
| Adhese Universal (Ivoclar Vivadent; Schaan, Lichtenstein) | MDP, bis-GMA, HEMA, MCAP, D3MA, ethanol, water, initiator, stabilizers, silicon dioxide |
| Z06NMY |
| Clearfil Universal Bond Quick (Kuraray Noritake, Tokyo, Japan) | Bis-GMA (10–25%), ethanol (10–25%), HEMA (2.5–10%), 10-MDP, hydrophilic amide monomer, colloidal silica, silane coupling agent, sodium fluoride, camphorquinone, water |
| CK0443 |
| Stela Primer (SDI Ltd., Bayswater, Victoria, Australia) | 10-MDP, dimethacrylates, methyl ethyl ketone (MEK), water, initiators, stabilizers |
| 1220447 |
| Tetric EvoCeram A3 (Ivoclar Vivadent, Schaan, Liechtenstein) (TE) | Filler: 75–76 wt.% (53–55 vol.%) inorganic fillers; barium glass, ytterbium trifluoride, mixed oxide (particle size of the inorganic fillers 40 nm–3000 nm, mean size 550 nm) and prepolymer (34 wt.%) Matrix: Bis-GMA, UDMA, ethoxylated Bis-EMA |
| Z071CG |
| Clearfil Majesty ES-2 A3 (Kuraray Noritake Dental; Tokyo, Japan) (CM) | Bis-GMA, Other methacrylic monomers, Surface treatment glass powder, Surface treatment organic composite filler, Photopolymerization catalyst, Pigments, etc. |
| 410173 |
| Stela Automix (SDI Ltd., Bayswater, Victoria, Australia) (ST) | UDMA, GDMA, fumed silica, barium aluminoborosilicate glass, fluoro aminosilicate glass, ytterbium trifluoride (YbF3), calcium aluminate, hydroperoxide-based initiators, stabilizers, pigments |
| 1226631 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laporte, C.; Bourgi, R.; Jmal, H.; Ben Ammar, T.; Hazko, S.; Addiego, F.; Sauro, S.; Haïkel, Y.; Kharouf, N. Mechanical, Antibacterial, and Physico-Chemical Properties of Three Different Polymer-Based Direct Restorative Materials: An In Vitro Study. Polymers 2025, 17, 1272. https://doi.org/10.3390/polym17091272
Laporte C, Bourgi R, Jmal H, Ben Ammar T, Hazko S, Addiego F, Sauro S, Haïkel Y, Kharouf N. Mechanical, Antibacterial, and Physico-Chemical Properties of Three Different Polymer-Based Direct Restorative Materials: An In Vitro Study. Polymers. 2025; 17(9):1272. https://doi.org/10.3390/polym17091272
Chicago/Turabian StyleLaporte, Chloé, Rim Bourgi, Hamdi Jmal, Teissir Ben Ammar, Sandy Hazko, Frédéric Addiego, Salvatore Sauro, Youssef Haïkel, and Naji Kharouf. 2025. "Mechanical, Antibacterial, and Physico-Chemical Properties of Three Different Polymer-Based Direct Restorative Materials: An In Vitro Study" Polymers 17, no. 9: 1272. https://doi.org/10.3390/polym17091272
APA StyleLaporte, C., Bourgi, R., Jmal, H., Ben Ammar, T., Hazko, S., Addiego, F., Sauro, S., Haïkel, Y., & Kharouf, N. (2025). Mechanical, Antibacterial, and Physico-Chemical Properties of Three Different Polymer-Based Direct Restorative Materials: An In Vitro Study. Polymers, 17(9), 1272. https://doi.org/10.3390/polym17091272











