MWCNT Localization and Electrical Percolation in Thin Films of Semifluorinated PMMA Block Copolymers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification of sMWCNT
2.3. Thermogravimetric Analysis
2.4. Characterization of CNT Dispersion in Solvents
2.5. Preparation of Thin Polymer and Nanocomposite Films
2.6. Evaluation of Film Thickness
2.7. Surface Spectroscopy of MWCNTs and BCP/MWCNT Layers
2.8. Morphological Characterization
2.9. Electrical Characterization
3. Results
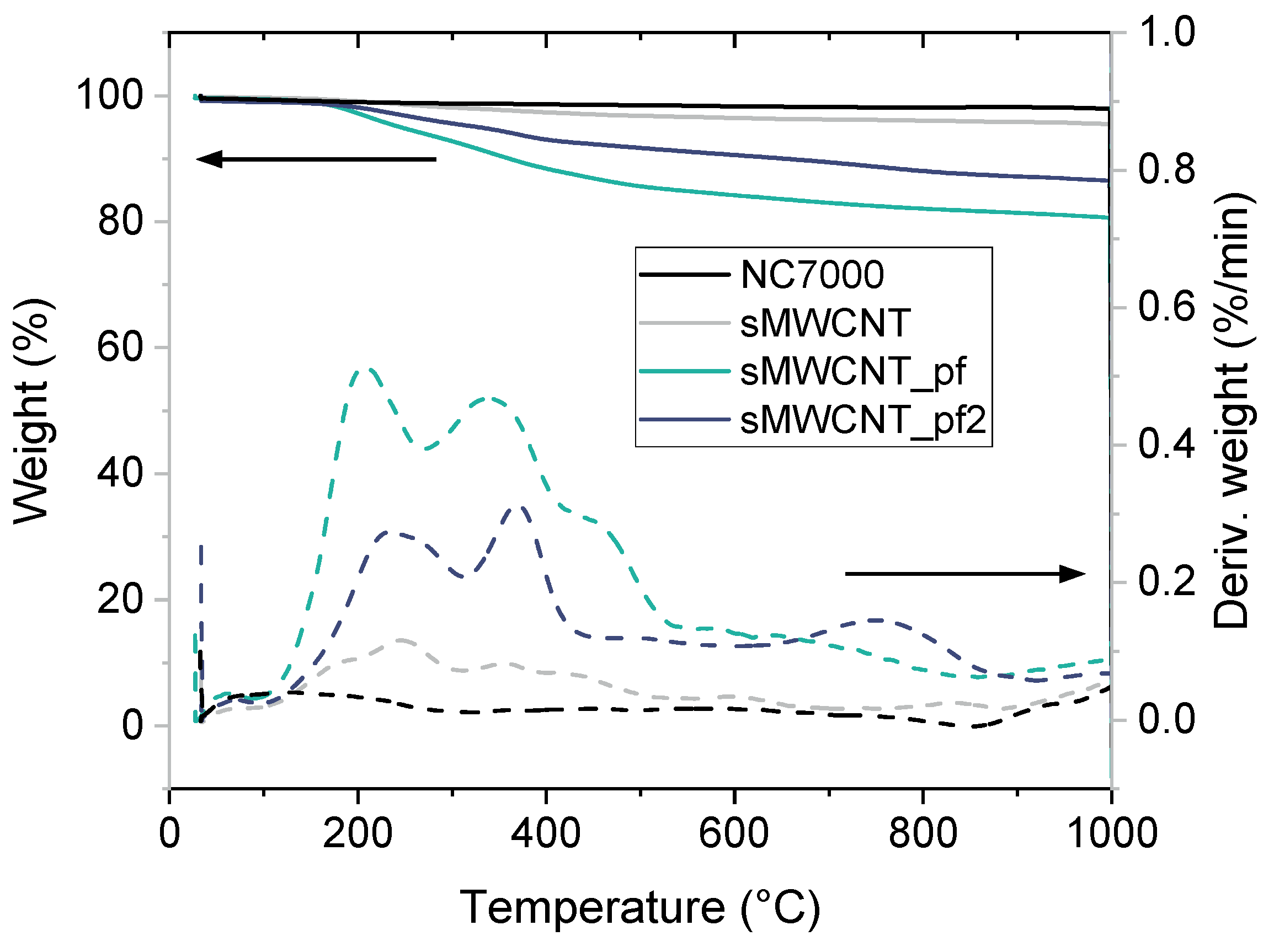
3.1. Characterization of the Modified MWCNT
3.2. Dispersability of the CNT in THF
3.3. Characterization of the BCP Used in This Study
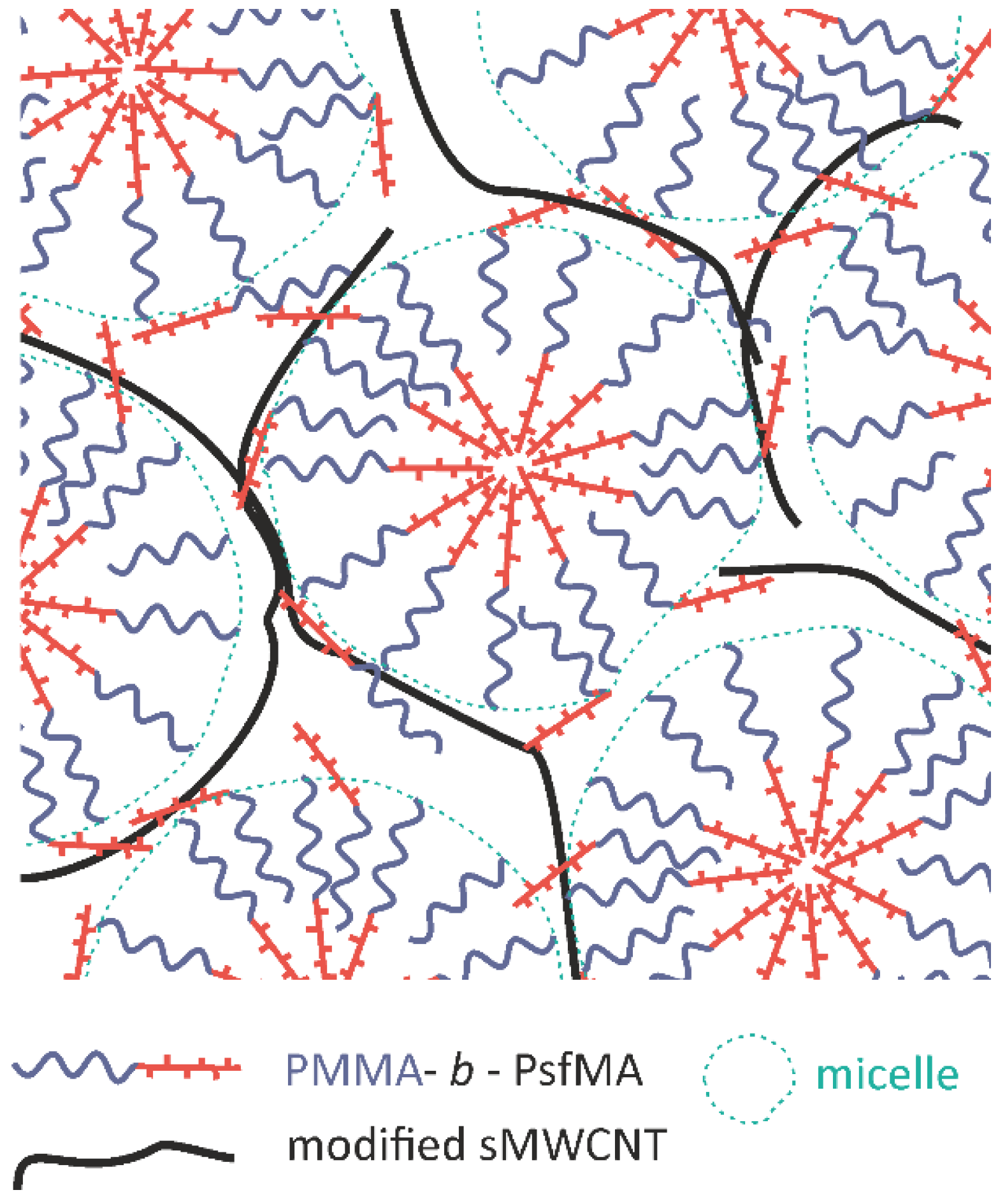
3.4. Characterization of BCP and BCP/CNT Nanocomposite Films
3.4.1. Film Thickness
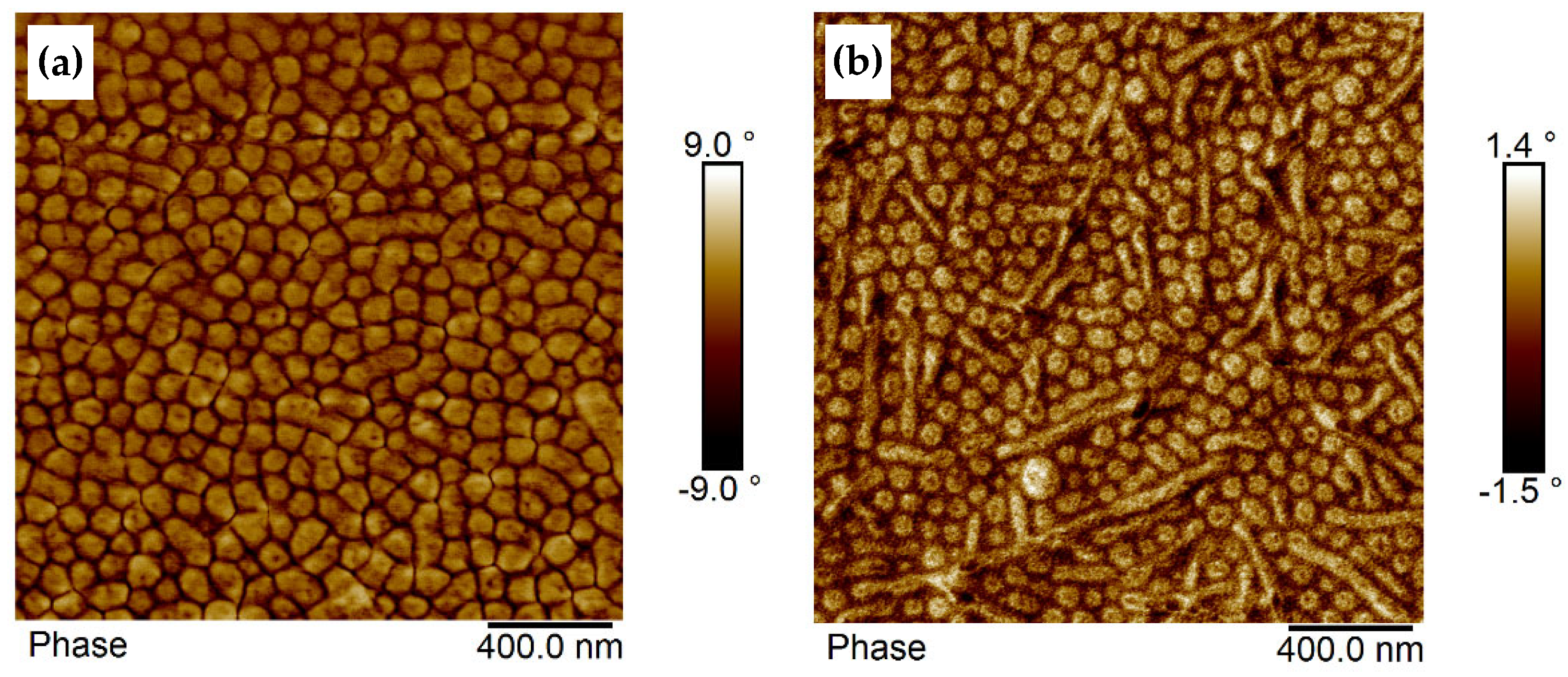
3.4.2. Film Morphology Investigated by AFM in Phase Contrast
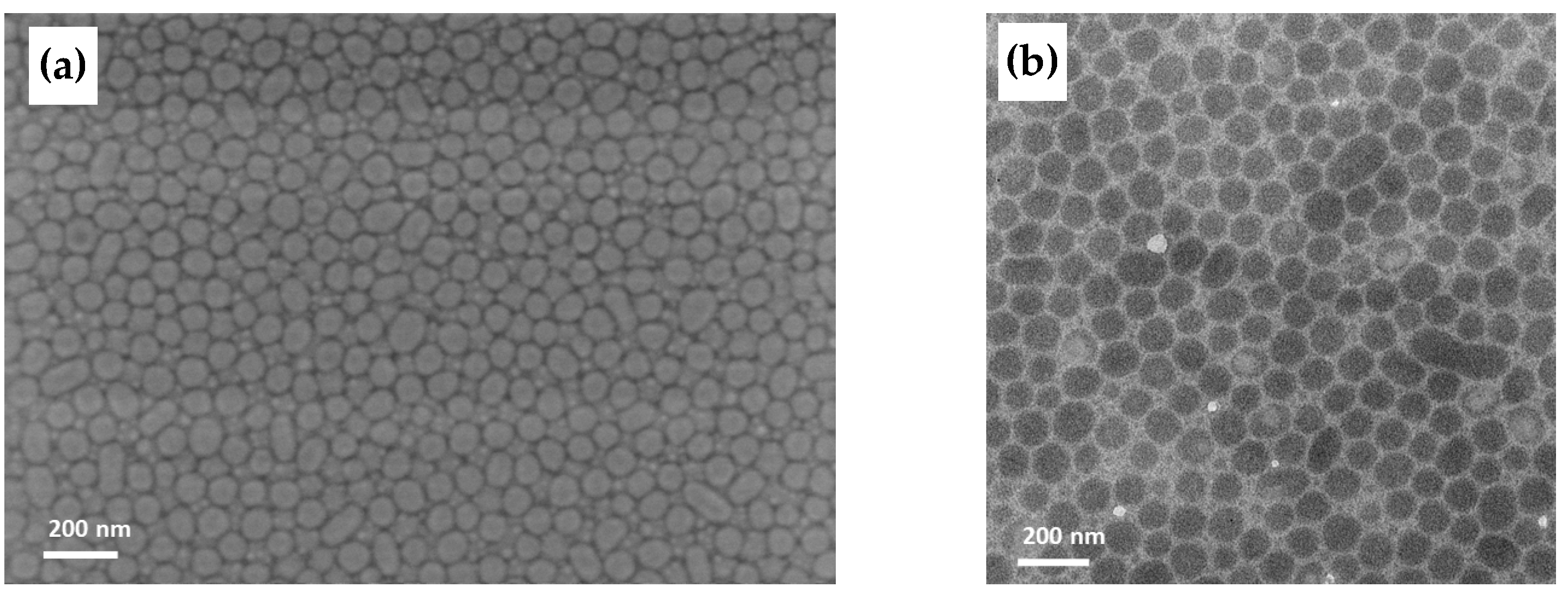
3.4.3. Film Morphology Investigated by TEM and SEM
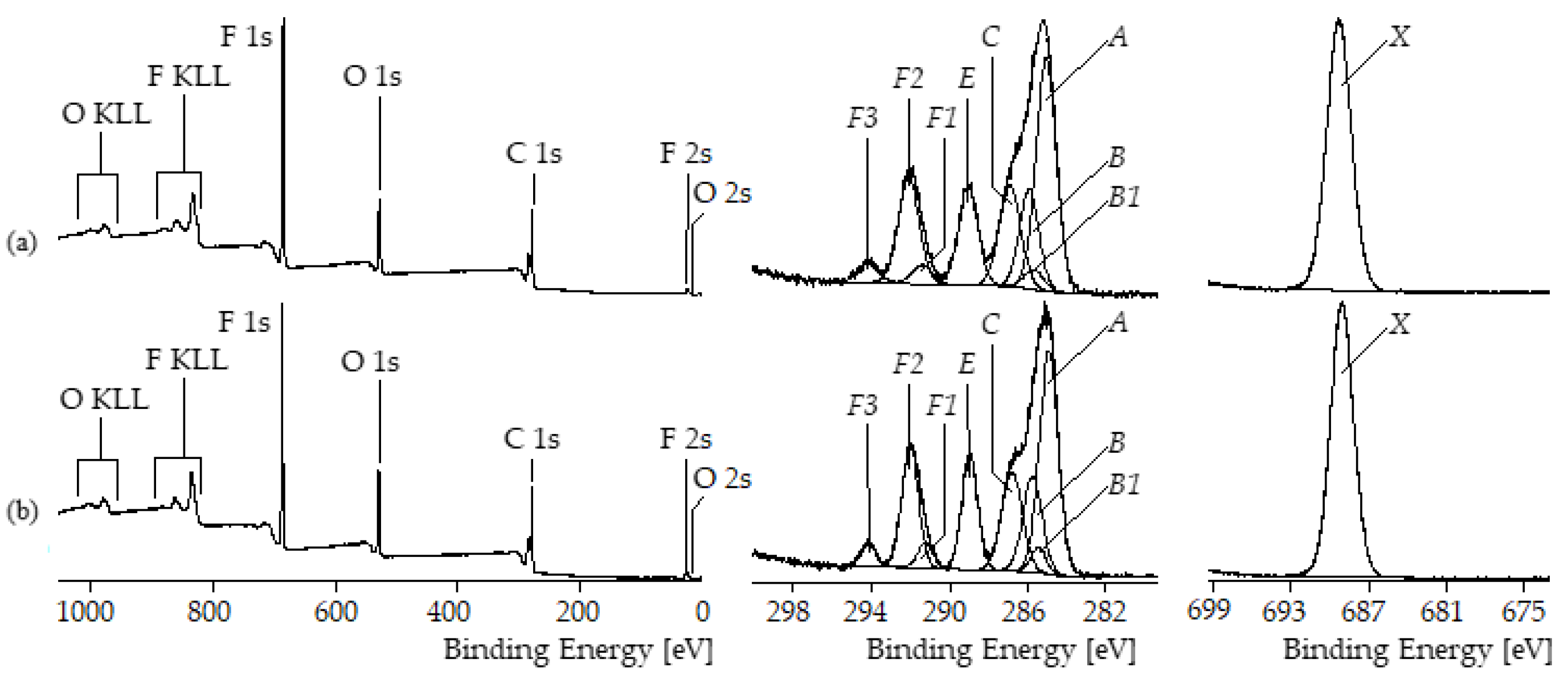
3.4.4. Chemical Composition of the Thin Films Surface Examined by XPS
3.5. Electrical Conductivity of Nanocomposite Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gopinath, S.C.B.; Lakshmipriya, T.; Md Arshad, M.K.; Uda, M.N.A.; Al-Douri, Y. 9—Nanoelectronics in Biosensing Applications. In Nanobiosensors for Biomolecular Targeting; Gopinath, S.C.B., Lakshmipriya, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 211–224. [Google Scholar] [CrossRef]
- Hu, L.B.; Hecht, D.S.; Gruner, G. Carbon Nanotube Thin Films: Fabrication, Properties, and Applications. Chem. Rev. 2010, 110, 5790–5844. [Google Scholar] [CrossRef] [PubMed]
- Naghdi, S.; Rhee, K.Y.; Hui, D.; Park, S.J. A Review of Conductive Metal Nanomaterials as Conductive, Transparent, and Flexible Coatings, Thin Films, and Conductive Fillers: Different Deposition Methods and Applications. Coatings 2018, 8, 278. [Google Scholar] [CrossRef]
- Clingerman, M.L.; King, J.A.; Schulz, K.H.; Meyers, J.D. Evaluation of electrical conductivity models for conductive polymer composites. J. Appl. Polym. Sci. 2002, 83, 1341–1356. [Google Scholar] [CrossRef]
- Payandehpeyman, J.; Mazaheri, M. Geometrical and physical effects of nanofillers on percolation and electrical conductivity of polymer carbon-based nanocomposites: A general micro-mechanical model. Soft Matter 2023, 19, 530–539. [Google Scholar] [CrossRef]
- Pospiech, D.; Jehnichen, D.; Starke, S.; Müller, F.; Bünker, T.; Wollenberg, A.; Häußler, L.; Simon, F.; Grundke, K.; Oertel, U.; et al. Multifunctional methacrylate-based coatings for glass and metal surfaces. Appl. Surf. Sci. 2017, 399, 205–214. [Google Scholar] [CrossRef]
- Walsh, C.B.; Franses, E.I. Ultrathin PMMA films spin-coated from toluene solutions. Thin Solid Film. 2003, 429, 71–76. [Google Scholar] [CrossRef]
- Pantano, M.F.; Pavlou, C.; Pastore Carbone, M.G.; Galiotis, C.; Pugno, N.M.; Speranza, G. Highly Deformable, Ultrathin Large-Area Poly(methyl methacrylate) Films. ACS Omega 2021, 6, 8308–8312. [Google Scholar] [CrossRef]
- Logakis, E.; Pandis, C.; Pissis, P.; Pionteck, J.; Pötschke, P. Highly conducting poly(methyl methacrylate)/carbon nanotubes composites: Investigation on their thermal, dynamic-mechanical, electrical and dielectric properties. Compos. Sci. Technol. 2011, 71, 854–862. [Google Scholar] [CrossRef]
- Pandis, C.; Peoglos, V.; Kyritsis, A.; Pissis, P. Gas sensing properties of conductive polymer nanocomposites. Procedia Eng. 2011, 25, 243–246. [Google Scholar] [CrossRef]
- Mir, M.; Jafari, S.H.; Khonakdar, H.A.; Krause, B.; Pötschke, P.; Taheri Qazvini, N. A promising approach to low electrical percolation threshold in PMMA nanocomposites by using MWCNT-PEO predispersions. Mater. Des. 2016, 111, 253–262. [Google Scholar] [CrossRef]
- Bates, F.S.; Fredrickson, G.H. Block Copolymer Thermodynamics: Theory and Experiment. Annu. Rev. Phys. Chem. 1990, 41, 525–557. [Google Scholar] [CrossRef] [PubMed]
- Leibler, L. Theory of Microphase Separation in Block Copolymers. Macromolecules 1980, 13, 1602–1617. [Google Scholar] [CrossRef]
- Khandpur, A.K.; Foerster, S.; Bates, F.S.; Hamley, I.W.; Ryan, A.J.; Bras, W.; Almdal, K.; Mortensen, K. Polyisoprene-Polystyrene Diblock Copolymer Phase Diagram near the Order-Disorder Transition. Macromolecules 1995, 28, 8796–8806. [Google Scholar] [CrossRef]
- Araki, T.; Shibayama, M.; Tran-Cong, Q. Structure and Properties of Multiphase Polymeric Materials; Taylor & Francis: London, UK, 1998. [Google Scholar]
- Matsen, M.W.; Schick, M. Microphase separation in starblock copolymer melts. Macromolecules 1994, 27, 6761–6767. [Google Scholar] [CrossRef]
- Matsen, M.W.; Bates, F.S. Unifying weak-and strong-segregation block copolymer theories. Macromolecules 1996, 29, 1091–1098. [Google Scholar] [CrossRef]
- Bates, F.S.; Fredrickson, G.H. Block Copolymers—Designer Soft Materials. Phys. Today 1999, 52, 32–38. [Google Scholar] [CrossRef]
- Cohen, R.E. Block copolymers as templates for functional materials. Curr. Opin. Solid State Mater. Sci. 1999, 4, 587–590. [Google Scholar] [CrossRef]
- Chiu, J.J.; Kim, B.J.; Yi, G.R.; Bang, J.; Kramer, E.J.; Pine, D.J. Distribution of nanoparticles in lamellar domains of block copolymers. Macromolecules 2007, 40, 3361–3365. [Google Scholar] [CrossRef]
- Listak, J.; Hakem, I.F.; Ryu, H.J.; Rangou, S.; Politakos, N.; Misichronis, K.; Avgeropoulos, A.; Bockstaller, M.R. Effect of Chain Architecture on the Compatibility of Block Copolymer/Nanoparticle Blends. Macromolecules 2009, 42, 5766–5773. [Google Scholar] [CrossRef]
- Yeh, S.-W.; Wei, K.-H.; Sun, Y.-S.; Jeng, U.S.; Liang, K.S. CdS Nanoparticles Induce a Morphological Transformation of Poly(styrene-b-4-vinylpyridine) from Hexagonally Packed Cylinders to a Lamellar Structure. Macromolecules 2005, 38, 6559–6565. [Google Scholar] [CrossRef]
- Jehnichen, D.; Pospiech, D.; Friedel, P.; Horechyy, A.; Korwitz, A.; Janke, A.; Näther, F.; Papadakis, C.M.; Perlich, J.; Neu, V. Effects of nanoparticles on phase morphology in thin films of phase-separated diblock copolymers. Powder Diffr. 2017, 32, S141–S150. [Google Scholar] [CrossRef]
- Zhang, Q.; Gupta, S.; Emrick, T.; Russell, T.P. Surface-Functionalized CdSe Nanorods for Assembly in Diblock Copolymer Templates. J. Am. Chem. Soc. 2006, 128, 3898–3899. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Zhang, Z.L.; Zhao, W.; Xie, X.M.; Ye, X.Y. Selective self-assembly of surface-functionalized carbon nanotubes in block copolymer template. Carbon 2009, 47, 1883–1885. [Google Scholar] [CrossRef]
- Garate, H.; Fascio, M.L.; Mondragon, I.; D’Accorso, N.B.; Goyanes, S. Surfactant-aided dispersion of polystyrene-functionalized carbon nanotubes in a nanostructured poly(styrene-b-isoprene-b-styrene) block copolymer. Polymer 2011, 52, 2214–2220. [Google Scholar] [CrossRef]
- Peponi, L.; Tercjak, A.; Gutierrez, J.; Cardinali, M.; Mondragon, I.; Valentini, L.; Kenny, J.M. Mapping of carbon nanotubes in the polystyrene domains of a polystyrene-b-polyisoprene-b-polystyrene block copolymer matrix using electrostatic force microscopy. Carbon 2010, 48, 2590–2595. [Google Scholar] [CrossRef]
- Park, I.; Lee, W.; Kim, J.; Park, M.; Lee, H. Selective sequestering of multi-walled carbon nanotubes in self-assembled block copolymer. Sens. Actuator B-Chem. 2007, 126, 301–305. [Google Scholar] [CrossRef]
- Albuerne, J.; Boschetti-de-Fierro, A.; Abetz, C.; Fierro, D.; Abetz, V. Block Copolymer Nanocomposites Based on Multiwall Carbon Nanotubes: Effect of the Functionalization of Multiwall Carbon Nanotubes on the Morphology of the Block Copolymer. Adv. Eng. Mater. 2012, 13, 803–810. [Google Scholar] [CrossRef]
- Wode, F.; Tzounis, L.; Kirsten, M.; Constantinou, M.; Georgopanos, P.; Rangou, S.; Zafeiropoulos, N.E.; Avgeropoulos, A.; Stamm, M. Selective localization of multi-wall carbon nanotubes in homopolymer blends and a diblock copolymer. Rheological orientation studies of the final nanocomposites. Polymer 2012, 53, 4438–4447. [Google Scholar] [CrossRef]
- Hasanabadi, N.; Nazockdast, H.; Gajewska, B.; Balog, S.; Gunkel, I.; Bruns, N.; Lattuada, M. Structural Behavior of Cylindrical Polystyrene-block-Poly(ethylene-butylene)-block-Polystyrene (SEBS) Triblock Copolymer Containing MWCNTs: On the Influence of Nanoparticle Surface Modification. Macromol. Chem. Phys. 2017, 218, 1700231. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, H.B.; Lim, S.T.; Kim, H.S.; Kwon, Y.K.; Choi, H.J. Ordering Behavior of Layered Silicate Nanocomposites with a Cylindrical Triblock Copolymer. Macromol. Chem. Phys. 2006, 207, 444–455. [Google Scholar] [CrossRef]
- Ganß, M.; Staudinger, U.; Satapathy, B.K.; Leuteritz, A.; Weidisch, R. Mechanism of strengthening and toughening of a nanostructured styrene-butadiene based block copolymer by oligostyrene-modified montmorillonites. Polymer 2021, 213, 123328. [Google Scholar] [CrossRef]
- Bockstaller, M.R.; Mickiewicz, R.A.; Thomas, E.L. Block Copolymer Nanocomposites: Perspectives for Tailored Functional Materials. Adv. Mater. 2005, 17, 1331–1349. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B.; Alexandridis, P. Block copolymer–nanoparticle composites: Structure, functional properties, and processing. Prog. Polym. Sci. 2015, 40, 33–62. [Google Scholar] [CrossRef]
- Lee, D.H.; Shin, D.O.; Lee, W.J.; Kim, S.O. Hierarchically Organized Carbon Nanotube Arrays from Self-Assembled Block Copolymer Nanotemplates. Adv. Mater. 2008, 20, 2480–2485. [Google Scholar] [CrossRef]
- Bennett, R.D.; Xiong, G.Y.; Ren, Z.F.; Cohen, R.E. Using Block Copolymer Micellar Thin Films as Templates for the Production of Catalysts for Carbon Nanotube Growth. Chem. Mater. 2004, 16, 5589–5595. [Google Scholar] [CrossRef]
- Staudinger, U.; Jakisch, L.; Hilbig, L. Dispersion and Localization Behavior of Modified MWCNTs in Immiscible Polymer Blends of Polystyrene and Polybutadiene and in Corresponding Nanostructured Block Copolymers. J. Compos. Sci. 2020, 4, 40. [Google Scholar] [CrossRef]
- Staudinger, U.; Janke, A.; Steinbach, C.; Reuter, U.; Ganß, M.; Voigt, O. Influence of CNT Length on Dispersion, Localization, and Electrical Percolation in a Styrene-Butadiene-Based Star Block Copolymer. Polymers 2022, 14, 2715. [Google Scholar] [CrossRef]
- Staudinger, U.; Satapathy, B.K.; Jehnichen, D. Nanofiller Dispersion, Morphology, Mechanical Behavior, and Electrical Properties of Nanostructured Styrene-Butadiene-Based Triblock Copolymer/CNT Composites. Polymers 2019, 11, 1831. [Google Scholar] [CrossRef]
- Ferreira Santos, J.P.; França Melo, G.H.; Gonçalves, A.M.; Eiras, J.A.; Suman Bretas, R.E. Flexible conductive poly(styrene-butadiene-styrene)/carbon nanotubes nanocomposites: Self-assembly and broadband electrical behavior. J. Appl. Polym. Sci. 2018, 135, 46650. [Google Scholar] [CrossRef]
- Topcu, G.; Reinoso Arenas, D.; McNally, T.; Becer, C.R. Microphase separation assisted reduction in the percolation threshold of MWCNT/block polymer composites. Soft Matter 2023, 19, 1109–1114. [Google Scholar] [CrossRef]
- Pospiech, D.; Jehnichen, D. Self-organizing semifluorinated methacrylate copolymers. In Handbook of Fluoropolymer Science & Technology; Smith, D.W., Iacono, S.T., Iyer, S.S., Eds.; John Wiley & Sons: New York, NY, USA, 2013; ISBN 978-0-470-07993-5. [Google Scholar]
- Pospiech, D.; Jehnichen, D.; Eckstein, K.; Scheibe, P.; Komber, H.; Sahre, K.; Janke, A.; Reuter, U.; Häußler, L.; Schellkopf, L.; et al. Semifluorinated PMMA Block Copolymers: Synthesis, Nanostructure, and Thin Film Properties. Macromol. Chem. Phys. 2017, 218, 1600599. [Google Scholar] [CrossRef]
- NANOCYL NC7000TM-Technical Datasheet: DM-TI-02-TDS-NC7000-V08. Available online: www.nanocyl.com (accessed on 30 February 2025).
- Krause, B.; Villmow, T.; Boldt, R.; Mende, M.; Petzold, G.; Pötschke, P. Influence of dry grinding in a ball mill on the length of multiwalled carbon nanotubes and their dispersion and percolation behaviour in melt mixed polycarbonate composites. Compos. Sci. Technol. 2011, 71, 1145–1153. [Google Scholar] [CrossRef]
- Jehnichen, D.; Pospiech, D.; Friedel, P.; Funari, S.S. Semifluorinated PMMA/PSFMA diblock copolymers with multiple phase separation. Z. Kristallogr. Proc. 2011, 1, 487–492. [Google Scholar] [CrossRef]
- Scheibe, P. Kontrollierte Synthese von Semifluorierten Methacrylatblockcopolymeren zur Präparation von Nanostrukturierten Filmen mit Kohlenstoffnanoröhren. Master’s Thesis, Technische Universität Dresden, Dresden, Germany, 2015. [Google Scholar]
- Hamilton, C.E.; Lomeda, J.R.; Sun, Z.; Tour, J.M.; Barron, A.R. Radical addition of perfluorinated alkyl iodides to multi-layered graphene and single-walled carbon nanotubes. Nano Res. 2010, 3, 138–145. [Google Scholar] [CrossRef]
- Bittrich, E.; Eichhorn, K.-J. 8 Application of spectroscopic ellipsometry in the analysis of thin polymer films/polymer interfaces. In Polymer Surface Characterization; Sabbatini, L., De Giglio, E., Eds.; De Gruyter: Berlin, Germany, 2022; pp. 281–322. [Google Scholar] [CrossRef]
- Kumar, L.; Horechyy, A.; Bittrich, E.; Nandan, B.; Uhlmann, P.; Fery, A. Amphiphilic Block Copolymer Micelles in Selective Solvents: The Effect of Solvent Selectivity on Micelle Formation. Polymers 2019, 11, 1882. [Google Scholar] [CrossRef]
- Shirley, D.A. High-Resolution X-Ray Photoemission Spectrum of the Valence Bands of Gold. Phys. Rev. B (Condens. Matter Mater. Phys.) 1972, 5, 4709–4714. [Google Scholar] [CrossRef]
- Magonov, S.N.; Elings, V.; Whangbo, M.H. Phase imaging and stiffness in tapping-mode atomic force microscopy. Surf. Sci. 1997, 375, L385–L391. [Google Scholar] [CrossRef]
- Thomas, P.J.; Midgley, P.A. An Introduction to Energy-Filtered Transmission Electron Microscopy. Top. Catal. 2002, 21, 109–138. [Google Scholar] [CrossRef]
- Gültner, M.; Boldt, R.; Formanek, P.; Fischer, D.; Simon, F.; Pötschke, P. The Localization Behavior of Different CNTs in PC/SAN Blends Containing a Reactive Component. Molecules 2021, 26, 1312. [Google Scholar] [CrossRef]
- Deng, J.; Cao, J.; Li, J.; Tan, H.; Zhang, Q.; Fu, Q. Mechanical and surface properties of polyurethane/fluorinated multi-walled carbon nanotubes composites. J. Appl. Polym. Sci. 2008, 108, 2023–2028. [Google Scholar] [CrossRef]
- Jehnichen, D.; Pospiech, D.; Ptacek, S.; Eckstein, K.; Friedel, P.; Janke, A.; Papadakis, C.M. Nanophase-separated diblock copolymers: Structure investigations on PPMA-b-PMMA using X-ray scattering methods. In Eleventh European Powder Diffraction Conference; Oldenbourg Wissenschaftsverlag: München, Germany, 2009; pp. 485–490. [Google Scholar] [CrossRef]
- Jang, S.G.; Khan, A.; Hawker, C.J.; Kramer, E.J. Morphology Evolution of PS-b-P2VP Diblock Copolymers via Supramolecular Assembly of Hydroxylated Gold Nanoparticles. Macromolecules 2012, 45, 1553–1561. [Google Scholar] [CrossRef]
- Pospiech, D.; Jehnichen, D.; Chunsod, P.; Friedel, P.; Simon, F.; Grundke, K. Structure-property relations in semifluorinated polymethacrylates. In Fluorinated Polymers: Volume 1: Synthesis, Properties, Processing and Simulation; Améduri, B., Sawada, H., Eds.; Royal Society of Chemistry: Cambridge, UK, 2016; pp. 235–275. [Google Scholar]
- Carabineiro, S.A.C.; Pereira, M.F.R.; Pereira, J.N.; Caparros, C.; Sencadas, V.; Lanceros-Mendez, S. Effect of the carbon nanotube surface characteristics on the conductivity and dielectric constant of carbon nanotube/poly(vinylidene fluoride) composites. Nanoscale Res. Lett. 2011, 6, 302. [Google Scholar] [CrossRef] [PubMed]
- Talaeemashhadi, S.; Sansotera, M.; Gambarotti, C.; Famulari, A.; Bianchi, C.L.; Antonio Guarda, P.; Navarrini, W. Functionalization of multi-walled carbon nanotubes with perfluoropolyether peroxide to produce superhydrophobic properties. Carbon 2013, 59, 150–159. [Google Scholar] [CrossRef]
- Staudinger, U.; Thoma, P.; Lüttich, F.; Janke, A.; Kobsch, O.; Gordan, O.D.; Pötschke, P.; Voit, B.; Zahn, D.R.T. Properties of thin layers of electrically conductive polymer/MWCNT composites prepared by spray coating. Compos. Sci. Technol. 2017, 138, 134–143. [Google Scholar] [CrossRef]
- Stauffer, D.; Aharony, A. Introduction in Percolation Theory; Taylor and Francis: London, UK, 1994. [Google Scholar]
- Sahimi, M. Applications of Percolation Theory, 1st ed.; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar] [CrossRef]










| BCP Sample | Composition MMA/sfMA (mol mol−1) | Mn (kg mol−1) | Đ = Mw/Mn | Morphology in Bulk (TEM, SAXS) | d-Spacing (SAXS) (nm) |
|---|---|---|---|---|---|
| BCP1 (PMF187) | 73.5/26.5 | 22.0 | 1.18 | Mixture of lamellae and cylinders | 41 (CYL/LAM) [48] |
| BCP2 (PMF102) | 73/27 | 26.2 | 1.10 | Mixture of lamellae and cylinders | 54 (CYL/LAM) [44] |
| BCP3 (PMF106) | 54/46 | 18.8 | 1.06 | Lamellae | 45 (LAM) [44,47] |
| BCP Sample | Film Thickness 1 (nm) | Morphology (AFM) | PMMA Domain Size 2 (nm) | Periodic Nanostructure 3 (nm) |
|---|---|---|---|---|
| BCP1 | 32 | spherical | 82 | 91 |
| BCP1/0.5 wt% sMWCNT_pf2 | 31 | spherical | 67 | 91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staudinger, U.; Janke, A.; Simon, F.; Jakisch, L.; Bittrich, E.; Formanek, P.; Mielke, L.; Schlicke, H.; Li, Q.; Eckstein, K.; et al. MWCNT Localization and Electrical Percolation in Thin Films of Semifluorinated PMMA Block Copolymers. Polymers 2025, 17, 1271. https://doi.org/10.3390/polym17091271
Staudinger U, Janke A, Simon F, Jakisch L, Bittrich E, Formanek P, Mielke L, Schlicke H, Li Q, Eckstein K, et al. MWCNT Localization and Electrical Percolation in Thin Films of Semifluorinated PMMA Block Copolymers. Polymers. 2025; 17(9):1271. https://doi.org/10.3390/polym17091271
Chicago/Turabian StyleStaudinger, Ulrike, Andreas Janke, Frank Simon, Lothar Jakisch, Eva Bittrich, Petr Formanek, Lukas Mielke, Hendrik Schlicke, Qiong Li, Kathrin Eckstein, and et al. 2025. "MWCNT Localization and Electrical Percolation in Thin Films of Semifluorinated PMMA Block Copolymers" Polymers 17, no. 9: 1271. https://doi.org/10.3390/polym17091271
APA StyleStaudinger, U., Janke, A., Simon, F., Jakisch, L., Bittrich, E., Formanek, P., Mielke, L., Schlicke, H., Li, Q., Eckstein, K., & Pospiech, D. (2025). MWCNT Localization and Electrical Percolation in Thin Films of Semifluorinated PMMA Block Copolymers. Polymers, 17(9), 1271. https://doi.org/10.3390/polym17091271







