An Analysis of Foams Produced from Recycled Polyolefins and Low-Cost Foaming Agents: Benchmarking Using Pore Size, Distribution, Shear Effects, and Thermal Properties
Abstract
:1. Introduction
- Temperatures in the extruder were found to have a weak influence on the density of extruded products.
- There was a strong dependence of the density of extrudates on the die temperature.
- In addition, the density of extrudates increased with screw speed.
- Foam/extrudate density decreased with die diameter or length if the ratio L/D was held constant.
- Density and foaming efficiencies (based on the quantity of unreacted CaSO4) both showed a decreasing trend with an increase in gypsum quantity in recycled HDPE.
- Adding recycled LDPE at various concentrations to recycled HDPE had a major influence on extrudate density, indicating a significant effect of comingled recycled raw material nature on the resultant foam properties.
2. Materials and Methods
- The pulverised recycled LDPE, recycled LLDPE, and V_LLDPE were dry blended in an Omni Macro ES for up to 15 min with 15% by weight of CBA (whether CA or NaB).
- A total of 6 g of the dry blended and pulverised mix was placed in a specially designed mould (Figure 2a).
- The entire assembly was placed in a Thermoline Scientific Oven at 200 °C for 15 min until foaming was achieved.
- The sample was withdrawn from the oven, removed from the mould, and cooled to RT and used for further analysis (Figure 2b).
- Recycled LDPE, recycled LLDPE, or V_LLDPE with 15% by weight of CBA (CA or NaB) were introduced into a Thermo Scientific internal mixer operated at 130 °C and 50 rpm for 5 min. However, ADC was added in 2.5, 5, and 10 wt.% to LDPE and was mixed for 7 min at 110 °C.
3. Results and Discussion
3.1. Morphology
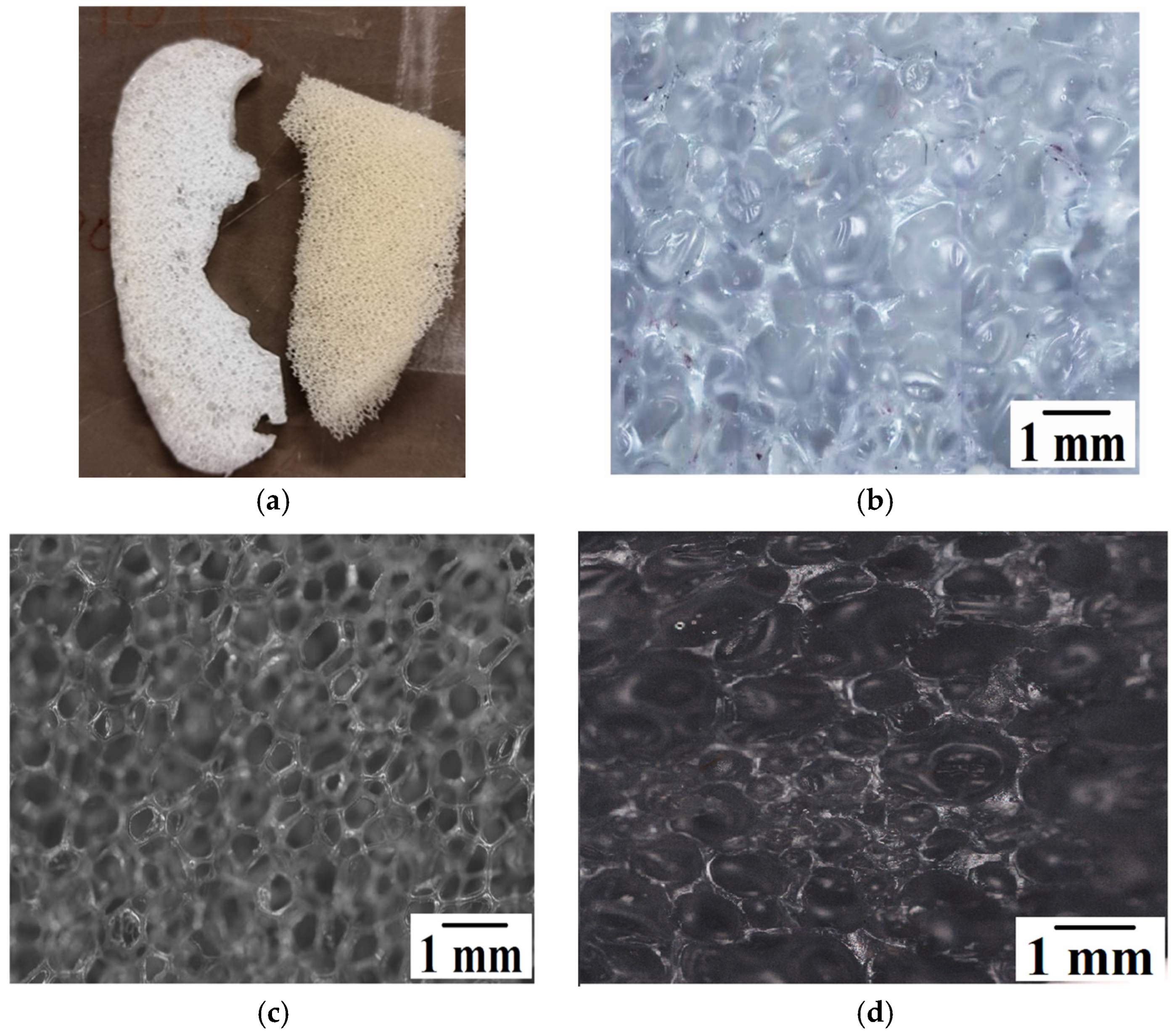
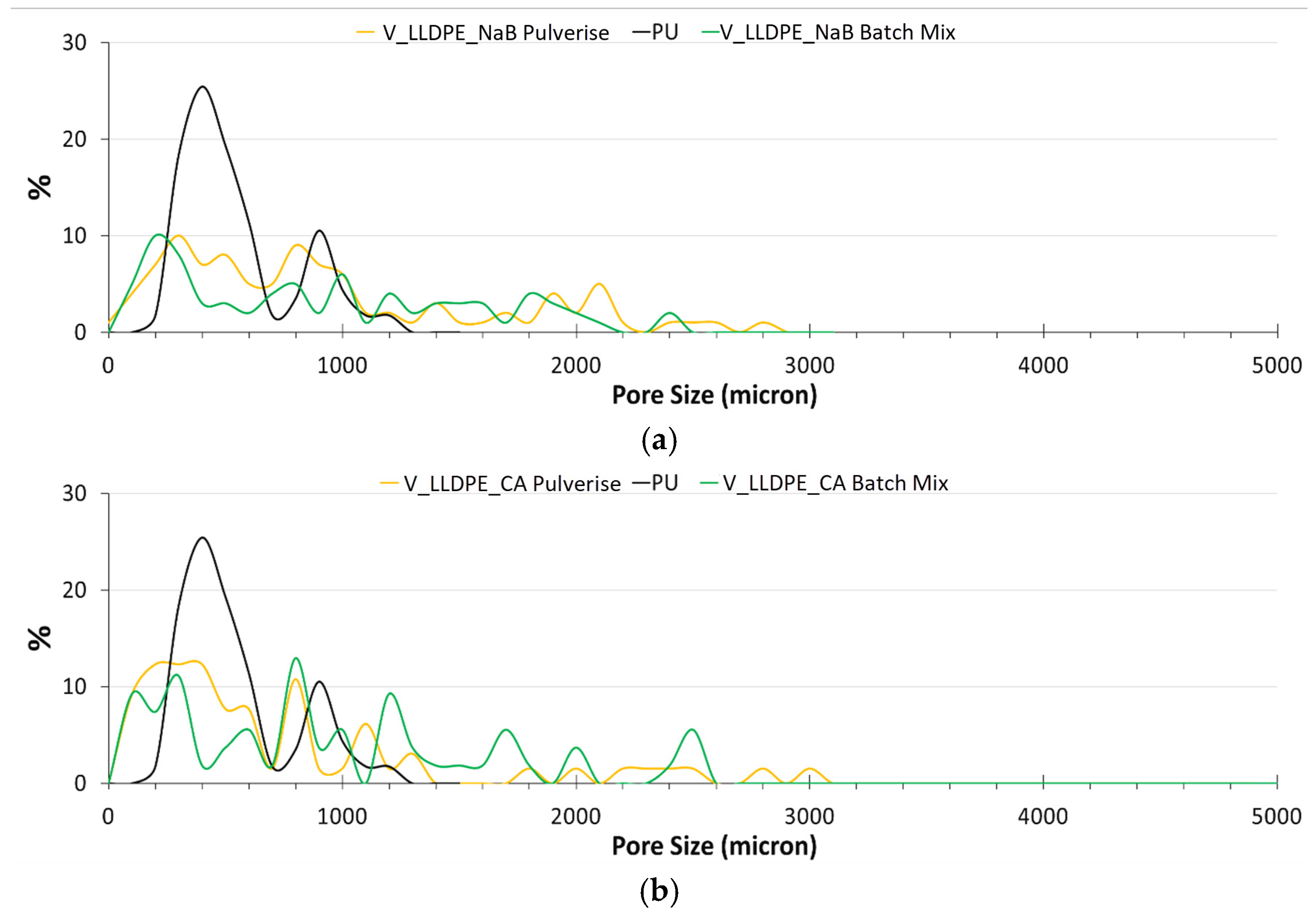
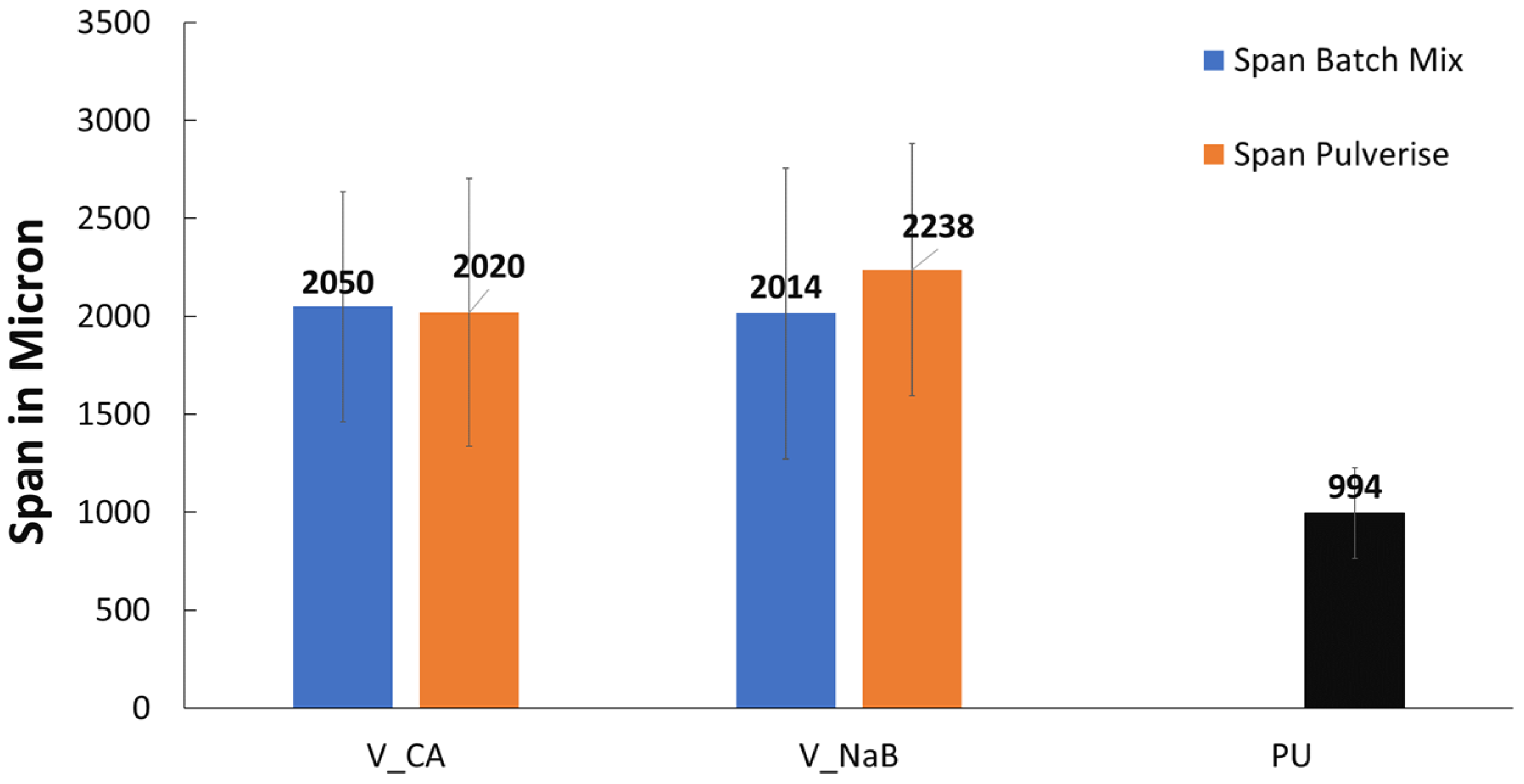
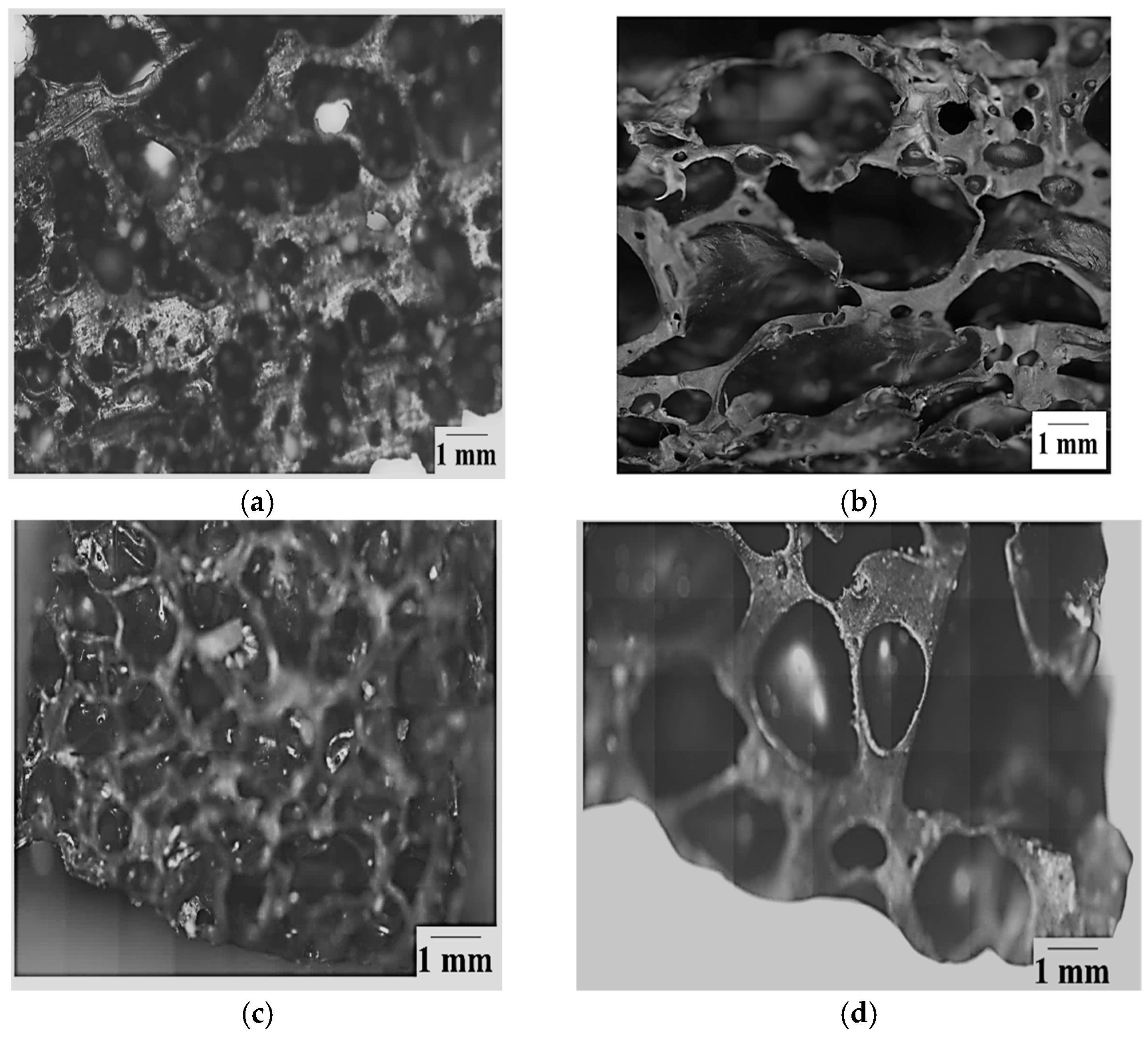
- The pores observed for the commercially available foamed PU sample, which were used in mattresses, were of an open cell variety, while the ones for the V_LLDPE foam were of the closed cell variety.
- The shapes of the pores in the commercial foam were polygonal in nature, while the pulverised V_LLDPE CA and batch mixed V_LLDPE CA foam exhibited mostly spherical pores.
3.2. Thermal Properties

- It can be clearly seen that melting peak became broader and less sharp, which indicated that a range of crystal sizes formed during the foaming process for all the polymers [28].
- The recrystallisation peaks showed changes in shape and sharpness and also exhibited new peaks indicative once again of the different crystal sizes and mechanisms initiated during and after the foaming process.
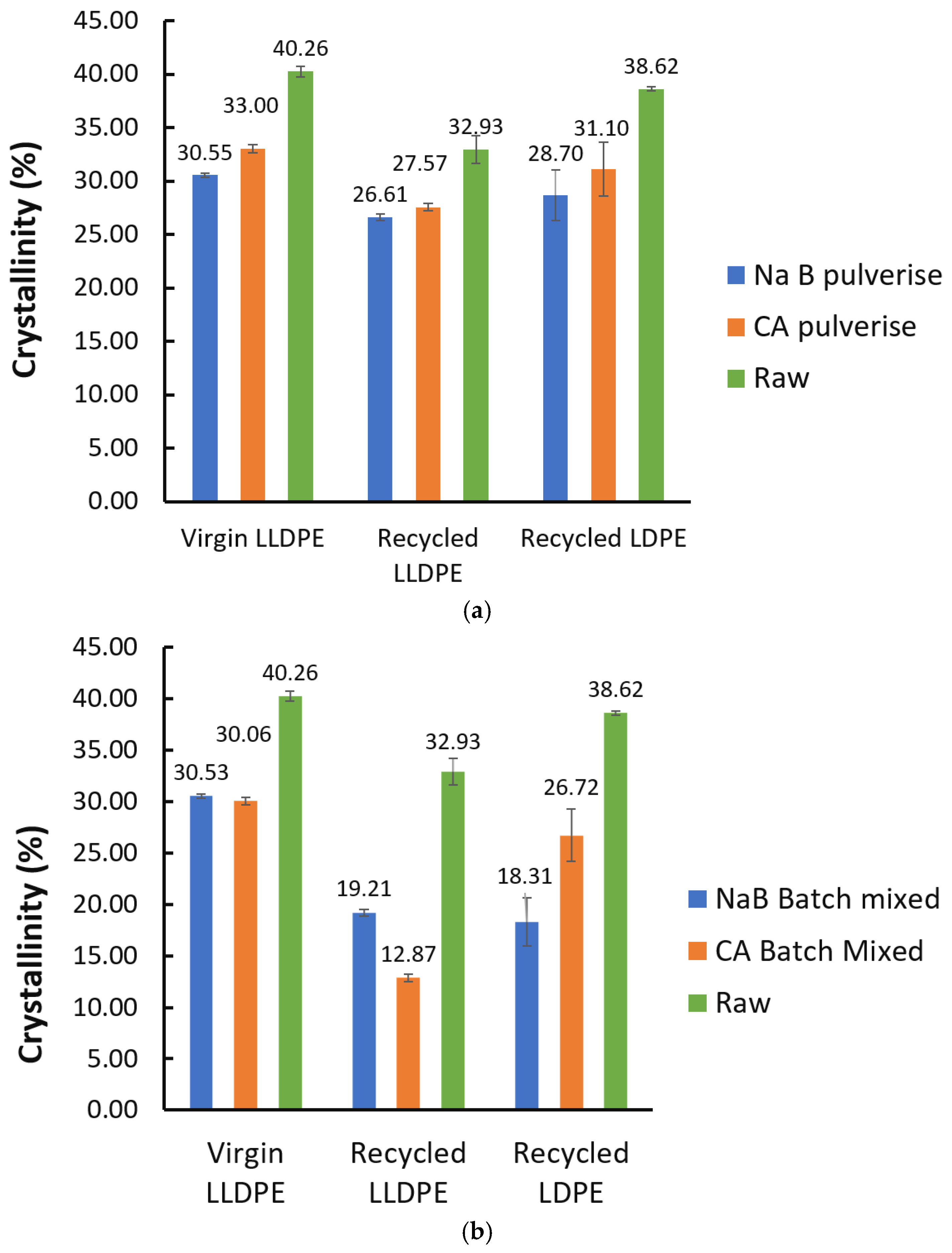
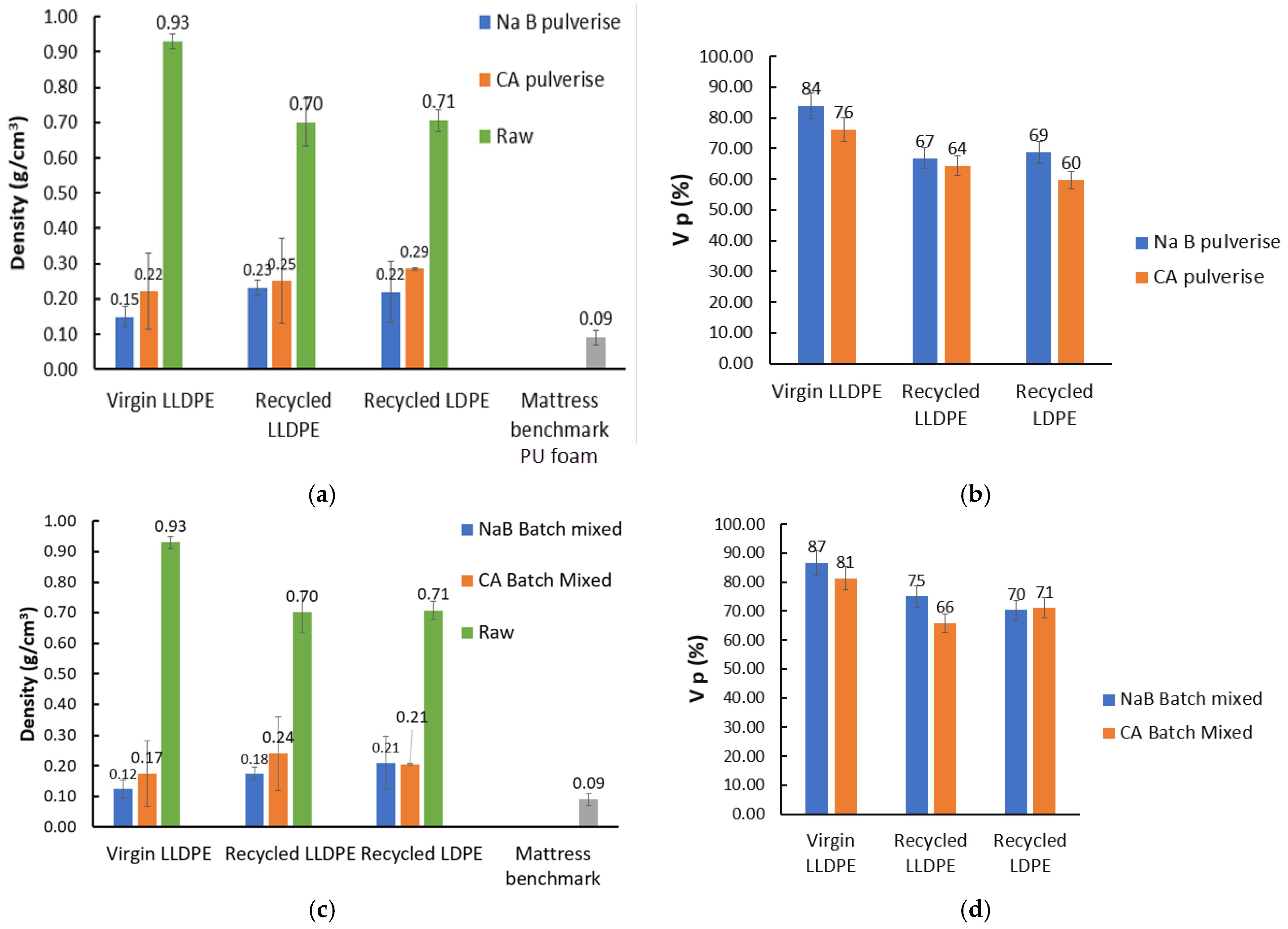
- This also affected the overall polymer crystallinity, as seen in Figure 13. It can be seen that there was a drop in crystallinity throughout after foaming, irrespective of the mixing method or foaming agent used. This was partly due to the widening of crystallite sizes and forms, as reported in [28]. In addition, the presence of the solid foaming agent particles can limit crystallite’s growth in spite of higher crystallite seed density. The quantity of reported work in this specific topic, i.e., the competition between cell nucleation in foaming and crystal nucleation for semi-crystalline polymers like LLDPE and LDPE is limited, and hence this is speculative at the moment [29]. However, it can be seen from our results in Figure 13 that the NaB foaming agent that stayed solid throughout the foaming process resulted in lowered crystallinity than CA, which underwent a phase change to liquid prior to decomposition and foaming.


3.3. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.T.; Park, C.B. Rheology of Thermoplastic Foam Extrusion Process; CRC Press: Boca Raton, FL, USA, 2014; pp. 56–99. [Google Scholar]
- Liu, S.; Huang, Q.; Chen, Q.; Huang, X.B. Application of Extensional Rheology in Polymer Characterization. Acta Polym. Sin. 2023, 54, 286–302. [Google Scholar] [CrossRef]
- Parky, C.P.; Garcia, G.A. Development of Polypropylene Plank Foam Products. J. Cell. Plast. 2002, 38, 219–228. [Google Scholar] [CrossRef]
- Jiang, Q.X.; Bismarck, A. A perspective: Is viscosity the key to open the next door for foam templating? React. Funct. Polym. 2021, 162, 104877. [Google Scholar] [CrossRef]
- Doyle, L.; Weidlich, I.; Di Maio, E. Developing Insulating Polymeric Foams: Strategies and Research Needs from a Circular Economy Perspective. Materials 2022, 15, 6212. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Hallmark, B.; Davidson, J.F. Highly viscous polymeric foam flowing through an orifice. In Proceedings of the 33rd Annual International Conference of the Polymer-Processing-Society (PPS), Cancun, Mexico, 10–14 December 2017. [Google Scholar]
- Münstedt, H. Extensional Rheology and Processing of Polymeric Materials. Int. Polym. Process. 2018, 33, 594–618. [Google Scholar] [CrossRef]
- Reignier, J.; Gendron, R.; Champagne, M.F. Autoclave Foaming of Poly(ε-Caprolactone) Using Carbon Dioxide: Impact of Crystallization on Cell Structure. J. Cell. Plast. 2007, 43, 459–489. [Google Scholar] [CrossRef]
- Rodriguez-Perez, M.A.; Álvarez-Láinez, M.; de Saja, J.A. Microstructure and physical properties of open-cell polyolefin foams. J. Appl. Polym. Sci. 2009, 114, 1176–1186. [Google Scholar] [CrossRef]
- Kim, K.-H.; Pandey, S.K.; Kim, Y.-H.; Sohn, J.R.; Oh, J.M. Emissions of amides (N,N-dimethylformamide and formamide) and other obnoxious volatile organic compounds from different mattress textile products. Ecotoxicol. Environ. Saf. 2015, 114, 350–356. [Google Scholar] [CrossRef]
- Pasquali, I.; Comi, L.; Pucciarelli, F.; Bettini, R. Swelling, melting point reduction and solubility of PEG 1500 in supercritical CO2. Int. J. Pharm. 2008, 356, 76–81. [Google Scholar] [CrossRef]
- Sarver, J.A.; Sumey, J.L.; Williams, M.L.; Bishop, J.P.; Dean, D.M.; Kiran, E. Foaming of poly(ethylene-co-vinyl acetate) and poly(ethylene-co-vinyl acetate-co-carbon monoxide) and their blends with carbon dioxide. J. Appl. Polym. Sci. 2018, 135, 45841. [Google Scholar] [CrossRef]
- Qin, X.; Thompson, M.R.; Hrymak, A.N. Rheology studies of foam flow during injection mold filling. Polym. Eng. Sci. 2007, 47, 522–529. [Google Scholar] [CrossRef]
- Djoumaliisky, S.; Christova, D.; Petrov, I.; Touleshkov, N.; Nedkov, E. Flow behaviour of gas-containing LDPE/i-PP melts. Macromol. Symp. 2002, 181, 493–498. [Google Scholar] [CrossRef]
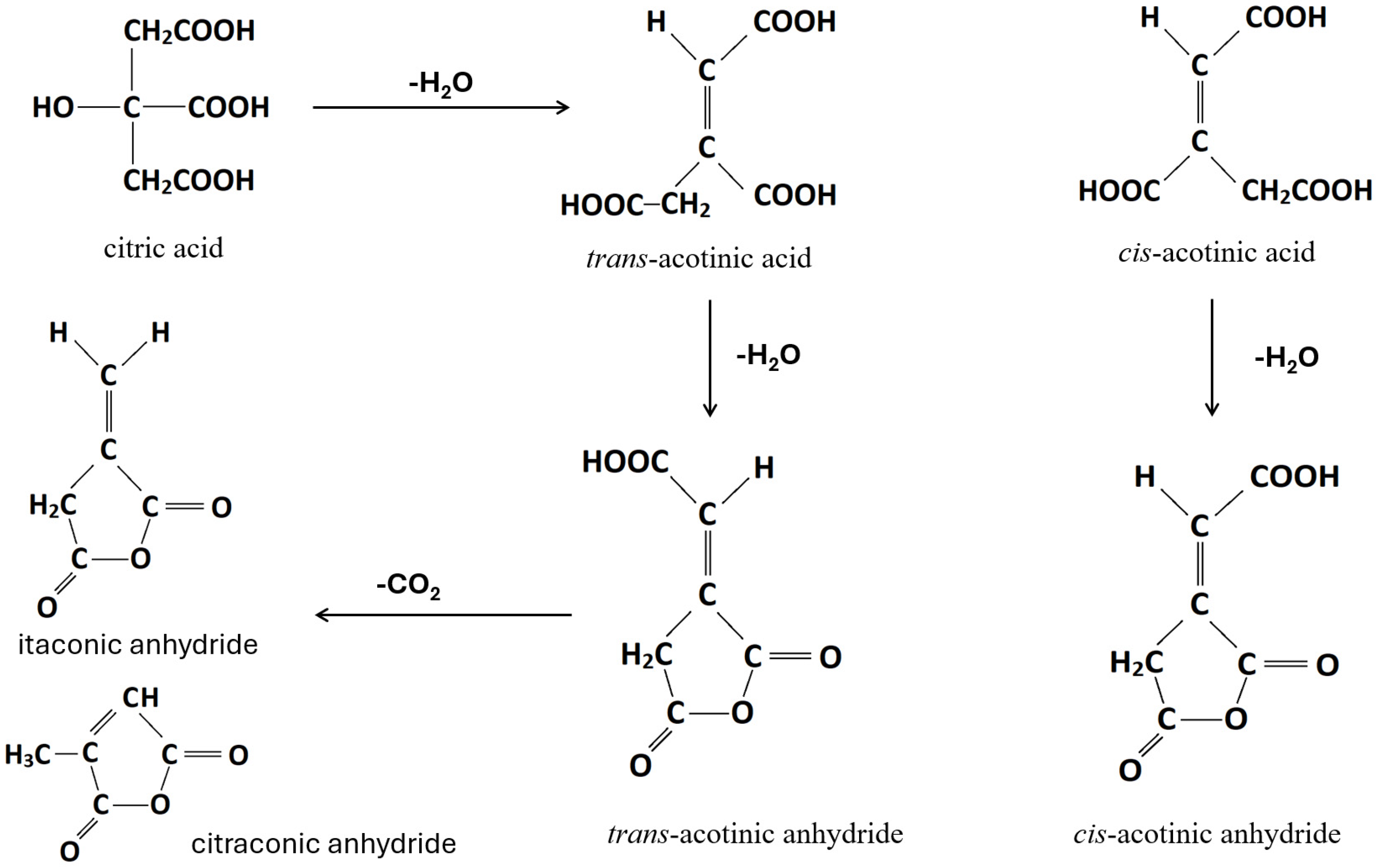
- Sadik, T.; Pillon, C.; Carrot, C.; Reglero Ruiz, J.A. DSC studies on the decomposition of chemical blowing agents based on citric acid and sodium bicarbonate. Thermochim. Acta 2018, 659, 74–81. [Google Scholar] [CrossRef]
- Wyrzykowski, D.; Hebanowska, E.; Nowak-Wiczk, G.; Makowski, M.; Chmurzyński, L. Thermal behaviour of citric acid and isomeric aconitic acids. J. Therm. Anal. Calorim. 2011, 104, 731–735. [Google Scholar] [CrossRef]
- Reglero Ruiz, J.A.; Vincent, M.; Agassant, J.-F.; Sadik, T.; Pillon, C.; Carrot, C. Polymer foaming with chemical blowing agents: Experiment and modeling. Polym. Eng. Sci. 2015, 55, 2018–2029. [Google Scholar] [CrossRef]
- Fasihi, M.; Targhi, A.A.; Bayat, H. The simultaneous effect of nucleating and blowing agents on the cellular structure of polypropylene foamed via the extrusion process. e-Polymers 2016, 16, 235–241. [Google Scholar] [CrossRef]
- Li, Q.; Matuana, L.M. Foam extrusion of high density polyethylene/wood-flour composites using chemical foaming agents. J. Appl. Polym. Sci. 2003, 88, 3139–3150. [Google Scholar] [CrossRef]
- Reglero Ruiz, J.; Vincent, M.; Agassant, J.-f. Numerical Modeling of Bubble Growth in Microcellular Polypropylene Produced in a Core-Back Injection Process Using Chemical Blowing Agents. Int. Polym. Process. J. Polym. Process. Soc. 2016, 31, 26–36. [Google Scholar] [CrossRef]
- Greco, A.; Maffezzoli, A.; Manni, O. Development of polymeric foams from recycled polyethylene and recycled gypsum. Polym. Degrad. Stab. 2005, 90, 256–263. [Google Scholar] [CrossRef]
- Atkinson, C.; Richardson, M. Thermodynamic properties of ideally crystalline polyethylene. Trans. Faraday Soc. 1969, 65, 1764–1773. [Google Scholar] [CrossRef]
- Prasad, K.; Nikzad, M.; Doherty, C.M.; Sbarski, I. Predicting trends in structural and physical properties of a model polymer with embedded natural fibers: Viability of molecular dynamics studies for a bottom up design. J. Appl. Polym. Sci. 2019, 136, 48189. [Google Scholar] [CrossRef]
- ASTM D3575-20; Standard Test Methods for Flexible Cellular Materials Made from Olefin Polymers. ASTM: West Conshohocken, PA, USA, 2020.
- Prasad, K.; Nikzad, M.; Sbarski, I. Using viscoelastic modeling and molecular dynamics based simulations to characterize polymer natural fiber composites. J. Appl. Polym. Sci. 2020, 137, 49220. [Google Scholar] [CrossRef]
- Prasad, K.; Nikzad, M.; Sbarski, I. Mechanical, viscoelastic and gas transport behaviour of rotationally molded polyethylene composites with hard- and soft-wood natural fibres. J. Polym. Res. 2022, 29, 131. [Google Scholar] [CrossRef]
- Aumnate, C.; Rudolph, N.; Sarmadi, M. Recycling of Polypropylene/Polyethylene Blends: Effect of Chain Structure on the Crystallisation Behaviors. Polymers 2019, 11, 1456. [Google Scholar] [CrossRef]
- Nofar, M.R.; Majithiya, K.; Kuboki, T.; Park, C.B. The foamability of low-melt-strength linear polypropylene with nanoclay and coupling agent. J. Cell. Plast. 2012, 48, 271–287. [Google Scholar] [CrossRef]
- Kabamba, E.; Rodrigue, D. The Effect of Recycling on LDPE Foam Properties. Prog. Rubber Plast. Recycl. Technol. 2008, 24, 1–17. [Google Scholar] [CrossRef]
- D’Arienzo, L.; Incarnato, L.; Rinaldi, S.; Scarfato, P. Comfort and fatigue performance of a Soya-derived flexible PU foam for mattresses. AIP Conf. Proc. 2019, 2196, 020028. [Google Scholar] [CrossRef]
- Scarfato, P.; Di Maio, L.; Incarnato, L. Structure and physical-mechanical properties related to comfort of flexible polyurethane foams for mattress and effects of artificial weathering. Compos. Part B Eng. 2017, 109, 45–52. [Google Scholar] [CrossRef]
- Shetage, S.S.; Traynor, M.J.; Brown, M.B.; Raji, M.; Graham-Kalio, D.; Chilcott, R.P. Effect of ethnicity, gender and age on the amount and composition of residual skin surface components derived from sebum, sweat and epidermal lipids. Ski. Res. Technol. 2014, 20, 97–107. [Google Scholar] [CrossRef]
- Toan, S.; O’Dell, W.; Russell, C.K.; Zhao, S.; Lai, Q.; Song, H.; Zhao, Y.; Fan, M. Thermodynamics of NaHCO3 decomposition during Na2CO3-based CO2 capture. J. Environ. Sci. 2019, 78, 74–80. [Google Scholar] [CrossRef]
- Apelblat, A. Citric Acid Chemistry. In Citric Acid; Springer International Publishing: Cham, Switzerland, 2014; pp. 213–266. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasad, K.; Jahromi, F.T.; Nisha, S.S.; Stehle, J.; Gad, E.; Nikzad, M. An Analysis of Foams Produced from Recycled Polyolefins and Low-Cost Foaming Agents: Benchmarking Using Pore Size, Distribution, Shear Effects, and Thermal Properties. Polymers 2025, 17, 1270. https://doi.org/10.3390/polym17091270
Prasad K, Jahromi FT, Nisha SS, Stehle J, Gad E, Nikzad M. An Analysis of Foams Produced from Recycled Polyolefins and Low-Cost Foaming Agents: Benchmarking Using Pore Size, Distribution, Shear Effects, and Thermal Properties. Polymers. 2025; 17(9):1270. https://doi.org/10.3390/polym17091270
Chicago/Turabian StylePrasad, Krishnamurthy, Fareed Tamaddoni Jahromi, Shammi Sultana Nisha, John Stehle, Emad Gad, and Mostafa Nikzad. 2025. "An Analysis of Foams Produced from Recycled Polyolefins and Low-Cost Foaming Agents: Benchmarking Using Pore Size, Distribution, Shear Effects, and Thermal Properties" Polymers 17, no. 9: 1270. https://doi.org/10.3390/polym17091270
APA StylePrasad, K., Jahromi, F. T., Nisha, S. S., Stehle, J., Gad, E., & Nikzad, M. (2025). An Analysis of Foams Produced from Recycled Polyolefins and Low-Cost Foaming Agents: Benchmarking Using Pore Size, Distribution, Shear Effects, and Thermal Properties. Polymers, 17(9), 1270. https://doi.org/10.3390/polym17091270






