Conjugated Polymer-Photosensitizers for Cancer Photodynamic Therapy and Their Multimodal Treatment Strategies
Abstract
1. Introduction
2. Design Strategy and Performance Optimization
2.1. D-A Structure Engineering
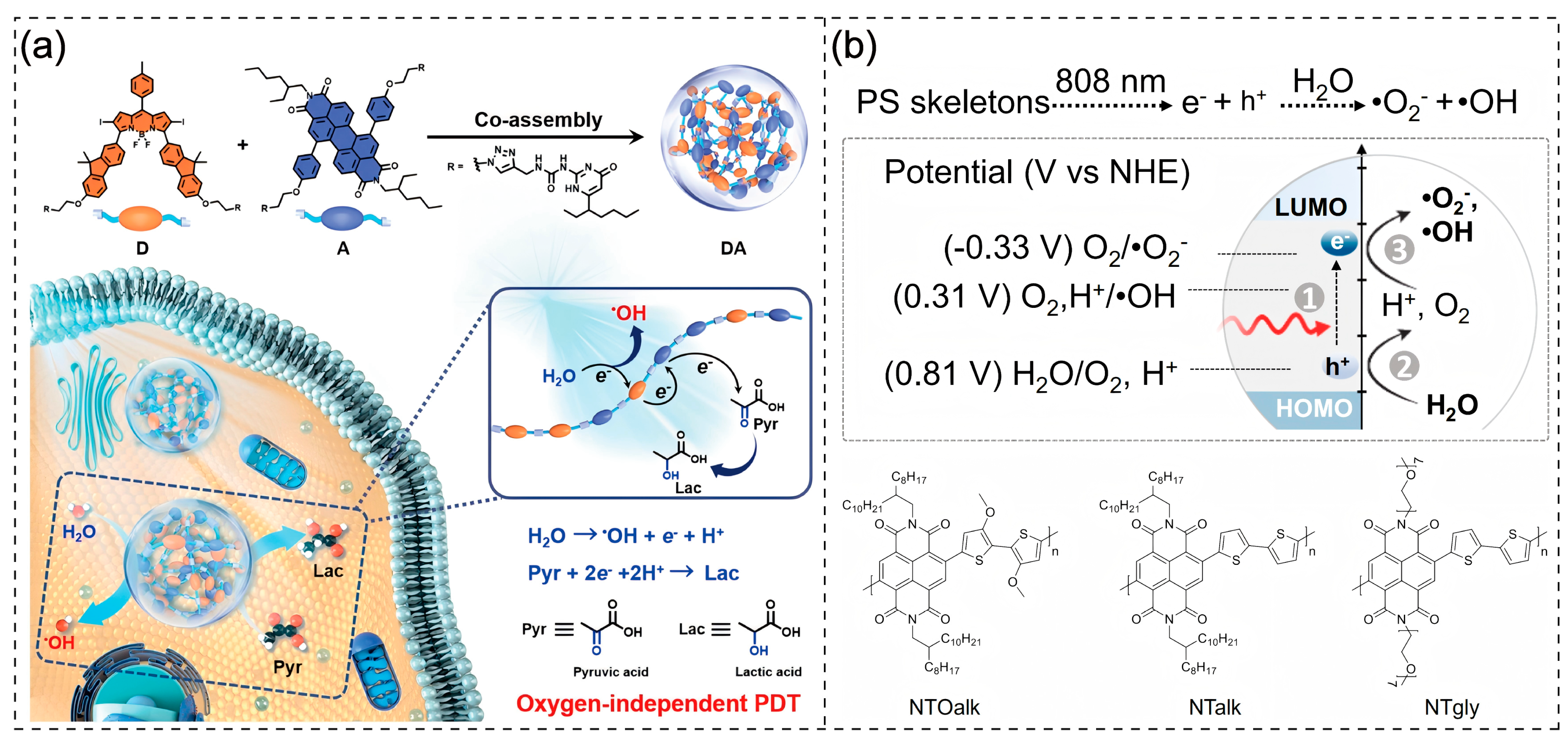
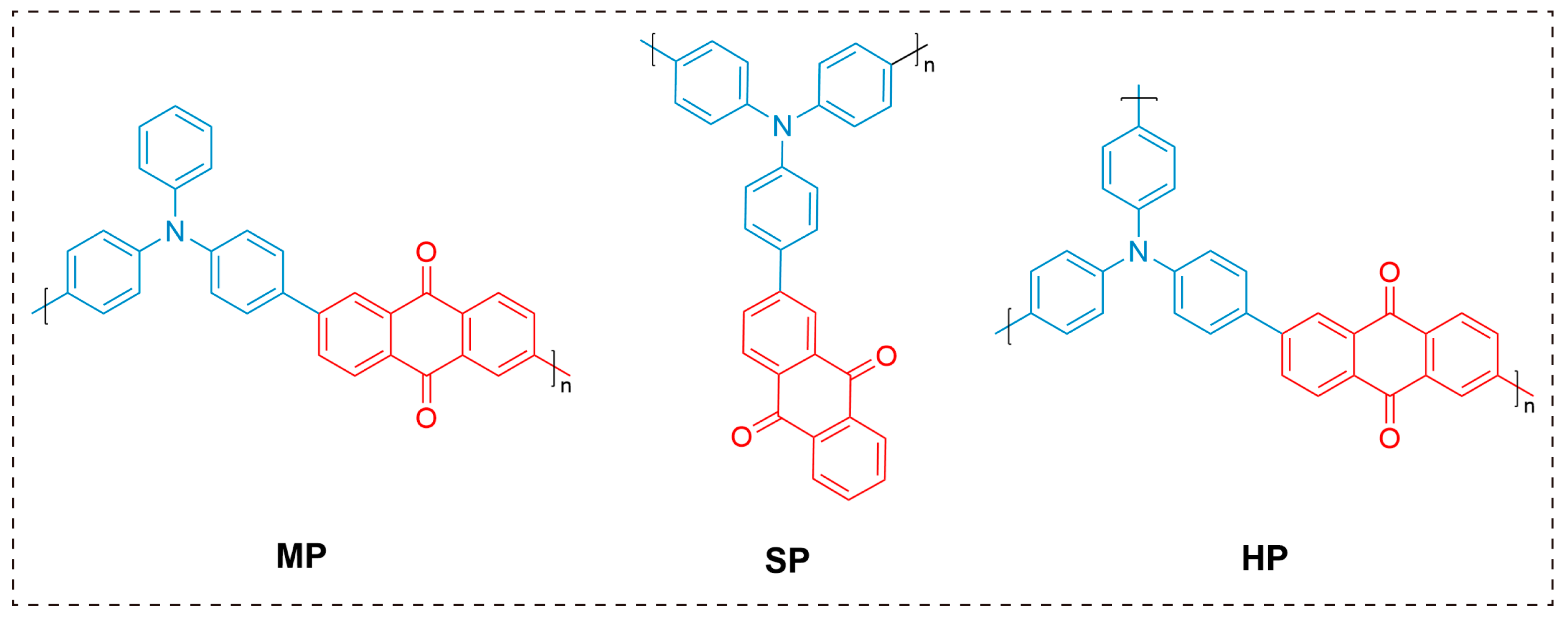
2.2. Incorporation of AIE Moieties
2.3. Heavy-Atom Effects

2.4. Hyperbranched
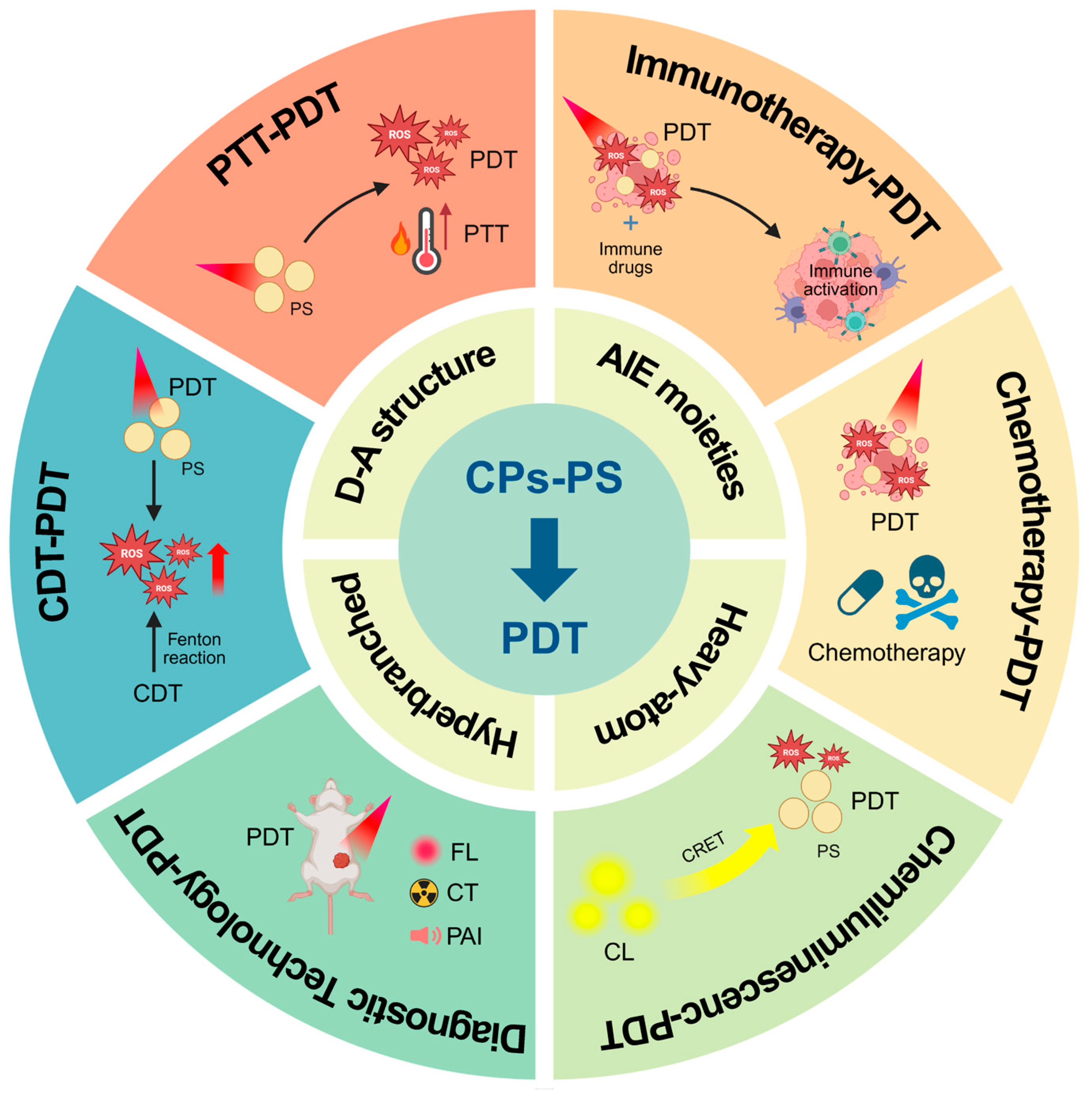
3. Multimodal Cancer Therapy Based on Conjugated Polymers
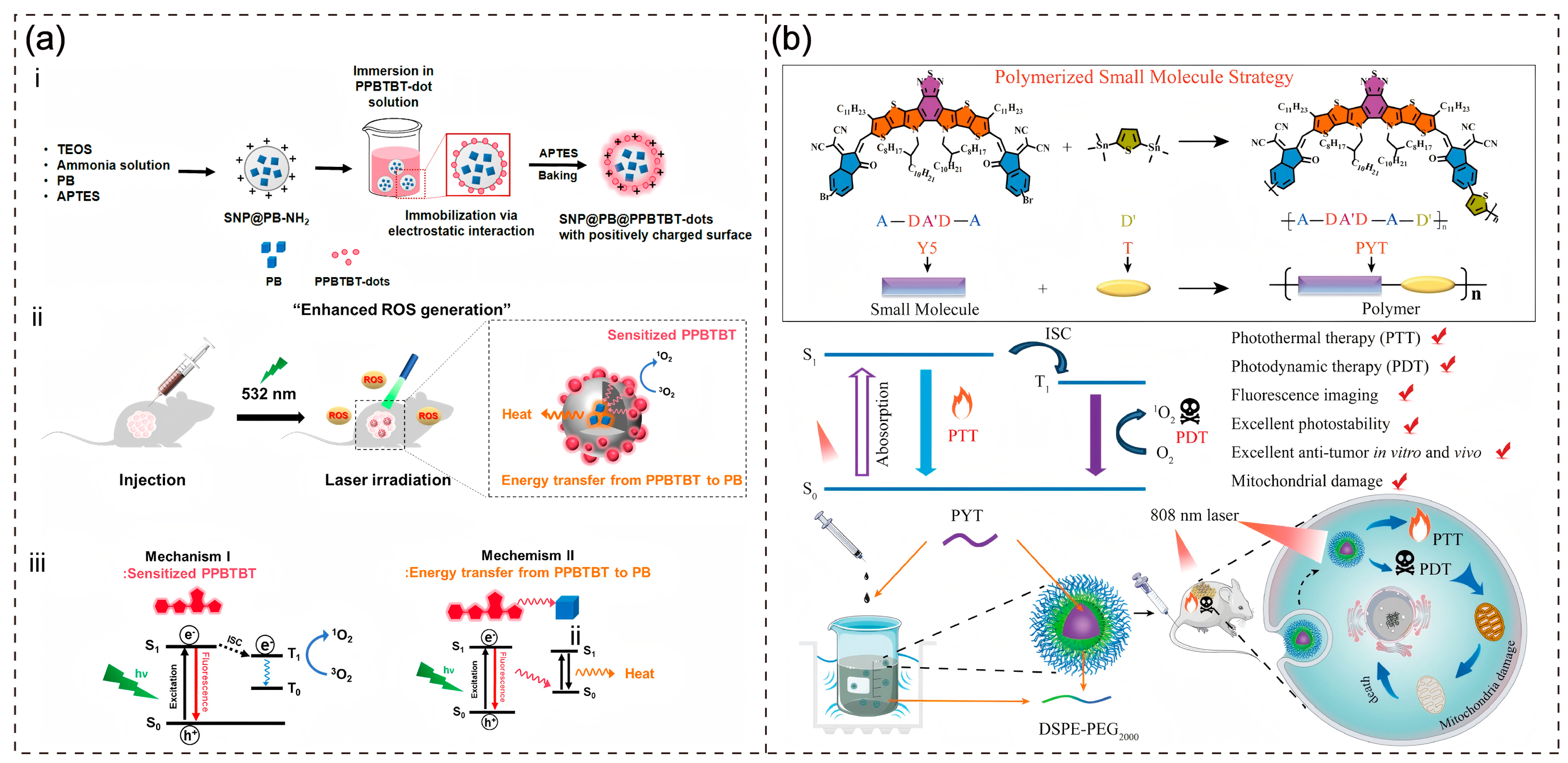
3.1. PTT-PDT
3.2. Photodynamic Immunotherapy
3.3. Chemotherapy-PDT
3.4. Chemiluminescence-Driven Light Source-Free PDT
3.5. Theranostics
3.6. CDT-PDT
4. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA-Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.; Massagué, J. Targeting metastatic cancer. Nat. Med. 2021, 27, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Jassim, A.; Rahrmann, E.P.; Simons, B.D.; Gilbertson, R.J. Cancers make their own luck: Theories of cancer origins. Nat. Rev. Cancer 2023, 23, 710–724. [Google Scholar] [CrossRef]
- Bhatia, S.; Tonorezos, E.S.; Landier, W. Clinical Care for People Who Survive Childhood Cancer. J. Am. Med. Assoc. 2023, 330, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Im, C.; Hasan, H.; Stene, E.; Monick, S.; Rader, R.K.; Sheade, J.; Wolfe, H.; Lu, Z.; Spector, L.G.; McDonald, A.J.; et al. Treatment, toxicity, and mortality after subsequent breast cancer in female survivors of childhood cancer. Nat. Commun. 2025, 16, 3088. [Google Scholar] [CrossRef]
- Gibson, T.M.; Karyadi, D.M.; Hartley, S.W.; Arnold, M.A.; de Gonzalez, A.B.; Conces, M.R.; Howell, R.M.; Kapoor, V.; Leisenring, W.M.; Neglia, J.P.; et al. Polygenic risk scores, radiation treatment exposures and subsequent cancer risk in childhood cancer survivors. Nat. Med. 2024, 30, 690. [Google Scholar] [CrossRef]
- Wu, Y.; Song, Y.Q.; Wang, R.Z.; Wang, T.L. Molecular mechanisms of tumor resistance to radiotherapy. Mol. Cancer 2023, 22, 21. [Google Scholar] [CrossRef]
- Xu, B.H.; Shao, Z.M.; Wang, S.; Jiang, Z.F.; Hu, X.C.; Zhang, X.H.; Li, X.R.; Liu, J.P.; Li, M.Q.; Wang, S. Treatment patterns and patient profiles for docetaxel-based adjuvant chemotherapy in early-stage breast cancer in China: A pooled analysis of four observational studies. J. Clin. Oncol. 2017, 35, 5. [Google Scholar] [CrossRef]
- Komarova, E.F.; Engibaryan, M.A.; Ulianova, Y.V.; Volkova, V.L.; Bauzhadze, M.V.; Pustovaya, I.V.; Chertova, N.A.; Goncharova, E.G.; Gvaramiya, A.K.; Maldonado, M.L.; et al. Experimental photodynamic therapy in the treatment of lung tumor. J. Clin. Oncol. 2022, 40, 1. [Google Scholar] [CrossRef]
- Ivanova, V.A.; Verenikina, E.V.; Nikitina, V.P.; Zhenilo, O.E.; Ardzha, A.Y. Photodynamic therapy for preinvasive vaginal cancer. J. Clin. Oncol. 2021, 39, 3. [Google Scholar] [CrossRef]
- Muroya, T.; Akiya, T.; Nakano, M.; Sakamoto, M.; Tenjin, Y. Photodynamic therapy for early cervical cancer. Nihon rinsho. Jpn. J. Clin. Med. 2004, 62 (Suppl. 10), 158–168. [Google Scholar]
- Li, M.L.; Xu, Y.J.; Peng, X.J.; Kim, J.S. From Low to No O2-Dependent Hypoxia Photodynamic Therapy (hPDT): A New Perspective. Acc. Chem. Res. 2022, 55, 3253–3264. [Google Scholar] [CrossRef] [PubMed]
- Laustriat, G. Molecular mechanisms of photosensitization. Biochimie 1986, 68, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Yaraki, M.T.; Liu, B.; Tan, Y.N. Emerging Strategies in Enhancing Singlet Oxygen Generation of Nano-Photosensitizers Toward Advanced Phototherapy. Nano-Micro Lett. 2022, 14, 49. [Google Scholar] [CrossRef]
- Baptista, M.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef]
- Matroule, J.Y.; Piette, J. Photosensitization and Redox Signaling. Antioxid. Redox Signal. 2000, 2, 301–315. [Google Scholar] [CrossRef]
- Poh-Fitzpatrick, M.B. Molecular and cellular mechanisms of porphyrin photosensitization. Photo-Dermatology 1986, 3, 148–157. [Google Scholar]
- Gedeon, C.; Del Rio, N.; Furlan, F.; Taddeucci, A.; Vanthuyne, N.; Gregoriou, V.G.; Fuchter, M.J.; Siligardi, G.; Gasparini, N.; Crassous, J.; et al. Rational Design of New Conjugated Polymers with Main Chain Chirality for Efficient Optoelectronic Devices: Carbo 6 Helicene and Indacenodithiophene Copolymers as Model Compounds. Adv. Mater. 2024, 36, 11. [Google Scholar] [CrossRef]
- Iwasaki, H.; Yamanaka, K.; Sato, Y.; Mikie, T.; Saito, M.; Ohkita, H.; Osaka, I. Efficient Derivatization of a Thienobenzobisthiazole-Based π-Conjugated Polymer Through Late-Stage Functionalization Towards High-Efficiency Organic Photovoltaic Cells. Angew. Chem.-Int. Ed. 2024, 63, 9. [Google Scholar] [CrossRef]
- Paleti, S.H.K.; Kim, Y.; Kimpel, J.; Craighero, M.; Haraguchi, S.; Müller, C. Impact of doping on the mechanical properties of conjugated polymers. Chem. Soc. Rev. 2024, 53, 1702–1729. [Google Scholar] [CrossRef]
- Liu, C.C.; Shao, L.; Chen, S.H.; Hu, Z.W.; Cai, H.J.; Huang, F. Recent Progress in π-Conjugated Polymers for Organic Photovoltaics: Solar Cells and Photodetectors. Prog. Polym. Sci. 2023, 143, 63. [Google Scholar] [CrossRef]
- MacFarlane, L.R.; Shaikh, H.; Garcia-Hernandez, J.D.; Vespa, M.; Fukui, T.; Manners, I. Functional nanoparticles through π-conjugated polymer self-assembly. Nat. Rev. Mater. 2021, 6, 7–26. [Google Scholar] [CrossRef]
- Yang, Z.W.; Shen, Q.; Xing, L.J.; Fu, X.C.; Qiu, Z.P.; Xiang, H.P.; Huang, Y.M.; Lv, F.T.; Bai, H.T.; Huo, Y.P.; et al. A biophotonic device based on a conjugated polymer and a macrophage-laden hydrogel for triggering immunotherapy. Mater. Horiz. 2023, 10, 2226–2236. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, H.; Yuan, H.; Lu, W.; Li, Z.; Wang, L.; Gao, L.-H. Conjugated Polymer and Triphenylamine Derivative Codoped Nanoparticles for Photothermal and Photodynamic Antimicrobial Therapy. ACS Appl. Bio Mater. 2020, 3, 3494–3499. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Yuan, Q.; Li, M.Q.; Tang, Y.L. Cationic Conjugated Microporous Polymers Coating for Dual-Modal Antimicrobial Inactivation with Self-Sterilization and Reusability Functions. Adv. Funct. Mater. 2023, 33, 9. [Google Scholar] [CrossRef]
- Pan, X.; Gao, A.T.; Hu, Y.N.; Hu, Z.Y.; Xie, C.; Lin, Z.T. Gadolinium-containing semiconducting polymer nanoparticles for magnetic resonance/fluorescence dual-modal imaging and photothermal therapy of oral squamous cell carcinoma. Nano Res. 2023, 16, 2808–2820. [Google Scholar] [CrossRef]
- Lubanska, D.; Alrashed, S.; Mason, G.T.; Nadeem, F.; Awada, A.; DiPasquale, M.; Sorge, A.; Malik, A.; Kojic, M.; Soliman, M.A.R.; et al. Impairing proliferation of glioblastoma multiforme with CD44+selective conjugated polymer nanoparticles. Sci. Rep. 2022, 12, 16. [Google Scholar] [CrossRef]
- Deng, G.J.; Peng, X.H.; Sun, Z.H.; Zheng, W.; Yu, J.; Du, L.L.; Chen, H.J.; Gong, P.; Zhang, P.F.; Cai, L.T.; et al. Natural-Killer-Cell-Inspired Nanorobots with Aggregation-Induced Emission Characteristics for Near-Infrared-II Fluorescence-Guided Glioma Theranostics. ACS Nano 2020, 14, 11452–11462. [Google Scholar] [CrossRef]
- Redmond, R.W.; Kochevar, I.E. Spatially resolved cellular responses to singlet oxygen. Photochem. Photobiol. 2006, 82, 1178–1186. [Google Scholar] [CrossRef]
- Niedre, M.; Patterson, M.S.; Wilson, B.C. Direct near-infrared luminescence detection of singlet oxygen generated by photodynamic therapy in cells in vitro and tissues in vivo. Photochem. Photobiol. 2002, 75, 382–391. [Google Scholar] [CrossRef]
- Mallidi, S.; Anbil, S.; Bulin, A.L.; Obaid, G.; Ichikawa, M.; Hasan, T. Beyond the Barriers of Light Penetration: Strategies, Perspectives and Possibilities for Photodynamic Therapy. Theranostics 2016, 6, 2458–2487. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Wen, X.; Zhang, S. Strategies to Improve Photodynamic Therapy Efficacy of Metal-Free Semiconducting Conjugated Polymers. Int. J. Nanomed. 2022, 17, 247–271. [Google Scholar] [CrossRef]
- Zhang, X.L.; Wu, M.; Li, J.; Lan, S.Y.; Zeng, Y.Y.; Liu, X.L.; Liu, J.F. Light-Enhanced Hypoxia-Response of Conjugated Polymer Nanocarrier for Successive Synergistic Photodynamic and Chemo-Therapy. ACS Appl. Mater. Interfaces 2018, 10, 21909–21919. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, Y.; Zhu, X.J.; Li, Y.F.; Cai, X.Y. Zwitterionic Conjugated Polymer as the Single Component for Photoacoustic-Imaging-Guided Dual-Modal Near-Infrared Phototherapy. ACS Biomater. Sci. Eng. 2020, 6, 4005–4011. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Jia, S.R.; Kang, X.Y.; Wu, X.Y.; Hong, Y.N.; Shan, K.; Kong, X.L.; Wang, Z.M.; Ding, D. Semiconducting Polymer Nanoparticles with Surface-Mimicking Protein Secondary Structure as Lysosome-Targeting Chimaeras for Self-Synergistic Cancer Immunotherapy. Adv. Mater. 2022, 34, 14. [Google Scholar] [CrossRef] [PubMed]
- Mikie, T.; Hayakawa, M.; Okamoto, K.; Iguchi, K.; Yashiro, S.; Koganezawa, T.; Sumiya, M.; Ishii, H.; Yamaguchi, S.; Fukazawa, A.; et al. Extended π-Electron Delocalization in Quinoid-Based Conjugated Polymers Boosts Intrachain Charge Carrier Transport. Chem. Mater. 2021, 33, 8183–8193. [Google Scholar] [CrossRef]
- Yu, Z.D.; Lu, Y.; Wang, J.Y.; Pei, J. Conformation Control of Conjugated Polymers. Chem.-Eur. J. 2020, 26, 16194–16205. [Google Scholar] [CrossRef]
- Grobelny, A.; Grobelny, A.; Zapotoczny, S. Precise Stepwise Synthesis of Donor-Acceptor Conjugated Polymer Brushes Grafted from Surfaces. Int. J. Mol. Sci. 2022, 23, 17. [Google Scholar] [CrossRef]
- Yue, Y.F.; Li, B.W.; Wang, D.D.; Wu, C.Z.; Li, Z.Y.; Liu, B. Optimizing Photosensitizers with Type I and Type II ROS Generation Through Modulating Triplet Lifetime and Intersystem Crossing Efficiency. Adv. Funct. Mater. 2025, 35, 2414542. [Google Scholar] [CrossRef]
- Pang, E.; Zhao, S.J.; Wang, B.H.; Niu, G.L.; Song, X.Z.; Lan, M.H. Strategies to construct efficient singlet oxygen-generating photosensitizers. Coord. Chem. Rev. 2022, 472, 16. [Google Scholar] [CrossRef]
- Li, X.Y.; Zheng, Q.A.; Wang, X.R.; Zheng, Q.Y.; Zhang, Y.; Cong, Y.Q.; Lv, S.W. Introduction of electron-deficient unit in resorcinol-formaldehyde resin to construct donor-acceptor conjugated polymer for enhancing photocatalytic H2O2 production. J. Mater. Chem. A 2024, 12, 8420–8428. [Google Scholar] [CrossRef]
- Liu, M.Y.; Shao, X.X.; Liu, J.; Wang, L.X. A Cyano-Substituted Organoboron Electron-deficient Building Block for D-A Type Conjugated Polymers. Chin. J. Polym. Sci. 2023, 41, 832–838. [Google Scholar] [CrossRef]
- Yan, H.J.; Deng, Y.C.; Shen, M.H.; Ye, Y.X.; Zhu, F.; Yang, X.; Ouyang, G.F. Regulation the reactive oxygen species on conjugated polymers for highly efficient photocatalysis. Appl. Catal. B-Environ. Energy 2022, 314, 9. [Google Scholar] [CrossRef]
- Tang, G.E.; Liu, G.H.; Song, T.; Hu, X.Y.; Long, B.; Deng, G.J. Design of D-A conjugated polymers for simultaneous selective bond breaking/formation and syngas production under visible light illumination. Chem. Eng. J. 2024, 481, 10. [Google Scholar] [CrossRef]
- Teng, K.X.; Niu, L.Y.; Yang, Q.Z. Supramolecular Photosensitizer Enables Oxygen-Independent Generation of Hydroxyl Radicals for Photodynamic Therapy. J. Am. Chem. Soc. 2023, 145, 4081–4087. [Google Scholar] [CrossRef]
- An, J.; Tang, S.L.; Hong, G.B.; Chen, W.L.; Chen, M.M.; Song, J.T.; Li, Z.L.; Peng, X.J.; Song, F.L.; Zheng, W.H. An unexpected strategy to alleviate hypoxia limitation of photodynamic therapy by biotinylation of photosensitizers. Nat. Commun. 2022, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wan, Y.P.; Cui, X.; Li, S.L.; Lee, C.S. Recent Advances in Hypoxia-Overcoming Strategy of Aggregation-Induced Emission Photosensitizers for Efficient Photodynamic Therapy. Adv. Healthc. Mater. 2021, 10, 14. [Google Scholar] [CrossRef]
- Yu, L.; Liu, Z.; Xu, W.; Jin, K.; Liu, J.L.; Zhu, X.H.; Zhang, Y.; Wu, Y.H. Towards overcoming obstacles of type II photodynamic therapy: Endogenous production of light, photosensitizer, and oxygen. Acta Pharm. Sin. B 2024, 14, 1111–1131. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.S.; Cadet, J.; Greer, A.; Thomas, A.H. Photosensitization Reactions of Biomolecules: Definition, Targets and Mechanisms. Photochem. Photobiol. 2021, 97, 1456–1483. [Google Scholar] [CrossRef]
- Li, M.L.; Xu, Y.J.; Pu, Z.J.; Xiong, T.; Huang, H.Q.; Long, S.; Son, S.; Yu, L.; Singh, N.; Tong, Y.; et al. Photoredox catalysis may be a general mechanism in photodynamic therapy. Proc. Natl. Acad. Sci. USA 2022, 119, 8. [Google Scholar] [CrossRef]
- Fu, S.W.; Chen, Z.X.; Li, L.; Wu, Y.W.; Liao, Y.L.; Li, X.S. Redox-activated photosensitizers for visualizing precise diagnosis and potentiating cancer therapy. Coord. Chem. Rev. 2024, 507, 16. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, L.L.; Li, Y.K.; Wang, Y.Y.; Chen, J.L.; Lin, B.Z.; Zheng, Y.Z.; Cheng, L.; Wang, S.B.; Chen, Y.L. Triptycene incorporated carbon nitride based donor-acceptor conjugated polymers with superior visible-light photocatalytic activities. J. Colloid Interface Sci. 2022, 622, 675–689. [Google Scholar] [CrossRef]
- Hayat, A.; Shaishta, N.; Mane, S.K.B.; Hayat, A.; Khan, J.; Rehman, A.U.; Li, T.H. Molecular engineering of polymeric carbon nitride based Donor-Acceptor conjugated copolymers for enhanced photocatalytic full water splitting. J. Colloid Interface Sci. 2020, 560, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.L.; Liu, S.Y.; Lin, J.Y.; Li, Y.; Chen, S.W.; Wang, P.K.; Zhu, S.M.; Han, X.J.; Wang, J. Donor-acceptor anchoring nanoarchitectonics in polymeric carbon nitride for rapid charge transfer and enhanced visible-light photocatalytic hydrogen evolution reaction. Carbon 2022, 197, 378–388. [Google Scholar] [CrossRef]
- Wang, H.Y.; Yang, Z.Y.; Zhang, K.M.; Wang, W.; Lin, F.X.; Huang, H.H.; Qin, W.; Chi, Z.G.; Yang, Z.Y. New Janus Organic Type-I Photosensitizer Capable of Transforming Glutathione from Opponent to Ally for Assisting Photodynamic Therapy. ACS Mater. Lett. 2024, 6, 1317–1326. [Google Scholar] [CrossRef]
- Fan, Z.; Teng, K.X.; Xu, Y.Y.; Niu, L.Y.; Yang, Q.Z. The Photodynamic Agent Designed by Involvement of Hydrogen Atom Transfer for Enhancing Photodynamic Therapy. Angew. Chem.-Int. Ed. 2025, 64, 7. [Google Scholar] [CrossRef]
- Teng, K.X.; Niu, L.Y.; Xie, N.; Yang, Q.Z. Supramolecular photodynamic agents for simultaneous oxidation of NADH and generation of superoxide radical. Nat. Commun. 2022, 13, 9. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Z.; Lin, L.; Chen, J.; Hao, K.; Tian, H.Y.; Chen, X.S. Covalent Organic Nanosheets Integrated Heterojunction with Two Strategies To Overcome Hypoxic-Tumor Photodynamic Therapy. Chem. Mater. 2019, 31, 3313–3323. [Google Scholar] [CrossRef]
- Zhao, J.H.; Wu, Y.M.; Liu, C.B.; Zhang, B.; Gao, Y. Enzyme-inspired molecular electrocatalysts for the oxygen reduction reaction. Electrochim. Acta 2024, 479, 11. [Google Scholar] [CrossRef]
- Bie, C.B.; Wang, L.X.; Yu, J.G. Challenges for photocatalytic overall water splitting. Chem 2022, 8, 1567–1574. [Google Scholar] [CrossRef]
- Tang, Y.F.; Li, Y.Y.; Li, B.W.; Song, W.T.; Qi, G.B.; Tian, J.W.; Huang, W.; Fan, Q.L.; Liu, B. Oxygen-independent organic photosensitizer with ultralow-power NIR photoexcitation for tumor-specific photodynamic therapy. Nat. Commun. 2024, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Fang, G.P.; Cao, D.R. Recent Advances of AIE-Active Conjugated Polymers: Synthesis and Application. J. Macromol. Sci. Part A-Pure Appl. Chem. 2014, 51, 668–681. [Google Scholar] [CrossRef]
- Wang, L.R.; Hu, R.; Qin, A.J.; Tang, B.Z. Conjugated Polymers with Aggregation-Induced Emission Characteristics for Fluorescence Imaging and Photodynamic Therapy. ChemMedChem 2021, 16, 2330–2338. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.M.; Wu, W.B.; Feng, G.X.; Wang, Z.M.; Liu, B.; Tang, B.Z. Size Optimization of Organic Nanoparticles with Aggregation-Induced Emission Characteristics for Improved ROS Generation and Photodynamic Cancer Cell Ablation. Small 2022, 18, 11. [Google Scholar] [CrossRef]
- Ding, D.; Tang, B. Advances in Improving Healthcare with Aggregation-Induced Emission. Adv. Healthc. Mater. 2021, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.C.; Yuan, H.X.; Hu, R. Design and structural regulation of AIE photosensitizers for imaging-guided photodynamic anti-tumor application. Biomater. Sci. 2022, 10, 4443–4457. [Google Scholar] [CrossRef]
- Xue, B.L.; Hou, A.D.; Du, Y.H.; Qi, Y.H.; Jiang, H.; Zhou, H.F.; Zhou, Z.; Chen, H. AIE donor-dependent photosensitizer for enhance photodynamic antibacterial interface. Surf. Interfaces 2023, 39, 8. [Google Scholar] [CrossRef]
- Cong, Z.S.; Xie, S.J.; Jiang, Z.R.; Zheng, S.; Wang, W.H.; Wang, W.Y.; Song, H. In vivo photodynamic therapy based on Near-Infrared AIE cationic polymers. Chem. Eng. J. 2022, 431, 8. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, J.; Zhang, X.; Ma, J.S.; Wang, S.S.; Li, Y.; Xie, X.L.; Jiao, X.Y.; Wang, X.; Tang, B. Cyano-substituted stilbene (CSS)-based conjugated polymers: Photophysical properties exploration and applications in photodynamic therapy. Biomaterials 2022, 291, 10. [Google Scholar] [CrossRef]
- Sheng, C.X.; Singh, S.; Gambetta, A.; Drori, T.; Tong, M.; Tretiak, S.; Vardeny, Z.V. Ultrafast intersystem-crossing in platinum containing π-conjugated polymers with tunable spin-orbit coupling. Sci. Rep. 2013, 3, 7. [Google Scholar] [CrossRef]
- Guo, J.J.; Yang, C.L.; Zhao, Y.L. Long-Lived Organic Room-Temperature Phosphorescence fromAmorphous Polymer Systems. Acc. Chem. Res. 2022, 55, 1160–1170. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.T.; Han, C.C.; Zhang, Q.H.; Zhang, Q.G.; Li, Z.T.; Gosztola, D.J.; Wiederrecht, G.P.; Wu, M.B. Functionalizing carbon nitride with heavy atom-free spin converters for enhanced 1O2 generation. J. Catal. 2018, 361, 222–229. [Google Scholar] [CrossRef]
- Wen, K.K.; Tan, H.; Peng, Q.; Chen, H.; Ma, H.; Wang, L.; Peng, A.D.; Shi, Q.Q.; Cai, X.D.; Huang, H. Achieving Efficient NIR-II Type-I Photosensitizers for Photodynamic/Photothermal Therapy upon Regulating Chalcogen Elements. Adv. Mater. 2022, 34, 12. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Pan, Y.Z.; Zhang, Z.J.; Zhu, J.; Sun, Y.; Zhang, Q.; Zhu, D.X.; Li, G.Z.; Bryce, M.R.; Wang, D.; et al. Leveraging Tumor Microenvironment to Boost Synergistic Photodynamic Therapy, Ferroptosis Anti-Tumor Efficiency Based on a Functional Iridium(III) Complex. Adv. Sci. 2025, 9, 2413879. [Google Scholar] [CrossRef]
- Qian, M.; Wang, K.; Yang, P.; Liu, Y.; Li, M.; Zhang, C.; Qi, H. Nonemissive Iridium(III) Solvent Complex as a Self-Reporting Photosensitizer for Monitoring Phototherapeutic Efficacy in a “Signal on” Mode. Chem. Biomed. Imaging 2024, 2, 808–816. [Google Scholar] [CrossRef]
- Yan, Y.F.; Li, X.L.; Zeng, L.Z.; Liu, Q.S.; Cai, Z.Y.; Ren, Y.R.; Ren, X.X.; Gao, F. Antitumor Cream: Transdermal Hydrogel Containing Liposome-Encapsulated Ruthenium Complex for Infrared-Controlled Multimodal Synergistic Therapy. Adv. Healthc. Mater. 2024, 13, 2403563. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Kang, D.W.; Lim, J.H.; An, J.M.; Kim, Y.; Kim, J.H.; Ji, M.S.; Park, S.; Kim, D.; Lee, J.Y.; et al. Wavelength engineerable porous organic polymer photosensitizers with protonation triggered ROS generation. Nat. Commun. 2023, 14, 13. [Google Scholar] [CrossRef]
- Balamurugan, A.; Reddy, M.L.P.; Jayakannan, M. Amphiphilic π-Conjugated Poly(m-phenylene) Photosensitizer for the Eu3+ Ion: The Role of Macromolecular Chain Aggregation on the Color Tunability of Lanthanides. J. Phys. Chem. B 2011, 115, 10789–10800. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, Z.X.; Guo, J.T.; Lyu, J.X.; Wang, B.Z.; Wang, G.; Wang, C.F.; Zhou, L.Q.; Yuan, Z.; Xing, G.C.; et al. Metallopolymer strategy to explore hypoxic active narrow-bandgap photosensitizers for effective cancer photodynamic therapy. Nat. Commun. 2024, 15, 14. [Google Scholar] [CrossRef]
- Liu, W.K.; He, S.; Ma, X.; Lv, C.Y.; Gu, H.; Cao, J.F.; Du, J.J.; Sun, W.; Fan, J.L.; Peng, X.J. Near-Infrared Heptamethine Cyanine Photosensitizers with Efficient Singlet Oxygen Generation for Anticancer Photodynamic Therapy. Angew. Chem.-Int. Ed. 2024, 63, 12. [Google Scholar] [CrossRef]
- Ma, X.S.; Tao, F.R.; Zhang, Y.; Li, T.D.; Raymo, F.M.; Cui, Y.Z. Detection of nitroaromatic explosives by a 3D hyperbranched σ-π conjugated polymer based on a POSS scaffold. J. Mater. Chem. A 2017, 5, 14343–14354. [Google Scholar] [CrossRef]
- Ma, X.S.; Cui, Y.Z.; Ding, Y.Q.; Tao, F.R.; Zheng, B.; Yu, R.H.; Huang, W. 2D hyperbranched conjugated polymer for detecting TNT with excellent exciton migration. Sens. Actuators B-Chem. 2017, 238, 48–57. [Google Scholar] [CrossRef]
- Liu, X.L.; Xiao, M.H.; Xue, K.; Li, M.Z.; Liu, D.M.; Wang, Y.; Yang, X.Z.; Hu, Y.B.; Kwok, R.T.K.; Qin, A.J.; et al. Heteroaromatic Hyperbranched Polyelectrolytes: Multicomponent Polyannulation and Photodynamic Biopatterning. Angew. Chem.-Int. Ed. 2021, 60, 19222–19231. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.X.; Zhou, Y.P.; Xu, S.D.; Xie, Y.J.; Mao, D.; Wu, W.B.; Li, Z. From main-chain conjugated polymer photosensitizer to hyperbranched polymer photosensitizer: Expansion of the polymerization-enhanced photosensitization effect for photodynamic therapy. J. Mat. Chem. B 2022, 10, 5008–5015. [Google Scholar] [CrossRef]
- Meng, Z.H.; Hou, W.Y.; Zhou, H.; Zhou, L.B.; Chen, H.B.; Wu, C.F. Therapeutic Considerations and Conjugated Polymer-Based Photosensitizers for Photodynamic Therapy. Macromol. Rapid Commun. 2018, 39, 15. [Google Scholar] [CrossRef]
- Zhang, G.X.; Zhang, D.Q. New Photosensitizer Design Concept: Polymerization-Enhanced Photosensitization. Chem. 2018, 4, 2013–2015. [Google Scholar] [CrossRef]
- Gao, J.; Chen, Z.J.; Li, X.M.; Yang, M.Y.; Lv, J.J.; Li, H.Y.; Yuan, Z.L. Chemiluminescence in Combination with Organic Photosensitizers: Beyond the Light Penetration Depth Limit of Photodynamic Therapy. Int. J. Mol. Sci. 2022, 23, 13. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Xie, Z.J.; Li, W.T.; Wu, X.Q.; Jiang, X.F.; Li, G.H.; Cao, L.Q.; Zhang, D.W.; Wang, Q.W.; Xue, P.; et al. Photodynamic immunotherapy of cancers based on nanotechnology: Recent advances and future challenges. J. Nanobiotechnology 2021, 19, 18. [Google Scholar] [CrossRef]
- Wang, L.H.; Song, K.Y.; Jiang, C.; Liu, S.P.; Huang, S.R.; Yang, H.; Li, X.L.; Zhao, F. Metal-Coordinated Polydopamine Structures for Tumor Imaging and Therapy. Adv. Healthc. Mater. 2024, 13, 23. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Zhao, T.Y.; Cao, Y.N.; Sun, J.; Zhou, Q.; Chen, H.Y.; Guo, S.T.; Wang, Y.F.; Zhen, Y.H.; Liang, X.J.; et al. Temperature-Sensitive Lipid-Coated Carbon Nanotubes for Synergistic Photothermal Therapy and Gene Therapy. ACS Nano 2021, 15, 6517–6529. [Google Scholar] [CrossRef]
- Ding, X.L.; Liu, M.D.; Cheng, Q.; Guo, W.H.; Niu, M.T.; Huang, Q.X.; Zeng, X.; Zhang, X.Z. Multifunctional liquid metal-based nanoparticles with glycolysis and mitochondrial metabolism inhibition for tumor photothermal therapy. Biomaterials 2022, 281, 10. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.P.; Chen, Z.Z.; Dai, J.N.; Pan, Y.J.; Tu, Y.K.; Meng, Q.; Diao, Y.Z.; Yang, S.B.; Guo, W.; Li, L.M.; et al. Recent Advances in Strategies to Enhance Photodynamic and Photothermal Therapy Performance of Single-Component Organic Phototherapeutic Agents. Adv. Sci. 2025, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Lee, H.; Park, J.H.; Yang, J.K.; Lee, W.J.; Lim, J.; Kim, S.; Lee, S.; Lee, T.S. Silica-Based Platform Decorated with Conjugated Polymer Dots and Prussian Blue for Improved Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2023, 15, 43455–43467. [Google Scholar] [CrossRef]
- Xie, X.; Wang, K.; Zeng, J.; Xu, M.Y.; Qu, X.H.; Xiang, Z.B.; Tou, F.F.; Huang, S.R.; Han, X.J. A novel polymer enabled by polymerized small molecule strategy for tumor photothermal and photodynamic therapy. J. Nanobiotechnology 2023, 21, 16. [Google Scholar] [CrossRef]
- Yang, T.; Liu, L.; Deng, Y.B.; Guo, Z.Q.; Zhang, G.B.; Ge, Z.S.; Ke, H.T.; Chen, H.B. Ultrastable Near-Infrared Conjugated-Polymer Nanoparticles for Dually Photoactive Tumor Inhibition. Adv. Mater. 2017, 29, 9. [Google Scholar] [CrossRef]
- Skalickova, M.; Vanova, K.H.; Uher, O.; Fialova, J.L.; Petrlakova, K.; Masarik, M.; Kejík, Z.; Martasek, P.; Pacak, K.; Jakubek, M. Injecting hope: The potential of intratumoral immunotherapy for locally advanced and metastatic cancer. Front. Immunol. 2025, 15, 20. [Google Scholar] [CrossRef]
- Martínez-Jiménez, F.; Priestley, P.; Shale, C.; Baber, J.; Rozemuller, E.; Cuppen, E. Genetic immune escape landscape in primary and metastatic cancer. Nat. Genet. 2023, 30, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.R.; Wang, Z.Y.; Li, Z.Y.; Zhang, X.G.; Zhang, H.T.; Zhang, T.; Meng, X.X.; Sheng, F.G.; Hou, Y.L. Amelioration of systemic antitumor immune responses in cocktail therapy by immunomodulatory nanozymes. Sci. Adv. 2022, 8, 17. [Google Scholar] [CrossRef]
- Warszy, M.; Repetowski, P.; Dabrowski, J.M. Photodynamic therapy combined with immunotherapy: Recent advances and future research directions. Coord. Chem. Rev. 2023, 495, 42. [Google Scholar] [CrossRef]
- Shen, L.J.; Zhou, T.J.; Fan, Y.T.; Chang, X.; Wang, Y.; Sun, J.G.; Xing, L.; Jiang, H.L. Recent progress in tumor photodynamic immunotherapy. Chin. Chem. Lett. 2020, 31, 1709–1716. [Google Scholar] [CrossRef]
- Ji, B.; Wei, M.J.; Yang, B. Recent advances in nanomedicines for photodynamic therapy (PDT)-driven cancer immunotherapy. Theranostics 2022, 12, 434–458. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, J.S.; Xu, M.K.; Yu, J.; Wei, X.; He, S.S.; Pu, K.Y. Eosinophil-Activating Semiconducting Polymer Nanoparticles for Cancer Photo-Immunotherapy. Angew. Chem.-Int. Ed. 2024, 63, 10. [Google Scholar] [CrossRef]
- Huang, H.Y.; Xie, W.S.; Hu, D.N.; He, X.Z.; Li, R.X.; Zhang, X.Y.; Wei, Y. Type I photodynamic therapy with a self-degradable conjugated polyelectrolyte in combination with CpG adjuvant for cancer immunotherapy. Chem. Eng. J. 2023, 451, 8. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 24. [Google Scholar] [CrossRef] [PubMed]
- Brianna; Lee, S.H. Chemotherapy: How to reduce its adverse effects while maintaining the potency? Med. Oncol. 2023, 40, 13. [Google Scholar] [CrossRef]
- Gutierrez, N.M.C.; Pujol-Solé, N.; Arifi, Q.; Coll, J.L.; le Clainche, T.; Broekgaarden, M. Increasing cancer permeability by photodynamic priming: From microenvironment to mechanotransduction signaling. Cancer Metastasis Rev. 2022, 41, 899–934. [Google Scholar] [CrossRef]
- El-Hussein, A.; Manoto, S.L.; Ombinda-Lemboumba, S.; Alrowaili, Z.A.; Mthunzi-Kufa, P. A Review of Chemotherapy and Photodynamic Therapy for Lung Cancer Treatment. Anti-Cancer Agents Med. Chem. 2021, 21, 149–161. [Google Scholar] [CrossRef]
- She, T.S.; Shi, Q.X.; Li, Z.; Feng, Y.R.; Yang, H.; Tao, Z.; Li, H.; Chen, J.; Wang, S.S.; Liang, Y.; et al. Combination of long-acting TRAIL and tumor cell-targeted photodynamic therapy as a novel strategy to overcome chemotherapeutic multidrug resistance and TRAIL resistance of colorectal cancer. Theranostics 2021, 11, 4281–4297. [Google Scholar] [CrossRef]
- Tang, D.S.; Yu, Y.J.; Zhang, J.B.; Dong, X.Y.; Liu, C.Y.; Xiao, H.H. Self-Sacrificially Degradable Pseudo-Semiconducting Polymer Nanoparticles that Integrate NIR-II Fluorescence Bioimaging, Photodynamic Immunotherapy, and Photo-Activated Chemotherapy. Adv. Mater. 2022, 34, 13. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, Q.; Zhang, Z.Q.; Tang, Y.L. A pH-Responsive Drug Delivery System Based on Conjugated Polymer for Effective Synergistic Chemo-/Photodynamic Therapy. Molecules 2023, 28, 13. [Google Scholar] [CrossRef]
- Li, B.H.; Lin, L. Internal light source for deep photodynamic therapy. Light-Sci. Appl. 2022, 11, 3. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, J.; Huang, J.; Yu, B.; Pu, K.; Xu, F.J. Chemiluminescence: From mechanism to applications in biological imaging and therapy. Aggregate 2021, 2, e140. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.Y.; Ren, Y.X.; Yuan, Q.; Wang, Y.Z.; Bao, B.K.; Li, M.Q.; Tang, Y.L. Chemiluminescent Conjugated Polymer Nanoparticles for Deep-Tissue Inflammation Imaging and Photodynamic Therapy of Cancer. J. Am. Chem. Soc. 2024, 146, 5927–5939. [Google Scholar] [CrossRef]
- Su, X.; Liu, R.H.; Li, Y.; Han, T.; Zhang, Z.J.; Niu, N.I.; Kang, M.M.; Fu, S.; Wang, D.L.; Wang, D.; et al. Aggregation-Induced Emission-Active Poly(phenyleneethynylene)s for Fluorescence and Raman Dual-Modal Imaging and Drug-Resistant Bacteria Killing. Adv. Healthc. Mater. 2021, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.X.; Shen, J.L.; Xu, Z.W.; Wang, W.Q.; Wu, Y.; Zhou, W.; Xie, C.; Fan, Q.L. Rational design of biodegradable semiconducting polymer nanoparticles for NIR-II fluorescence imaging-guided photodynamic therapy. Nano Res. 2024, 17, 5399–5408. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, Y.; Zhang, Y.T.; Xin, X.Y.; Li, R.T.; Xie, C.; Fan, Q.L. Iodine-Rich Semiconducting Polymer Nanoparticles for CT/Fluorescence Dual-Modal Imaging-Guided Enhanced Photodynamic Therapy. Small 2020, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, J.G.; Zhang, Z.J.; Jiang, S.S.; Kang, M.M.; Wang, D.L.; Li, X.; Huang, P.; Gao, X.K.; Wang, D.; Tang, B.Z. NIR-II Excitable Semiconducting Polymers with AIE Characteristics for Fluorescence-Photoacoustic Imaging-Guided Synergistic Phototherapy. Adv. Funct. Mater. 2024, 34, 12. [Google Scholar] [CrossRef]
- Sun, D.Q.; Sun, X.X.; Zhang, X.; Wu, J.P.; Shi, X.B.; Sun, J.; Luo, C.; He, Z.G.; Zhang, S.W. Emerging Chemodynamic Nanotherapeutics for Cancer Treatment. Adv. Healthc. Mater. 2024, 13, 26. [Google Scholar] [CrossRef]
- Di, X.J.; Pei, Z.C.; Pei, Y.X.; James, T.D. Tumor microenvironment-oriented MOFs for chemodynamic therapy. Coord. Chem. Rev. 2023, 484, 21. [Google Scholar] [CrossRef]
- Shen, J.; Yu, H.Z.; Shu, Y.M.; Ma, M.; Chen, H.R. A Robust ROS Generation Strategy for Enhanced Chemodynamic/Photodynamic Therapy via H2O2/O2 Self-Supply and Ca2+ Overloading. Adv. Funct. Mater. 2021, 31, 12. [Google Scholar] [CrossRef]
- Li, W.Y.; Li, R.T.; Ye, Q.; Zou, Y.M.; Lu, X.; Zhang, W.H.; Chen, J.X.; Zhao, Y.H. Mn3O4 Nanoshell Coated Metal-Organic Frameworks with Microenvironment-Driven O2 Production and GSH Exhaustion Ability for Enhanced Chemodynamic and Photodynamic Cancer Therapies. Adv. Healthc. Mater. 2023, 12, e2202280. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wu, Y.N.; Wu, M.; Zhao, Q.F.; Li, H.S.; Liu, J.F.; Liu, X.L.; Zhang, X.L.; Zeng, Y.Y. Engineered Red Blood Cell Biomimetic Nanovesicle with Oxygen Self-Supply for Near-Infrared-II Fluorescence-Guided Synergetic Chemo-Photodynamic Therapy against Hypoxic Tumors. ACS Appl. Mater. Interfaces 2021, 13, 52435–52449. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Li, L.L.; Zhang, M.; Yu, C.W.; Pan, Y.H.; Cheng, F.F.; Hu, W.B.; Lu, X.M.; Wang, Q.; Fan, Q.L. Confined semiconducting polymers with boosted NIR light-triggered H2O2 production for hypoxia-tolerant persistent photodynamic therapy. Chem. Sci. 2024, 15, 12086–12097. [Google Scholar] [CrossRef]


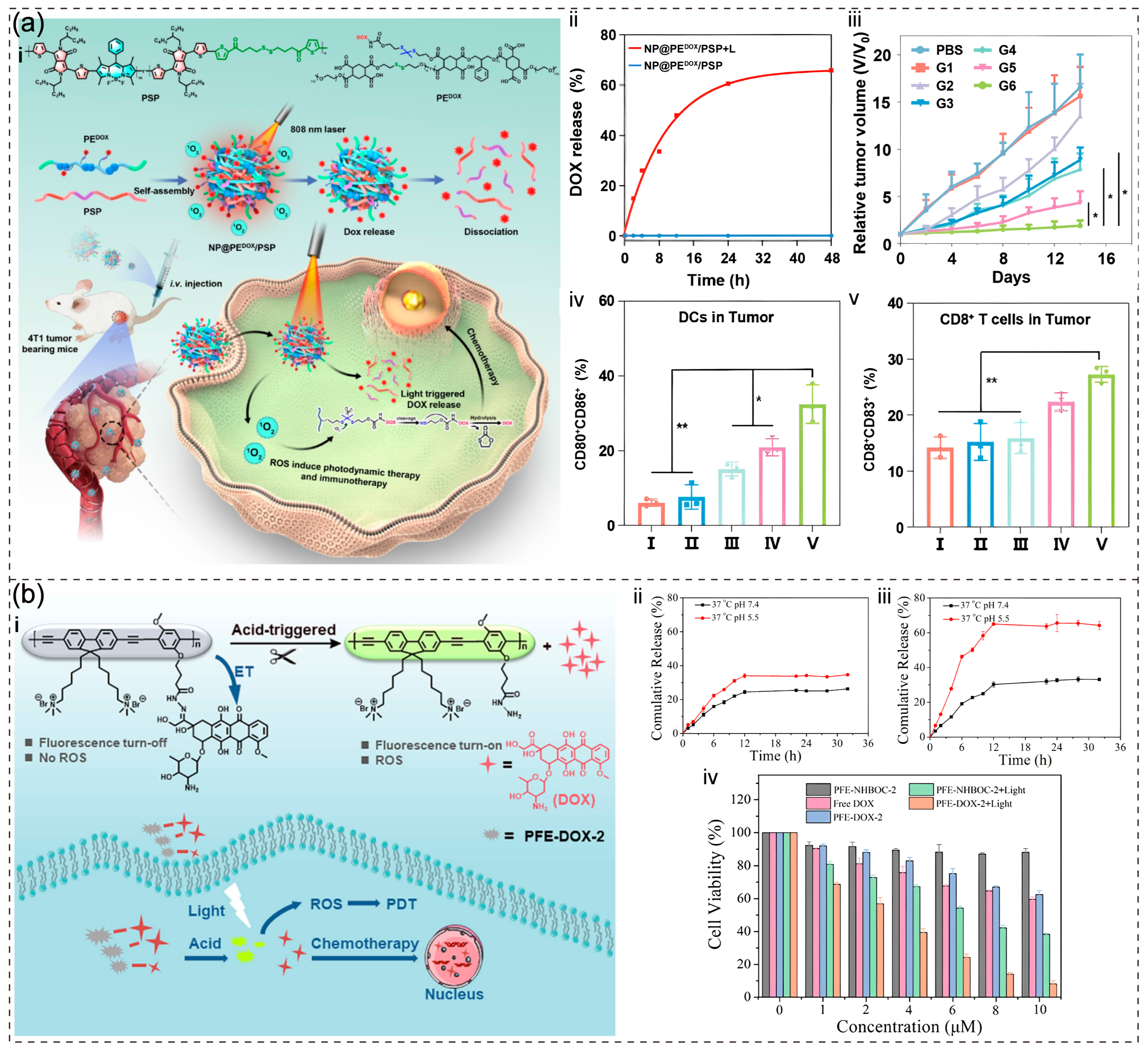
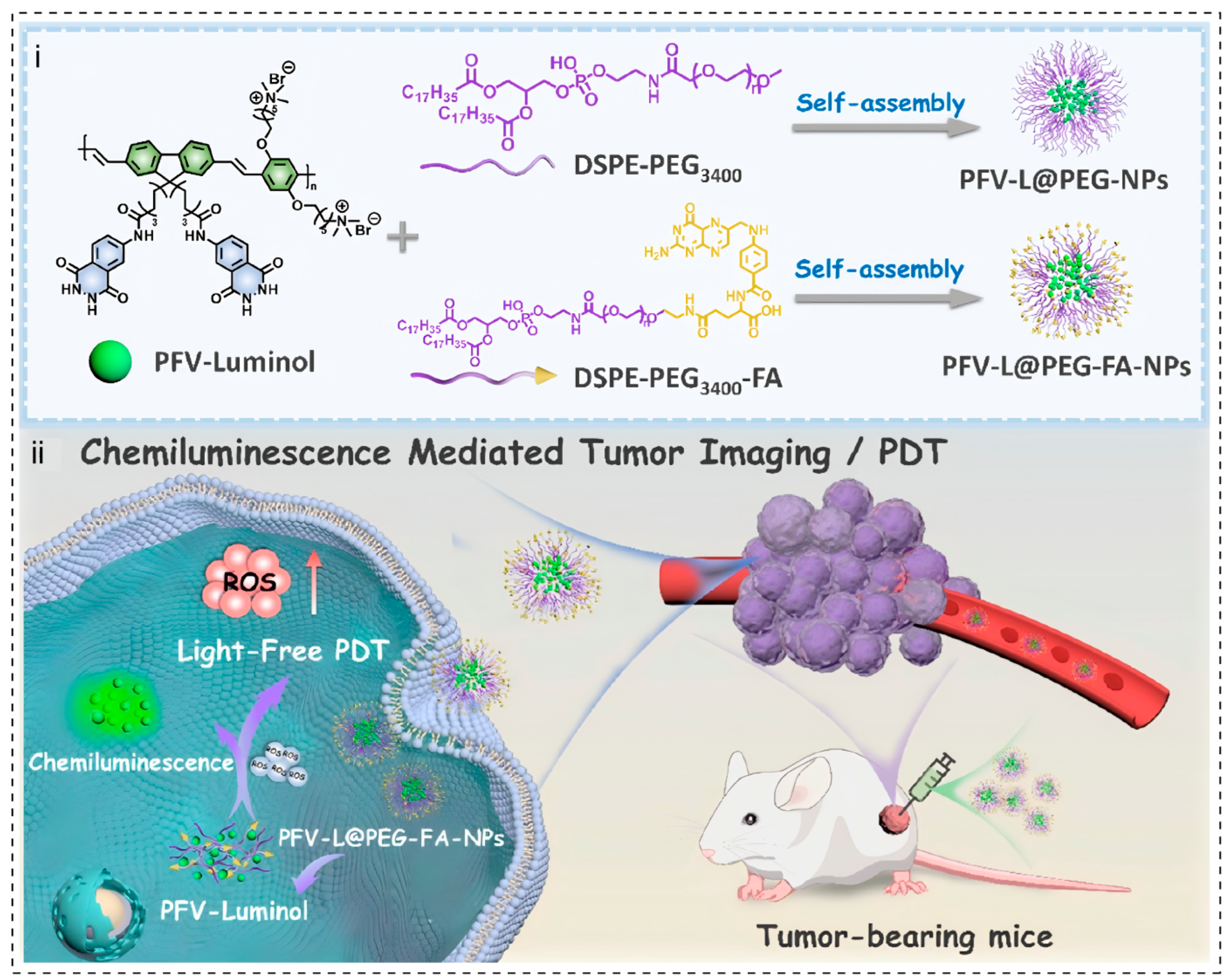
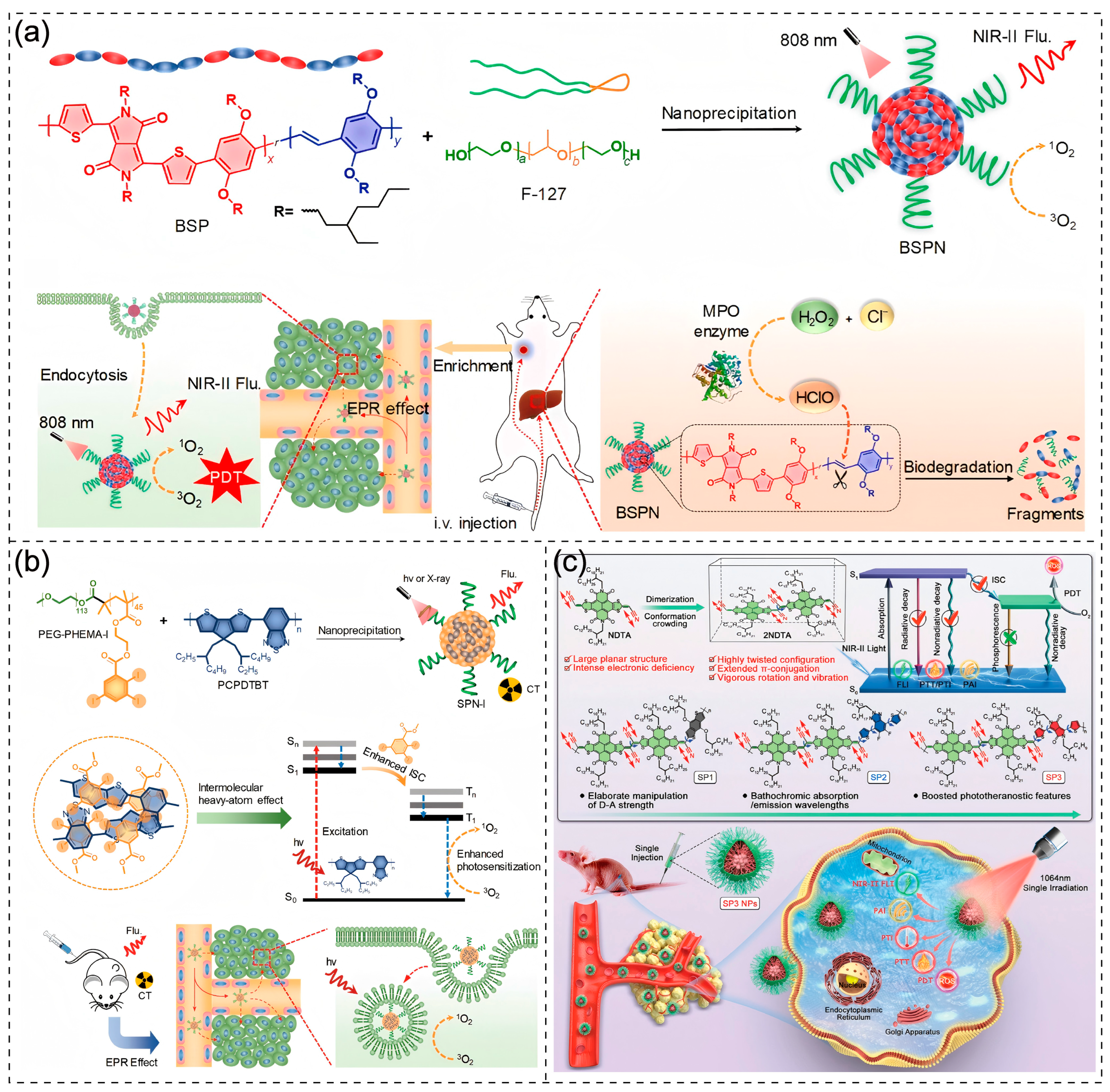

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Z.; Ye, Q.; Lao, J.; Liu, X.; Wu, P. Conjugated Polymer-Photosensitizers for Cancer Photodynamic Therapy and Their Multimodal Treatment Strategies. Polymers 2025, 17, 1258. https://doi.org/10.3390/polym17091258
Cheng Z, Ye Q, Lao J, Liu X, Wu P. Conjugated Polymer-Photosensitizers for Cancer Photodynamic Therapy and Their Multimodal Treatment Strategies. Polymers. 2025; 17(9):1258. https://doi.org/10.3390/polym17091258
Chicago/Turabian StyleCheng, Zhengqing, Qiuting Ye, Jieling Lao, Xiyu Liu, and Pan Wu. 2025. "Conjugated Polymer-Photosensitizers for Cancer Photodynamic Therapy and Their Multimodal Treatment Strategies" Polymers 17, no. 9: 1258. https://doi.org/10.3390/polym17091258
APA StyleCheng, Z., Ye, Q., Lao, J., Liu, X., & Wu, P. (2025). Conjugated Polymer-Photosensitizers for Cancer Photodynamic Therapy and Their Multimodal Treatment Strategies. Polymers, 17(9), 1258. https://doi.org/10.3390/polym17091258




