Obtaining Polyacrylonitrile Carbon Nanofibers by Electrospinning for Their Application as Flame-Retardant Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents Used
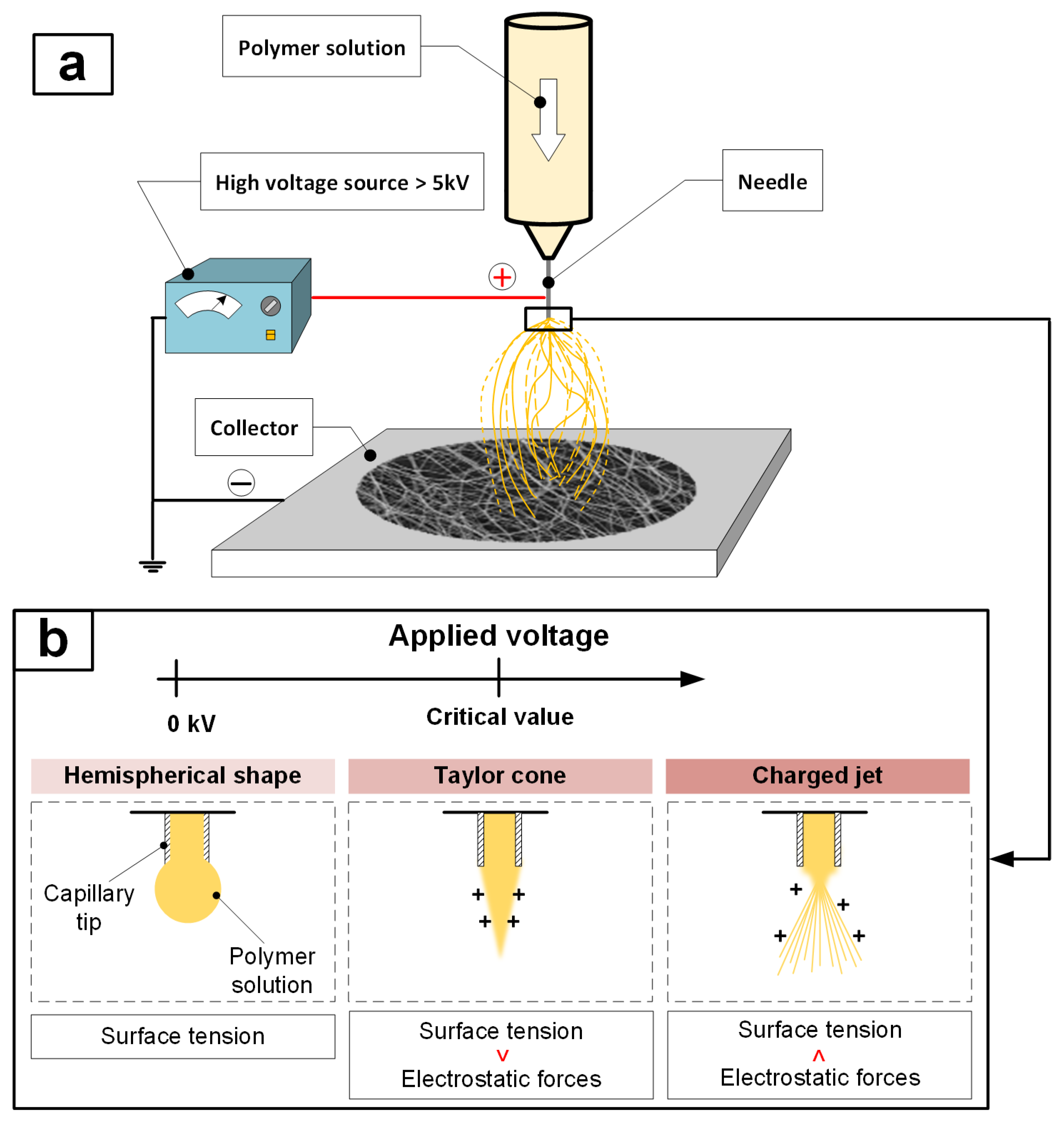
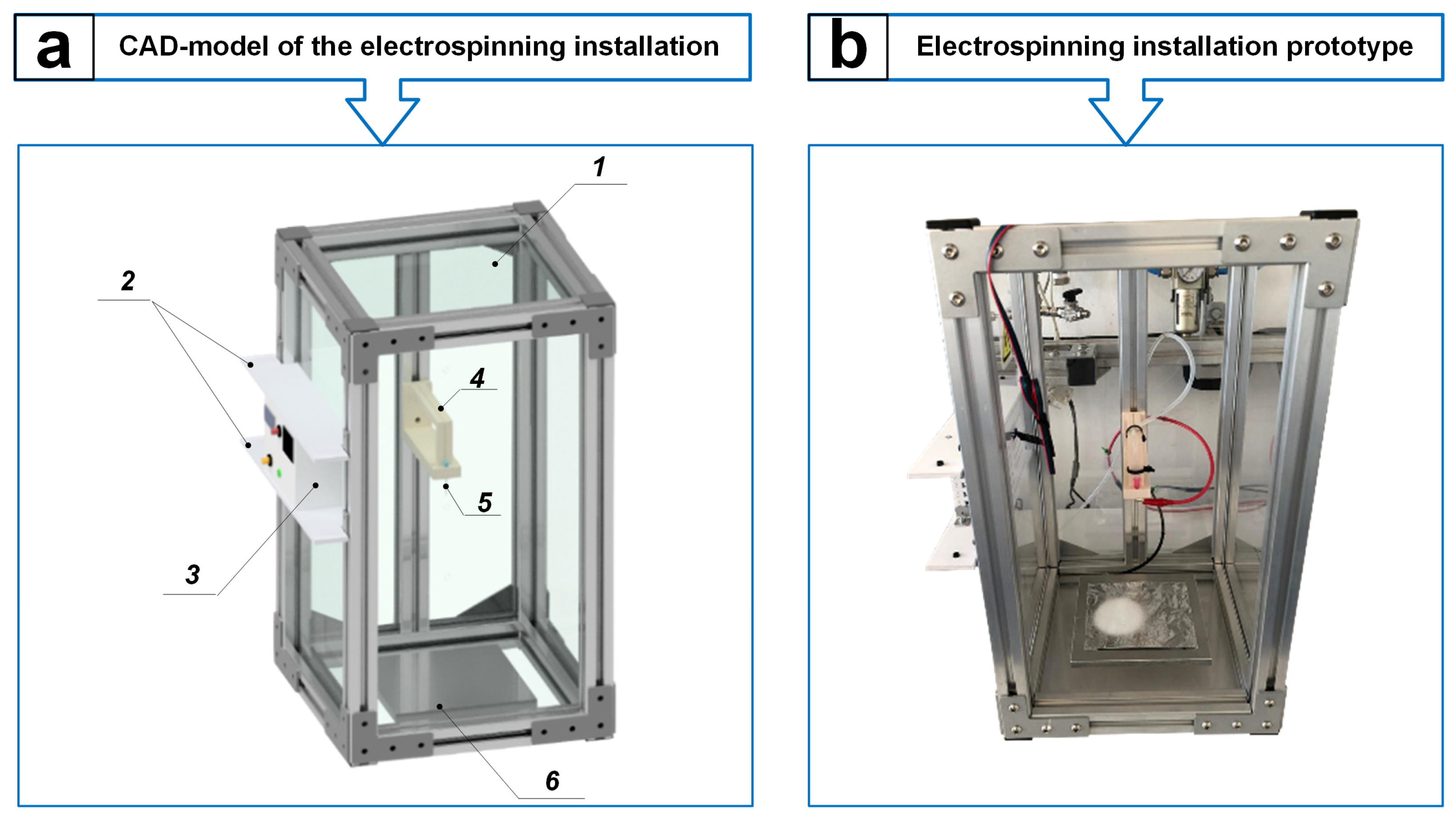
2.2. Development of an Electrospinning Installation
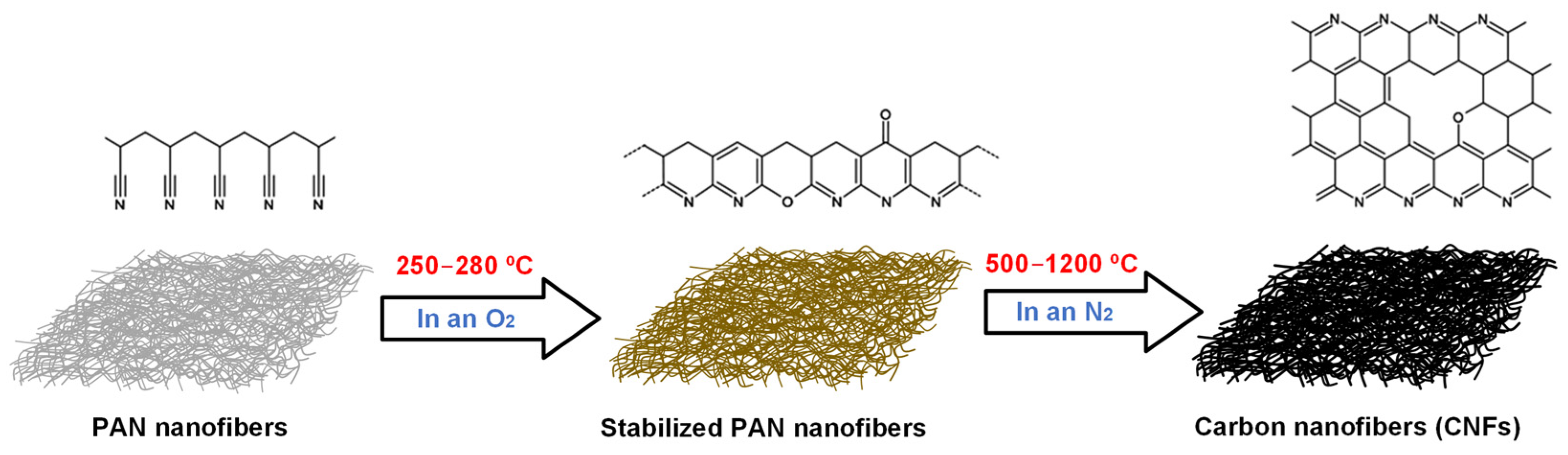
2.3. Methodology for Obtaining CNFs Based on PAN
2.4. Study of the Effect of Temperature Treatment of PAN Nanofibers on Their Resistance to DMSO
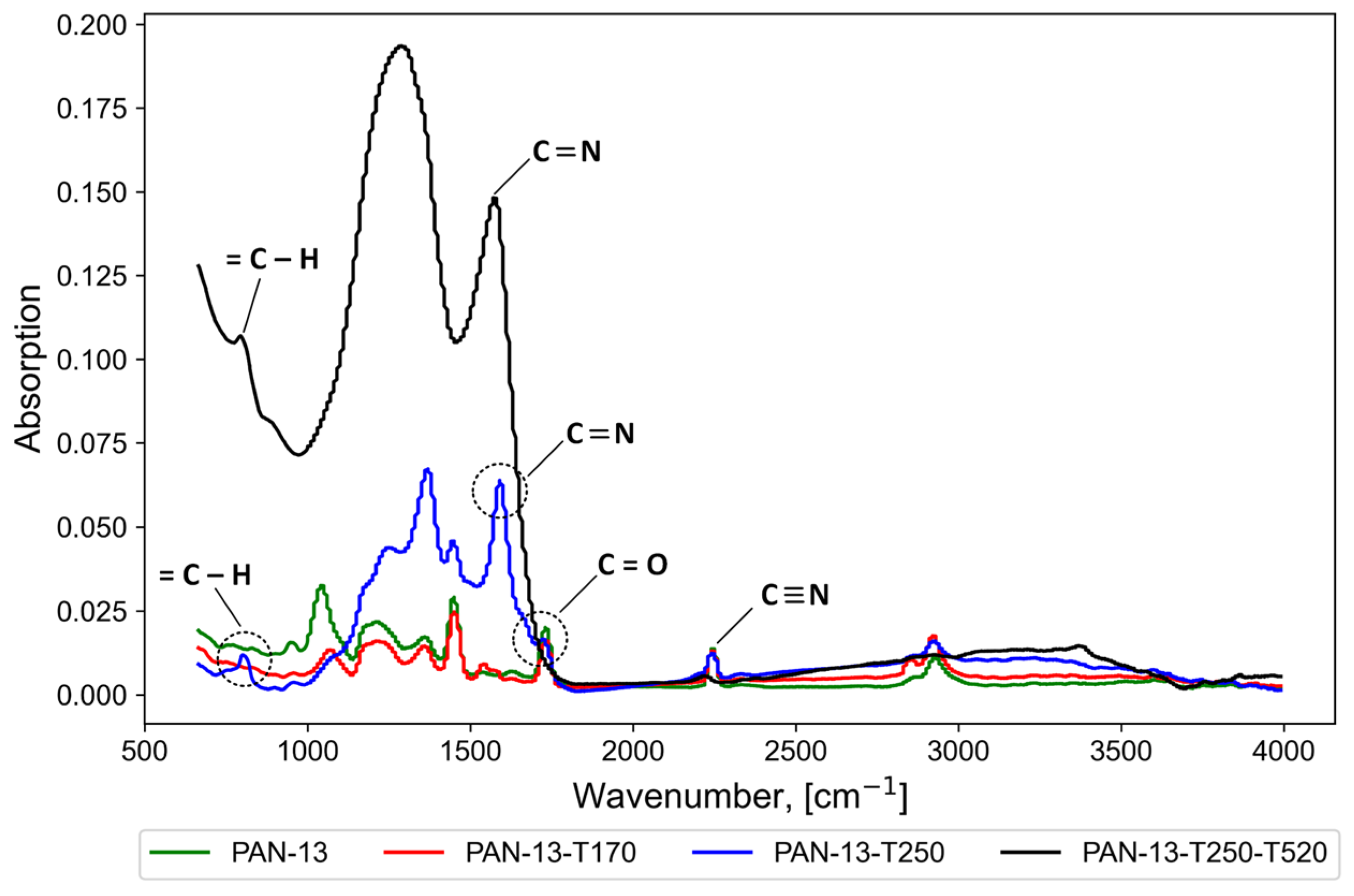
2.5. Infrared Spectroscopy (IR Spectroscopy)
2.6. Scanning Electron Microscopy (SEM)
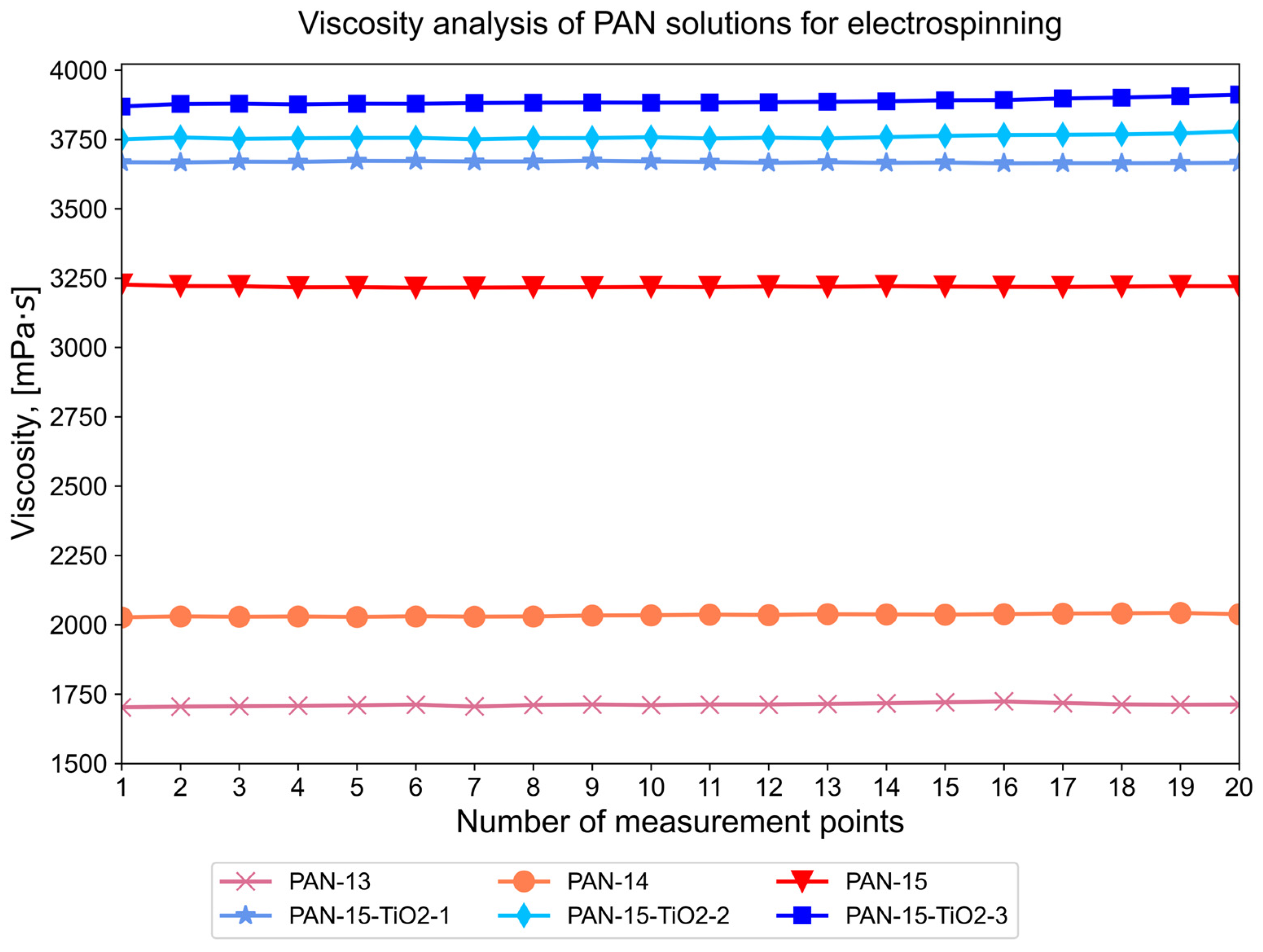
2.7. Study of Viscosity of Initial PAN Solutions
2.8. Elemental Analysis of the Obtained Samples (EDS Analysis)
2.9. Differential Scanning Calorimetry and Thermogravimetric Analysis
2.10. Visualization of Flammability of PAN Nanofibers
3. Results and Discussion
3.1. Effect of Temperature Treatment of Nanofibers on the Stabilization of PAN
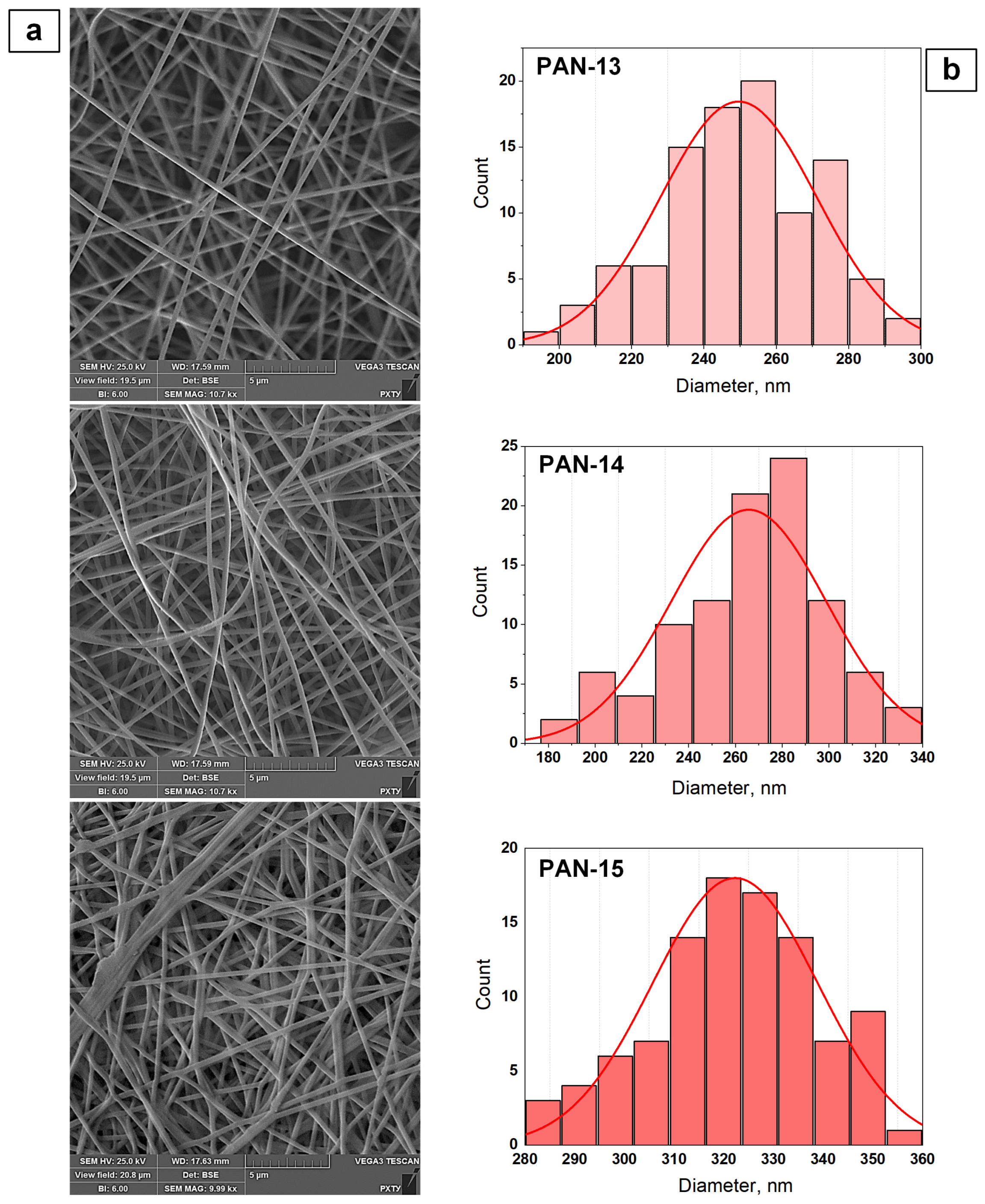
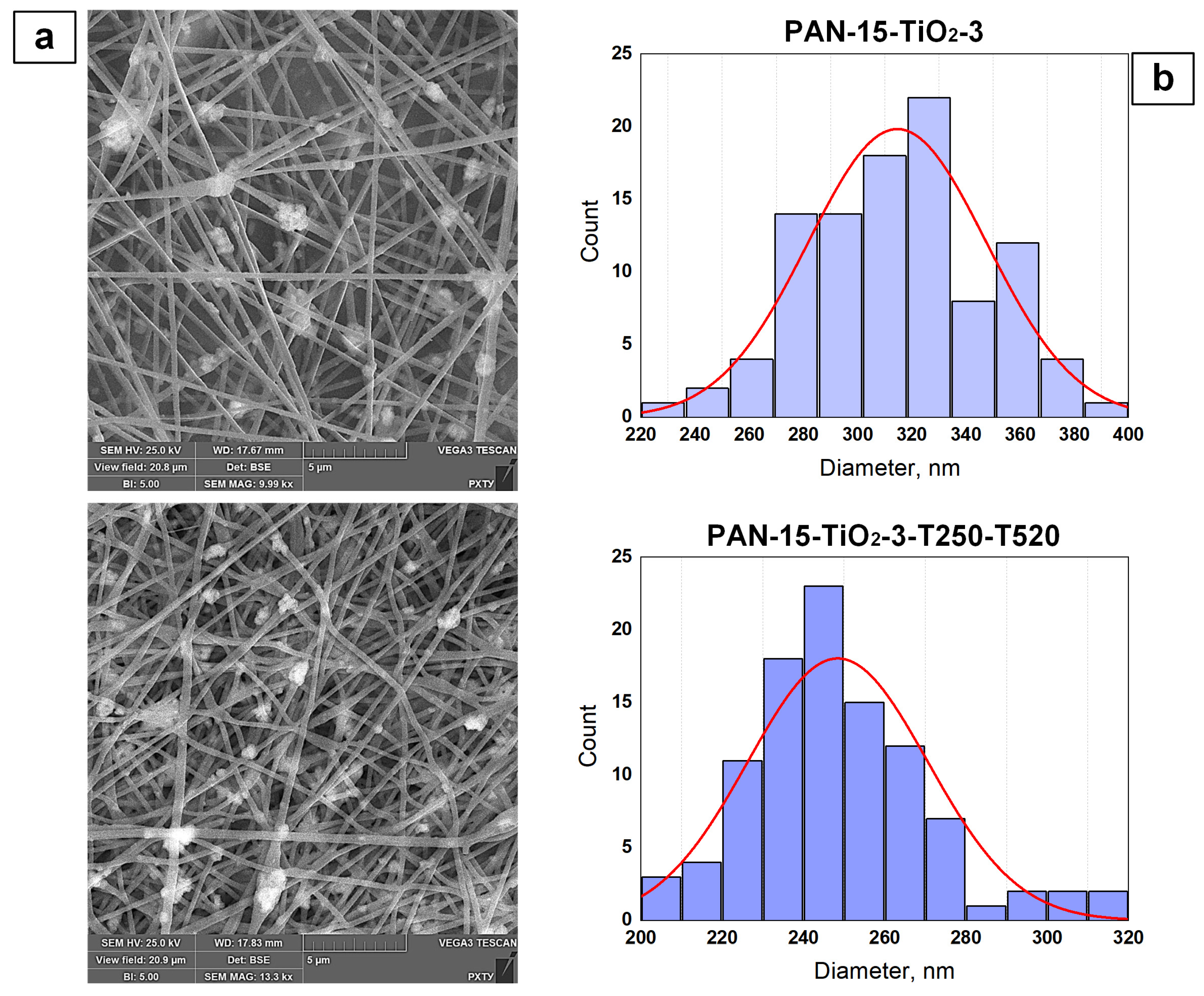
3.2. Results of the Study of the Structure of PAN Nanofibers
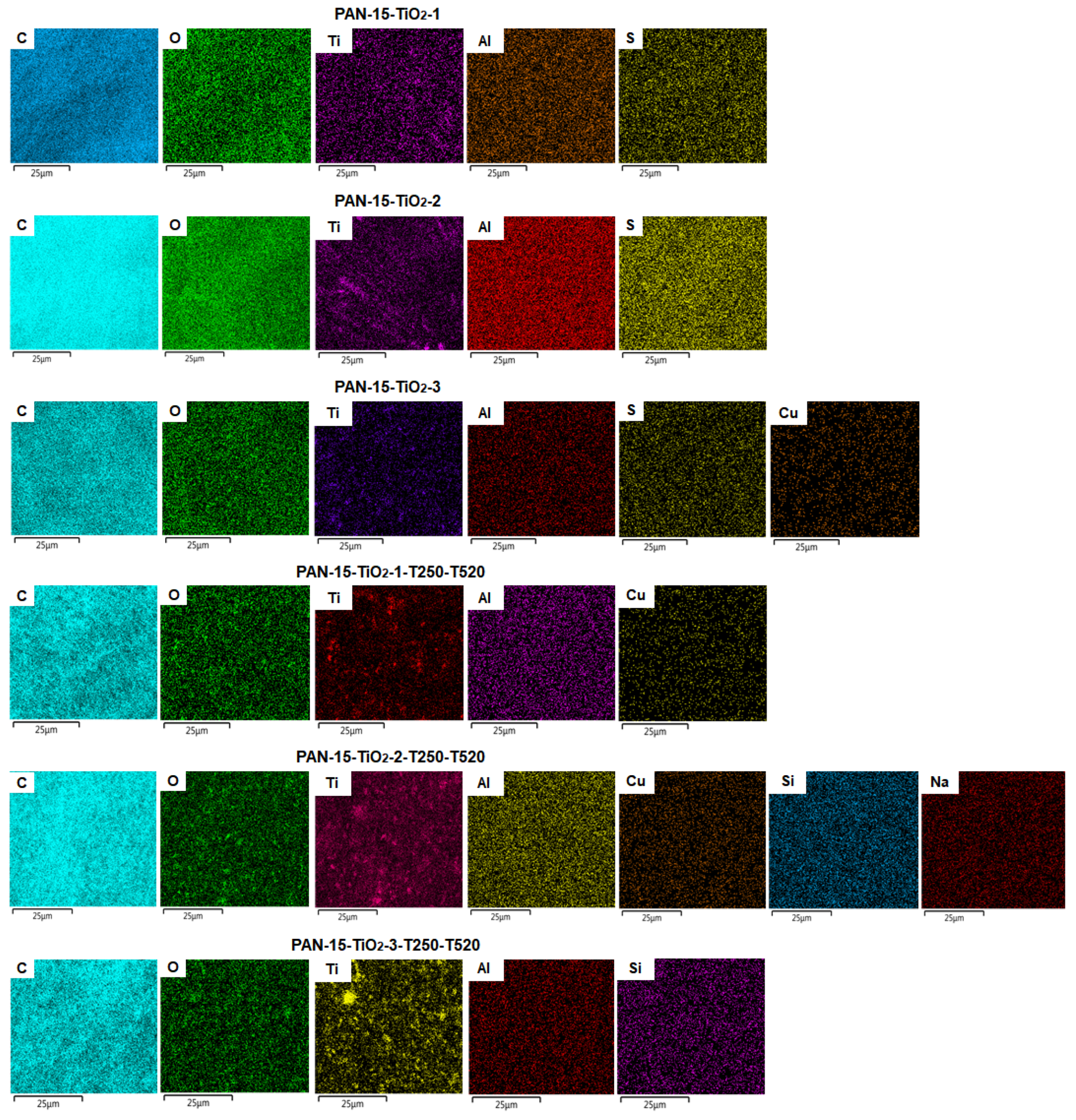
3.3. Results of Elemental Analysis of PAN Nanofiber Samples
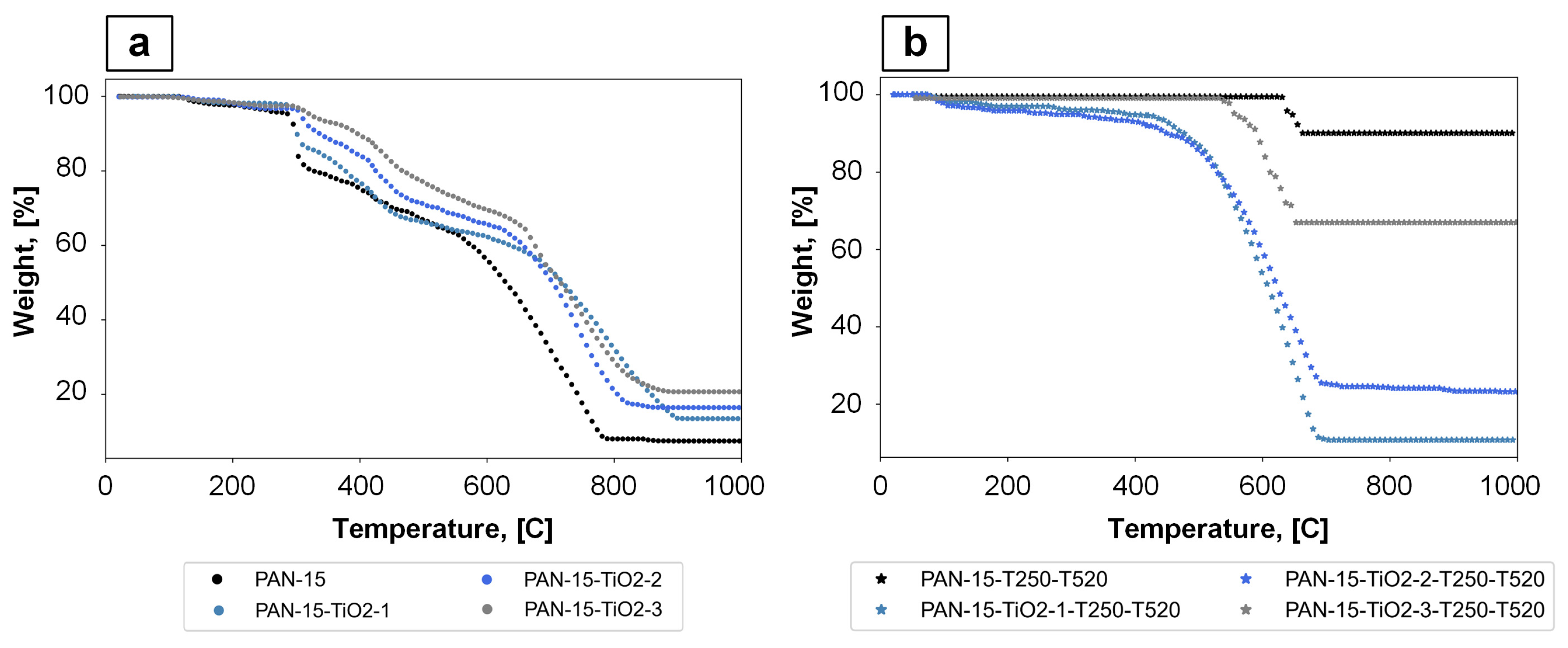
3.4. Characteristics of DSC and TGA for PAN Nanofibers
3.5. Results of Visual Comparison of Flammability of PAN Nanofibers
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MUCTR | Mendeleev University of Chemical Technology of Russia |
| PAN | Polyacrylonitrile |
| CNFs | Carbon nanofibers |
| LOI | Limiting oxygen index |
| PI | Polyimide |
| PAA | Polyamic acid |
| DMSO | Dimethyl sulfoxide |
| DMF | Dimethylformamide |
| DMA | Dimethylacetamide |
| CAD | Computer-aided design |
| V | Voltage |
| PVC | Polyvinyl chloride |
| IR | Infrared spectroscopy |
| SEM | Scanning Electron Microscopy |
| EDS | Energy-dispersive X-ray spectroscopy |
| DSC | Differential scanning calorimetry |
| TGA | Thermogravimetric analysis |
References
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. J. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Nadaf, A.; Gupta, A.; Hasan, N.; Ahmad, S.; Kesharwani, P.; Ahmad, F.J. Recent update on electrospinning and electrospun nanofibers: Current trends and their applications. RSC Adv. 2022, 12, 23808–23828. [Google Scholar] [CrossRef] [PubMed]
- Baji, A.; Mai, Y.W.; Wong, S.C.; Abtahi, M.; Chen, P. Electrospinning of Polymer Nanofibers: Effects on Oriented Morphology, Structures and Tensile Properties. Compos. Sci. Technol. 2010, 70, 703–718. [Google Scholar] [CrossRef]
- Bonakdar, A.M.; Rodrigue, D. Electrospinning: Processes, Structures, and Materials. Macromol 2024, 4, 58–103. [Google Scholar] [CrossRef]
- Mitchell, G.R.; Ahn, K.H.; Davis, F.J. The Potential of Electrospinning in Rapid Manufacturing Processes: The Fundamentals of Electrospinning, Key Process Parameters, Materials and Potential Application in Rapid Manufacturing Are Presented in This Paper. Virtual Phys. Prototyp. 2011, 6, 63–77. [Google Scholar] [CrossRef]
- Garg, K.; Bowlin, G.L. Electrospinning Jets and Nanofibrous Structures. Biomicrofluidics 2011, 5, 013403. [Google Scholar] [CrossRef]
- Ballengee, J.; Pintauro, P. Morphological Control of Electrospun Nafion Nanofiber Mats. ECS Trans. 2010, 33, 647–658. [Google Scholar] [CrossRef]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Beck Tan, N.C. The Effect of Processing Variables on the Morphology of Electrospun. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Subbiah, T.; Bhat, G.S.; Tock, R.W.; Parameswaran, S.; Ramkumar, S.S. Electrospinning of Nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar] [CrossRef]
- Peng, H.; Liu, Y.; Ramakrishna, S. Recent development of centrifugal electrospinning. J. Appl. Polym. Sci. 2017, 134, 44578. [Google Scholar] [CrossRef]
- Cherwin, A.; Namen, S.; Rapacz, J.; Kusik, G.; Anderson, A.; Wang, Y.; Kaltchev, M.; Schroeder, R.; O’Connell, K.; Stephens, S. Design of a Novel Oxygen Therapeutic Using Polymeric Hydrogel Microcapsules Mimicking Red Blood Cells. Pharmaceutics 2019, 11, 583. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Zhou, F.; Williams, G.R. Developing and scaling up fast-dissolving electrospun formulations based on poly(vinylpyrrolidone) and ketoprofen. J. Drug Deliv. Sci. Technol. 2021, 61, 102138. [Google Scholar] [CrossRef]
- Song, J.; Li, Z.; Wu, H. Blowspinning: A New Choice for Nanofibers. Appl. Mater. Interfaces 2020, 12, 33447–33464. [Google Scholar] [CrossRef] [PubMed]
- Barhoum, A.; Pal, K.; Rahier, H.; Uludag, H.; Kim, I.S.; Bechelany, M. Nanofibers as new-generation materials: From spinning and nano-spinning fabrication techniques to emerging applications. Appl. Mater. Today 2019, 17, 1–35. [Google Scholar] [CrossRef]
- Aminyan, R.; Bazgir, S. Fabrication and characterization of nanofibrous polyacrylic acid superabsorbent using gas-assisted electrospinning technique. React. Funct. Polym. 2019, 141, 133–144. [Google Scholar] [CrossRef]
- Zaarour, B.; Zhu, L.; Huang, C.; Jin, X. A mini review on the generation of crimped ultrathin fibers via electrospinning: Materials, strategies, and applications. Polym. Adv. Technol. 2020, 31, 1449–1462. [Google Scholar] [CrossRef]
- Keirouz, A.; Wang, Z.; Reddy, V.S.; Kristóf Nagy, Z.; Vass, P.; Buzgo, M.; Ramakrishna, S.; Radacsi, N. The History of Electrospinning: Past, Present, and Future Developments. Adv. Mater. Technol. 2023, 8, 2–34. [Google Scholar] [CrossRef]
- Frank, E.; Steudle, L.M.; Ingildeev, D.; Sporl, J.M.; Buchmeiser, M.R. Carbon fibers: Precursor systems, processing, structure, and properties. Angew. Chem. Int. Ed. 2014, 53, 5262–5298. [Google Scholar] [CrossRef]
- Kopeć, M.; Lamson, M.; Yuan, R.; Tang, C.; Kruk, M.; Zhong, M.; Matyjaszewski, K.; Kowalewski, T. Polyacrylonitrile-derived nanostructured carbon materials. Prog. Polym. Sci. 2019, 92, 89–134. [Google Scholar] [CrossRef]
- Nataraj, S.K.; Yang, K.S.; Aminabhavi, T.M. Polyacrylonitrile-based nanofibers—A state-of-the-art review. Prog. Polym. Sci. 2012, 37, 487–513. [Google Scholar] [CrossRef]
- Morgan, P. Carbon Fibers and Their Composites; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Chand, S. Review carbon fibers for composites. J. Mater. Sci. 2000, 35, 1303–1313. [Google Scholar] [CrossRef]
- McGree, T. Fire in Industrial or Manufacturing Properties. NFPA Research, 2023. Available online: https://www.nfpa.org/education-and-research/research/nfpa-research/fire-statistical-reports/fires-in-us-industrial-or-manufacturing-properties (accessed on 17 April 2025).
- Manufacturing and Industrial Fires Responsible for $1 Billion in Property Damage Annually. Voss Law Firm. Available online: https://www.vosslawfirm.com/blog/fire-statistics-for-industrial-and-manufacturing-properties.cfm (accessed on 17 April 2025).
- Workplace Fires: Common Causes and Statistics. MorganClark. 2024. Available online: https://www.morganclark.co.uk/about-us/blog/common-causes-of-workplace-fires-in-the-uk/ (accessed on 17 April 2025).
- The Latest Data Confirms: Forest Fires Are Getting Worse. World Resources Institute. 2024. Available online: https://www.wri.org/insights/global-trends-forest-fires (accessed on 17 April 2025).
- Ogale, A.A.; Zhang, M.; Jin, J. Recent advances in carbon fibers derived from biobased precursors. J. Appl. Polym. Sci. 2016, 133, 43794. [Google Scholar] [CrossRef]
- Jadhav, A.S.; Dhavale, B.S.; Patil, H.A.; Patil, S.P. Brief overview of electrospun polyacrylonitrile carbon nanofibers: Preparation process with applications and recent trends. Mater. Des. Process. Commun. 2019, 1, e83. [Google Scholar] [CrossRef]
- Jeon, B.; Ha, T.; Lee, D.Y.; Choi, M.-S.; Lee, S.W.; Jung, K.-H. Preparation and Electrochemical Properties of Porous Carbon Nanofiber Electrodes Derived from New Precursor Polymer: 6FDA-TFMB. Polymers 2020, 12, 1851. [Google Scholar] [CrossRef]
- Chen, S.; Han, D.; Hou, H. High strength electrospun fibers. Polym. Adv. Technol. 2011, 22, 295–303. [Google Scholar] [CrossRef]
- Xi, X.; Chung, D.D.L. Colossal electric permittivity discovered in polyacrylonitrile (PAN) based carbon fiber, with comparison of PAN-based and pitch-based carbon fibers. Carbon 2019, 145, 734–739. [Google Scholar] [CrossRef]
- Inagaki, M.; Kaneko, K.; Nishizawa, T. Nanocarbons–recent research in Japan. Carbon 2004, 42, 1401–1417. [Google Scholar] [CrossRef]
- Deng, J.; Liu, C.; Madou, M. Continuously superior-strong carbon nanofibers by additive nanostructuring and carbonization of polyacrylonitrile jetting. Microsyst. Nanoeng. 2024, 10, 185. [Google Scholar] [CrossRef]
- Dang, W.; Liu, J.; Wang, X.; Yan, K.; Zhang, A.; Yang, J.; Chen, L.; Liang, J. Structural Transformation of Polyacrylonitrile (PAN) Fibers during Rapid Thermal Pretreatment in Nitrogen Atmosphere. Polymers 2020, 12, 63. [Google Scholar] [CrossRef]
- Storck, J.L.; Brockhagen, B.; Grothe, T.; Sabantina, L.; Kaltschmidt, B.; Tuvshinbayar, K.; Braun, L.; Tanzli, E.; Hütten, A.; Ehrmann, A. Stabilization and Carbonization of PAN Nanofiber Mats Electrospun on Metal Substrates. J. Carbon Res. 2021, 7, 12. [Google Scholar] [CrossRef]
- Ismar, E.; Sarac, A.S. Nonflammable pre-carbonized polyacrylonitrile nanofiber webs. SN Appl. Sci. 2020, 2, 589. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, C.; Liao, W.; Peng, J. Mechanical and electrochemical properties of TiO2 modified polyurethane nanofibers. Appl. Phys. A 2023, 129, 162. [Google Scholar] [CrossRef]
- Tanski, T.; Matysiak, W.; Krzeminski, L. Analysis of optical properties of TiO2 nanoparticles and PAN/TiO2 composite nanofibers. Mater. Manuf. Process. 2017, 32, 1218–1224. [Google Scholar] [CrossRef]
- Kayaci, F.; Ozgit-Akgun, C.; Biyikli, N.; Uyar, T. Surface-decorated ZnO nanoparticles and ZnO nanocoating on electrospun polymeric nanofibers by atomic layer deposition for flexible photocatalytic nanofibrous membranes. RSC Adv. 2013, 3, 6817–6820. [Google Scholar] [CrossRef]
- Ning, C.; Li, C.; Han, C.; Luo, X.; Shen, L.; Xue, Y.; Yu, F. Tailoring mechanical and antibacterial properties of chitosan/gelatin nanofiber membranes with Fe3O4 nanoparticles for potential wound dressing application. Appl. Surf. Sci. 2016, 369, 492–500. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, N.; Fan, L.; Ding, A.; Dong, Q. Polyacrylonitrile (PAN)/TiO2 mixed matrix membrane synthesis by thermally induced self-crosslinking for thermal and organic-solvent resistant filtration. Chem. Eng. Sci. 2020, 228, 115993. [Google Scholar] [CrossRef]
- Sabantina, L.; Klöcker, M.; Wortmann, M.; Mirasol, J.R.; Cordero, T.; Moritzer, E.; Finsterbusch, K.; Ehrmann, A. Stabilization of polyacrylonitrile nanofiber mats obtained by needleless electrospinning using dimethyl sulfoxide as solvent. J. Ind. Text. 2020, 50, 224–239. [Google Scholar] [CrossRef]
- Khan, Z.; Kafiah, F.; Shafi, H.Z.; Nufaiei, F.; Furquan, S.A.; Matin, A. Morphology, Mechanical Properties and Surface Characteristics of Electrospun Polyacrylonitrile (PAN) Nanofiber Mats. Int. J. Adv. Eng. Nano Technol. 2015, 2, 15–22. [Google Scholar]
- Brunchi, C.E.; Bercea, M.; Morariu, S. Viscometric and rheological study of polyacrylonitrile solutions. e-Polymers 2009, 9, 065. [Google Scholar] [CrossRef]
- Brenner, M.; Popescu, C.; Weichold, O. Anti-Frothing Effect of Poultry Feathers in Bio-Based, Polycondensation-Type Thermoset Composites. Appl. Sci. 2020, 10, 2150. [Google Scholar] [CrossRef]
- van Krevelen, D.W. Some Basic Aspects of Flame Resistance of Polymeric Materials. Polymer 1975, 16, 615–620. [Google Scholar] [CrossRef]





| Sample | Compound | Distance from Needle to Collector, mm | Flow Rate, mL/h | V, kV | |
|---|---|---|---|---|---|
| PAN, % | TiO2, % | ||||
| PAN-13 | 13 | – | 15 | 0.07 | 15 |
| PAN-14 | 14 | – | |||
| PAN-15 | 15 | – | |||
| PAN-15-TiO2-1 | 15 | 1 | |||
| PAN-15-TiO2-2 | 15 | 2 | |||
| PAN-15-TiO2-3 | 15 | 3 | |||
| Sample | Compound | Temperature Treatment, °C | Time, min | |
|---|---|---|---|---|
| PAN, % | TiO2, % | |||
| PAN-13-T170 | 13 | – | 170 | 20 |
| PAN-13-T210 | – | 210 | ||
| PAN-13-T250 | – | 250 | ||
| PAN-15-T250 | 15 | – | ||
| Sample | Compound | Temperature Treatment, °C | Time, min | |
|---|---|---|---|---|
| PAN, % | TiO2, % | |||
| PAN-13-T250-T520 | 13 | – | 520 | 60 |
| PAN-15-T250-T520 | 15 | – | ||
| PAN-15-TiO2-1-T250-T520 | 15 | 1 | ||
| PAN-15-TiO2-2-T250-T520 | 15 | 2 | ||
| PAN-15-TiO2-3-T250-T520 | 15 | 3 | ||
| Elements | C | O | Ti | Al | S | Cu | Si | Na |
|---|---|---|---|---|---|---|---|---|
| Sample | PAN-15-TiO2-1 | |||||||
| w., % | 83.07 | 16.21 | 0.21 | 0.36 | 0.15 | – | – | – |
| σ w.% | 0.09 | 0.09 | 0.01 | 0.01 | 0.01 | – | – | – |
| Sample | PAN-15-TiO2-2 | |||||||
| w., % | 82.81 | 16.82 | 0.14 | 0.18 | 0.05 | – | – | – |
| σ w.% | 0.05 | 0.05 | 0.00 | 0.00 | 0.00 | – | – | – |
| Sample | PAN-15-TiO2-3 | |||||||
| w., % | 81.45 | 17.96 | 0.30 | 0.11 | 0.07 | 0.10 | – | – |
| σ w.% | 0.10 | 0.10 | 0.01 | 0.01 | 0.01 | 0.01 | – | – |
| Sample | PAN-15-TiO2-1-T250-T520 | |||||||
| w., % | 86.78 | 12.06 | 0.96 | 0.09 | – | 0.11 | – | – |
| σ w.% | 0.10 | 0.10 | 0.01 | 0.01 | – | 0.01 | – | – |
| Sample | PAN-15-TiO2-2-T250-T520 | |||||||
| w., % | 83.21 | 12.45 | 4.14 | 0.08 | – | 0.12 | – | – |
| σ w.% | 0.08 | 0.08 | 0.01 | 0.01 | – | 0.01 | – | – |
| Sample | PAN-15-TiO2-3-T250-T520 | |||||||
| w., % | 82.27 | 16.02 | 1.50 | 0.19 | – | – | 0.03 | – |
| σ w.% | 0.11 | 0.11 | 0.01 | 0.01 | – | – | 0.01 | – |
| Sample | SR, % | LOI, % |
|---|---|---|
| PAN-15 | 7.45 | 20.48 |
| PAN-15-TiO2-1 | 13.48 | 22.89 |
| PAN-15-TiO2-2 | 16.44 | 24.08 |
| PAN-15-TiO2-3 | 20.68 | 25.77 |
| PAN-15-T250-T520 | 90.05 | 53.52 |
| PAN-15-TiO2-1-T250-T520 | 10.73 | 21.79 |
| PAN-15-TiO2-2-T250-T520 | 23.25 | 26.80 |
| PAN-15-TiO2-3-T250-T520 | 66.96 | 44.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokhova, E.; Gordienko, M.; Menshutina, N.; Serkina, K.; Avetissov, I. Obtaining Polyacrylonitrile Carbon Nanofibers by Electrospinning for Their Application as Flame-Retardant Materials. Polymers 2025, 17, 1255. https://doi.org/10.3390/polym17091255
Mokhova E, Gordienko M, Menshutina N, Serkina K, Avetissov I. Obtaining Polyacrylonitrile Carbon Nanofibers by Electrospinning for Their Application as Flame-Retardant Materials. Polymers. 2025; 17(9):1255. https://doi.org/10.3390/polym17091255
Chicago/Turabian StyleMokhova, Elizaveta, Mariia Gordienko, Natalia Menshutina, Ksenia Serkina, and Igor Avetissov. 2025. "Obtaining Polyacrylonitrile Carbon Nanofibers by Electrospinning for Their Application as Flame-Retardant Materials" Polymers 17, no. 9: 1255. https://doi.org/10.3390/polym17091255
APA StyleMokhova, E., Gordienko, M., Menshutina, N., Serkina, K., & Avetissov, I. (2025). Obtaining Polyacrylonitrile Carbon Nanofibers by Electrospinning for Their Application as Flame-Retardant Materials. Polymers, 17(9), 1255. https://doi.org/10.3390/polym17091255







