A Silane Cross-Linked Cellulose-Based Separator for Long-Life Lithium Metal Batteries Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GPTMS-Crosslinked Propionylated Bagasse Fiber-Based Separator (PBF-GPTMS)
2.3. Characterization
2.4. Cell Assembly
2.5. Molecular Simulation
3. Results and Discussion
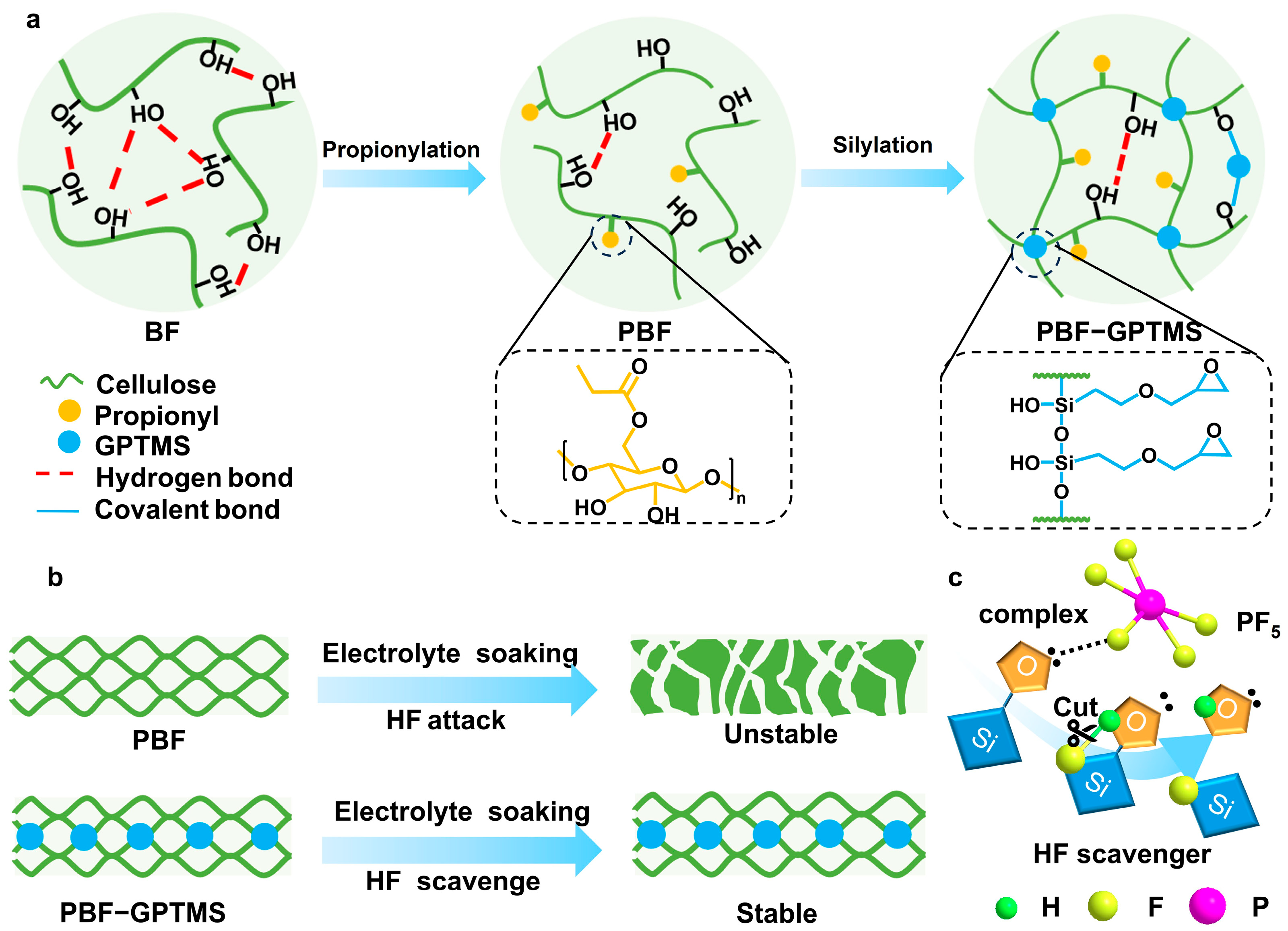
3.1. Design Idea
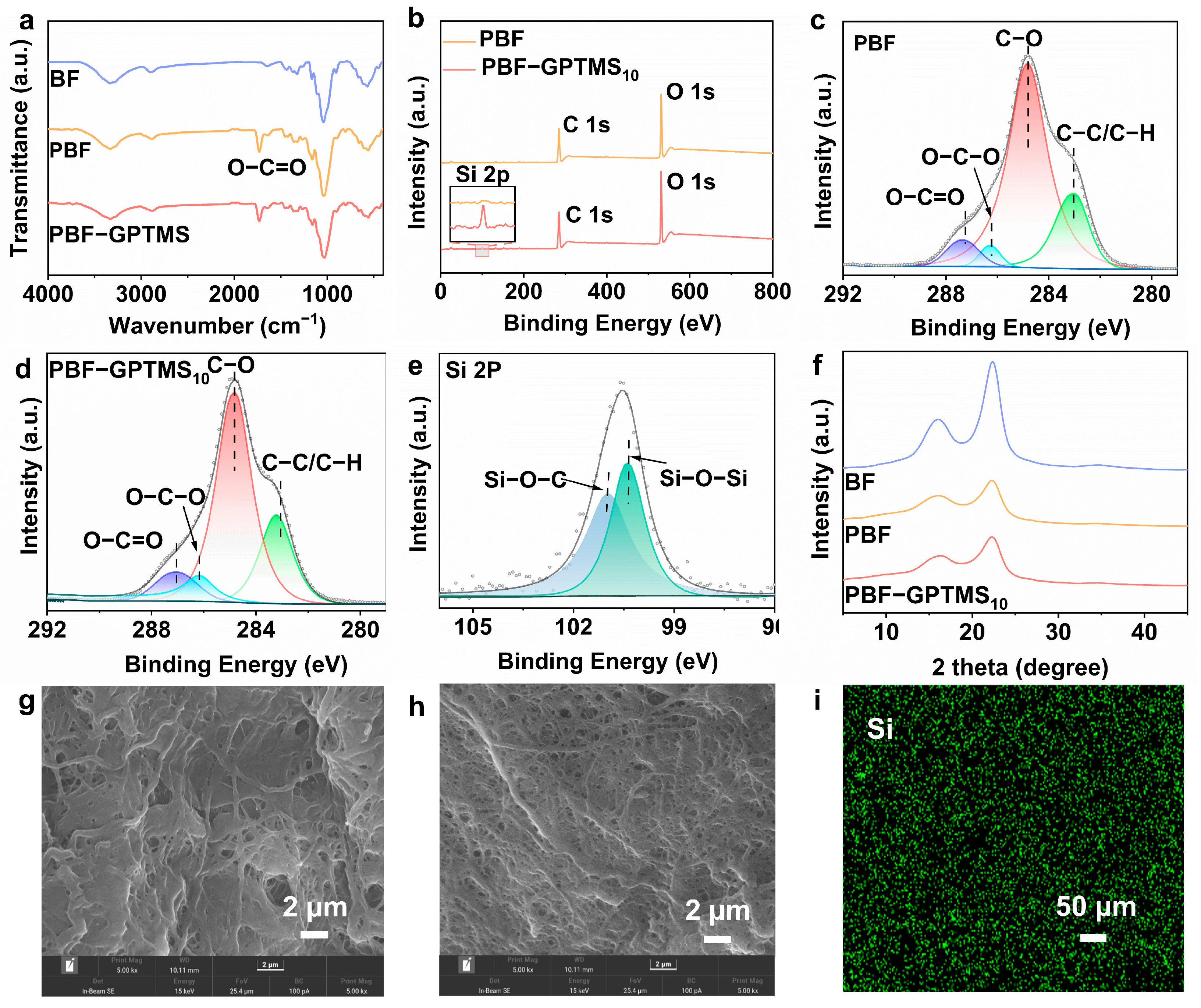
3.2. Chemical Structure and Morphology
3.3. Physicochemical Properties
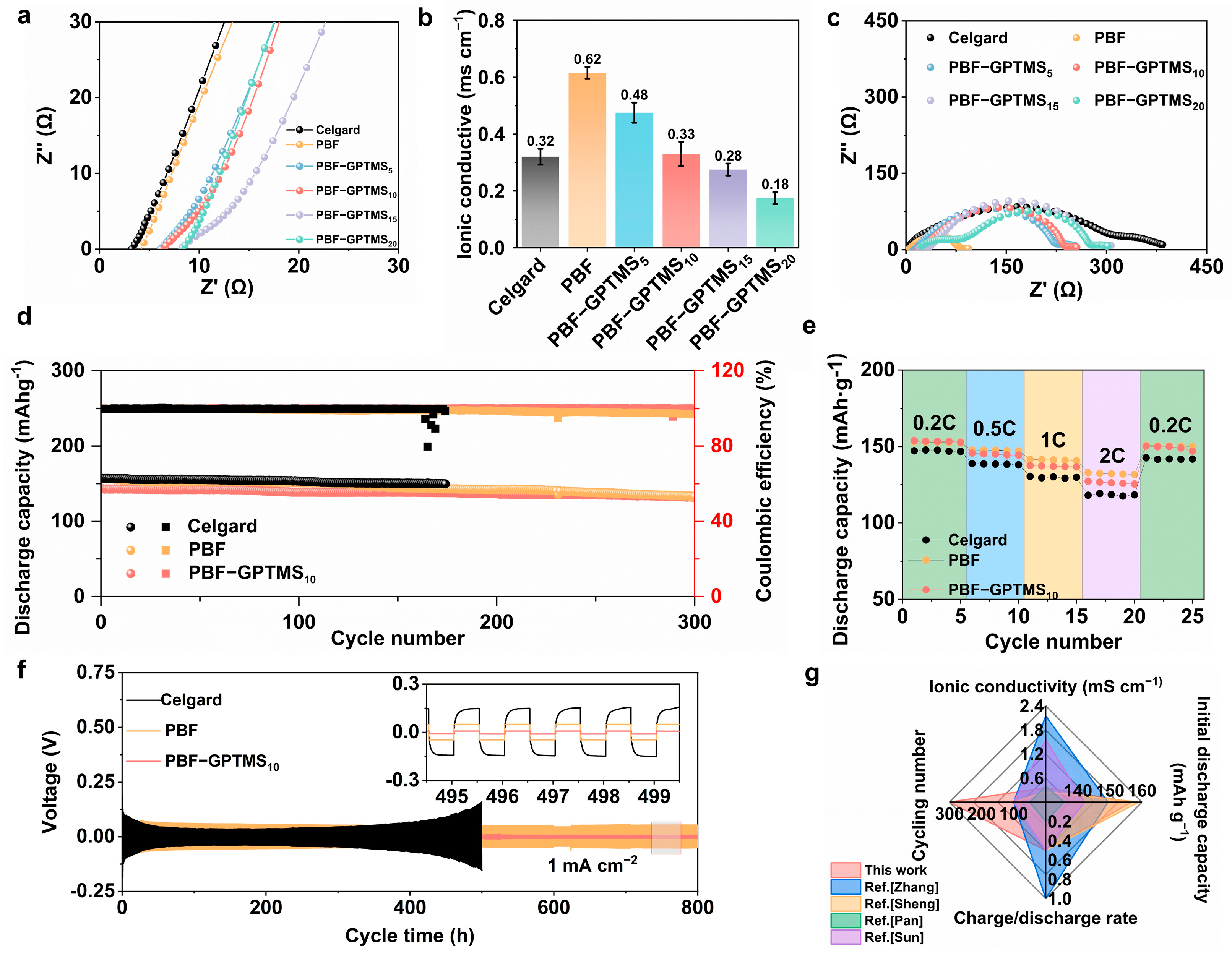
3.4. Electrochemical Properties
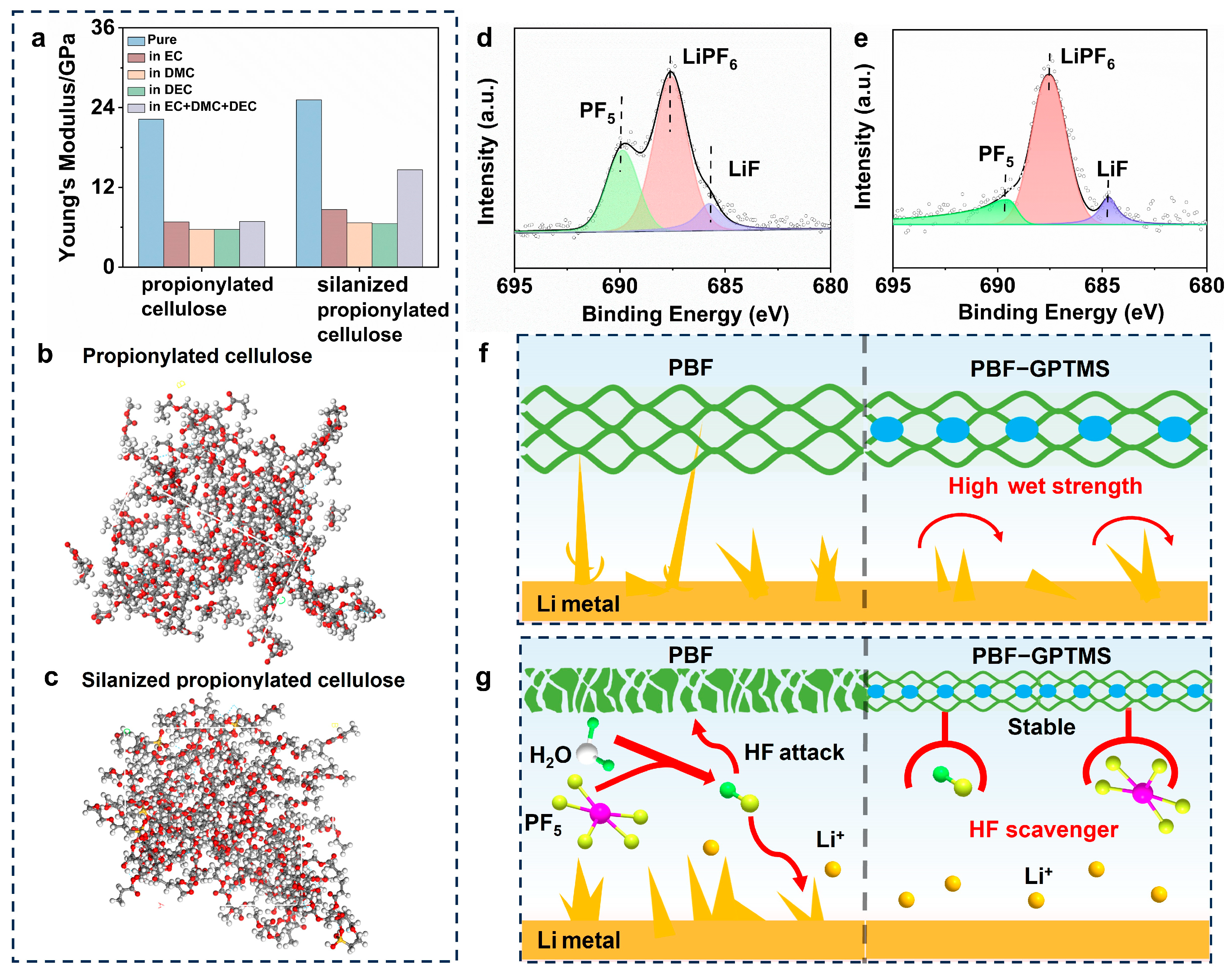
3.5. Working Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Acebedo, B.; Morant-Minana, M.C.; Gonzalo, E.; de Larramendi, I.R.; Villaverde, A.; Rikarte, J.; Fallarino, L. Current Status and Future Perspective on Lithium Metal Anode Production Methods. Adv. Energy Mater. 2023, 13, 2203744. [Google Scholar] [CrossRef]
- Lee, S.; Jeon, Y.J.; Kim, K.; Yoon, J.A.; Yim, T. Bis(Fluorosulfonyl)Imide- and Allyl-Functionalized Electrolyte Additive as an Interface Stabilizer for Li-Metal Batteries. Appl. Surf. Sci. 2023, 614, 156140. [Google Scholar] [CrossRef]
- Ji, C.; Wu, S.; Tang, F.; Yu, Y.; Hung, F.; Wei, Q. Cationic Cellulose Nanofiber Solid Electrolytes: A Pathway to High Lithium-Ion Migration and Polysulfide Adsorption for Lithium-Sulfur Batteries. Carbohydr. Polym. 2024, 335, 122075. [Google Scholar] [CrossRef] [PubMed]
- Joshi, J.S.; Langwald, S.V.; Ehrmann, A.; Sabantina, L. Algae-Based Biopolymers for Batteries and Biofuel Applications in Comparison with Bacterial Biopolymers—A Review. Polymers 2024, 16, 16050610. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ma, Z.; Zhao, W.; Guo, Z.; Zhao, H.; Ren, L. Deterioration Mechanism of the Wettability of a Lithium-Ion Battery Separator Induced by Low-Temperature Discharge. Appl. Energy 2024, 364, 123136. [Google Scholar] [CrossRef]
- Turossi, T.C.; Júnior, H.L.O.; Monticeli, F.M.; Dias, O.T.; Zattera, A.J. Cellulose-Derived Battery Separators: A Minireview on Advances towards Environmental Sustainability. Polymers 2025, 17, 17040456. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, Y.; Gong, W.; Zhou, J.; Ye, D.; Fan, L.; Xu, J. Redox-Active Janus Separator Based on Polyaniline Nanosheets and Bacterial Cellulose Nanofibers for Lithium-Ion Batteries. Int. J. Biol. Macromol. 2025, 302, 140536. [Google Scholar] [CrossRef]
- Ait Benhamou, A.; Moumen, E.; Aqil, M.; Amine, R.; El Fallah, H.; Son, S.-B.; Boukind, S.; El Achaby, M.; El Hankari, S.; Alami, J.; et al. In Situ Synthesis of Phosphate-Based CelloMOF as a Promising Separator for Li–Ion Batteries. ACS Appl. Energy Mater. 2025, 8, 3379–3391. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Z.; Feng, Y.; Cai, S.; Gao, H.; Wei, Z.; Zhao, Y. Cellulose-Based Separators for Lithium Batteries: Source, Preparation and Performance. Chem. Eng. J. 2023, 471, 144593. [Google Scholar] [CrossRef]
- Xie, W.; Liu, W.; Dang, Y.; Tang, A.; Deng, T.; Qiu, W. Investigation on Electrolyte-Immersed Properties of Lithium-Ion Battery Cellulose Separator through Multi-Scale Method. J. Power Sources 2019, 417, 150–158. [Google Scholar] [CrossRef]
- Xie, W.; Dang, Y.; Wu, L.; Liu, W.; Tang, A.; Luo, Y. Experimental and Molecular Simulating Study on Promoting Electrolyte-Immersed Mechanical Properties of Cellulose/Lignin Separator for Lithium-Ion Battery. Polym. Test. 2020, 90, 106773. [Google Scholar] [CrossRef]
- Lv, D.; Chai, J.; Wang, P.; Zhu, L.; Liu, C.; Nie, S.; Li, B.; Cui, G. Pure Cellulose Lithium-Ion Battery Separator with Tunable Pore Size and Improved Working Stability by Cellulose Nanofibrils. Carbohydr. Polym. 2021, 251, 116975. [Google Scholar] [CrossRef]
- Zhang, F.; Lan, X.; Peng, H.; Hu, X.; Zhao, Q. A “Trojan Horse” Camouflage Strategy for High-performance Cellulose Paper and Separators. Adv. Funct. Mater. 2020, 30, 2002169. [Google Scholar] [CrossRef]
- Zhang, H.; An, X.; Liu, L.; Lu, Z.; Liu, H.; Ni, Y. Preparation of Cellulose-Based Lithium Ion Battery Membrane Enhanced with Alkali-Treated Polysulfonamide Fibers and Cellulose Nanofibers. J. Membr. Sci. 2019, 591, 117346. [Google Scholar] [CrossRef]
- Son, H.B.; Cho, S.; Baek, K.; Jung, J.; Nam, S.; Han, D.-Y.; Kang, S.J.; Moon, H.R.; Park, S. All-Impurities Scavenging, Safe Separators with Functional Metal-Organic-Frameworks for High-Energy-Density Li-Ion Battery. Adv. Funct. Mater. 2023, 33, 2302563. [Google Scholar] [CrossRef]
- Kim, H.; Mattinen, U.; Guccini, V.; Liu, H.; Salazar-Alvarez, G.; Lindström, R.W.; Lindbergh, G.; Cornell, A. Feasibility of Chemically Modified Cellulose Nanofiber Membranes as Lithium-Ion Battery Separators. ACS Appl. Mater. Interfaces 2020, 12, 41211–41222. [Google Scholar] [CrossRef]
- Li, Z.; Wang, W.; Liang, X.; Wang, J.; Xu, Y.; Li, W. Fiber Swelling to Improve Cycle Performance of Paper-Based Separator for Lithium-Ion Batteries Application. J. Energy Chem. 2023, 79, 92–100. [Google Scholar] [CrossRef]
- Wang, X.; Tang, C.; Wang, Q.; Lu, Y.; Liu, X. Thermal Stability Improvement of Polysiloxane-Grafted Insulating Paper Cellulose in Micro-Water Environment. AIP Adv. 2018, 8, 105007. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, S.; Yang, B.; Hao, M.; Chen, Z.; Liu, Y.; Wang, X.; Yao, J. Preparation of Ambient-Dried Multifunctional Cellulose Aerogel by Freeze-Linking Technique. Chem. Eng. J. 2023, 477, 147044. [Google Scholar] [CrossRef]
- Han, J.; Kim, K.; Lee, Y.; Choi, N. Scavenging Materials to Stabilize LiPF6-containing Carbonate-based Electrolytes for Li-ion Batteries. Adv. Mater. 2019, 31, 1804822. [Google Scholar] [CrossRef]
- Wang, D.; Xie, H.; Liu, Q.; Mu, K.; Song, Z.; Xu, W.; Tian, L.; Zhu, C.; Xu, J. Low-Cost, High-Strength Cellulose-Based Quasi-Solid Polymer Electrolyte for Solid-State Lithium-Metal Batteries. Angew. Chem. Int. Edit. 2023, 62, e202302767. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, D.; Shao, Z.; Liu, S. Cellulosic Materials-Enhanced Sandwich Structure-like Separator via Electrospinning towards Safer Lithium-Ion Battery. Carbohydr. Polym. 2019, 214, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Mei, B.; Jiao, P.; Xie, Y.; Zhao, Y.; Li, Y.; Liu, H. Wood Derived Conductive Aerogel with Ultrahigh Specific Surface Area and Exceptional Mechanical Flexibility for Pressure Sensing. Chem. Eng. J. 2024, 500, 157020. [Google Scholar] [CrossRef]
- Yan, C.; Zhu, P.; Jia, H.; Du, Z.; Zhu, J.; Orenstein, R.; Cheng, H.; Wu, N.; Dirican, M.; Zhang, X. Garnet-Rich Composite Solid Electrolytes for Dendrite-Free, High-Rate, Solid-State Lithium-Metal Batteries. Energy Storage Mater. 2020, 26, 448–456. [Google Scholar] [CrossRef]
- Yu, Y.; Jia, G.; Zhao, L.; Xiang, H.; Hu, Z.; Xu, G.; Zhu, M. Flexible and Heat-Resistant Polyphenylene Sulfide Ultrafine Fiber Hybrid Separators for High-Safety Lithium-Ion Batteries. Chem. Eng. J. 2023, 452, 139112. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, Q.; Mo, J.; Lu, Y.; Cai, C.; Luo, B.; Nie, S. Chemically Tailored Molecular Surface Modification of Cellulose Nanofibrils for Manipulating the Charge Density of Triboelectric Nanogenerators. Nano Energy 2021, 89, 106369. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.; Huang, H.; Li, W.; Wang, J. Facile Design of Novel Nanocellulose-Based Gel Polymer Electrolyte for Lithium-Ion Batteries Application. Chem. Eng. J. 2022, 445, 136568. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, L.; Wang, Y.; Guan, X.; Zhang, Z.; Li, H.; Wang, F.; Zhang, H.; Zhang, Z.; Yang, Z.; et al. Starfish-Inspired Solid-State Li-Ion Conductive Membrane with Balanced Rigidity and Flexibility for Ultrastable Lithium Metal Batteries. Angew. Chem. Int. Ed. 2025, 64, e202420001. [Google Scholar] [CrossRef]
- Tian, G.; Duan, C.; Lu, W.; Liu, X.; Zhao, B.; Meng, Z.; Wang, Q.; Nie, S. Cellulose Acetate-Based Electrospun Nanofiber Aerogel with Excellent Resilience and Hydrophobicity for Efficient Removal of Drug Residues and Oil Contaminations from Wastewater. Carbohydr. Polym. 2024, 329, 121794. [Google Scholar] [CrossRef]
- Lee, K.; Sim, Y.L.; Jeong, H.; Kim, A.; Lee, Y.; Shim, S.E.; Qian, Y. Mechanochemically Functionalized and Fibrillated Microcrystalline Cellulose as a Filler in Silicone Foam: An Integrated Experimental and Simulation Investigation. Carbohydr. Polym. 2024, 327, 121660. [Google Scholar] [CrossRef]
- Zhou, S.; Yu, L.; Yu, L.; Li, L.; Wu, Y.; Zheng, H. A Study of High-Strength and Durable Cotton Fabric Joining via Laser-Induced Polymerisation of Silica-Sol Photosensitive Resin. J. Mater. Res. Technol. 2023, 27, 5507–5517. [Google Scholar] [CrossRef]
- Tong, M.; Kuang, S.; Wang, Q.; Li, X.; Yu, H.; Zeng, S.; Yu, X. Dual Cross-Linked Cellulose-Based Hydrogel for Dendrites-Inhibited Flexible Zinc-Ion Energy Storage Devices with Ultra-Long Cycles and High Energy Density. Carbohydr. Polym. 2024, 343, 122444. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, J.; Xu, J.; Yin, X.; Wu, J.; Chen, S.; Zhu, Z.; Wang, L.; Wang, H. Facile Fabrication of Cellulose/Polyphenylene Sulfide Composite Separator for Lithium-Ion Batteries. Carbohydr. Polym. 2020, 248, 116753. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, I.C.; Berlioz, S.; Fahs, A.; Louarn, G.; Carriere, P. Chemical Functionalization of Nano Fibrillated Cellulose by Glycidyl Silane Coupling Agents: A Grafted Silane Network Characterization Study. Int. J. Biol. Macromol. 2020, 165, 1773–1782. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, Z.; Liu, S.; Pei, Y.; Luo, X. Polydopamine-Modified Cellulose-Based Composite Separator for Inhibiting Dendritic Growth of Lithium Metal Batteries. Electrochim. Acta 2024, 475, 143661. [Google Scholar] [CrossRef]
- Cao, M.; Chen, W.; Ma, Y.; Huang, H.; Luo, S.; Zhang, C. Cross-Linked K2Ti4O9 Nanoribbon Arrays with Superior Rate Capability and Cyclability for Lithium-Ion Batteries. Mater. Lett. 2020, 279, 128495. [Google Scholar] [CrossRef]
- Sun, X.; Xu, W.; Zhang, X.; Lei, T.; Lee, S.-Y.; Wu, Q. ZIF-67@Cellulose Nanofiber Hybrid Membrane with Controlled Porosity for Use as Li-Ion Battery Separator. J. Energy Chem. 2021, 52, 170–180. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, J.; Guo, B.; Li, Z.; Wang, W.; Li, W. Cellulose-Based Eutectogel Electrolyte with High Ionic Conductivity for Solid-State Lithium-Ion Batteries. Chem. Eng. J. 2024, 491, 151783. [Google Scholar] [CrossRef]
- Syed, M.A.; Salehabadi, M.; Obrovac, M.N. High Energy Density Large Particle LiFePO4. Chem. Mater. 2024, 36, 803–814. [Google Scholar] [CrossRef]
- Sheng, J.; Chen, T.; Wang, R.; Zhang, Z.; Hua, F.; Yang, R. Ultra-Light Cellulose Nanofibril Membrane for Lithium-Ion Batteries. J. Membr. Sci. 2020, 595, 117550. [Google Scholar] [CrossRef]
- Pan, R.; Cheung, O.; Wang, Z.; Tammela, P.; Huo, J.; Lindh, J.; Edström, K.; Strømme, M.; Nyholm, L. Mesoporous Cladophora Cellulose Separators for Lithium-Ion Batteries. J. Power Sources 2016, 321, 185–192. [Google Scholar] [CrossRef]
- Ding, C.; Liu, Y.; Ono, L.K.; Tong, G.; Zhang, C.; Zhang, J.; Lan, J.; Yu, Y.; Chen, B.; Qi, Y.B. Ion-Regulating Hybrid Electrolyte Interface for Long-Life and Low N/P Ratio Lithium Metal Batteries. Energy Storage Mater. 2022, 50, 417–425. [Google Scholar] [CrossRef]
- Duan, H.; Yin, Y.-X.; Shi, Y.; Wang, P.-F.; Zhang, X.-D.; Yang, C.-P.; Shi, J.-L.; Wen, R.; Guo, Y.-G.; Wan, L.-J. Dendrite-Free Li-Metal Battery Enabled by a Thin Asymmetric Solid Electrolyte with Engineered Layers. J. Am. Chem. Soc. 2018, 140, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Yu, Z.; Cui, Y.; Bao, Z. Degradation and Speciation of Li Salts during XPS Analysis for Battery Research. ACS Energy Lett. 2022, 7, 3270–3275. [Google Scholar] [CrossRef]
- Shin, M.; Song, W.-J.; Han, J.-G.; Hwang, C.; Lee, S.; Yoo, S.; Park, S.; Song, H.-K.; Yoo, S.; Choi, N.-S.; et al. Metamorphosis of Seaweeds into Multitalented Materials for Energy Storage Applica-tions. Adv. Energy Mater. 2019, 9, 1900570. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.; Meng, H.; Li, W. A Silane Cross-Linked Cellulose-Based Separator for Long-Life Lithium Metal Batteries Application. Polymers 2025, 17, 1203. https://doi.org/10.3390/polym17091203
Cui J, Meng H, Li W. A Silane Cross-Linked Cellulose-Based Separator for Long-Life Lithium Metal Batteries Application. Polymers. 2025; 17(9):1203. https://doi.org/10.3390/polym17091203
Chicago/Turabian StyleCui, Jinghao, Hongliang Meng, and Wei Li. 2025. "A Silane Cross-Linked Cellulose-Based Separator for Long-Life Lithium Metal Batteries Application" Polymers 17, no. 9: 1203. https://doi.org/10.3390/polym17091203
APA StyleCui, J., Meng, H., & Li, W. (2025). A Silane Cross-Linked Cellulose-Based Separator for Long-Life Lithium Metal Batteries Application. Polymers, 17(9), 1203. https://doi.org/10.3390/polym17091203





