Poly(3-hydroxybutyrate)/Clay/Essential Oils Bionanocomposites Incorporating Biochar: Thermo-Mechanical and Antioxidant Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Films of Bionanocomposites
2.3. Physicochemical Characterizations
Nanoindentation
2.4. Antioxidant Activity via DPPH and ABTS
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MMT-OM | Organic montmorillonite Shelsite 30B |
| EO | Tepa:eugenol (70:30) essential oil mixture |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl radical |
| ABTS | 2,2′ azino-bis (3-ethylbenzothiazoline)-6-sulfonic acid |
| PHB | Polyhydroxybutyrate |
| Td | Degradation temperature |
| Tm | Melting temperature |
| Tc | Crystallization temperature |
| ΔHm | Enthalpies of fusion |
| ΔHc | Enthalpies of crystallization |
| Xc | Degree of crystallinity |
References
- Ishangulyyev, R.; Kim, S.; Lee, S.H. Understanding Food Loss and Waste—Why Are We Losing and Wasting Food? Foods 2019, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- Guillard, V.; Gaucel, S.; Fornaciari, C.; Angellier-Coussy, H.; Buche, P.; Gontard, N. The Next Generation of Sustainable Food Packaging to Preserve Our Environment in a Circular Economy Context. Front. Nutr. 2018, 5, 412270. [Google Scholar] [CrossRef]
- Michaliszyn-Gabryś, B.; Krupanek, J.; Kalisz, M.; Smith, J. Challenges for Sustainability in Packaging of Fresh Vegetables in Organic Farming. Sustainability 2022, 14, 5346. [Google Scholar] [CrossRef]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S.R. A Concise Guide to Active Agents for Active Food Packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Jachuła, J.; Konarska, A.; Denisow, B. Micromorphological and Histochemical Attributes of Flowers and Floral Reward in Linaria Vulgaris (Plantaginaceae). Protoplasma 2018, 255, 1763–1776. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Allagui, M.B.; Moumni, M.; Romanazzi, G. Antifungal Activity of Thirty Essential Oils to Control Pathogenic Fungi of Postharvest Decay. Antibiotics 2024, 13, 28. [Google Scholar] [CrossRef]
- Embuscado, M.E. Spices and Herbs: Natural Sources of Antioxidants—A Mini Review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Chen, X.; Shang, S.; Yan, F.; Jiang, H.; Zhao, G.; Tian, S.; Chen, R.; Chen, D.; Dang, Y. Antioxidant Activities of Essential Oils and Their Major Components in Scavenging Free Radicals, Inhibiting Lipid Oxidation and Reducing Cellular Oxidative Stress. Molecules 2023, 28, 4559. [Google Scholar] [CrossRef]
- Bibow, A.; Oleszek, W. Essential Oils as Potential Natural Antioxidants, Antimicrobial, and Antifungal Agents in Active Food Packaging. Antibiotics 2024, 13, 1168. [Google Scholar] [CrossRef]
- Gibriel, A.Y.; Al-Sayed, H.M.A.; Rady, A.H.; Abdelaleem, M.A. Synergistic Antibacterial Activity of Irradiated and Nonirradiated Cumin, Thyme and Rosemary Essential Oils. J. Food Saf. 2013, 33, 222–228. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Nikkhah, M.; Hashemi, M.; Najafi, M.B.H.; Farhoosh, R. Synergistic Effects of Some Essential Oils against Fungal Spoilage on Pear Fruit. Int. J. Food Microbiol. 2017, 257, 285–294. [Google Scholar] [CrossRef]
- Benamar-Aissa, B.; Gourine, N.; Ouinten, M.; Harrat, M.; Benarfa, A.; Yousfi, M. Synergistic Effects of Essential Oils and Phenolic Extracts on Antioxidant Activities Responses Using Two Artemisia Species (A. Campestris and A. Herba Alba) Combined with Citrus Aurantium. Biocatal. Agric. Biotechnol. 2023, 47, 102570. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential Oils in Combination and Their Antimicrobial Properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef]
- Basavegowda, N.; Baek, K.H. Synergistic Antioxidant and Antibacterial Advantages of Essential Oils for Food Packaging Applications. Biomolecules 2021, 11, 1267. [Google Scholar] [CrossRef]
- Norambuena, C.; Silva, G.; Urbina, A.; Figueroa, I.; Rodríguez-Maciel, J.C. Insecticidal Activity of Laureliopsis Philippiana (Looser) Schodde (Atherospermataceae) Essential Oil against Sitophilus Spp. (Coleoptera Curculionidae). Chil. J. Agric. Res. 2016, 76, 330–336. [Google Scholar] [CrossRef]
- Toledo, D.; Mutis, A.; Hormazabal, E.; Quiroz, A.; Palma, R.; Parada, M.; Scheuermann, E. Chemical Composition and Antibacterial Activity of Laureliopsis Philippiana (Looser) Essential Oil. Bol. Latinoam. Caribe Plantas Med. Aromat. 2014, 13, 117–126. [Google Scholar]
- Pérez-San Martín, A.; Uribe, K.; Hernández-Montelongo, J.; Naveas, N.; Manso-Silván, M.; Oyarzún, P.; Díaz-García, V.; Contreras, B.; Recio-Sánchez, G. Antibacterial Activity and Kinetic Release of Laureliopsis Philippiana (Looser) Essential Oil from Nanostructured Porous Silicon with Surface-Functionalization Alternatives. Appl. Sci. 2022, 12, 8258. [Google Scholar] [CrossRef]
- Basumatary, I.B.; Mukherjee, A.; Katiyar, V.; Kumar, S. Biopolymer-Based Nanocomposite Films and Coatings: Recent Advances in Shelf-Life Improvement of Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2020, 2020, 1848789. [Google Scholar] [CrossRef]
- Hussain, S.; Akhter, R.; Maktedar, S.S. Advancements in Sustainable Food Packaging: From Eco-Friendly Materials to Innovative Technologies. Sustain. Food Technol. 2024, 2, 1297–1364. [Google Scholar] [CrossRef]
- Panayotidou, E.; Baklavaridis, A.; Zuburtikudis, I.; Achilias, D.S. Nanocomposites of Poly(3-Hydroxybutyrate)/Organomodified Montmorillonite: Effect of the Nanofiller on the Polymer’s Biodegradation. J. Appl. Polym. Sci. 2015, 132, 41656. [Google Scholar] [CrossRef]
- Zhou, W.; Bergsma, S.; Colpa, D.I.; Euverink, G.J.W.; Krooneman, J. Polyhydroxyalkanoates (PHAs) Synthesis and Degradation by Microbes and Applications towards a Circular Economy. J. Environ. Manag. 2023, 341, 118033. [Google Scholar] [CrossRef]
- Westlake, J.R.; Tran, M.W.; Jiang, Y.; Zhang, X.; Burrows, A.D.; Xie, M. Biodegradable Biopolymers for Active Packaging: Demand, Development and Directions. Sustain. Food Technol. 2023, 1, 50–72. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-Based Films and Coatings for Food Packaging: A Review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent Advances in Biodegradable Polymers for Sustainable Applications. NPJ Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Lenz, R.W.; Marchessault, R.H. Bacterial Polyesters: Biosynthesis, Biodegradable Plastics and Biotechnology. Biomacromolecules 2004, 6, 1–8. [Google Scholar] [CrossRef]
- Chen, G.Q. A Microbial Polyhydroxyalkanoates (PHA) Based Bio- and Materials Industry. Chem. Soc. Rev. 2009, 38, 2434–2446. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Villano, M.; Oliveira, C.; Albuquerque, M.G.E.; Majone, M.; Reis, M.; Lopez-Rubio, A.; Lagaron, J.M. Characterization of Polyhydroxyalkanoates Synthesized from Microbial Mixed Cultures and of Their Nanobiocomposites with Bacterial Cellulose Nanowhiskers. New Biotechnol. 2014, 31, 364–376. [Google Scholar] [CrossRef]
- Follain, N.; Chappey, C.; Dargent, E.; Chivrac, F.; Crétois, R.; Marais, S. Structure and Barrier Properties of Biodegradable Polyhydroxyalkanoate Films. J. Phys. Chem. C 2014, 118, 6165–6177. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Ionita, E.R.; Nicolae, C.A.; Gabor, A.R.; Ionita, M.D.; Trusca, R.; Lixandru, B.E.; Codita, I.; Dinescu, G. Poly(3-Hydroxybutyrate) Modified by Nanocellulose and Plasma Treatment for Packaging Applications. Polymers 2018, 10, 1249. [Google Scholar] [CrossRef] [PubMed]
- Kishore, A.; Mithul Aravind, S.; Singh, A. Bionanocomposites for Active and Smart Food Packaging: A Review on Its Application, Safety, and Health Aspects. J. Food Process Eng. 2023, 46, e14320. [Google Scholar] [CrossRef]
- Franco-Urquiza, E.A. Clay-Based Polymer Nanocomposites: Essential Work of Fracture. Polymers 2021, 13, 2399. [Google Scholar] [CrossRef]
- Naskar, A.; Sanyal, I.; Nahar, N.; Ghosh, D.D.; Chakraborty, S. Bionanocomposites Films Applied as Active and Smart Food Packaging: A Review. Polym. Eng. Sci. 2023, 63, 2675–2699. [Google Scholar] [CrossRef]
- Puiggalí, J.; Katsarava, R. Bionanocomposites. In Clay-Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 239–272. ISBN 9780323461610. [Google Scholar]
- Babu Valapa, R.; Loganathan, S.; Pugazhenthi, G.; Thomas, S.; Varghese, T.O. An Overview of Polymer-Clay Nanocomposites; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 29–81. ISBN 9780323461610. [Google Scholar]
- Ashfaq, A.; Khursheed, N.; Fatima, S.; Anjum, Z.; Younis, K. Application of Nanotechnology in Food Packaging: Pros and Cons. J. Agric. Food Res. 2022, 7, 100270. [Google Scholar] [CrossRef]
- Garrido-Miranda, K.A.; Rivas, B.L.; Pérez -Rivera, M.A.; Sanfuentes, E.A.; Peña-Farfal, C. Antioxidant and Antifungal Effects of Eugenol Incorporated in Bionanocomposites of Poly(3-Hydroxybutyrate)-Thermoplastic Starch. LWT-Food Sci. Technol. 2018, 98, 260–267. [Google Scholar] [CrossRef]
- Botana, A.; Mollo, M.; Eisenberg, P.; Torres Sanchez, R.M. Effect of Modified Montmorillonite on Biodegradable PHB Nanocomposites. Appl. Clay Sci. 2010, 47, 263–270. [Google Scholar] [CrossRef]
- D’Amico, D.A.; Manfredi, L.B.; Cyras, V.P. Crystallization Behavior of Poly(3-Hydroxybutyrate) Nanocomposites Based on Modified Clays: Effect of Organic Modifiers. Thermochim. Acta 2012, 544, 47–53. [Google Scholar] [CrossRef]
- Mohan, A.; Girdhar, M.; Kumar, R.; Chaturvedi, H.S.; Vadhel, A.; Solanki, P.R.; Kumar, A.; Kumar, D.; Mamidi, N. Polyhydroxybutyrate-Based Nanocomposites for Bone Tissue Engineering. Pharmaceuticals 2021, 14, 1163. [Google Scholar] [CrossRef]
- Mohapatra, A.K.; Aswathy, N.R. Mechanical, Thermal, and Morphological Properties of Poly(3-Hydroxy Butyrate) Nanocomposites Prepared by Melt Mixing Method. J. Polym. Eng. 2024, 44, 263–274. [Google Scholar] [CrossRef]
- Campos-Requena, V.H.; Rivas, B.L.; Pérez, M.A.; Garrido-Miranda, K.A.; Pereira, E.D. Release of Essential Oil Constituent from Thermoplastic Starch/Layered Silicate Bionanocomposite Film as a Potential Active Packaging Material. Eur. Polym. J. 2018, 109, 64–71. [Google Scholar] [CrossRef]
- Das, O.; Kim, N.K.; Hedenqvist, M.S.; Lin, R.J.T.; Sarmah, A.K.; Bhattacharyya, D. An Attempt to Find a Suitable Biomass for Biochar-Based Polypropylene Biocomposites. Environ. Manag. 2018, 62, 403–413. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Zhang, H.; Bellmer, D.; Huhnke, R. Recent Advances in Utilization of Biochar. Renew. Sustain. Energy Rev. 2015, 42, 1055–1064. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Noreen, S.; Abd-Elsalam, K.A. Biochar-Based Nanocomposites: A Sustainable Tool in Wastewater Bioremediation; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 185–200. ISBN 9780128211410. [Google Scholar]
- Haeldermans, T.; Samyn, P.; Cardinaels, R.; Vandamme, D.; Vanreppelen, K.; Cuypers, A.; Schreurs, S. Bio-Based Poly(3-Hydroxybutyrate)/Thermoplastic Starch Composites as a Host Matrix for Biochar Fillers. J. Polym. Environ. 2021, 29, 2478–2491. [Google Scholar] [CrossRef]
- Corrado, I.; Di Girolamo, R.; Regalado-González, C.; Pezzella, C. Polyhydroxyalkanoates-Based Nanoparticles as Essential Oil Carriers. Polymers 2022, 14, 166. [Google Scholar] [CrossRef]
- González, M.E.; Romero-Hermoso, L.; González, A.; Hidalgo, P.; Meier, S.; Navia, R.; Cea, M. Effects of Pyrolysis Conditions on Physicochemical Properties of Oat Hull Derived Biochar. BioResources 2017, 12, 2040–2057. [Google Scholar] [CrossRef]
- Garrido-Miranda, K.A.; Rivas, B.L.; Pérez, M.A. Poly(3-Hydroxybutyrate)-Thermoplastic Starch-Organoclay Bionanocomposites: Surface Properties. J. Appl. Polym. Sci. 2017, 134, 45217. [Google Scholar] [CrossRef]
- Bruzaud, S.; Bourmaud, A. Thermal Degradation and (Nano)Mechanical Behavior of Layered Silicate Reinforced Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Nanocomposites. Polym. Test. 2007, 26, 652–659. [Google Scholar] [CrossRef]
- Kojom, J.J.W.; Bogning, C.Z.; Lappa, E.L.; Sonfack, C.S.; Kuinze, A.N.; Etamé-Loé, G.; Dongmo, A.B. Antioxidant Properties and Vasorelaxant Mechanism of Aqueous Extract of Ricinodendron Heudelotii (Euphorbiaceae). Biomed Res. Int. 2024, 2024, 3435974. [Google Scholar] [CrossRef]
- Liu, S.; Peng, S.; Zhang, B.; Xue, B.; Yang, Z.; Wang, S.; Xu, G. Effects of Biochar Pyrolysis Temperature on Thermal Properties of Polyethylene Glycol/Biochar Composites as Shape-Stable Biocomposite Phase Change Materials. RSC Adv. 2022, 12, 9587–9598. [Google Scholar] [CrossRef] [PubMed]
- Das, O.; Bhattacharyya, D.; Hui, D.; Lau, K.T. Mechanical and Flammability Characterisations of Biochar/Polypropylene Biocomposites. Compos. Part B Eng. 2016, 106, 120–128. [Google Scholar] [CrossRef]
- Lehmann, M.; Liepins, K.; Volperts, A.; Dobele, G.; Plavniece, A.; Bikovens, O.; Sansonetti, E.; Zhurinsh, A. Enhancing the Wetting Properties of Activated Biochar by Oxidation with Hydrogen Peroxide. Chemistry 2024, 6, 911–921. [Google Scholar] [CrossRef]
- Aboughaly, M.; Babaei-Ghazvini, A.; Dhar, P.; Patel, R.; Acharya, B. Enhancing the Potential of Polymer Composites Using Biochar as a Filler: A Review. Polymers 2023, 15, 3981. [Google Scholar] [CrossRef]
- Abdelmalek, F.; Steinbüchel, A.; Rofeal, M. The Hyperproduction of Polyhydroxybutyrate Using Bacillus Mycoides ICRI89 through Enzymatic Hydrolysis of Affordable Cardboard. Polymers 2022, 14, 2810. [Google Scholar] [CrossRef]
- Seoane, I.T.; Fortunati, E.; Puglia, D.; Cyras, V.P.; Manfredi, L.B. Development and Characterization of Bionanocomposites Based on Poly(3-Hydroxybutyrate) and Cellulose Nanocrystals for Packaging Applications. Polym. Int. 2016, 65, 1046–1053. [Google Scholar] [CrossRef]
- Tapadiya, A.; Vasanthan, N. Crystallization and Alkaline Hydrolysis of Poly(3- Hydroxybutyrate) Films Probed by Thermal Analysis and Infrared Spectroscopy. Int. J. Biol. Macromol. 2017, 102, 1130–1137. [Google Scholar] [CrossRef]
- Campa-Siqueiros, P.I.; Madera-Santana, T.J.; Ayala-Zavala, J.F.; López-Cervantes, J.; Castillo-Ortega, M.M.; Herrera-Franco, P.J.; Quintana-Owen, P. Co-Electrospun Nanofibers of Gelatin and Chitosan–Polyvinyl Alcohol–Eugenol for Wound Dressing Applications. Polym. Bull. 2023, 80, 3611–3632. [Google Scholar] [CrossRef]
- Kumari, S.V.G.; Pakshirajan, K.; Pugazhenthi, G. Development and Characterization of Active Poly (3-Hydroxybutyrate) Based Composites with Grapeseed Oil and MgO Nanoparticles for Shelf-Life Extension of White Button Mushrooms (Agaricus Bisporus). Int. J. Biol. Macromol. 2024, 260, 129521. [Google Scholar] [CrossRef]
- Mokhtar, A.; Asli, B.; Abdelkrim, S.; Hachemaoui, M.; Boukoussa, B.; Sassi, M.; Viscusi, G.; Abboud, M. Polymer/Clay Nanocomposites as Advanced Adsorbents for Textile Wastewater Treatment. Minerals 2024, 14, 1216. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Ko, S. Nanoclays in Food and Beverage Packaging. J. Nanomater. 2019, 2019, 8927167. [Google Scholar] [CrossRef]
- Zhu, Y.; Kottarath, S.; Iroh, J.O.; Vaia, R.A. Progressive Intercalation and Exfoliation of Clay in Polyaniline–Montmorillonite Clay Nanocomposites and Implication to Nanocomposite Impedance. Energies 2022, 15, 5366. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Nicolae, C.A.; Frone, A.N.; Chiulan, I.; Stanescu, P.O.; Draghici, C.; Iorga, M.; Mihailescu, M. Plasticized Poly(3-Hydroxybutyrate) with Improved Melt Processing and Balanced Properties. J. Appl. Polym. Sci. 2017, 134, 44810. [Google Scholar] [CrossRef]
- Abdelwahab, M.A.; Flynn, A.; Chiou, B.-S.; Imam, S.; Orts, W.; Chiellini, E. Thermal, Mechanical and Morphological Characterization of Plasticized PLA–PHB Blends. Polym. Degrad. Stab. 2012, 97, 1822–1828. [Google Scholar] [CrossRef]
- Zhang, M.; Thomas, N.L. Preparation and Properties of Polyhydroxybutyrate Blended with Different Types of Starch. J. Appl. Polym. Sci. 2009, 116, 688–694. [Google Scholar] [CrossRef]
- Anbukarasu, P.; Sauvageau, D.; Elias, A. Tuning the Properties of Polyhydroxybutyrate Films Using Acetic Acid via Solvent Casting. Sci. Rep. 2015, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Alghyamah, A.A.; Yagoub Elnour, A.; Shaikh, H.; Haider, S.; Manjaly Poulose, A.; Al-Zahrani, S.M.; Almasry, W.A.; Young Park, S. Biochar/Polypropylene Composites: A Study on the Effect of Pyrolysis Temperature on Crystallization Kinetics, Crystalline Structure, and Thermal Stability. J. King Saud Univ.-Sci. 2021, 33, 101409. [Google Scholar] [CrossRef]
- Musioł, M.; Rydz, J.; Janeczek, H.; Andrzejewski, J.; Cristea, M.; Musioł, K.; Kampik, M.; Kowalczuk, M. (Bio)Degradable Biochar Composites of PLA/P(3HB-Co-4HB) Commercial Blend for Sustainable Future—Study on Degradation and Electrostatic Properties. Polymers 2024, 16, 2331. [Google Scholar] [CrossRef]
- Fernández, M.J.; Fernández, M.D. Effect of Organic Modifier and Clay Content on Non-Isothermal Cold Crystallization and Melting Behavior of Polylactide/Organovermiculite Nanocomposites. Polymers 2020, 12, 364. [Google Scholar] [CrossRef]
- Srihanam, P.; Pakkethati, K.; Srisuwan, Y.; Phromsopha, T.; Manphae, A.; Phinyocheep, P.; Yamaguchi, M.; Baimark, Y. Utilization of Bamboo Biochar as a Multi-Functional Filler of Flexible Poly(L-Lactide)-b-Poly(Ethylene Glycol)-b-Poly(L-Lactide) Bioplastic. Sci. Rep. 2024, 14, 1–15. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, H.; Zhu, J.; Chang, L.; Ye, L. Nanoindentation and Thermal Study of Polyvinylalcohol/Graphene Oxide Nanocomposite Film through Organic/Inorganic Assembly. Appl. Surf. Sci. 2015, 349, 27–34. [Google Scholar] [CrossRef]
- Kuai, L.; Liu, F.; Chiou, B.S.; Avena-Bustillos, R.J.; McHugh, T.H.; Zhong, F. Controlled Release of Antioxidants from Active Food Packaging: A Review. Food Hydrocoll. 2021, 120, 106992. [Google Scholar] [CrossRef]
- Orlo, E.; Stanzione, M.; Lavorgna, M.; Isidori, M.; Ruffolo, A.; Sinagra, C.; Buonocore, G.G.; Lavorgna, M. Novel Eugenol-Based Antimicrobial Coatings on Aluminium Substrates for Food Packaging Applications. J. Appl. Polym. Sci. 2023, 140, 53519. [Google Scholar] [CrossRef]
- Woźniak, M.; Młodziejewska, J.; Stefanowska, K.; Mrówczyńska, L.; Sip, A.; Dobrucka, R.; Ratajczak, I. Chitosan-Based Films with Essential Oil Components for Food Packaging. Coatings 2024, 14, 830. [Google Scholar] [CrossRef]
- Bortolomeazzi, R.; Verardo, G.; Liessi, A.; Callea, A. Formation of Dehydrodiisoeugenol and Dehydrodieugenol from the Reaction of Isoeugenol and Eugenol with DPPH Radical and Their Role in the Radical Scavenging Activity. Food Chem. 2010, 118, 256–265. [Google Scholar] [CrossRef]



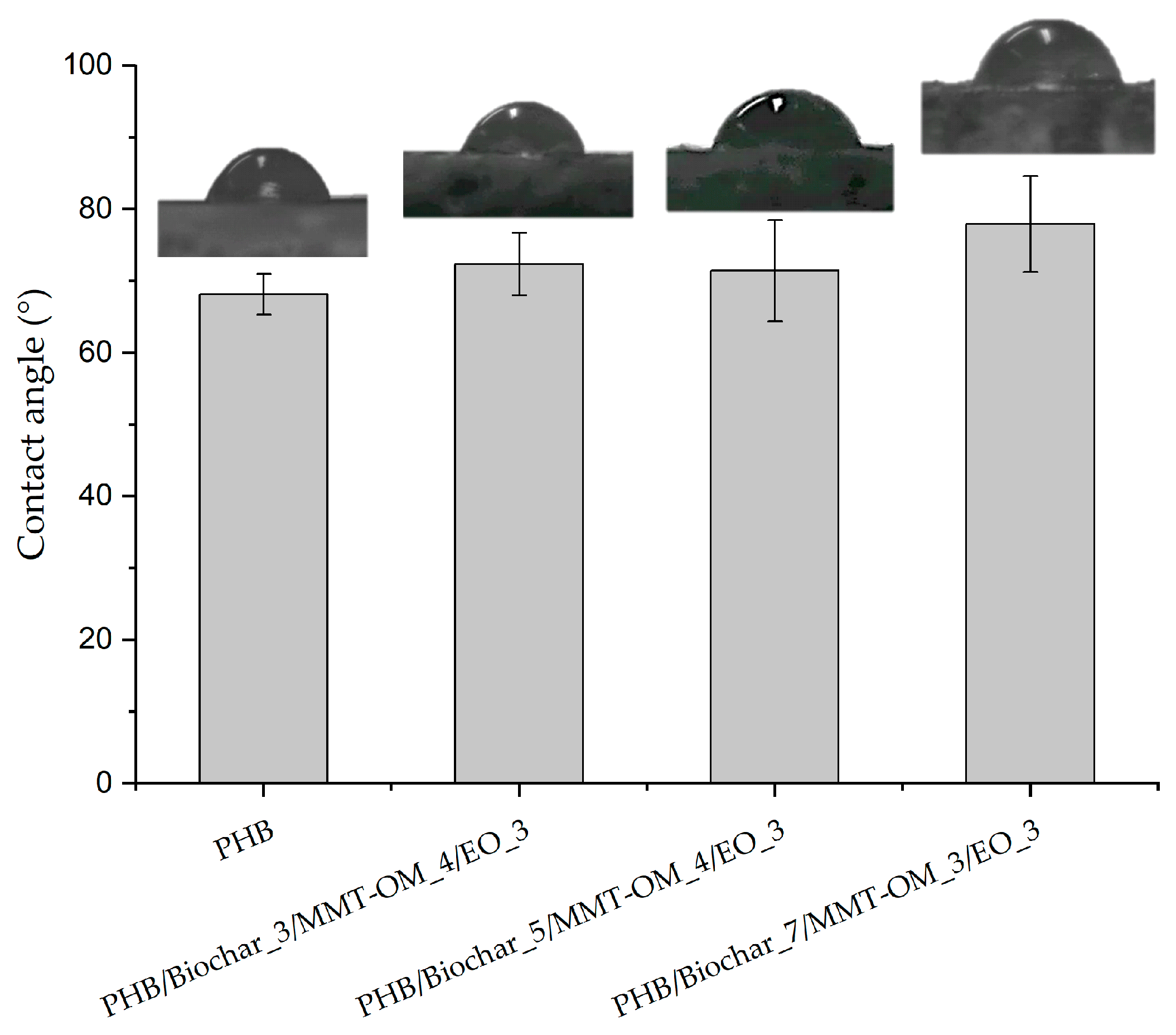
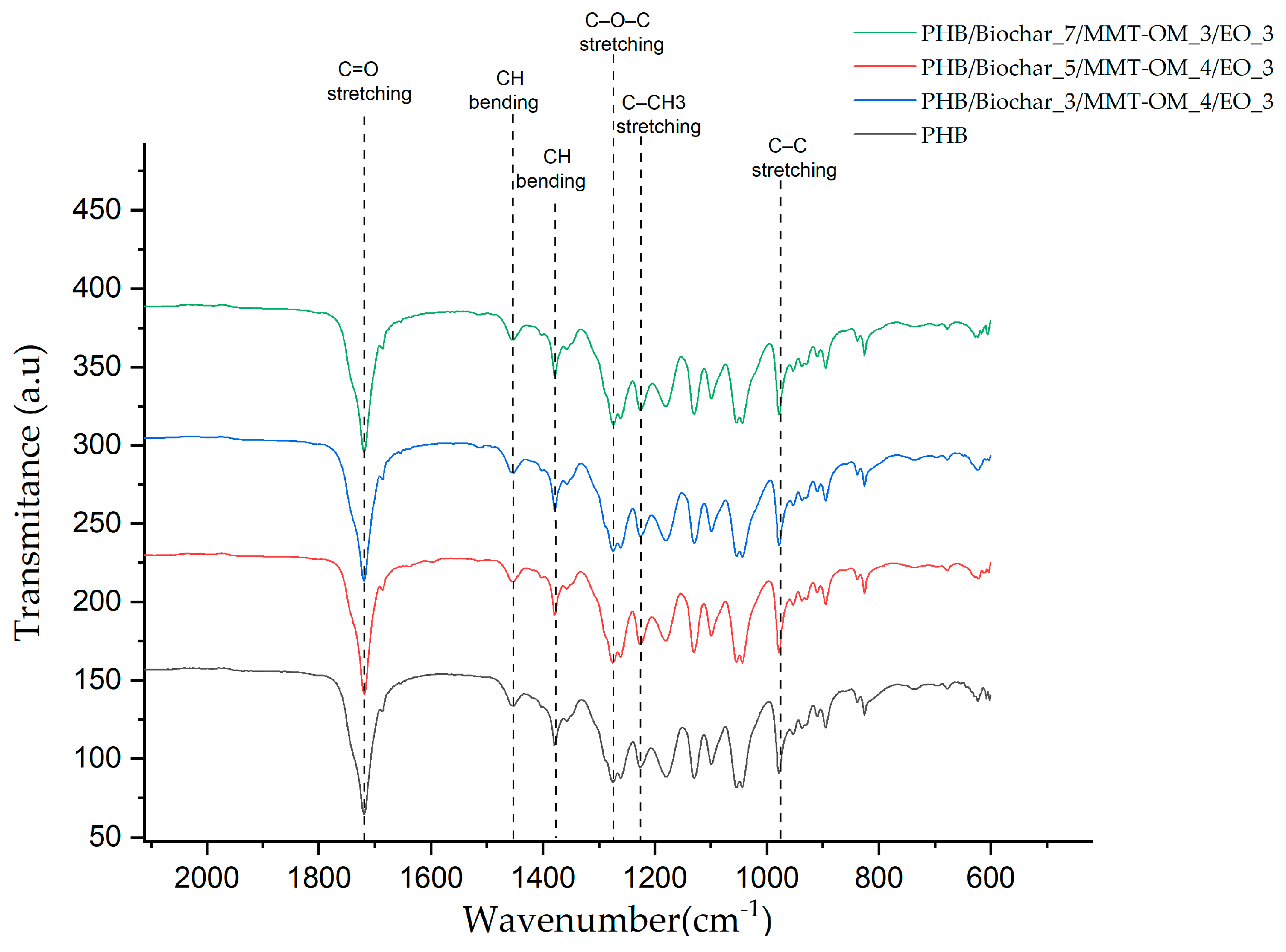

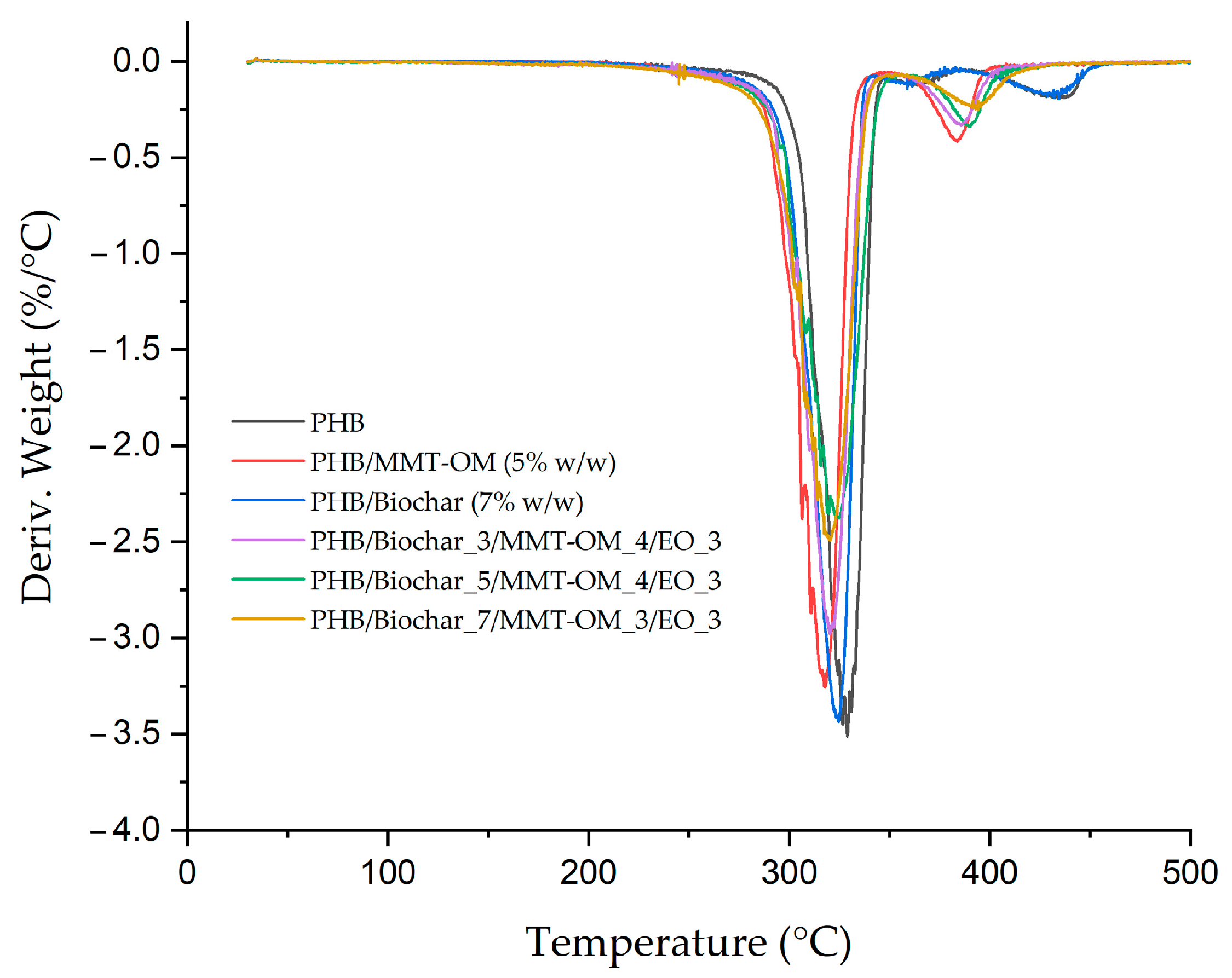

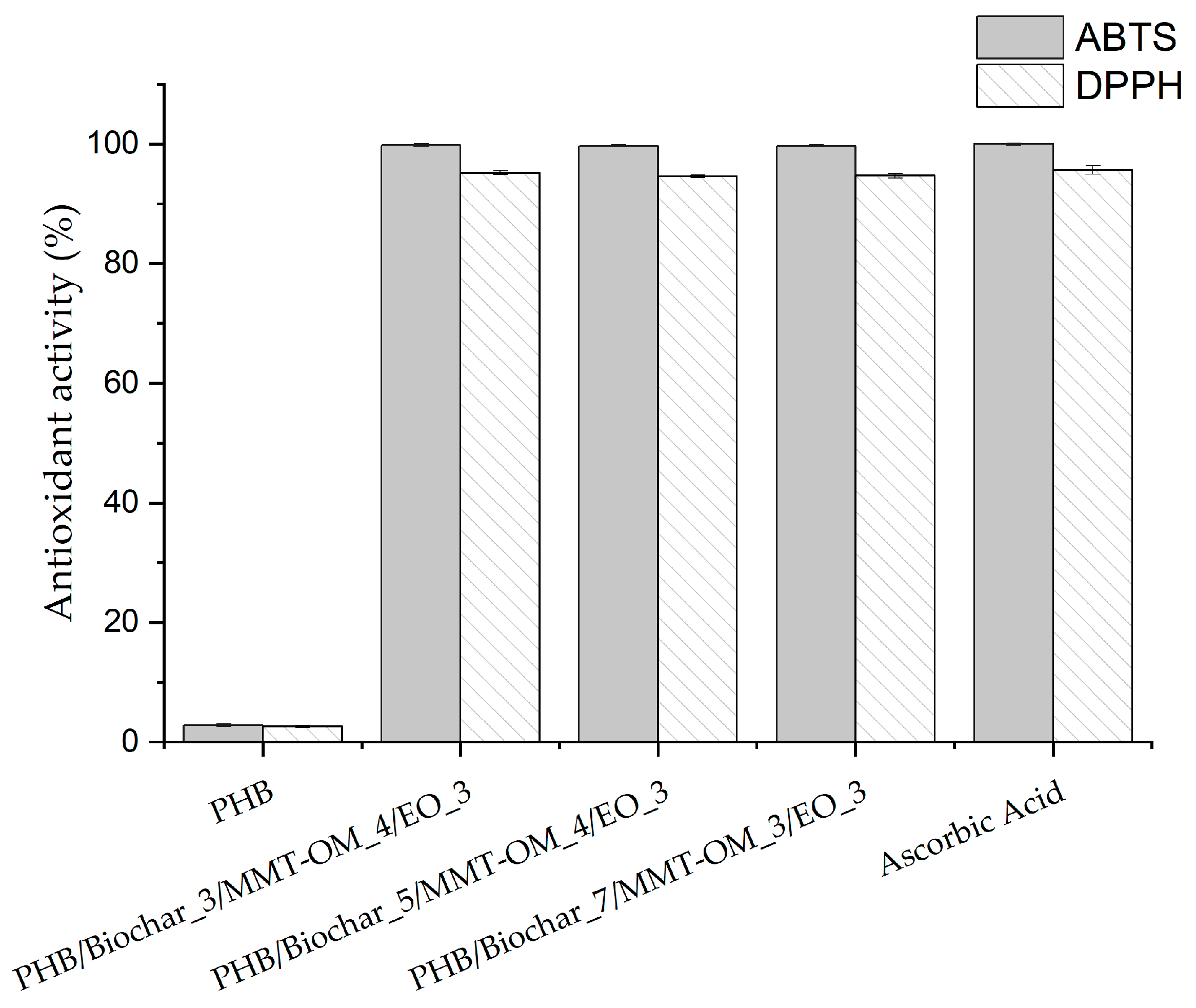
| Sample | Biochar (% w/w) | MMT-OM (% w/w) | EO (% w/w) |
|---|---|---|---|
| PHB | 0 | 0 | 0 |
| PHB/biochar_3/MMT-OM_4/EO_3 | 3 | 4 | 3 |
| PHB/biochar_5/MMT-OM_4/EO_3 | 5 | 4 | 3 |
| PHB/biochar_7/MMT-OM_3/EO_3 | 7 | 3 | 3 |
| Sample | Tm1 (°C) | Tm2 (°C) | Tc (°C) | ΔHm (J/g) | ΔHc (J/g) | Xc (%) | Td (°C) | T5% (°C) | T10% (°C) | T50% (°C) |
|---|---|---|---|---|---|---|---|---|---|---|
| PHB | 168.3 | - | 112.9 | 72.70 | 63.03 | 49.79 | 316.2 | 289.4 | 300.5 | 315.5 |
| PHB/biochar_3/MMT-OM_4/EO_3 | 154.8 | 162.8 | 110.8 | 70.13 | 63.13 | 53.37 | 320.6 | 277.6 | 296.8 | 319.7 |
| PHB/biochar_5/MMT-OM_4/EO_3 | 150.3 | 158.7 | 108.8 | 62.65 | 60.49 | 48.76 | 324.9 | 275.1 | 296.5 | 324.4 |
| PHB/biochar_7/MMT-OM_3/EO_3 | 152.4 | 159.5 | 109.7 | 63.91 | 58.41 | 50.32 | 320.1 | 272.2 | 293.0 | 320.0 |
| Sample | Elastic Modulus (GPa) | Hardness (MPa) |
|---|---|---|
| PHB | 2.08 ± 0.23 | 134.05 ± 12.68 |
| PHB/biochar_3/MMT-OM_4/EO_3 | 1.86 ± 0.19 | 85.56 ± 5.19 |
| PHB/biochar_5/MMT-OM_4/EO_3 | 1.47 ± 0.16 | 66.80 ± 6.02 |
| PHB/biochar_7/MMT-OM_3/EO_3 | 2.40 ± 0.96 | 128.96 ± 30.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrido-Miranda, K.A.; Gonzalez, M.E.; Hernandez-Montelongo, J.; Jaramillo, A.; Oñate, A.; Burgos-Díaz, C.; Manso-Silvan, M. Poly(3-hydroxybutyrate)/Clay/Essential Oils Bionanocomposites Incorporating Biochar: Thermo-Mechanical and Antioxidant Properties. Polymers 2025, 17, 1157. https://doi.org/10.3390/polym17091157
Garrido-Miranda KA, Gonzalez ME, Hernandez-Montelongo J, Jaramillo A, Oñate A, Burgos-Díaz C, Manso-Silvan M. Poly(3-hydroxybutyrate)/Clay/Essential Oils Bionanocomposites Incorporating Biochar: Thermo-Mechanical and Antioxidant Properties. Polymers. 2025; 17(9):1157. https://doi.org/10.3390/polym17091157
Chicago/Turabian StyleGarrido-Miranda, Karla A., María Eugenia Gonzalez, Jacobo Hernandez-Montelongo, Andrés Jaramillo, Angelo Oñate, César Burgos-Díaz, and Miguel Manso-Silvan. 2025. "Poly(3-hydroxybutyrate)/Clay/Essential Oils Bionanocomposites Incorporating Biochar: Thermo-Mechanical and Antioxidant Properties" Polymers 17, no. 9: 1157. https://doi.org/10.3390/polym17091157
APA StyleGarrido-Miranda, K. A., Gonzalez, M. E., Hernandez-Montelongo, J., Jaramillo, A., Oñate, A., Burgos-Díaz, C., & Manso-Silvan, M. (2025). Poly(3-hydroxybutyrate)/Clay/Essential Oils Bionanocomposites Incorporating Biochar: Thermo-Mechanical and Antioxidant Properties. Polymers, 17(9), 1157. https://doi.org/10.3390/polym17091157











