Recent Progress in Cellulose-Based Conductive Hydrogels
Abstract
1. Introduction
2. Development and Use of Cellulose
2.1. Overview of Cellulose
2.2. Structure of Cellulose
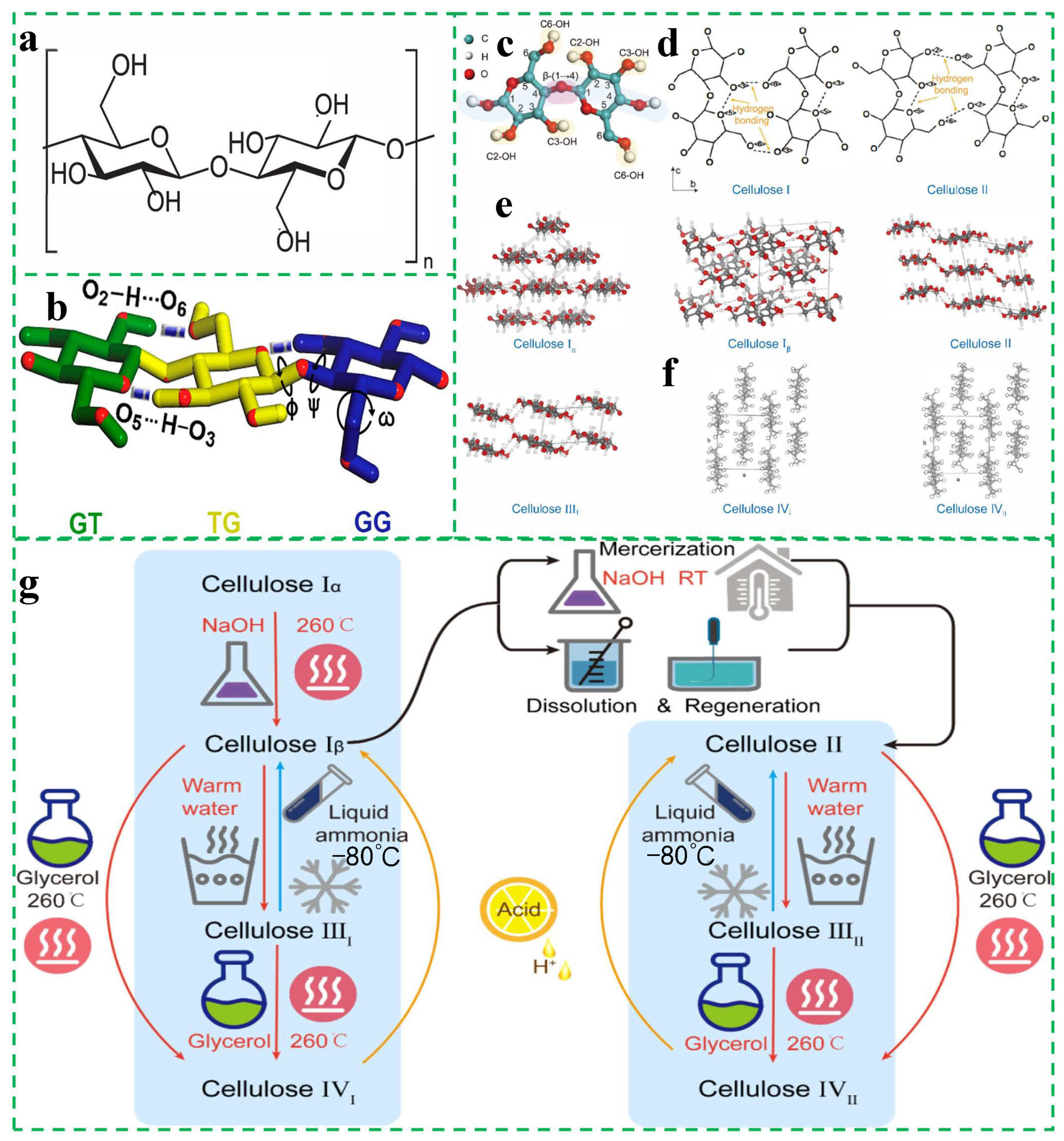
2.3. Sources and Classification of Cellulose in Practical Applications
3. Cellulose-Based Conductive Hydrogels
3.1. Overview of Cellulose-Based Hydrogels
3.1.1. Overview of Hydrogels
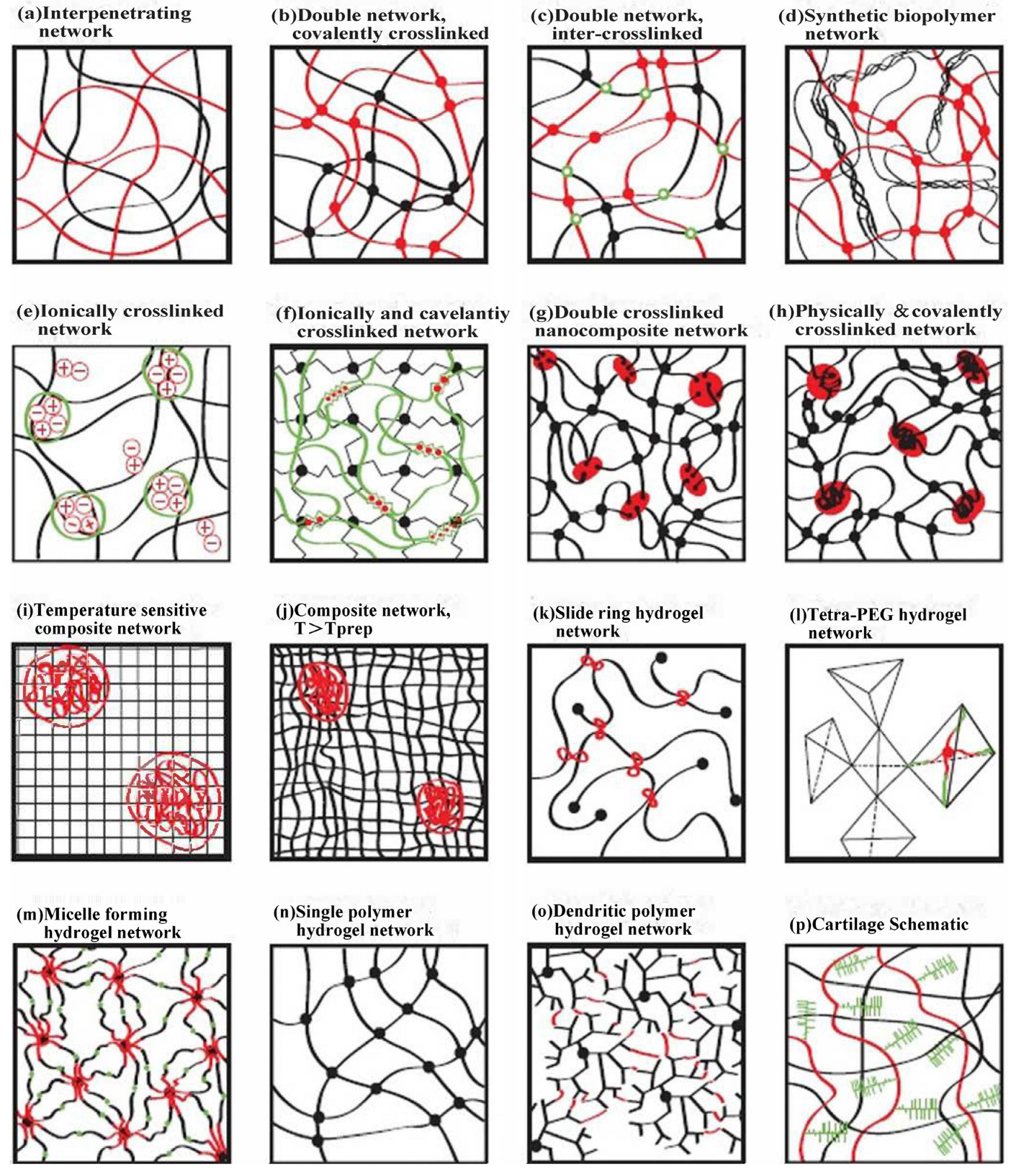
3.1.2. Structure of Cellulose-Based Hydrogel Networks
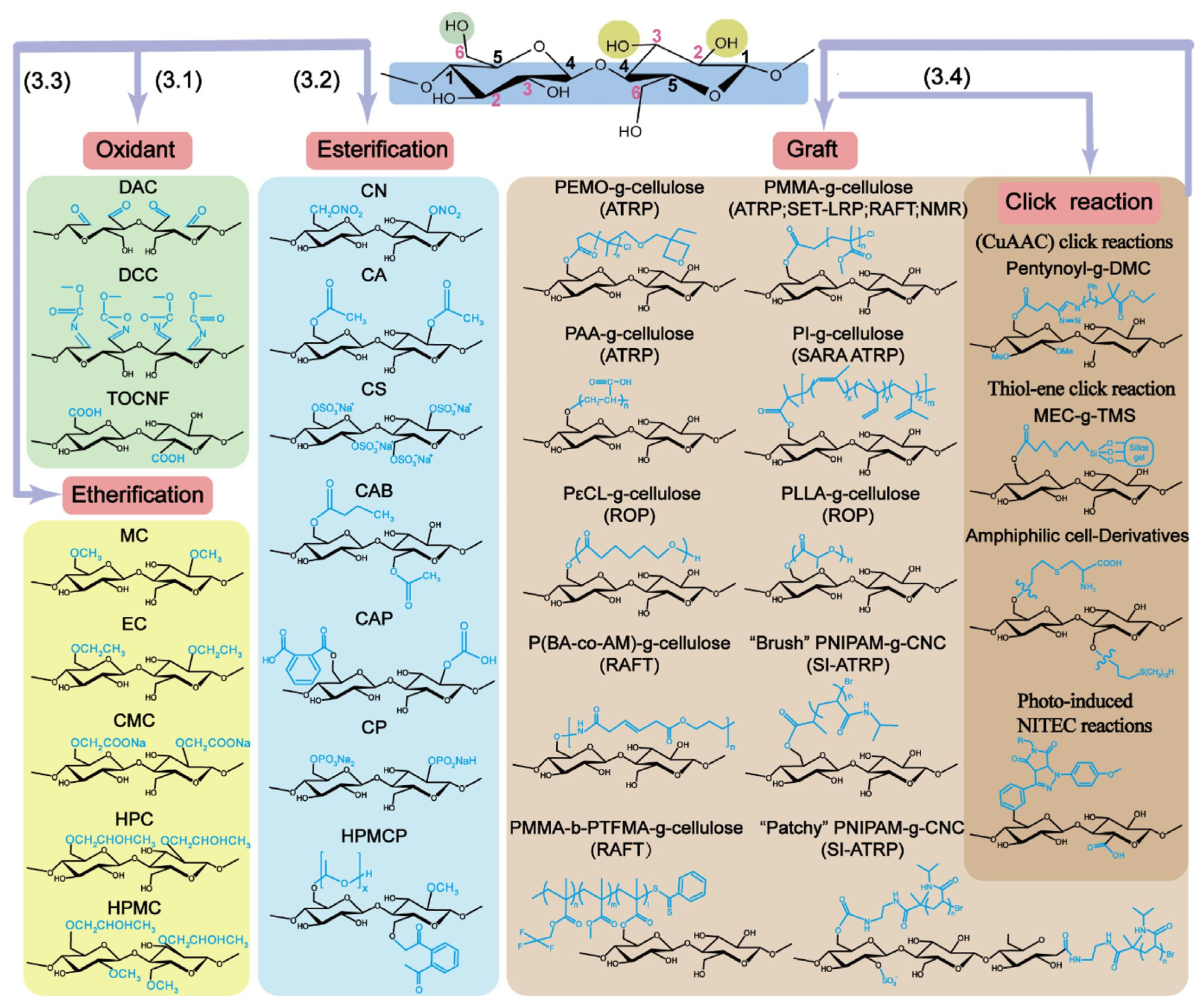
3.2. Construction of Cellulose-Based Conductive Hydrogels
3.2.1. Conductive Forms of Cellulose-Based Conductive Hydrogels
- Electronically Conductive Cellulose-Based Hydrogels
- Metal-Based Cellulose Conductive Hydrogels
- Carbon-Based Cellulose Conductive Hydrogels
- Conductive Polymer-Based Cellulose Conductive Hydrogels
- Ionically Conductive Cellulose-Based Hydrogels
- Electrolyte-Based Cellulose Conductive Hydrogels
- Ionic Liquid-Based Cellulose Conductive Hydrogels
3.2.2. Performance Requirements for Cellulose-Based Conductive Hydrogels
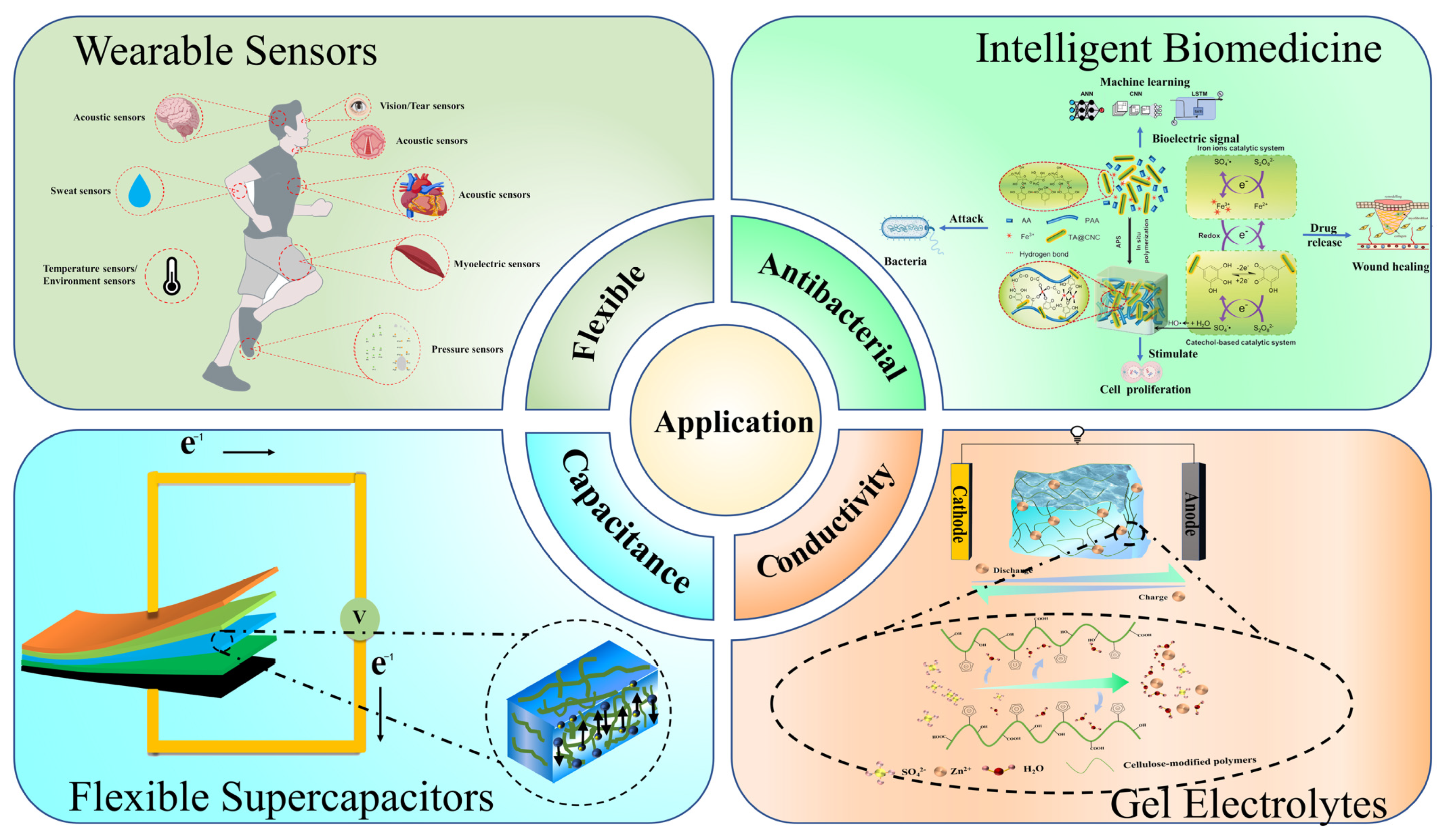
4. Applications of Cellulose-Based Conductive Hydrogels
4.1. Wearable Sensors
4.2. Intelligent Biomedicine
4.3. Flexible Supercapacitors
4.4. Application of Gel Electrolytes in Conventional Batteries
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Xing, S.; Yin, H.; Weisbecker, H.; Tran, H.T.; Guo, Z.; Han, T.; Wang, Y.; Liu, Y.; Wu, Y.; et al. Skin-inspired, sensory robots for electronic implants. Nat. Commun. 2024, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Min, J.; Ko, S.H. Recent Developments and Future Directions of Wearable Skin Biosignal Sensors. Adv. Sens. Res. 2024, 3, 2300118. [Google Scholar] [CrossRef]
- Duan, S.; Zhang, H.; Liu, L.; Lin, Y.; Zhao, F.; Chen, P.; Cao, S.; Zhou, K.; Gao, C.; Liu, Z.; et al. A comprehensive review on triboelectric sensors and AI-integrated systems. Mater. Today 2024, 80, 450–480. [Google Scholar] [CrossRef]
- Sunwoo, S.-H.; Ha, K.-H.; Lee, S.; Lu, N.; Kim, D.-H. Wearable and Implantable Soft Bioelectronics: Device Designs and Material Strategies. Annu. Rev. Chem. Biomol. Eng. 2021, 12, 359–391. [Google Scholar] [CrossRef]
- Xu, P.; Wang, S.; Lin, A.; Min, H.-K.; Zhou, Z.; Dou, W.; Sun, Y.; Huang, X.; Tran, H.; Liu, X. Conductive and elastic bottlebrush elastomers for ultrasoft electronics. Nat. Commun. 2023, 14, 12. [Google Scholar] [CrossRef]
- Dai, S.; Dai, Y.; Zhao, Z.; Xia, F.; Li, Y.; Liu, Y.; Cheng, P.; Strzalka, J.; Li, S.; Li, N.; et al. Intrinsically stretchable neuromorphic devices for on-body processing of health data with artificial intelligence. Matter 2022, 5, 3375–3390. [Google Scholar] [CrossRef]
- Xue, Z.; Gai, Y.; Wu, Y.; Liu, Z.; Li, Z. Wearable mechanical and electrochemical sensors for real-time health monitoring. Commun. Mater. 2024, 5, 211. [Google Scholar] [CrossRef]
- Zhou, Q.; Lu, S.; Huang, C.; Puglia, D.; Xu, P.; Niu, D.; Yang, W.; Ma, P. Polyvinyl alcohol/sodium alginate hydrogels with tunable mechanical and conductive properties for flexible sensing applications. Int. J. Biol. Macromol. 2024, 283, 137822. [Google Scholar] [CrossRef]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.D.; Cranston, E.D.; Eichhorn, S.J.; et al. Current characterization methods for cellulose nanomaterials. Chem. Soc. Rev. 2018, 47, 2609–2679. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, L.; Ding, C.; Pan, M. Eco-friendly wood-plastic composites from laminate sanding dust and waste poly(propylene) food pails. Waste Manag. 2022, 149, 96–104. [Google Scholar] [CrossRef]
- Grigoras, A. Investigation of Cellulose-Based Materials Applied in Life Sciences Using Laser Light Scattering Methods. Polymers 2024, 16, 39. [Google Scholar] [CrossRef] [PubMed]
- PR Newswire. Global Precious Metals E-Waste Recovery Research Report 2024–2030 Featuring Johnson Matthey, Sims, EnviroLeach Technologies, Umicore, Materion, Boliden, and DOWA; PR Newswire: New York, NY, USA, 2024. [Google Scholar]
- Dutta, S.D.; Patel, D.K.; Lim, K. Functional cellulose-based hydrogels as extracellular matrices for tissue engineering. J. Biol. Eng. 2019, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Zabed, H.; Sahu, J.; Boyce, A.; Faruq, G. Fuel ethanol production from lignocellulosic biomass: An overview on feedstocks and technological approaches. Renew. Sustain. Energy Rev. 2016, 66, 751–774. [Google Scholar] [CrossRef]
- Liang, J.; Li, Z.; Dai, S.; Tian, G.; Wang, Z. Production of hemicelluloses sugars, cellulose pulp, and lignosulfonate surfactant using corn stalk by prehydrolysis and alkaline sulfite cooking. Ind. Crops Prod. 2023, 192, 115880. [Google Scholar] [CrossRef]
- Fitria; Ruan, H.; Fransen, S.C.; Carter, A.H.; Tao, H.; Yang, B. Selecting winter wheat straw for cellulosic ethanol production in the Pacific Northwest, U.S.A. Biomass Bioenergy 2019, 123, 59–69. [Google Scholar]
- Wildschut, J.; Smit, A.J.; Reith, J.H.; Huijgen, W.J.J. Ethanol-based organosolv fractionation of wheat straw for the production of lignin and enzymatically digestible cellulose. Bioresour. Technol. 2013, 135, 58–66. [Google Scholar] [CrossRef]
- Saputra, A.M.A.; Ibadurrahman, M.; Piliang, A.F.R.; Marpongahtun; Ong, A.J.; Goei, R.; Tok, A.I.Y.; Ikhtiari, R.; Gea, S.; Zuhra, C.F. Optimising cellulose nanofiber extraction from water hyacinth (Eichhornia crassipes) stems: Effects of steam explosion pretreatment and ultrasonication time. JCIS Open 2025, 17, 100129. [Google Scholar] [CrossRef]
- Hemida, M.H.; Moustafa, H.; Mehanny, S.; Morsy, M.; Dufresne, A.; EL Rahman, E.N.A.; Ibrahim, M. Cellulose nanocrystals from agricultural residues (Eichhornia crassipes): Extraction and characterization. Heliyon 2023, 9, e16436. [Google Scholar] [CrossRef]
- Hietala, M.; Varrio, K.; Berglund, L.; Soini, J.; Oksman, K. Potential of municipal solid waste paper as raw material for production of cellulose nanofibres. Waste Manag. 2018, 80, 319–326. [Google Scholar] [CrossRef]
- Bregado, J.L.; Secchi, A.R.; Tavares, F.W.; de Sousa Rodrigues, D.; Gambetta, R. Amorphous paracrystalline structures from native crystalline cellulose: A molecular dynamics protocol. Fluid Phase Equilibr. 2019, 491, 56–76. [Google Scholar] [CrossRef]
- Muddasar, M.; Beaucamp, A.; Culebras, M.; Collins, M.N. Cellulose: Characteristics and applications for rechargeable batteries. Int. J. Biol. Macromol. 2022, 219, 788–803. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Zhang, H.; Su, Z.; Zhang, X.; Zhou, H.; Yu, L.; Chen, C.; Wang, X. A Biodegradable, Waterproof, and Thermally Processable Cellulosic Bioplastic Enabled by Dynamic Covalent Modification. Adv. Mater. 2023, 35, 10. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Zhu, Y.; Jiang, G.; Cao, K.; Zeng, S.; Chen, W.; Zhao, D.; Yu, H. Sustainable cellulose and its derivatives for promising biomedical applications. Prog. Mater. Sci. 2023, 138, 101152. [Google Scholar] [CrossRef]
- Yamanaka, S.; Sugiyama, J. Structural modification of bacterial cellulose. Cellulose 2000, 7, 213–225. [Google Scholar] [CrossRef]
- Petersen, N.; Gatenholm, P. Bacterial cellulose-based materials and medical devices: Current state and perspectives. Appl. Microbiol. Biotechnol. 2011, 91, 1277–1286. [Google Scholar] [CrossRef]
- Wang, X.; Fang, J.; Zhu, W.; Zhong, C.; Ye, D.; Zhu, M.; Lu, X.; Zhao, Y.; Ren, F. Bioinspired Highly Anisotropic, Ultrastrong and Stiff, and Osteoconductive Mineralized Wood Hydrogel Composites for Bone Repair. Adv. Funct. Mater. 2021, 31, 10. [Google Scholar] [CrossRef]
- Yamada, Y.; Yukphan, P.; Vu, H.T.L.; Muramatsu, Y.; Ochaikul, D.; Tanasupawat, S.; Nakagawa, Y. Description of Komagataeibacter gen. nov., with proposals of new combinations (Acetobacteraceae). J. Gen. Appl. Microbiol. 2012, 58, 397–404. [Google Scholar] [CrossRef]
- Keshk, S.; Sameshima, K. Evaluation of different carbon sources for bacterial cellulose production. Afr. J. Biotechnol. 2005, 4, 478–482. [Google Scholar]
- Zhang, T.; Zhang, S.; Wang, Y.; Peng, Z.; Xin, B.; Zhong, C. Tandem GGDEF-EAL Domain Proteins Pleiotropically Modulate c-di-GMP Metabolism Enrolled in Bacterial Cellulose Biosynthesis. J. Agric. Food Chem. 2025, 73, 1982–1993. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Tsai, M.-L.; Chen, C.-W.; Di Dong, C. Genetic modification for enhancing bacterial cellulose production and its applications. Bioengineered 2021, 12, 6793–6807. [Google Scholar] [CrossRef]
- Przygrodzka, K.; Charęza, M.; Banaszek, A.; Zielińska, B.; Ekiert, E.; Drozd, R. Bacterial Cellulose Production by Komagateibacter xylinus with the Use of Enzyme-Degraded Oligo- and Polysaccharides as the Substrates. Appl. Sci. 2022, 12, 16. [Google Scholar] [CrossRef]
- Sumini, M.; Andrade, G.J.S.; Tischer, C.A.; Kobayashi, R.K.T.; Nakazato, G. Production of bacterial cellulose by Komagataeibacter xylinus: Biochemistry, synthesis and applications. Cellulose 2025, 32, 81–94. [Google Scholar] [CrossRef]
- Li, W.; Huang, X.; Liu, H.; Lian, H.; Xu, B.; Zhang, W.; Sun, X.; Wang, W.; Jia, S.; Zhong, C. Improvement in bacterial cellulose production by co-culturing Bacillus cereus and Komagataeibacter xylinus. Carbohydr. Polym. 2023, 313, 10. [Google Scholar] [CrossRef] [PubMed]
- Thongsuk, K.; Tippayasak, U.; Sukkasem, T.; Naloka, K.; Puangsin, B.; Chonudomkul, D.; Yakushi, T.; Theeragool, G. Production of probiotic bacterial cellulose with improved yield, mechanical properties, and antibacterial activity from cost-effective coculture and mixed-culture fermentation in coconut water by Komagataeibacter xylinus MSKU 12. Int. J. Biol. Macromol. 2025, 291, 14. [Google Scholar] [CrossRef]
- de Oliveira Barud, H.G.; Da Silva, R.R.; Da Silva Barud, H.; Tercjak, A.; Gutierrez, J.; Lustri, W.R.; de Oliveira Junior, O.B.; Ribeiro, S.J.L. A multipurpose natural and renewable polymer in medical applications: Bacterial cellulose. Carbohydr. Polym. 2016, 153, 406–420. [Google Scholar] [CrossRef]
- Lin, S.-P.; Kung, H.-N.; Tsai, Y.-S.; Tseng, T.-N.; Hsu, K.-D.; Cheng, K.-C. Novel dextran modified bacterial cellulose hydrogel accelerating cutaneous wound healing. Cellulose 2017, 24, 4927–4937. [Google Scholar] [CrossRef]
- Shi, Z.; Zang, S.; Jiang, F.; Huang, L.; Lu, D.; Ma, Y.; Yang, G. In situ nano—Assembly of bacterial cellulose—Polyaniline composites. RSC Adv. 2012, 2, 1040–1046. [Google Scholar] [CrossRef]
- Yusuf, J.; Firdaus, A.; Sapuan, S.; Rashid, U.; Ilyas, R.; Hassan, M.; Ansari, M.A. Nanocellulose-graphene hybrid composites: Fabrication, characterization, applications and environmental impact. Int. J. Biol. Macromol. 2024, 282, 137244. [Google Scholar] [CrossRef]
- Sobhiga, G.; Maria, H.J.; Mozetič, M.; Thomas, S. A review on green materials: Exploring the potential of poly(vinyl alcohol) (PVA) and nanocellulose composites. Int. J. Biol. Macromol. 2024, 283, 137176. [Google Scholar] [CrossRef]
- Ouyang, S.; Wang, F.; Liu, Y.; Ma, S.; Li, M.; Wu, Y.; Hu, Z.; Zhang, S.; Wang, L. Advances in the enhancement of mechanical and hydrophobic properties of nanocellulose-based packaging materials: A review. Int. J. Biol. Macromol. 2024, 282, 137392. [Google Scholar] [CrossRef]
- Ilyas, R.; Asyraf, M.; Rajeshkumar, L.; Awais, H.; Siddique, A.; Shaker, K.; Nawab, Y.; Wahit, M.U. A review of bio-based nanocellulose epoxy composites. J. Environ. Chem. Eng. 2024, 12, 113835. [Google Scholar] [CrossRef]
- Sun, H.; Lu, Y.; Chen, Y.; Yue, Y.; Jiang, S.; Xu, X.; Mei, C.; Xiao, H.; Han, J. Flexible environment-tolerant electroluminescent devices based on nanocellulose-mediated transparent electrodes. Carbohydr. Polym. 2022, 296, 119891. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Qian, Y.; Zhu, P.; Xiang, L.; Lei, C.; Qiu, G.; Wang, C.; Liu, Y.; Liu, Y.; Chen, G. Nanocellulose-templated carbon nanotube enhanced conductive organohydrogel for highly-sensitive strain and temperature sensors. Cellulose 2022, 29, 3829–3844. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, R.; Yuan, M.; He, H. Nano Ag/PPy Biocomposites Based on Graphene Oxide Modified Bacterial Cellulose from the Juice of Xinhui Citrus and Its Antibacterial Activity. Micromachines 2023, 14, 13. [Google Scholar] [CrossRef]
- Shang, X.; Li, C.; Xi, X.; Wei, Z.; Wu, Z.; Lei, H.; Du, G. Biomass cellulose-based wood adhesive with improved water resistance and bonding strength by esterification reaction. Ind. Crops Prod. 2024, 222, 119667. [Google Scholar] [CrossRef]
- Nguyen, D.T. Insight into etherification of 1,3-dimethylol-2-imidazolidinone with the primary alcohols and the hydroxyl groups of cellulose chain (n = 1–4) in acidic condition. Comput. Theor. Chem. 2023, 1226, 114208. [Google Scholar] [CrossRef]
- Roberts, M.G.; Yu, Q.; Keunen, R.; Liu, J.; Wong, E.C.N.; Rastogi, C.K.; Reilly, R.M.; Allen, C.; Winnik, M.A. Functionalization of Cellulose Nanocrystals with POEGMA Copolymers via Copper-Catalyzed Azide–Alkyne Cycloaddition for Potential Drug-Delivery Applications. Biomacromolecules 2020, 21, 2014–2023. [Google Scholar] [CrossRef]
- Alshammari, O.A.; Alhar, M.S.; Elsayed, N.H.; Monier, M.; Youssef, I. Synthesis of furan-modified cationic cellulose for stereo-specific imprinting and separation of S-indacrinone via Diels-Alder reaction. Int. J. Biol. Macromol. 2024, 275, 133384. [Google Scholar] [CrossRef]
- Liang, H.; Yin, D.; Shi, L.; Liu, Y.; Hu, X.; Zhu, N.; Guo, K. Surface modification of cellulose via photo-induced click reaction. Carbohydr. Polym. 2023, 301, 120321. [Google Scholar] [CrossRef]
- Guo, C.; Zhu, A.; Wang, X.; Dai, J.; Luo, L.; Xu, Y.; Zeng, B.; Chen, G.; Dai, L. Ultra-stretchable and anti-freezing conductive organohydrogel reinforced with ionic clusters for wearable strain sensors. Sens. Actuators B Chem. 2022, 362, 131796. [Google Scholar] [CrossRef]
- Dehkordi, N.K.; Shojaei, S.; Asefnejad, A.; Hassani, K.; Benisi, S.Z. Investigation of mechanical properties and the effect of volume fraction of polyacrylamide hydrogel with molecular dynamics simulation. Results Phys. 2024, 57, 107440. [Google Scholar] [CrossRef]
- Moura, L.I.; Dias, A.M.; Carvalho, E.; de Sousa, H.C. Recent advances on the development of wound dressings for diabetic foot ulcer treatment—A review. Acta Biomater. 2013, 9, 7093–7114. [Google Scholar] [CrossRef] [PubMed]
- Ghamari, M.; Sun, D.; Dai, Y.; See, C.H.; Yu, H.; Edirisinghe, M.; Sundaram, S. Valorization of diverse waste-derived nanocellulose for multifaceted applications: A review. Int. J. Biol. Macromol. 2024, 280, 136130. [Google Scholar] [CrossRef]
- Peak, C.W.; Wilker, J.J.; Schmidt, G. A review on tough and sticky hydrogels. Colloid Polym. Sci. 2013, 291, 2031–2047. [Google Scholar] [CrossRef]
- Yang, F.; He, W.; Li, H.; Zhang, X.; Feng, Y. Role of acid treatment combined with the use of urea in forming cellulose hydrogel. Carbohydr. Polym. 2019, 223, 115059. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, J.; Zhong, Y.; Li, K.; Zhang, L.; Cai, J. High-Strength and High-Toughness Double-Cross-Linked Cellulose Hydrogels: A New Strategy Using Sequential Chemical and Physical Cross-Linking. Adv. Funct. Mater. 2018, 28, 1. [Google Scholar] [CrossRef]
- Jeong, D.; Joo, S.-W.; Hu, Y.; Shinde, V.V.; Cho, E.; Jung, S. Carboxymethyl cellulose-based superabsorbent hydrogels containing carboxymehtyl β-cyclodextrin for enhanced mechanical strength and effective drug delivery. Eur. Polym. J. 2018, 105, 17–25. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, X.; Yuk, H.; Lin, S.; Liu, X.; Parada, G. Soft Materials by Design: Unconventional Polymer Networks Give Extreme Properties. Chem. Rev. 2021, 121, 4309–4372. [Google Scholar] [CrossRef]
- Dragan, E.S. Design and applications of interpenetrating polymer network hydrogels. A review. Chem. Eng. J. 2014, 243, 572–590. [Google Scholar] [CrossRef]
- Mu, Q.; Cui, K.; Wang, Z.J.; Matsuda, T.; Cui, W.; Kato, H.; Namiki, S.; Yamazaki, T.; Frauenlob, M.; Nonoyama, T.; et al. Force-triggered rapid microstructure growth on hydrogel surface for on-demand functions. Nat. Commun. 2022, 13, 10. [Google Scholar] [CrossRef]
- Wang, K.; Ma, Q.; Zhang, Y.-M.; Han, G.-T.; Qu, C.-X.; Wang, S.-D. Preparation of bacterial cellulose/silk fibroin double-network hydrogel with high mechanical strength and biocompatibility for artificial cartilage. Cellulose 2020, 27, 1845–1852. [Google Scholar] [CrossRef]
- Song, L.; Liu, F.; Zhu, C.; Li, A. Facile one-step fabrication of carboxymethyl cellulose based hydrogel for highly efficient removal of Cr(VI) under mild acidic condition. Chem. Eng. J. 2019, 369, 641–651. [Google Scholar] [CrossRef]
- Du, M.; Lu, W.; Zhang, Y.; Mata, A.; Fang, Y. Natural polymer-sourced interpenetrating network hydrogels: Fabrication, properties, mechanism and food applications. Trends Food Sci. Technol. 2021, 116, 342–356. [Google Scholar] [CrossRef]
- Kang, M.; Liang, H.; Hu, Y.; Wei, Y.; Huang, D. Gelatin-based hydrogels with tunable network structure and mechanical property for promoting osteogenic differentiation. Int. J. Biol. Macromol. 2024, 281, 136312. [Google Scholar] [CrossRef]
- Zhu, B.; Ma, D.; Wang, J.; Zhang, S. Structure and properties of semi-interpenetrating network hydrogel based on starch. Carbohydr. Polym. 2015, 133, 448–455. [Google Scholar] [CrossRef]
- Abel, S.B.; Busatto, C.A.; Karp, F.; Estenoz, D.; Calderón, M. Weaving the next generation of (bio)materials: Semi-interpenetrated and interpenetrated polymeric networks for biomedical applications. Adv. Colloid Interface Sci. 2023, 321, 103026. [Google Scholar] [CrossRef]
- Cai, Z.; Hou, C.; Yang, G. Preparation and Characteristics of Cellulose/Polyurethane Semi-Interpenetrating Polymer Networks. Polym. Mater. Sci. Eng. 2011, 27, 154–157. [Google Scholar]
- Gomez, I.; Alesanco, Y.; Blázquez, J.A.; Viñuales, A.; Colmenares, L.C. Room-Temperature Self-Standing Cellulose-Based Hydrogel Electrolytes for Electrochemical Devices. Polymers 2020, 12, 17. [Google Scholar] [CrossRef]
- Yi, K.; Huang, J.; Pang, H.; Li, S.; Liu, Z.; Wang, X.; Zhang, W.; Zhang, C.; Liu, S.; Gu, Y. Semi-interpenetrating network hydrogels-based microcapsule for quorum quenching bacteria biocontainment to enhance biofouling control in membrane bioreactor. Chem. Eng. J. 2024, 486, 150103. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, Y.; Lv, M.; Qiao, X.; Fan, G.C.; Luo, X. An anti-fouling wearable molecular imprinting sensor based on semi-interpenetrating network hydrogel for the detection of tryptophan in sweat. Anal. Chim. Acta 2023, 1283, 341948. [Google Scholar] [CrossRef]
- Pal, A.; Nayak, J.; Roy, B.; Maiti, S.; Chowdhury, S.N.; Das, P.; Katheria, A.; Ray, S.K.; Chattopadhyay, S.; Das, N.C. Dual crosslinked interpenetrating polymer network-based porous hydrogel membrane for solid-state supercapacitor applications. Polymer 2024, 308, 127408. [Google Scholar] [CrossRef]
- Liu, J.; Lv, S.; Mu, Y.; Tong, J.; Liu, L.; He, T.; Zeng, Q.; Wei, D. Applied research and recent advances in the development of flexible sensing hydrogels from cellulose: A review. Int. J. Biol. Macromol. 2024, 281, 136100. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fu, S.; Jin, K.; Cheng, Y.; Li, Y.; Zhao, Y.; Liu, R.; Tian, Y. Advances in polysaccharide-based conductive hydrogel for flexible electronics. Carbohydr. Polym. 2025, 348, 122836. [Google Scholar] [CrossRef]
- Lim, B.H.; Kim, J.M.; Nguyen, V.T.; Kim, H.; Park, C.W.; Lee, J.K.; Lee, C.H.; Yoo, J.; Min, B.K.; Kim, S.K. Functionalized methyl cellulose/LiClO4 composite as an environmentally friendly quasi-solid polymer electrolyte for solid-state electrochromic devices and cellulose-based supercapacitors. Mater. Today Energy 2023, 33, 101263. [Google Scholar] [CrossRef]
- Xiao, G.; Wang, Y.; Zhang, H.; Zhu, Z.; Fu, S. Cellulose nanocrystal mediated fast self-healing and shape memory conductive hydrogel for wearable strain sensors. Int. J. Biol. Macromol. 2021, 170, 272–283. [Google Scholar] [CrossRef]
- Huang, F.; Wei, W.; Fan, Q.; Li, L.; Zhao, M.; Zhou, Z. Super-stretchable and adhesive cellulose Nanofiber-reinforced conductive nanocomposite hydrogel for wearable Motion-monitoring sensor. J. Colloid Interface Sci. 2022, 615, 215–226. [Google Scholar] [CrossRef]
- Lou, Y.; Wang, J.; Peng, Y.; Wang, X.; Zhang, J.; Chen, L.; Gao, W.; Gao, Z.; Li, X.; Chen, W.; et al. Double network hydrogel confined MXene/Liquid metal by dynamic hydrogen bond for high-performance wearable sensors. Chem. Eng. J. 2024, 500, 156884. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, H.; Li, S.; Huang, L.; Zhang, R.; Zhang, L.; Yu, A.; Duan, B. Polyphenol-driving assembly for constructing chitin-polyphenol-metal hydrogel as wound dressing. Carbohydr. Polym. 2022, 290, 119444. [Google Scholar] [CrossRef]
- Lin, F.; Wang, Z.; Shen, Y.; Tang, L.; Zhang, P.; Wang, Y.; Chen, Y.; Huang, B.; Lu, B. Natural skin-inspired versatile cellulose biomimetic hydrogels. J. Mater. Chem. A 2019, 7, 26442–26455. [Google Scholar] [CrossRef]
- Zou, Y.; Liao, Z.; Zhang, R.; Song, S.; Yang, Y.; Xie, D.; Liu, X.; Wei, L.; Liu, Y.; Song, Y. Cellulose nanofibers/liquid metal hydrogels with high tensile strength, environmental adaptability and electromagnetic shielding for temperature monitoring and strain sensors. Carbohydr. Polym. 2025, 348, 122788. [Google Scholar] [CrossRef]
- Erol, O.; Uyan, I.; Hatip, M.; Yilmaz, C.; Tekinay, A.B.; Guler, M.O. Recent advances in bioactive 1D and 2D carbon nanomaterials for biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2433–2454. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Lu, K.; Lu, Y.; Zhu, S.; Yue, Y.; Xu, X.; Mei, C.; Xiao, H.; Wu, Q.; Han, J. A stretchable, self-healing conductive hydrogels based on nanocellulose supported graphene towards wearable monitoring of human motion. Carbohydr. Polym. 2020, 250, 116905. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Liu, F.; Abdiryim, T.; Chen, J.; Liu, X. Sodium carboxymethyl cellulose and MXene reinforced multifunctional conductive hydrogels for multimodal sensors and flexible supercapacitors. Carbohydr. Polym. 2024, 327, 121677. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Lu, Z.; Hu, C.; Gao, Y.; Zhu, J.; Hu, W. Research progress on conductive polymer actuators: Mechanisms, performance improvement, and applications. Mater. Today Commun. 2024, 41, 110828. [Google Scholar] [CrossRef]
- Chen, C.; Li, Y.; Qian, C.; Liu, X.; Yang, Y.; Han, L.; Han, Q. Carboxymethyl cellulose assisted PEDOT in polyacrylamide hydrogel for high performance supercapacitors and self-powered sensing system. Eur. Polym. J. 2022, 179, 111563. [Google Scholar] [CrossRef]
- Li, Y.; Gong, Q.; Liu, X.; Xia, Z.; Yang, Y.; Chen, C.; Qian, C. Wide temperature-tolerant polyaniline/cellulose/polyacrylamide hydrogels for high-performance supercapacitors and motion sensors. Carbohydr. Polym. 2021, 267, 118207. [Google Scholar] [CrossRef]
- Suneetha, M.; Moo, O.S.; Choi, S.M.; Zo, S.; Rao, K.M.; Han, S.S. Tissue-adhesive, stretchable, and self-healable hydrogels based on carboxymethyl cellulose-dopamine/PEDOT:PSS via mussel-inspired chemistry for bioelectronic applications. Chem. Eng. J. 2021, 426, 130847. [Google Scholar] [CrossRef]
- Li, H.; Jia, Y.; Bai, S.; Peng, H.; Li, J. Metal-chelated polydopamine nanomaterials: Nanoarchitectonics and applications in biomedicine, catalysis, and energy storage. Adv. Colloid Interface Sci. 2024, 334, 103316. [Google Scholar] [CrossRef]
- Wei, D.; Lv, S.; Zuo, J.; Liu, J.; Wang, J.; Liu, L.; Zeng, Q. Engineering versatile bi-network ionic conductive hydrogels wearable sensors via on demand graft modification for real-time human movement monitoring. Chem. Eng. J. 2024, 496, 154176. [Google Scholar] [CrossRef]
- Zhou, L.; Fan, L.; Yi, X.; Zhou, Z.; Liu, C.; Fu, R.; Dai, C.; Wang, Z.; Chen, X.; Yu, P.; et al. Soft Conducting Polymer Hydrogels Cross-Linked and Doped by Tannic Acid for Spinal Cord Injury Repair. ACS Nano 2018, 12, 10957–10967. [Google Scholar] [CrossRef]
- Wang, L.; Luo, M.; Zhang, Z.; Ji, D.; Chang, X.; Zhu, Y. Ultra-stretchable, robust, self-healable conductive hydrogels enabled by the synergistic effects of hydrogen bonds and ionic coordination bonds toward high-performance e-skins. Chem. Eng. J. 2024, 500, 156800. [Google Scholar] [CrossRef]
- Dong, Z.; Sheng, Z.; Shen, Z.; Qu, S.; Jia, Z. Mechanics of electroadhesion of polyelectrolyte hydrogel heterojunctions enabled by ionic double layers. J. Mech. Phys. Solids 2024, 195, 105960. [Google Scholar] [CrossRef]
- Yu, J.; Feng, Y.; Sun, D.; Ren, W.; Shao, C.; Sun, R. Highly Conductive and Mechanically Robust Cellulose Nanocomposite Hydrogels with Antifreezing and Antidehydration Performances for Flexible Humidity Sensors. ACS Appl. Mater. Interfaces 2022, 14, 10886–10897. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Zhang, Y.; Ma, J.; Fan, M.; Zhang, S.; Wang, J. Ionic liquids for advanced materials. Mater. Today Nano 2022, 17, 100159. [Google Scholar] [CrossRef]
- Sheng, H.; Li, R.; Li, R.; Li, L.; Li, S.; Li, Y.; Yuan, J.; Huang, J.; Xu, Q.; Zheng, Q.; et al. Molecularly engineered chitosan-derived poly(aprotic/protic ionic liquids) double-network hydrogel electrolytes for flexible supercapacitors and wearable strain sensors. Chem. Eng. J. 2024, 497, 154994. [Google Scholar] [CrossRef]
- Lee, H.; Erwin, A.; Buxton, M.L.; Kim, M.; Stryutsky, A.V.; Shevchenko, V.V.; Sokolov, A.P.; Tsukruk, V.V. Shape persistent, highly conductive ionogels from ionic liquids reinforced with cellulose nanocrystal network. Adv. Funct. Mater. 2021, 31, 2103083. [Google Scholar] [CrossRef]
- Zhu, M.; Gong, D.; Ji, Z.; Yang, J.; Wang, M.; Wang, Z.; Tao, S.; Wang, X.; Xu, M. Cellulose-reinforced poly(Ionic Liquids) composite hydrogel for infected wounds therapy and real-time reliable bioelectronic. Chem. Eng. J. 2023, 476, 146816. [Google Scholar] [CrossRef]
- Li, M.; Chen, D.; Sun, X.; Xu, Z.; Yang, Y.; Song, Y.; Jiang, F. An environmentally tolerant, highly stable, cellulose nanofiber-reinforced, conductive hydrogel multifunctional sensor. Carbohydr. Polym. 2022, 284, 10. [Google Scholar] [CrossRef]
- Zhang, X.F.; Ma, X.; Hou, T.; Guo, K.; Yin, J.; Wang, Z.; Shu, L.; He, M.; Yao, J. Inorganic Salt Induced Thermal Reversible and Anti-Freezing Cellulose Hydrogels. Angew. Chem. Int. Ed. 2019, 58, 7366–7370. [Google Scholar] [CrossRef]
- Giachini, P.A.; Gupta, S.S.; Wang, W.; Wood, D.; Yunusa, M.; Baharlou, E.; Sitti, M.; Menges, A. Additive manufacturing of cellulose-based materials with continuous, multidirectional stiffness gradients. Sci. Adv. 2020, 6, 11. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, S.; Sheng, N.; Wang, B.; Wu, Z.; Liang, Q.; Wang, H. Anisotropic bacterial cellulose hydrogels with tunable high mechanical performances, non-swelling and bionic nanofluidic ion transmission behavior. Nanoscale 2021, 13, 8126–8136. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, H.; Wang, R.; Zhou, J.; Zhao, L.; Li, Q.; Liu, Z. Preparation and application of environmentally-responsive hydrogels in tissue engineering. Mater. Today Commun. 2024, 40, 109493. [Google Scholar] [CrossRef]
- Kanafi, N.M.; Rahman, N.A.; Rosdi, N.H. Citric acid cross-linking of highly porous carboxymethyl cellulose/poly(ethylene oxide) composite hydrogel films for controlled release applications. Mater. Today Proc. 2019, 7, 721–731. [Google Scholar] [CrossRef]
- Oechsle, A.-L.; Lewis, L.; Hamad, W.Y.; Hatzikiriakos, S.G.; MacLachlan, M.J. CO2-Switchable Cellulose Nanocrystal Hydrogels. Chem. Mater. 2018, 30, 376–385. [Google Scholar] [CrossRef]
- Wu, M.; Han, L.; Yan, B.; Zeng, H. Self-healing hydrogels based on reversible noncovalent and dynamic covalent interactions: A short review. Supramol. Mater. 2023, 2, 100045. [Google Scholar] [CrossRef]
- Chen, M.; Chen, J.; Zhou, W.; Xu, J.; Wong, C.-P. High-performance flexible and self-healable quasi-solid-state zinc-ion hybrid supercapacitor based on borax-crosslinked polyvinyl alcohol/nanocellulose hydrogel electrolyte. J. Mater. Chem. A 2019, 7, 26524–26532. [Google Scholar] [CrossRef]
- Quan, Y.; Zhou, W.; Wu, T.; Chen, M.; Han, X.; Tian, Q.; Xu, J.; Chen, J. Sorbitol-modified cellulose hydrogel electrolyte derived from wheat straws towards high-performance environmentally adaptive flexible zinc-ion batteries. Chem. Eng. J. 2022, 446, 137056. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, F.; Chen, L.; Wei, X.; Xue, M.; Yang, F.; Jiang, S. 3D printing hydrogels for actuators: A review. Chin. Chem. Lett. 2021, 32, 2923–2932. [Google Scholar] [CrossRef]
- Gong, S.; Lu, Y.; Yin, J.; Levin, A.; Cheng, W. Materials-Driven Soft Wearable Bioelectronics for Connected Healthcare. Chem. Rev. 2024, 124, 455–553. [Google Scholar] [CrossRef]
- Ma, J.; Zhong, J.; Sun, F.; Liu, B.; Peng, Z.; Lian, J.; Wu, X.; Li, L.; Hao, M.; Zhang, T. Hydrogel sensors for biomedical electronics. Chem. Eng. J. 2024, 481, 148317. [Google Scholar] [CrossRef]
- Pan, X.; Li, J.; Ma, N.; Ma, X.; Gao, M. Bacterial cellulose hydrogel for sensors. Chem. Eng. J. 2023, 461, 142062. [Google Scholar] [CrossRef]
- Guo, W.; Ma, M. Conductive nanocomposite hydrogels for flexible wearable sensors. J. Mater. Chem. A 2024, 12, 9371–9399. [Google Scholar] [CrossRef]
- Song, M.; Yu, H.; Zhu, J.; Ouyang, Z.; Abdalkarim, S.Y.H.; Tam, K.C.; Li, Y. Constructing stimuli-free self-healing, robust and ultrasensitive biocompatible hydrogel sensors with conductive cellulose nanocrystals. Chem. Eng. J. 2020, 398, 125547. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, Y.; Zhu, J.; Le, K.; Servati, P.; Jiang, F. Tough and Ultrastretchable Liquid-Free Ion Conductor Strengthened by Deep Eutectic Solvent Hydrolyzed Cellulose Microfibers. Adv. Funct. Mater. 2022, 32, 10. [Google Scholar] [CrossRef]
- Qin, Y.; Mo, J.; Liu, Y.; Zhang, S.; Wang, J.; Fu, Q.; Wang, S.; Nie, S. Stretchable Triboelectric Self-Powered Sweat Sensor Fabricated from Self-Healing Nanocellulose Hydrogels. Adv. Funct. Mater. 2022, 32, 10. [Google Scholar] [CrossRef]
- Shi, H.; Huo, H.; Yang, H.; Li, H.; Shen, J.; Wan, J.; Du, G.; Yang, L. Cellulose-Based Dual-Network Conductive Hydrogel with Exceptional Adhesion. Adv. Funct. Mater. 2024, 34, 12. [Google Scholar] [CrossRef]
- Shi, S.; Wang, J.; Wang, W.; Chen, H.; Bai, L.; Yang, H.; Yang, L.; Wei, D.; Yin, K. Design of antifreeze, self-healing nanocomposite hydrogels with proline and functional cellulose nanocrystals for the application in wearable flexible sensors. Eur. Polym. J. 2024, 220, 113452. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, W.; Cao, X.; Li, B. Induction of polymer-grafted cellulose nanocrystals in hydrogel nanocomposites to increase anti-swelling, mechanical properties and conductive self-recovery for underwater strain sensing. Int. J. Biol. Macromol. 2024, 274, 133410. [Google Scholar] [CrossRef]
- Nada, A.A.; Šišková, A.O.; Kleinová, A.; Andicsová, A.E.; Šimon, E.; Mosnáček, J. Ionic conductive cellulose-based hydrogels for Al-air batteries: Influence of the charged-functional groups on the electrochemical properties. J. Power Sources 2023, 572, 233089. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, W.; Jin, L.; Chen, N.; Zhang, Y.; Ni, Y. Highly efficient room temperature grafting polymerization of cellulose for the synthesis of cellulose-g-polypeptide biological macromolecules. Int. J. Biol. Macromol. 2025, 306, 141685. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Armstrong, J.P.K.; Scarpa, F.; Perriman, A.W. Hydrogel-Based Artificial Synapses for Sustainable Neuromorphic Electronics. Adv. Mater. 2024, 36, 21. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Song, P.; Li, G.; Han, Y.; Ren, X.; Bai, L.; Su, J. AI energized hydrogel design, optimization and application in biomedicine. Mater. Today Bio 2024, 25, 19. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Sun, S.; Cheng, Y.; Ma, J.; Hu, Y.; Yang, Z.; Yao, H.; Zhang, Z. Opportunities and challenges of nanomaterials in wound healing: Advances, mechanisms, and perspectives. Chem. Eng. J. 2024, 495, 153640. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Saleh, B.; Dhaliwal, H.K.; Portillo-Lara, R.; Shirzaei Sani, E.; Abdi, R.; Amiji, M.M.; Annabi, N. Local Immunomodulation Using an Adhesive Hydrogel Loaded with miRNA-Laden Nanoparticles Promotes Wound Healing. Small 2019, 15, 15. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Peng, Y.; Liu, X.; Du, J.; Liao, J. Rapid Synthesis of Functions-Integrated Hydrogel as a Self-Powered Wound Dressing for Real-Time Drug Release and Health Monitoring. Adv. Healthc. Mater. 2024, 15, 2401704. [Google Scholar] [CrossRef]
- Patel, D.K.; Ganguly, K.; Hexiu, J.; Dutta, S.D.; Patil, T.V.; Lim, K.-T. Functionalized chitosan/spherical nanocellulose-based hydrogel with superior antibacterial efficiency for wound healing. Carbohydr. Polym. 2022, 284, 119202. [Google Scholar] [CrossRef]
- Yan, L.; Zhou, T.; Han, L.; Zhu, M.; Cheng, Z.; Li, D.; Ren, F.; Wang, K.; Lu, X. Conductive Cellulose Bio-Nanosheets Assembled Biostable Hydrogel for Reliable Bioelectronics. Adv. Funct. Mater. 2021, 31, 12. [Google Scholar] [CrossRef]
- Xu, D.; Fan, L.; Gao, L.; Xiong, Y.; Wang, Y.; Ye, Q.; Yu, A.; Dai, H.; Yin, Y.; Cai, J.; et al. Micro-Nanostructured Polyaniline Assembled in Cellulose Matrix via Interfacial Polymerization for Applications in Nerve Regeneration. ACS Appl. Mater. Interfaces 2016, 8, 17090–17097. [Google Scholar] [CrossRef]
- Li, Q.; Tian, B.; Tang, G.; Zhan, H.; Liang, J.; Guo, P.; Liu, Q.; Wu, W. Multifunctional conductive hydrogels for wearable sensors and supercapacitors. J. Mater. Chem. A 2024, 12, 3589–3600. [Google Scholar] [CrossRef]
- Zhou, S.; Zhao, Y.; Zhang, K.; Xun, Y.; Tao, X.; Yan, W.; Zhai, W.; Ding, J. Impact-resistant supercapacitor by hydrogel-infused lattice. Nat. Commun. 2024, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Chen, A. Application of supramolecular hydrogel in supercapacitors: Opportunities and challenges. Aggregate 2024, 5, e581. [Google Scholar] [CrossRef]
- Wan, H.; Qin, C.; Lu, A. A flexible, robust cellulose/phytic acid/polyaniline hydrogel for all-in-one supercapacitors and strain sensors. J. Mater. Chem. A 2022, 10, 17279–17287. [Google Scholar] [CrossRef]
- Lin, X.; Wang, M.; Zhao, J.; Wu, X.; Xie, J.; Yang, J. Super-tough and self-healable all-cellulose-based electrolyte for fast degradable quasi-solid-state supercapacitor. Carbohydr. Polym. 2023, 304, 120502. [Google Scholar] [CrossRef]
- Zhang, K.; Pang, Y.; Chen, C.; Wu, M.; Liu, Y.; Yu, S.; Li, L.; Ji, Z.; Pang, J. Stretchable and conductive cellulose hydrogel electrolytes for flexible and foldable solid-state supercapacitors. Carbohydr. Polym. 2022, 293, 119673. [Google Scholar] [CrossRef] [PubMed]
- Zu, C.; Li, J.; Cai, B.; Qiu, J.; Zhao, Y.; Yang, Q.; Li, H.; Yu, H. Separators with reactive metal oxide coatings for dendrite-free lithium metal anodes. J. Power Sources 2023, 555, 232336. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, H.; Li, L.; Chen, R.; Guo, S. Ionogel Electrolytes for High-Performance Lithium Batteries: A Review. Adv. Energy Mater. 2018, 8, 1702675. [Google Scholar] [CrossRef]
- Zhao, L.; Fu, J.; Du, Z.; Jia, X.; Qu, Y.; Yu, F.; Du, J.; Chen, Y. High-strength and flexible cellulose/PEG based gel polymer electrolyte with high performance for lithium ion batteries. J. Membr. Sci. 2020, 593, 117428. [Google Scholar] [CrossRef]
- Pu, J.; Gao, Y.; Geng, Z.; Zhang, Y.; Cao, Q.; Yang, J.; Zhao, X.; Wang, Y.; Wang, J.; Guan, C. Grafted MXene Assisted Bifunctional Hydrogel for Stable and Highly Sensitive Self-Powered Fibrous System. Adv. Funct. Mater. 2024, 34, 10. [Google Scholar] [CrossRef]
- Park, J.-H.; Park, S.H.; Joung, D.; Kim, C. Sustainable biopolymeric hydrogel interphase for dendrite-free aqueous zinc-ion batteries. Chem. Eng. J. 2022, 433, 133532. [Google Scholar] [CrossRef]
- Xu, L.; Meng, T.; Zheng, X.; Li, T.; Brozena, A.H.; Mao, Y.; Zhang, Q.; Clifford, B.C.; Rao, J.; Hu, L. Nanocellulose-Carboxymethylcellulose Electrolyte for Stable, High-Rate Zinc-Ion Batteries. Adv. Funct. Mater. 2023, 33, 2302098. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Z.; Wang, N.; Du, J. Recent Progress in Cellulose-Based Conductive Hydrogels. Polymers 2025, 17, 1089. https://doi.org/10.3390/polym17081089
Du Z, Wang N, Du J. Recent Progress in Cellulose-Based Conductive Hydrogels. Polymers. 2025; 17(8):1089. https://doi.org/10.3390/polym17081089
Chicago/Turabian StyleDu, Zhenrui, Na Wang, and Jie Du. 2025. "Recent Progress in Cellulose-Based Conductive Hydrogels" Polymers 17, no. 8: 1089. https://doi.org/10.3390/polym17081089
APA StyleDu, Z., Wang, N., & Du, J. (2025). Recent Progress in Cellulose-Based Conductive Hydrogels. Polymers, 17(8), 1089. https://doi.org/10.3390/polym17081089





