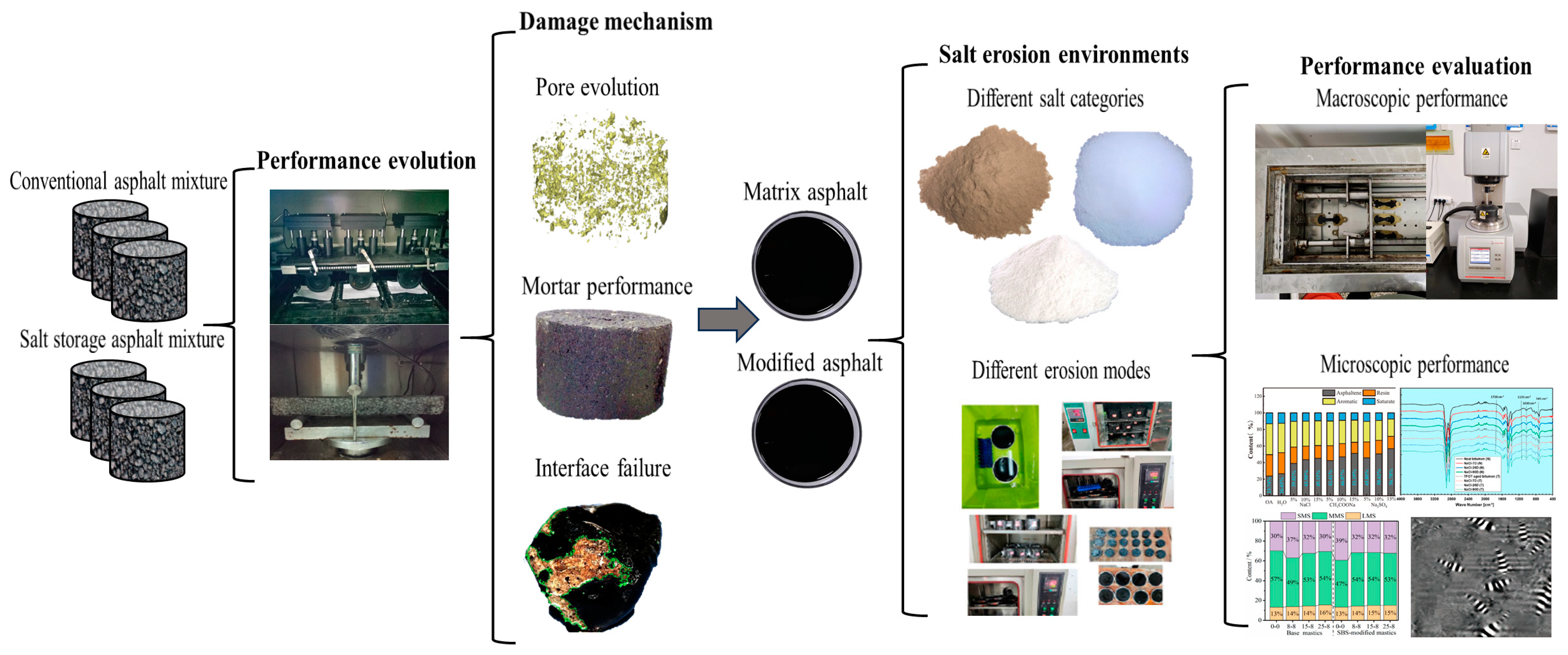
Mechanical Performance of Asphalt Materials Under Salt Erosion Environments: A Literature Review
Abstract
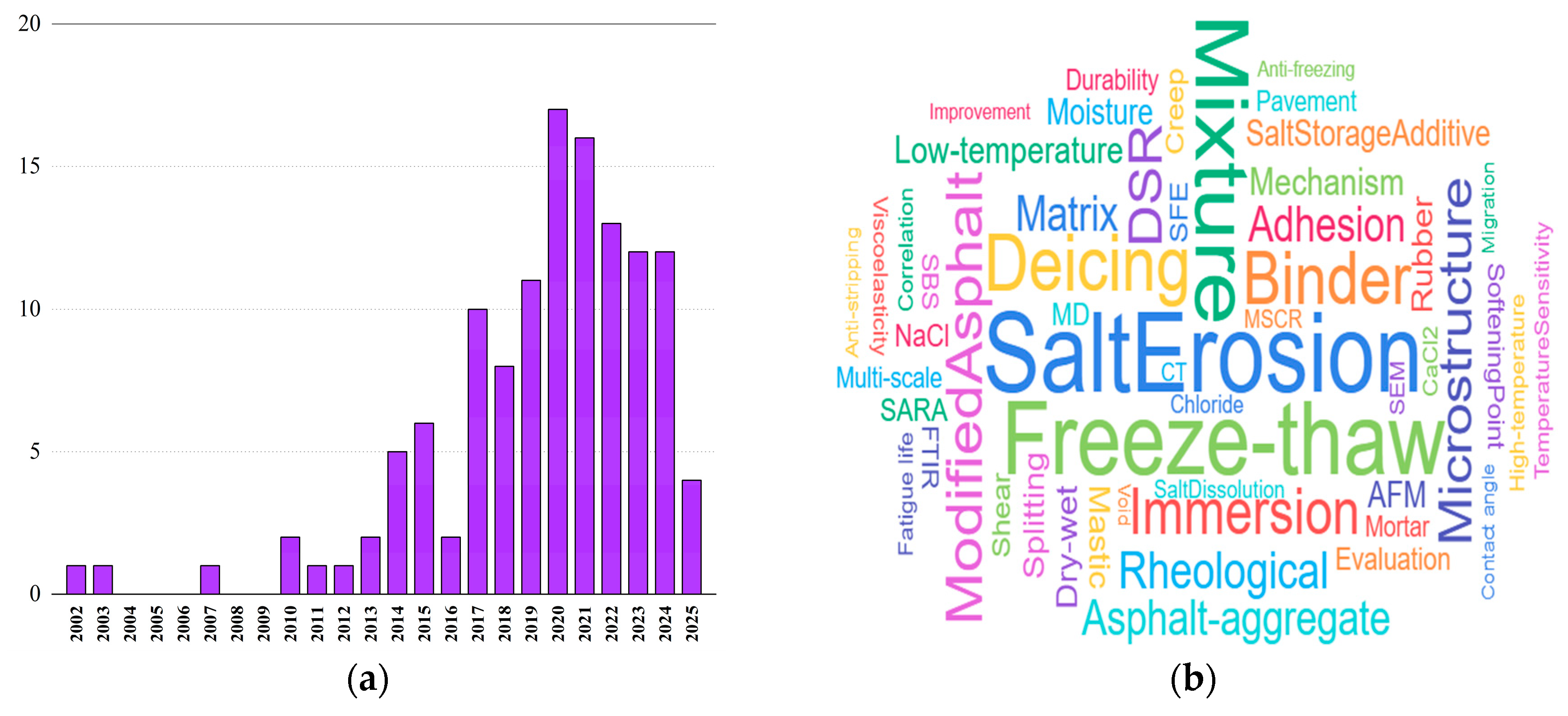
1. Introduction
2. Damage Characteristics of Asphalt Mixture Under Salt Erosion Environments
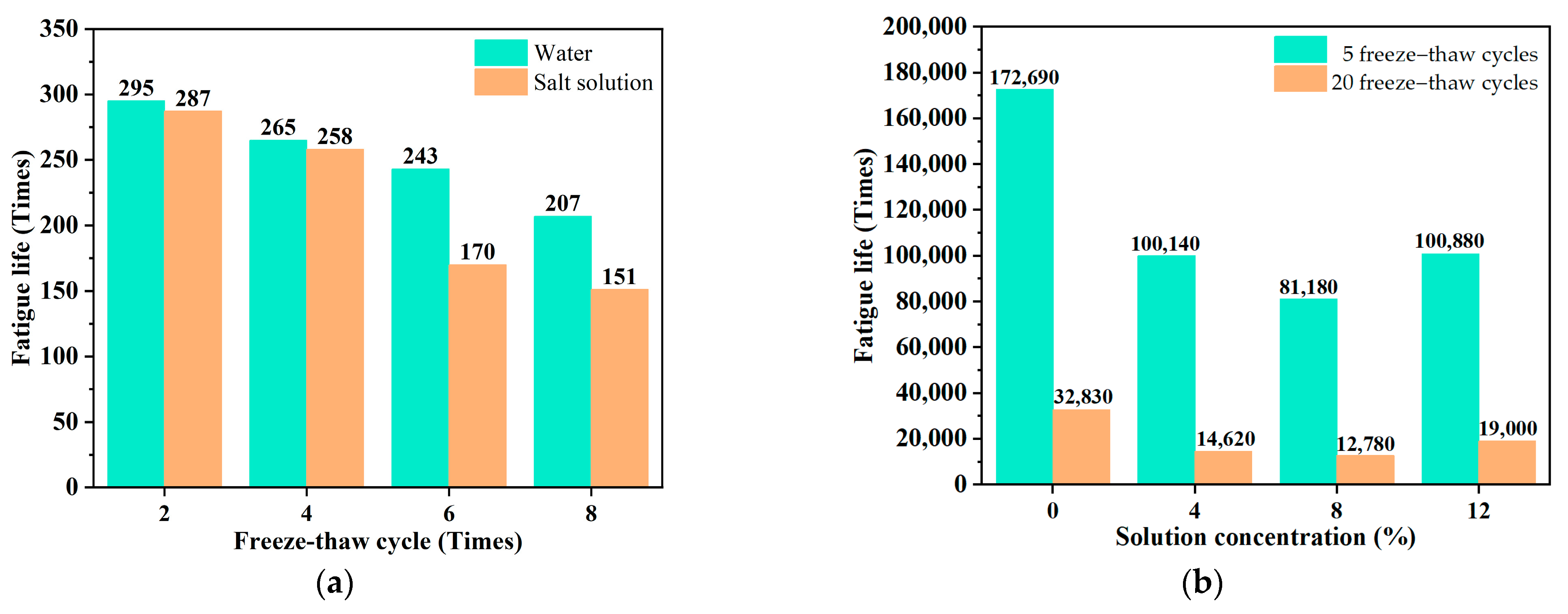
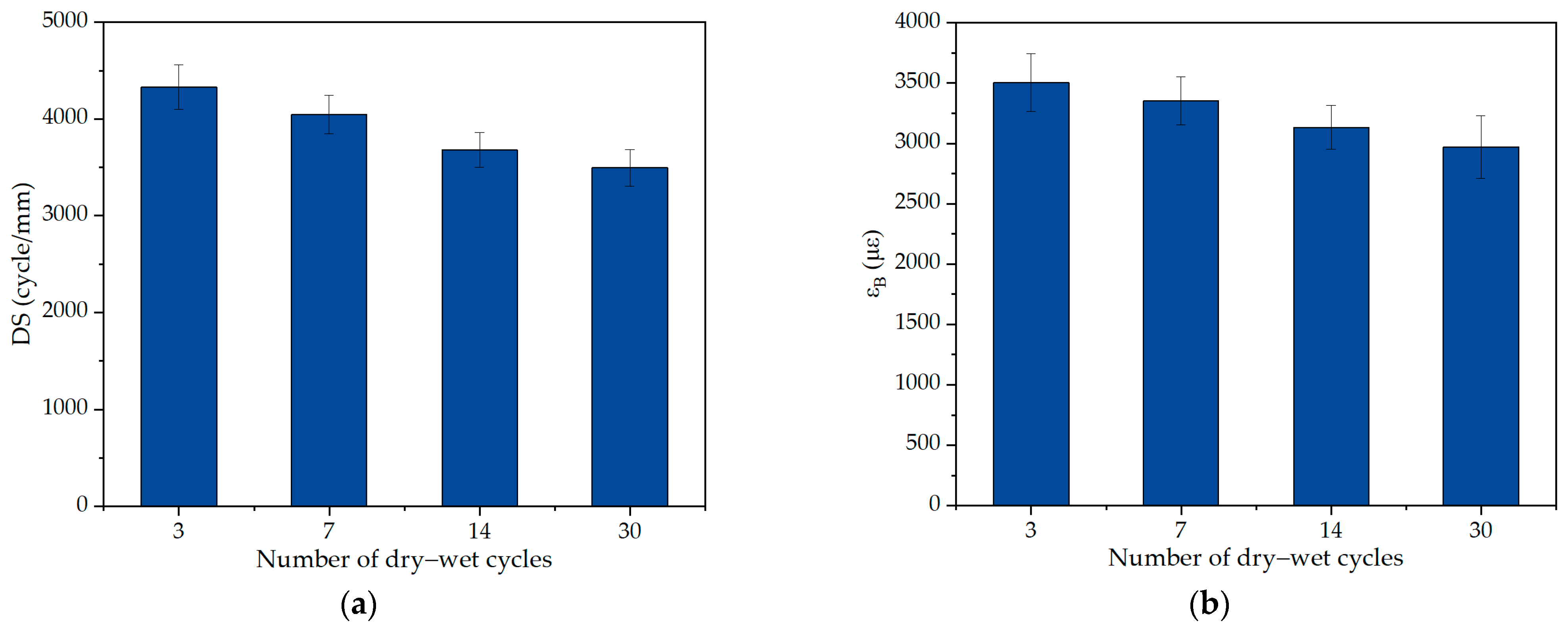
2.1. Performance Evolution of Asphalt Mixture Under Salt Erosion Environments
2.2. Discussion of the Pore Evolution Under Salt Erosion Environments
2.3. Discussion of Asphalt–Aggregate Adhesion Property Under Salt Erosion Environments
2.4. Discussion of Asphalt Mortar Property Under Salt Erosion Environments
3. Performance Degradation for Asphalt Binder in Salt Erosion Environments
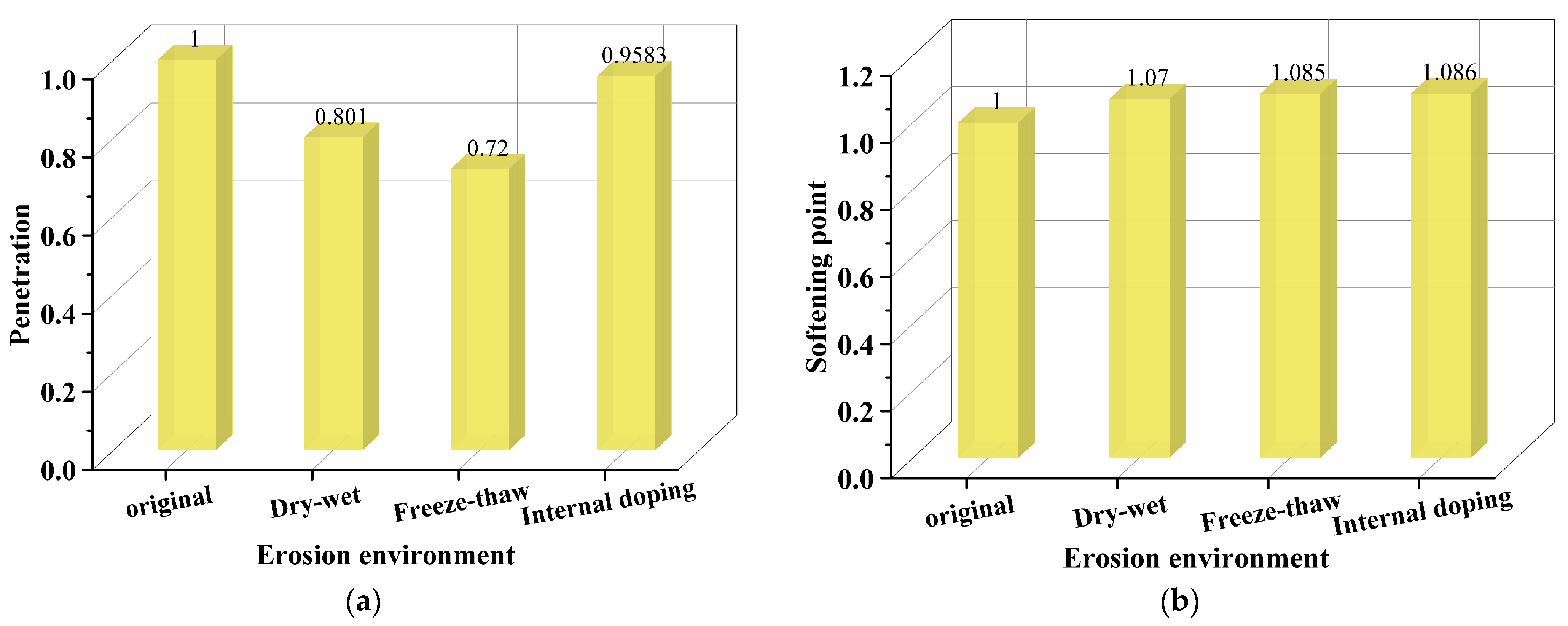
3.1. Asphalt Binder Performance Under the Erosion of Various Salt Categories
3.2. Influence of Salt Erosion Mode on the Asphalt Binder Performance
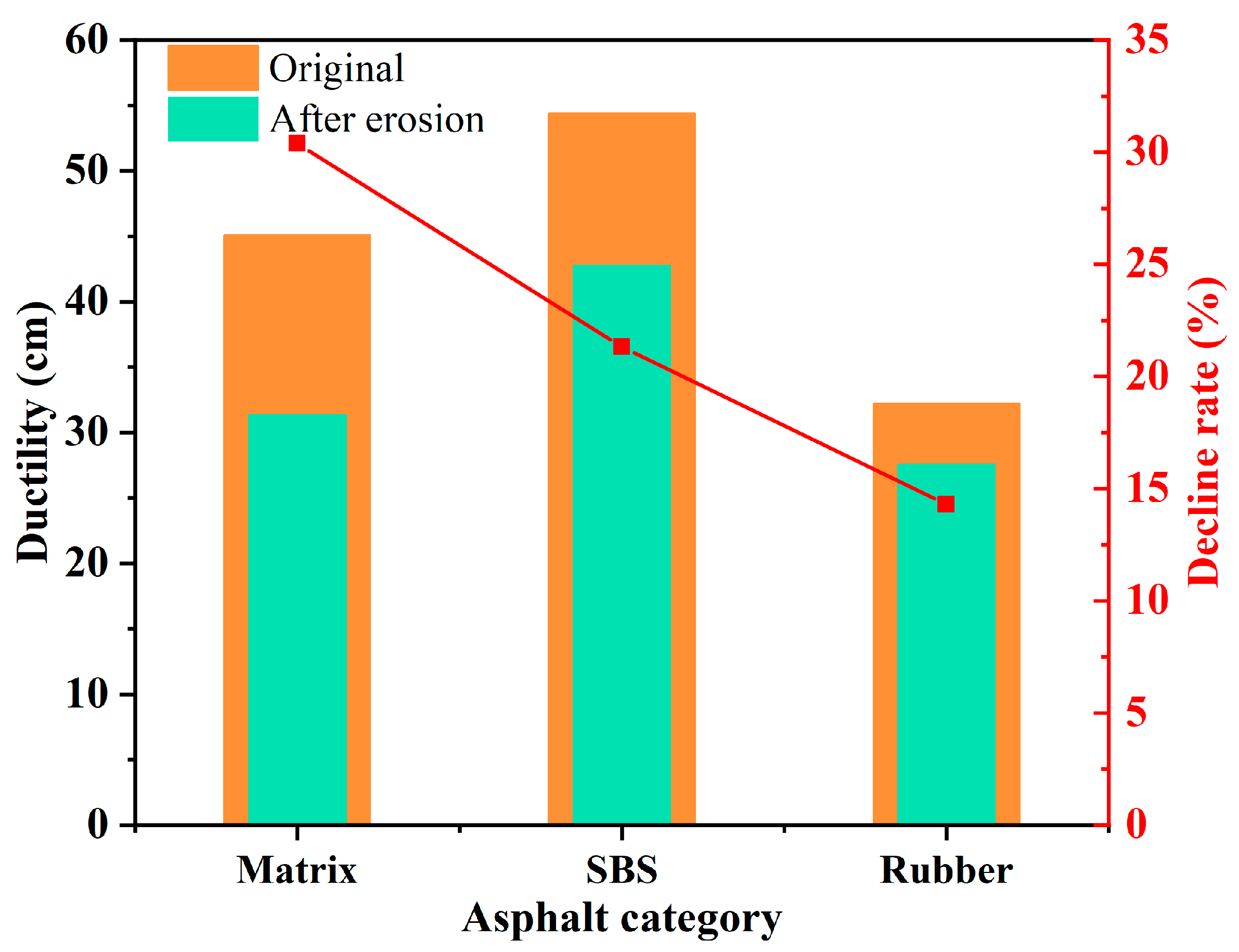
3.3. Salt Erosion Resistance of Different Asphalt Binders
4. Evolution of Chemical Compositions and Microscopic Morphology of Asphalt Binder Under Salt Erosion Environments
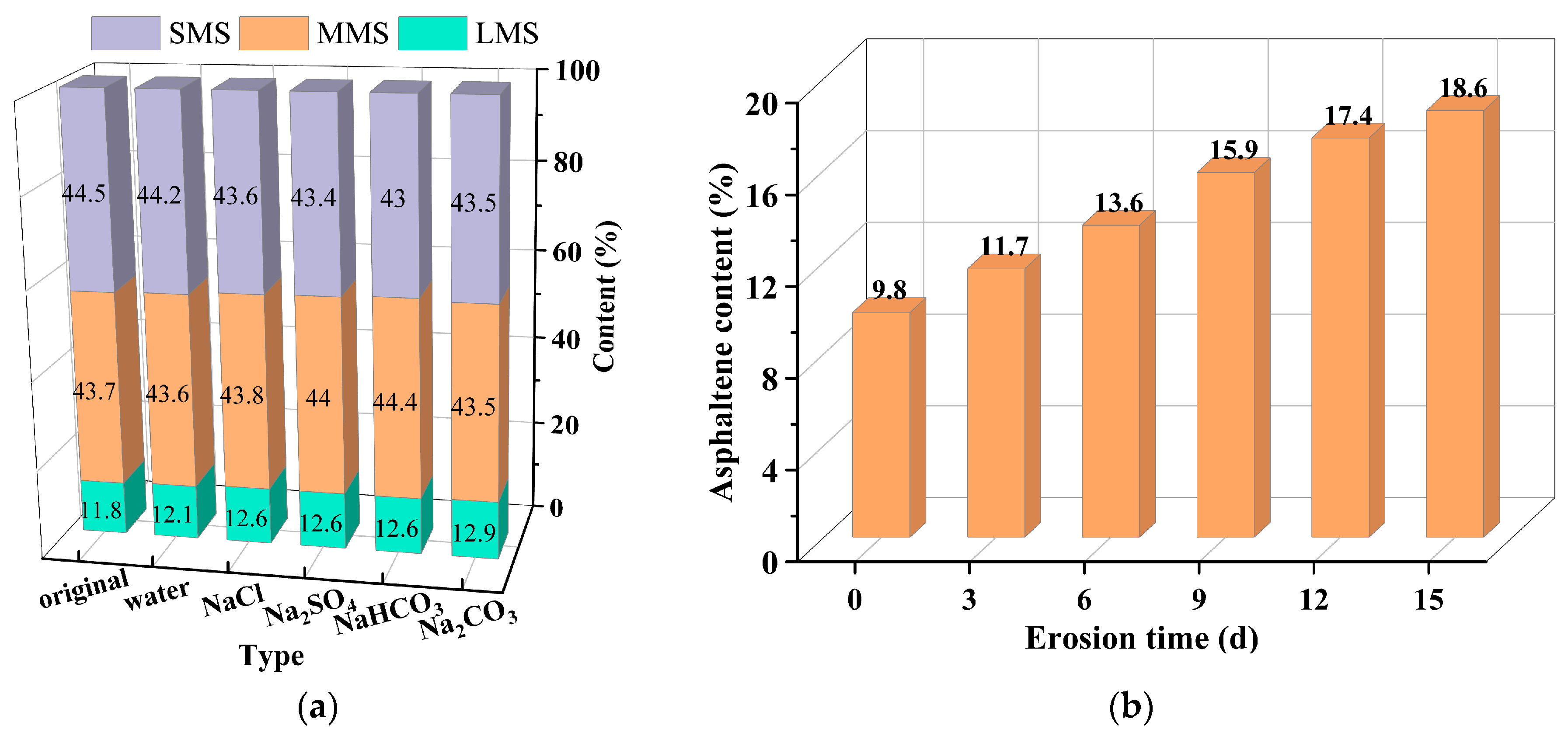
4.1. Effect of Salt-Erosion Environments on Chemical Compositions of Asphalt Binder
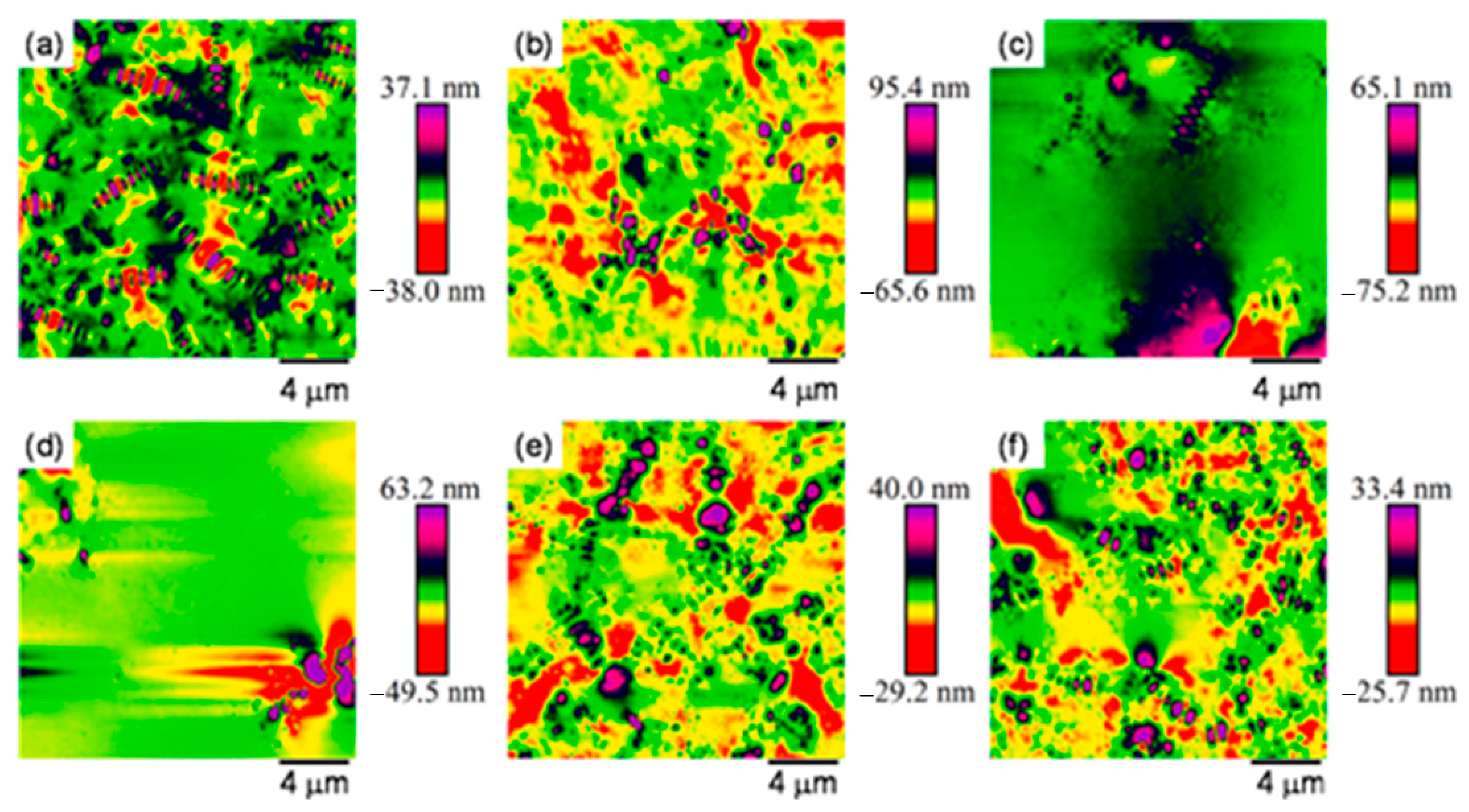
4.2. Microscopic Morphology of Asphalt Binder Under Salt Erosion Environments
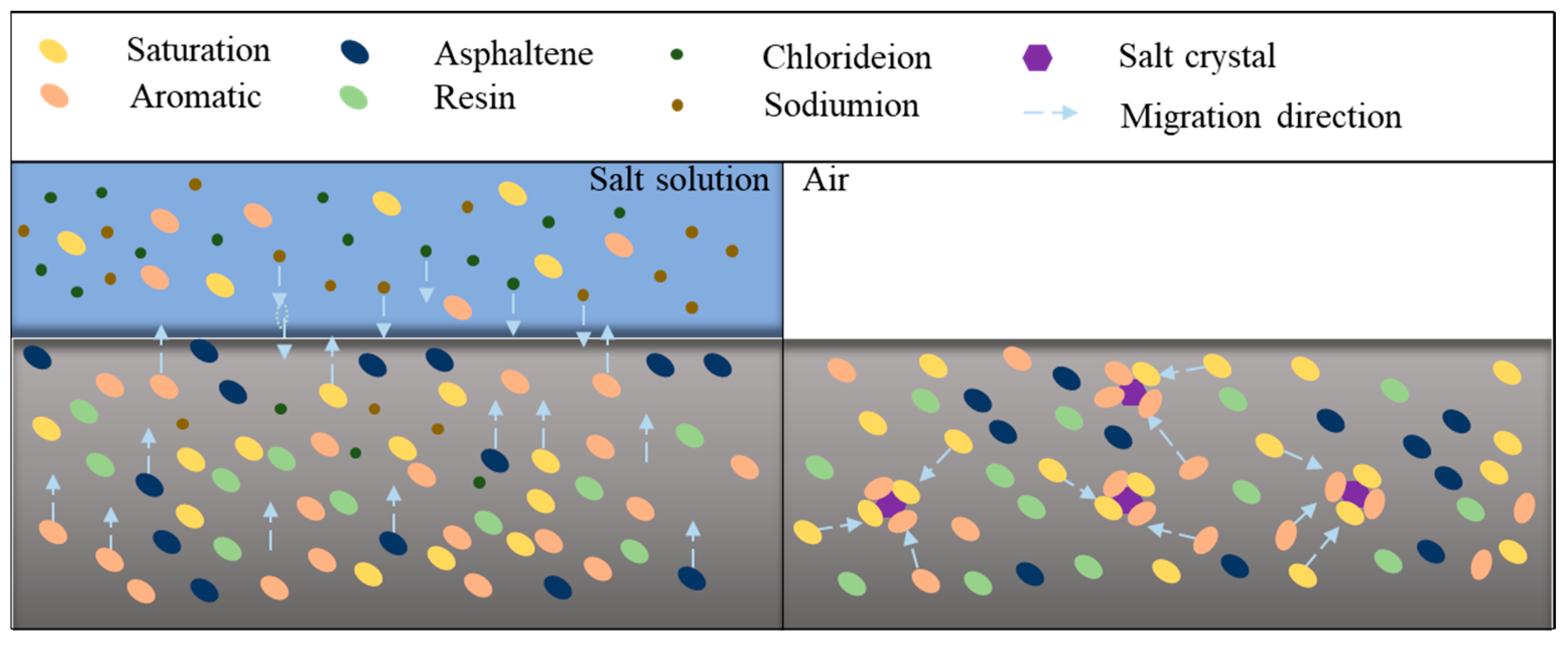
4.3. Micro-Scale Damage Mechanism of Salt-Eroded Asphalt Binder
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Wu, D.L.; Zhang, X.J.; Chang, K.L.; Wang, Y.B. Effect of organic deicing agents on asphalt rheology and analysis of the mechanism. Constr. Build. Mater. 2021, 284, 122649. [Google Scholar] [CrossRef]
- Busang, S.; Maina, J. Laboratory simulation and mechanical performance of asphalt materials under the action of saline. Constr. Build. Mater. 2021, 313, 125387. [Google Scholar] [CrossRef]
- Cui, Y.N.; Wang, L. Viscoelastic variation and microscopic analysis of asphalt mortar after salt freezing. J. China Foreign Highw. 2014, 2014, 215–220. [Google Scholar] [CrossRef]
- Cui, Y.N.; Chen, D.S.; Feng, L.; Wang, L. Effects of salt freeze damage on the viscoelastic performance of asphalt mortar. Ceram. Ceram. Ceram. Silik. 2017, 61, 257–266. [Google Scholar] [CrossRef]
- Han, J.W.; Cui, Y.N.; Wang, L.; Zhang, S.Y.; Zhen, L. Grey relation entropy analysis on salt freezing performances of asphalt and asphalt mortar. J. Funct. Mater. 2017, 48, 3139–3144. [Google Scholar]
- Wu, S.Y.; Yang, J.; Yang, R.C.; Zhu, J.P.; Liu, S.; Wang, C.Y. Investigation of microscopic air void structure of anti-freezing asphalt pavement with X-ray CT and MIP. Constr. Build. Mater. 2018, 178, 473–483. [Google Scholar] [CrossRef]
- Xiao, Q.Y.; Hu, H.X.; Wang, L.J.; Li, L.N. Study on erosion of new de-icing salt on asphalt mixture based on surface energy theory. J. Hebei Univ. Technol. 2012, 41, 64–68. [Google Scholar] [CrossRef]
- Wu, J.R.; Ma, Q.Y.; Wang, W.J. Influence of temperature and corrosion on freezing-thawing fatigue life of asphalt concrete. J. Glaciol. Geocryol. 2015, 37, 422–427. [Google Scholar]
- Xiong, R.; Jiang, W.Y.; Yang, F.; Li, K.H.; Guan, B.W.; Zhao, H. Investigation of Voids Characteristics in an Asphalt Mixture Exposed to Salt Erosion Based on CT Images. Materials 2019, 12, 3774. [Google Scholar] [CrossRef]
- Ma, T.; Geng, L.; Ding, X.H.; Zhang, D.Y.; Huang, X.M. Experimental study of deicing asphalt mixture with anti-icing additives. Constr. Build. Mater. 2016, 127, 653–662. [Google Scholar] [CrossRef]
- Zhang, R.; Tang, N.P.; Deng, X.K.; Zhu, H.Z.; Su, C.L.; Xi, Y. Erosion mechanism of sea salt solution on the performance of SBS-modified asphalt mixtures. Int. J. Pavement Eng. 2023, 28, 2120991. [Google Scholar] [CrossRef]
- Zhang, X.M.; Chen, H.; Hoff, I. The mutual effect and reaction mechanism of bitumen and de-icing salt solution. Constr. Build. Mater. 2021, 302, 124213. [Google Scholar] [CrossRef]
- Cui, Y.N.; Sun, G.N.; Wei, H.J.; Zhao, L. Microstructure and high-and-low temperature performance of two kinds of asphalt under salt freezing cycle. Acta Mater. Compos. Sin. 2017, 34, 906–914. [Google Scholar] [CrossRef]
- Wang, L.; Guo, Z.X.; Zhang, B.X.; Song, C.Z.; He, L.Q. High and Low Temperature Performance of Warm/Hot-Mixed Crumb Rubber Modified Asphalt Mixture under Salt Freezing-Thawing Cycles. Bull. Chin. Ceram. Soc. 2021, 40, 1395–1404. [Google Scholar] [CrossRef]
- Sansalone, J.J.; Glenn, D.W. Metal distributions in soil receiving urban pavement runoff and snowmelt. Water Environ. Res. 2007, 79, 736–752. [Google Scholar] [CrossRef]
- Feng, D.C.; Yi, J.Y.; Wang, D.S.; Chen, L.L. Impact of salt and freeze-thaw cycles on performance of asphalt mixtures in coastal frozen region of China. Cold Reg. Sci. Technol. 2010, 62, 34–41. [Google Scholar] [CrossRef]
- Zhou, X.D.; Chang, C.Q.; Wang, L. Effect of Salt Freeze-Thaw Cycle on Fatigue Performance of Warm Mix Rubber Modified Asphalt Mixture. J. Build. Mater. 2020, 23, 650–656. Available online: https://caod.oriprobe.com/articles/59407891/Effect_of_Salt_Freeze_Thaw_Cycle_on_Fatigue_Perfor.htm (accessed on 24 March 2025).
- Fakhri, M.; Javadi, S.; Sedghi, R.; Arzjani, D.; Zarrinpour, Y. Effects of deicing agents on moisture susceptibility of the WMA containing recycled crumb rubber. Constr. Build. Mater. 2019, 227, 116581. [Google Scholar] [CrossRef]
- Vega-Zamanillo, A.; Juli-Gandara, L.; Calzada-Perez, M.A.; Teijon-Lopez-Zuazo, E. Impact of Temperature Changes and Freeze-Thaw Cycles on the Behaviour of Asphalt Concrete Submerged in Water with Sodium Chloride. Appl. Sci. 2020, 10, 1241. [Google Scholar] [CrossRef]
- Wang, F.Y.; Qin, X.Y.; Pang, W.C.; Wang, W.S. Performance Deterioration of Asphalt Mixture under Chloride Salt Erosion. Materials 2021, 14, 3339. [Google Scholar] [CrossRef]
- Chen, H.L.; Sha, A.M.; Jiang, W.; Wang, Y.N.; Liu, Z.Z. Mechanical Behaviors of Asphalt Mixtures in Salt-wet-heat Cycling. J. Highw. Transp. Res. Dev. 2016, 33, 42–47. [Google Scholar]
- Han, J.W.; Cui, Y.N.; Wang, L.; Li, Z.; Zhang, S.Y. The mechanical properties analysis of SBS modified asphalt mortar under salt freezing cycle. J. Funct. Mater. 2015, 2015, 12141–12145. [Google Scholar] [CrossRef]
- Li, R.X.; Li, X.; Yue, J.C.; Yang, C.W. Effects of salt solution-aging coupling on viscoelastic, fatigue and self-healing properties of asphalt binder. Constr. Build. Mater. 2024, 419, 135514. [Google Scholar] [CrossRef]
- Yan, J.C.; Ma, M.M.; Wang, G.L. Mesoscopic and macroscopic analysis of fatigue cracking in Buton Rock Asphalt Concrete. Constr. Build. Mater. 2025, 472, 140821. [Google Scholar] [CrossRef]
- Guo, D.D.; Sun, X.P.; Tian, J.H.; Xu, M.; Yang, S.H.; Zou, H.Y.; Li, J.; Wang, T.; Riccardi, C. Salt release and performance of self-ice-melting epoxy asphalt pavement under accelerated loading simulation conditions. Constr. Build. Mater. 2025, 467, 140360. [Google Scholar] [CrossRef]
- Ogbon, W.A.; Jiang, W.; Yuan, D.D.; Xing, C.W.; Xiao, J.J. Asphalt mixture performance deterioration in the salty environment based on theoretical calculation. Constr. Build. Mater. 2023, 393, 132096. [Google Scholar] [CrossRef]
- Wu, S.Y.; Yang, J.; Sun, X.Y.; Wang, C.Y.; Yang, R.C.; Zhu, J.P. Preparation and characterization of anti-freezing asphalt pavement. Constr. Build. Mater. 2020, 236, 117579. [Google Scholar] [CrossRef]
- Guo, R.H.; Zhang, H.H.; Tan, Y.X. Influence of salt dissolution on durable performance of asphalt and Self-ice-melting asphalt mixture. Constr. Build. Mater. 2022, 346. [Google Scholar] [CrossRef]
- Xu, O.M.; Yang, X.H.; Xiang, S.L.; Zhang, H. Migration characteristic and model of chloride ions for NaCl-based salt storage asphalt mixtures. Constr. Build. Mater. 2021, 280, 122482. [Google Scholar] [CrossRef]
- Cao, Y.S.; Li, J.R.; Liu, Z.Z.; Li, X.Z.; Zhang, F.; Shan, B.Z. Rheological Properties of Styrene-Butadiene-Styrene Asphalt Mastic Containing High Elastic Polymer and Snow Melting Salt. Polymers 2022, 14, 3651. [Google Scholar] [CrossRef]
- Zhou, J.; Li, J.; Liu, G.Q.; Yang, T.; Zhao, Y.L. Long-Term Performance and Deicing Effect of Sustained-Release Snow Melting Asphalt Mixture. Adv. Civ. Eng. 2019, 2019, 1940692. [Google Scholar] [CrossRef]
- Wang, Z.J.; Zhang, T.; Shao, M.Y.; Ai, T.; Zhao, P. Investigation on snow-melting performance of asphalt mixtures incorporating with salt-storage aggregates. Constr. Build. Mater. 2017, 142, 187–198. [Google Scholar] [CrossRef]
- Zhang, K.; Luo, Y.F.; Li, Z.H.; Zhao, Y.L.; Zhao, Y. Evaluation of Performance Deterioration Characteristics of Asphalt Mixture in Corrosion Environment Formed by Snow-Melting Agents. J. Mater. Civ. Eng. 2022, 34, 4021481. [Google Scholar] [CrossRef]
- Zhou, Z.G.; Li, H.J.; Liu, X.; He, W.L. Analysis of the Damage Effect of Sea Salt Erosion on Asphalt-Aggregate Interfacial System in Hygrothermal Environment. J. Build. Mater. 2020, 23, 1430–1439. [Google Scholar] [CrossRef]
- Xiong, R.; Chu, C.; Qiao, N.; Wang, L.; Yang, F.; Sheng, Y.P.; Guan, B.W.; Niu, D.Y.; Geng, J.G.; Chen, H.X. Performance evaluation of asphalt mixture exposed to dynamic water and chlorine salt erosion. Constr. Build. Mater. 2019, 201, 121–126. [Google Scholar] [CrossRef]
- Wang, J.W.; Shen, A.Q.; Guo, Y.C.; Zhou, X.H.; Zhou, T.Y. Road Performance of Asphalt Mixture with Anti-Stripping Agent in Salt Freeze-Thaw Environment. Bull. Chin. Ceram. Soc. 2019, 38, 2770–2776. [Google Scholar] [CrossRef]
- Yang, H.; Pang, L.; Zou, Y.X.; Liu, Q.T.; Xie, J. The effect of water solution erosion on rheological, cohesion and adhesion properties of asphalt. Constr. Build. Mater. 2020, 246, 118465. [Google Scholar] [CrossRef]
- Ji, X.P.; Sun, E.Y.; Zou, H.W.; Hou, Y.Q.; Chen, B. Study on the multiscale adhesive properties between asphalt and aggregate. Constr. Build. Mater. 2020, 249I, 118693. [Google Scholar] [CrossRef]
- Liang, S.; Liang, C.; Li, M.; Cui, H.; Wang, Z.; Wang, S. Investigation of nanoscale interfacial bonding properties in foamed asphalt cold recycled mixtures under chloride salt erosion. Case Stud. Constr. Mater. 2024, 21, e03390. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, J.; Zhao, Y.; Xie, W.; Wang, Y. Interfacial bonding property and microscopic damage mechanism of bitumen-aggregate in salt-freeze-thawing environment. Mater. Today Commun. 2023, 36, 106664. [Google Scholar] [CrossRef]
- Xu, O.M.; Xiang, S.L.; Yang, X.H.; Liu, Y.M. Estimation of the surface free energy and moisture susceptibility of asphalt mastic and aggregate system containing salt storage additive. Constr. Build. Mater. 2022, 318I, 125814. [Google Scholar] [CrossRef]
- Cui, S.C.; Wang, L. Effect of Ultraviolet Aging on Cracking Characteristics of Warm Mix Crumb Rubber Modified Asphalt Mortar. J. Build. Mater. 2022, 25, 285–293. [Google Scholar] [CrossRef]
- Niu, D.Y.; Chen, H.X.; RenQian, L.Z.; Geng, J.G.; He, R.; Xiong, R. Mechanical damage of freeze-thaw cycle on asphalt mortar and life prediction. J. Hefei Univ. Technol. (Nat. Sci.) 2018, 41, 943–948. [Google Scholar] [CrossRef]
- Ding, X.H.; Ma, T.; Gu, L.H.; Zhang, Y. Investigation of surface micro-crack growth behavior of asphalt mortar based on the designed innovative mesoscopic test. Mater. Des. 2020, 185, 108238. [Google Scholar] [CrossRef]
- Feng, L.; Chang, J.P. Study on Low Temperature Performance of Warm Mix Rubber Powder Modified Asphalt Mortar under Salt Freeze-Thaw Cycles. J. Inn. Mong. Univ. Technol. (Nat. Sci. Ed.) 2020, 39, 141–146. [Google Scholar] [CrossRef]
- Guo, K. Marine Manual; Marit. Press: Beijing China, 1984. (In Chinese) [Google Scholar]
- Zhang, Q. Study on Water Damage Mechanism of Asphalt Mixture in Multi-Factor Environment of the South Coast; Zhejiang University: Hohhot, China, 2020. (In Chinese) [Google Scholar]
- Feng, R.L.; Wang, S.Z.; Wu, L.J.; Lu, H.C.; Shen, Y.P. Bulging deformation mechanism of asphalt pavement in sulfate saline soil areas of Xinjiang. Chin. J. Geotech. Eng. 2021, 43, 1739–1745. [Google Scholar] [CrossRef]
- Dai, Z.Y.; Mao, X.S.; Xu, W.; Tang, K.; Wu, Q.; Tang, W.J. Investigation and analysis of the causes of arch expansion of cement stabilized macadam base asphalt pavement in Gobi saline soil area. China Sci. 2021, 16, 444–449. [Google Scholar] [CrossRef]
- Su, B.; Meng, Y.; Hu, S.; Qin, Y. Study on self-healing performance of asphalt under sodium salt erosion. Case Stud. Constr. Mater. 2024, 20, e02921. [Google Scholar] [CrossRef]
- Qiu, X.; Deng, J.; Fu, Q.; Lou, Y.; Ye, Y.; Zhang, D. Investigation of Chloride Salt Erosion on Asphalt Binders and Mixtures: Performance Evaluation and Correlation Analysis. Materials 2025, 18, 156. [Google Scholar] [CrossRef]
- Xue, B.Y.; Yao, P.; Zou, X.L.; Liu, Q.; Zhao, Y.L. Damage characteristics of recycled asphalt mixtures under the erosion effect of snow-melting salt. Dyna 2021, 96, 379–387. [Google Scholar] [CrossRef]
- Pang, L.; Zhang, X.M.; Wu, S.P.; Ye, Y.; Li, Y.Y. Influence of Water Solute Exposure on the Chemical Evolution and Rheological Properties of Asphalt. Materials 2018, 11, 983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Tang, N.P.; Zhu, H.Z. The effect of sea salt solution erosion on cohesion, chemical and rheological properties of SBS modified asphalt. Constr. Build. Mater. 2022, 318, 125923. [Google Scholar] [CrossRef]
- Hung, A.M.; Goodwin, A.; Fini, E.H. Effects of water exposure on bitumen surface microstructure. Constr. Build. Mater. 2017, 135, 682–688. [Google Scholar] [CrossRef]
- Li, Y.Y.; Li, H.C.; Nie, S.; Wu, S.P.; Liu, Q.T.; Li, C.M.; Shu, B.A.; Li, C.; Song, W.; Zou, Y.X.; et al. Negative impacts of environmental factors (UV radiation, water and different solutions) on bitumen and its mechanism. Constr. Build. Mater. 2020, 265, 120288. [Google Scholar] [CrossRef]
- Xiao, Q.Y.; Wang, Y.B.; Hu, H.X.; Liu, M.T. Mechanism of acetate-based deicer eroding asphalt mixture. Eng. J. Wuhan Univ. 2015, 2015, 187–190. [Google Scholar] [CrossRef]
- Qiao, N.; Xiong, R.; Li, L.D.; Yang, F.; Guan, B.W.; Sheng, Y.P.; Zhao, H. Investigation on Performance of Asphalt Mortar Mixed with Salts. Bull. Chin. Ceram. Soc. 2018, 37, 2224–2230. [Google Scholar] [CrossRef]
- Xu, S.J.; Zhou, Z.L.; Feng, L.C.; Cui, N.; Xie, N. Durability of Pavement Materials with Exposure to Various Anti-Icing Strategies. Processes 2021, 9, 291. [Google Scholar] [CrossRef]
- Zheng, M.L.; Wu, S.J.; Wang, C.T.; Li, Y.F.; Ma, Z.H.; Peng, L. A Study on Evaluation and Application of Snowmelt Performance of Anti-Icing Asphalt Pavement. Appl. Sci. 2017, 7, 583. [Google Scholar] [CrossRef]
- Meng, Y.J.; Xu, R.G.; Guo, H.Y.; Ling, L.S.; Xi, C.C.; Qin, Y.; Liu, Z.R.; Rong, H.L. Influences of sodium salt erosion on the self-healing ability and fatigue life of bitumen. Constr. Build. Mater. 2021, 286, 122884. [Google Scholar] [CrossRef]
- Meng, Y.Y.; Hu, C.C.; Tang, Y.K.; Grossegger, D.; Qin, W.H. Investigation on the erosion mechanism of simulated salt conditions on bitumen. Constr. Build. Mater. 2022, 334, 127267. [Google Scholar] [CrossRef]
- Cui, Y.N.; Yu, Q.N.; Han, J.W.; Chen, C. Low Temperature Performance of Rubber-modified Asphalt Under Complex Climate. Mater. Rev. 2018, 32, 2078–2084. [Google Scholar] [CrossRef]
- Yin, J.X.; Ma, Q.W.; Zhang, S.Q.; Guo, P. Effect of Additives on Rheological Properties of Asphalt Under Salt Freezing Cycles. Road Mach. Constr. Mech. 2020, 37, 25–30. [Google Scholar] [CrossRef]
- Cui, Y.N.; Chen, R.P.; Han, J.W.; Li, Z. Microstructure and Low Temperature Creep Properties of SBS Modified Asphalt and Its Mixture under Salt Freezing Cycle. Bull. Chin. Ceram. Soc. 2018, 37, 1467–1473. [Google Scholar] [CrossRef]
- Ma, T.; Ding, X.H.; Wang, H.; Zhang, W.G. Experimental Study of High-Performance Deicing Asphalt Mixture for Mechanical Performance and Anti-Icing Effectiveness. J. Mater. Civ. Eng. 2018, 30, 4018180. [Google Scholar] [CrossRef]
- Cui, Y.N.; Zhao, L.; Han, J.W.; Sun, G.N. High temperature rheological properties and microstructures of asphalt under salt freezing cycles. Acta Mater. Compos. Sin. 2017, 34, 1839–1846. [Google Scholar] [CrossRef]
- Li, Z.; Cui, Y.N.; Han, J.W.; Wang, L.; Liu, Z.Z. The Effect of Salt Freezing Circulation to the Performance of Asphalt. Highw. Eng. 2015, 2015, 120–123. [Google Scholar] [CrossRef]
- Cui, Y.N.; Han, J.W.; Feng, L.; Li, J.D.; Wang, L. Microstructure of asphalt under salt freezing cycles. J. Jilin Univ. (Eng. Technol. Ed.) 2017, 47, 452–458. [Google Scholar] [CrossRef]
- Cui, Y.N.; Yu, Q.N.; Han, J.W.; Zhang, S.Y.; Feng, L. The microstructure and repeat creep property of modified asphalt under the salt freezing cycle. Acta Mater. Compos. Sin. 2017, 34, 1137–1145. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, W.; Wu, S.; Gou, X. Study on the mechanical performance damage in laboratory-simulated periodic salt environment for asphalt concrete. Constr. Build. Mater. 2024, 411, 134306. [Google Scholar] [CrossRef]
- Zhang, K.; Luo, Y.F.; Xie, W.; Wu, J.J. Evaluation of road performance and adhesive characteristic of asphalt binder in salt erosion environment. Mater. Today Commun. 2020, 25, 101593. [Google Scholar] [CrossRef]
- Que, Y.; Fei, W.Y.; Xu, S.; Fan, Y.; Zhang, C.L. Influence of SBS content on asphalt aging performance. J. Fuzhou Univ. (Nat. Sci. Ed.) 2022, 50, 848–855. [Google Scholar] [CrossRef]
- Xu, S.; Fan, Y.; Feng, Z.G.; Ke, Y.B.; Zhang, C.L.; Huang, H.Y. Comparison of quantitative determination for SBS content in SBS modified asphalt. Constr. Build. Mater. 2021, 282, 122733. [Google Scholar] [CrossRef]
- Zhou, H.; Holikatti, S.; Vacura, P. Caltrans use of scrap tires in asphalt rubber products: A comprehensive review. J. Traffic Transp. Eng. (Engl. Ed.) 2014, 1, 39–48. [Google Scholar] [CrossRef]
- Li, X.J.; Xu, X.J.; Wang, Q.Q. Study on Performance of Desulfurized Rubber Modified Asphalt with Different Original Asphalt. J. China Foreign Highw. 2022, 42, 156–160. [Google Scholar] [CrossRef]
- Fu, G.W. Research on Influence of Snowmelt Agent to Performances of Asphalt and Asphalt Mixture; Changsha University of Science & Technology: Changsha, China, 2010. [Google Scholar]
- Li, C.; Han, J.W.; Liu, Z.Z.; Li, Z. Research On The Performance Of Modified Asphalt Under The Salt Frost Thawing Cycle. J. Inn. Mong. Univ. Technol. (Nat. Sci. Ed.) 2014, 33, 56–61. [Google Scholar] [CrossRef]
- Cui, Y.N.; Chen, R.P.; Han, J.W.; Yu, Q.N. High and low temperature performance of asphalt under salt freezing cycle by the grey relation entropy analysis. Acta Mater. Compos. Sin. 2017, 34, 1130–1136. [Google Scholar] [CrossRef]
- Yu, X.; Leng, Z.; Wei, T.Z. Investigation of the Rheological Modification Mechanism of Warm-Mix Additives on Crumb-Rubber-Modified Asphalt. J. Mater. Civ. Eng. 2014, 26, 312–319. [Google Scholar] [CrossRef]
- Cui, Y.N.; Han, J.J.; Li, Z.; Hang, S.Y.; Liu, Z.Z. Research on the performance and microstructure of asphalt under salt freezing cycle. J. Funct. Mater. 2015, 2015, 18037–18042. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y.; Luo, Y.L.; Yin, L. The Effects of Salt on Rheological Properties of Asphalt after Long-Term Aging. Sci. World J. 2013, 2013, 921090. [Google Scholar] [CrossRef]
- Meng, Y.J.; Wei, G.P.; Li, Z.H.; Qin, Y.; Duan, Y.H.; Jun, Z.H.; Jiang, W. Performance of TLA modified asphalt and mixture under chloride attack. J. Guangxi Univ. (Nat. Sci. Ed.) 2020, 45, 61–66. [Google Scholar] [CrossRef]
- Zhang, X.M.; Hoff, I.; Chen, H. Characterization of various bitumen exposed to environmental chemicals. J. Clean. Prod. 2022, 337, 130610. [Google Scholar] [CrossRef]
- Ma, J.M.; Sun, G.Q.; Sun, D.Q.; Yu, F.; Hu, M.J.; Lu, T. Application of gel permeation chromatography technology in asphalt materials: A review. Constr. Build. Mater. 2021, 278, 122386. [Google Scholar] [CrossRef]
- Fini, E.H.; Kalberer, E.W.; Shahbazi, A.; Basti, M.; You, Z.; Ozer, H.; Aurangzeb, Q. Chemical Characterization of Biobinder from Swine Manure: Sustainable Modifier for Asphalt Binder. J. Mater. Civ. Eng. 2011, 23, 1506–1513. [Google Scholar] [CrossRef]
- Huang, S.C.; Robertson, R.E.; Branthaver, J.F.; Petersen, J.C. Impact of lime modification of asphalt and freeze-thaw cycling on the asphalt-aggregate interaction and moisture resistance to moisture damage. J. Mater. Civ. Eng. 2005, 17, 711–718. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Hao, P.W.; Dong, S.; Li, Y.; Yuan, G.A. Asphalt binder micro-characterization and testing approaches: A review. Measurement 2020, 151, 107255. [Google Scholar] [CrossRef]
- Lyne, A.L.; Wallqvist, V.; Birgisson, B. Adhesive surface characteristics of bitumen binders investigated by Atomic Force Microscopy. Fuel 2013, 113, 248–256. [Google Scholar] [CrossRef]
- Xiong, R.; Qiao, N.; Chu, C.; Yang, F.; Guan, B.W.; Sheng, Y.P.; Niu, D.Y. Investigation on low-temperature rheology and adhesion properties of salt-doped asphalt mortars. J. Jilin Univ. (Eng. Technol. Ed.) 2020, 50, 183–190. [Google Scholar] [CrossRef]
- Zhou, P.L.; Wang, W.S.; Zhu, L.L.; Wang, H.Y.; Ai, Y.M. Study on Performance Damage and Mechanism Analysis of Asphalt under Action of Chloride Salt Erosion. Materials 2021, 14, 3089. [Google Scholar] [CrossRef]
- Hou, X.; Lv, S.; Chen, Z.; Xiao, F. Applications of Fourier transform infrared spectroscopy technologies on asphalt materials. Measurement 2018, 121, 304–316. [Google Scholar] [CrossRef]
- Kambham, B.S.; Ram, V.V.; Raju, S. Investigation of laboratory and field aging of bituminous concrete with and without anti-aging additives using FESEM and FTIR. Constr. Build. Mater. 2019, 222, 193–202. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Huang, Z.Y. Investigation of the Microcharacteristics of Asphalt Mastics under Dry-Wet and Freeze-Thaw Cycles in a Coastal Salt Environment. Materials 2019, 12, 2627. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Cai, S.; Huang, R.; Xu, K.; Ma, Z.; Fang, L.; Zhang, C. Investigation of aging behaviors of asphalt under the coupling conditions of salt and water. Fuel 2024, 365, 131191. [Google Scholar] [CrossRef]
- Fini, E.H.; Hung, A.M.; Roy, A. Active Mineral Fillers Arrest Migrations of Alkane Acids to the Interface of Bitumen and Siliceous Surfaces. ACS Sustain. Chem. Eng. 2019, 7, 10340–10348. [Google Scholar] [CrossRef]
- Koyun, A.N.; Zakel, J.; Kayser, S.; Stadler, H.; Keutsch, F.N.; Grothe, H. High resolution nanoscale chemical analysis of bitumen surface microstructures. Sci. Rep. 2021, 11, 13554. [Google Scholar] [CrossRef]
- Yang, J.; Gong, M.H.; Pauli, T.; Wei, J.M.; Wang, X.T. Study on Micro-Structures of Asphalt by Using Atomic Force Microscopy. Acta Pet. Sin. (Pet. Process. Sect.) 2015, 2015, 959–965. [Google Scholar] [CrossRef]
- Fischer, H.R.; Dillingh, E.C.; Hermse, C.G.M. On the microstructure of bituminous binders. Road Mater. Pavement Des. 2014, 15, 1–15. [Google Scholar] [CrossRef]
- Long, Z.W.; Guo, N.N.; Tang, X.Q.; Ding, Y.H.; You, L.Y.; Xu, F. Microstructural evolution of asphalt induced by chloride salt erosion. Constr. Build. Mater. 2022, 343, 128056. [Google Scholar] [CrossRef]
- Xu, F.; Li, Z.; Liu, Y.; Xie, H.; Long, Z.; Dai, B.; Yang, H.; Zhu, C.; You, L.; Jin, D. Microscopic morphology and adhesion performance of SBS/OMMT modified asphalt under chloride salt erosion. Constr. Build. Mater. 2025, 458, 139578. [Google Scholar] [CrossRef]
- Yuan, D.-D.; Jiang, W.; Xiao, J.-J.; Lu, H.; Wu, W.-J. Thermal-oxygen aging effects on viscoelastic properties of high viscosity modified asphalt. J. Chang. Univ. (Nat. Sci. Ed.) 2020, 40, 1–11. [Google Scholar] [CrossRef]
- Shi, K.; Fu, Z.; Song, R.M.; Liu, F.L.; Ma, F.; Dai, J.S. Waste chicken fat oil as a biomass regenerator to restore the performance of aged asphalt: Rheological properties and regeneration mechanism. Road Mater. Pavement Des. 2023, 24, 191–215. [Google Scholar] [CrossRef]
- Song, R.M.; Sha, A.M.; Shi, K.; Li, J.R.; Li, X.Z.; Zhang, F. Polyphosphoric acid and plasticizer modified asphalt: Rheological properties and modification mechanism. Constr. Build. Mater. 2021, 309, 125158. [Google Scholar] [CrossRef]
- Zhang, R.; You, Z.P.; Wang, H.N.; Ye, M.X.; Yap, Y.K.; Si, C.D. The impact of bio-oil as rejuvenator for aged asphalt binder. Constr. Build. Mater. 2019, 196, 134–143. [Google Scholar] [CrossRef]
- Li, Z.Z.; Fa, C.G.; Zhao, H.Y.; Zhang, Y.Y.; Chen, H.X.; Xie, H.W. Investigation on evolution of bitumen composition and micro-structure during aging. Constr. Build. Mater. 2020, 244, 118322. [Google Scholar] [CrossRef]
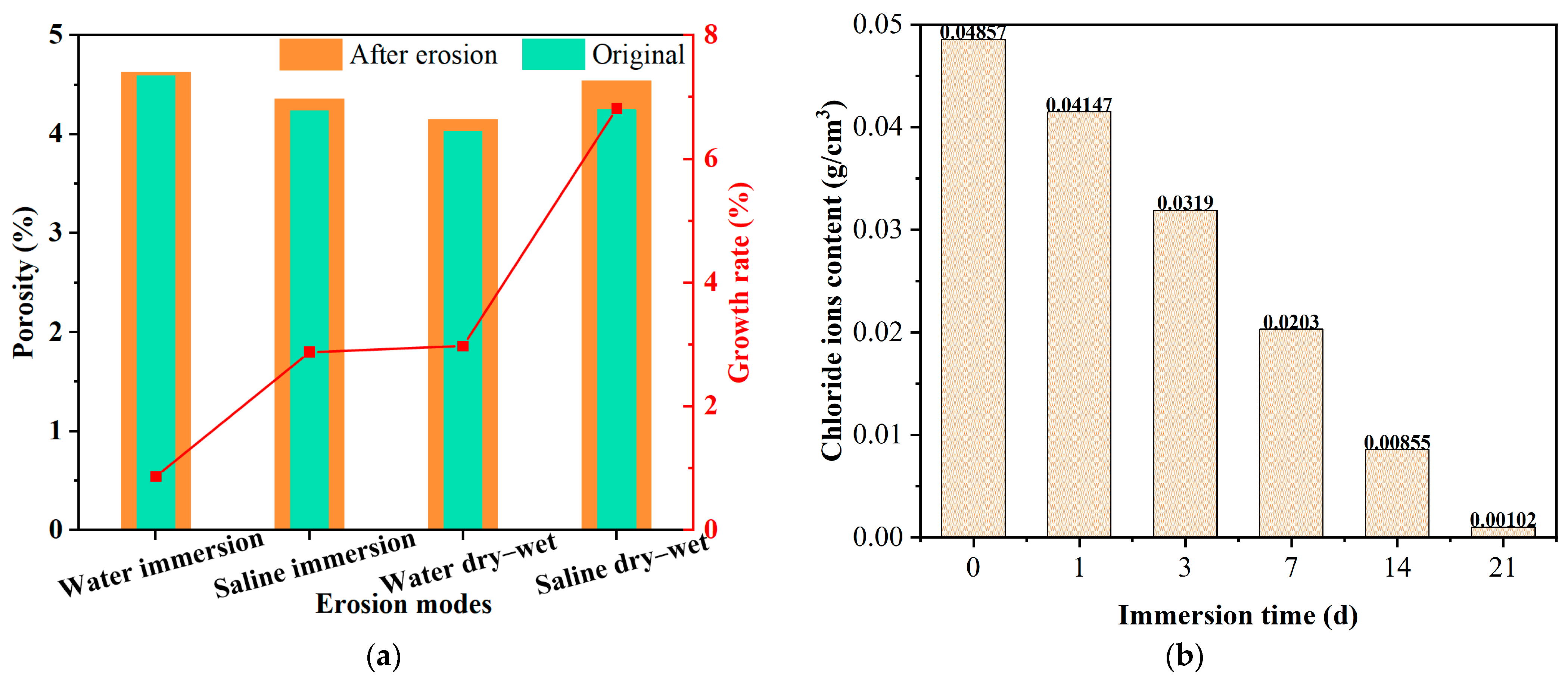

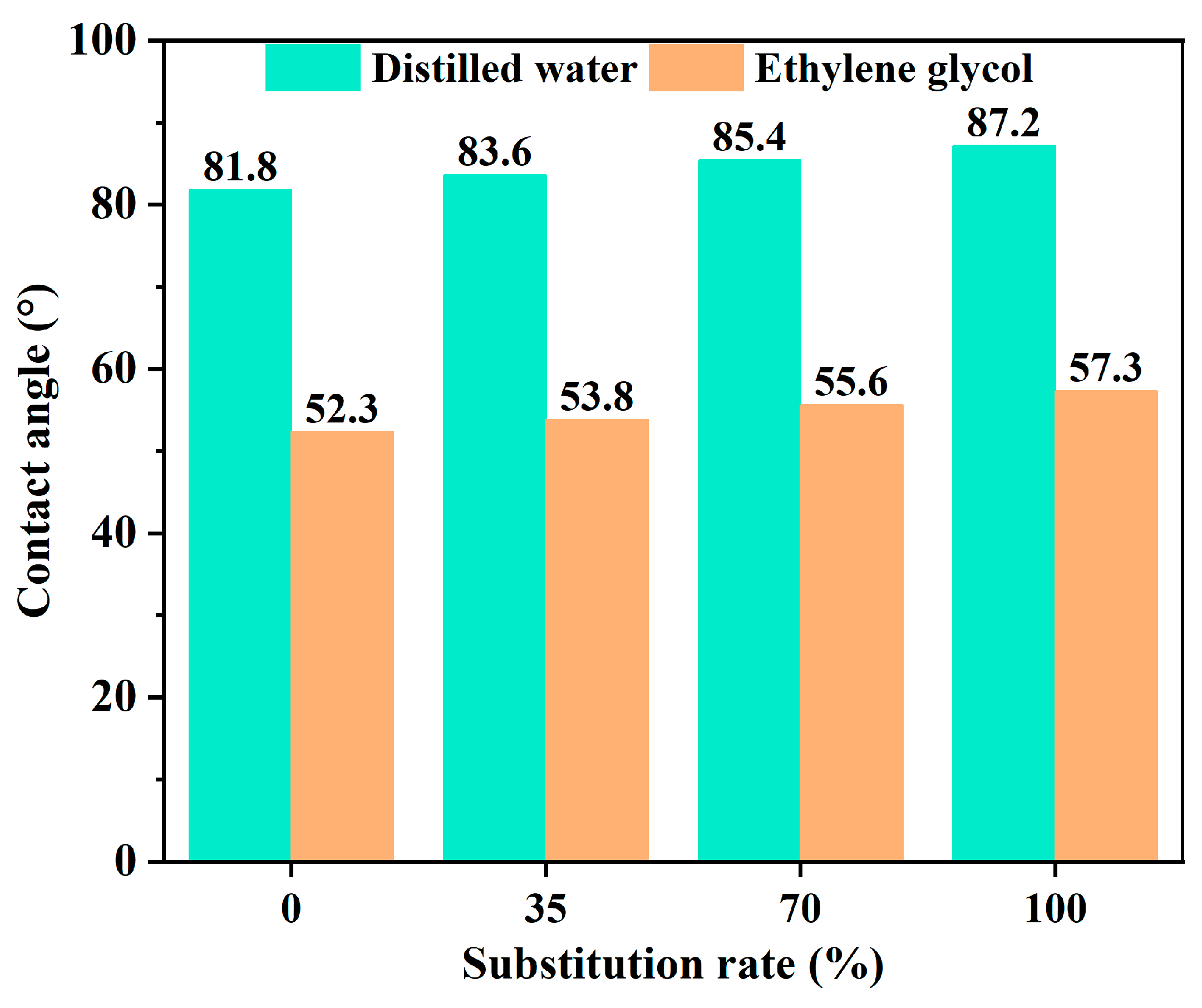
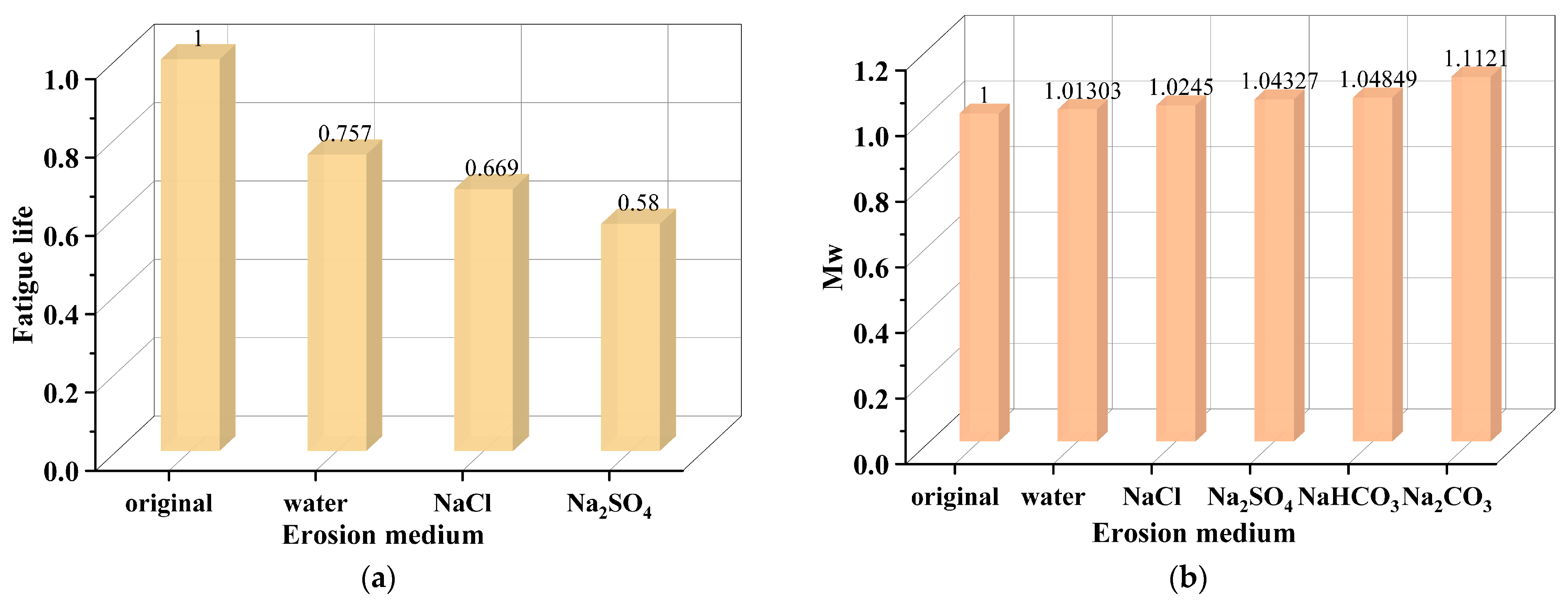
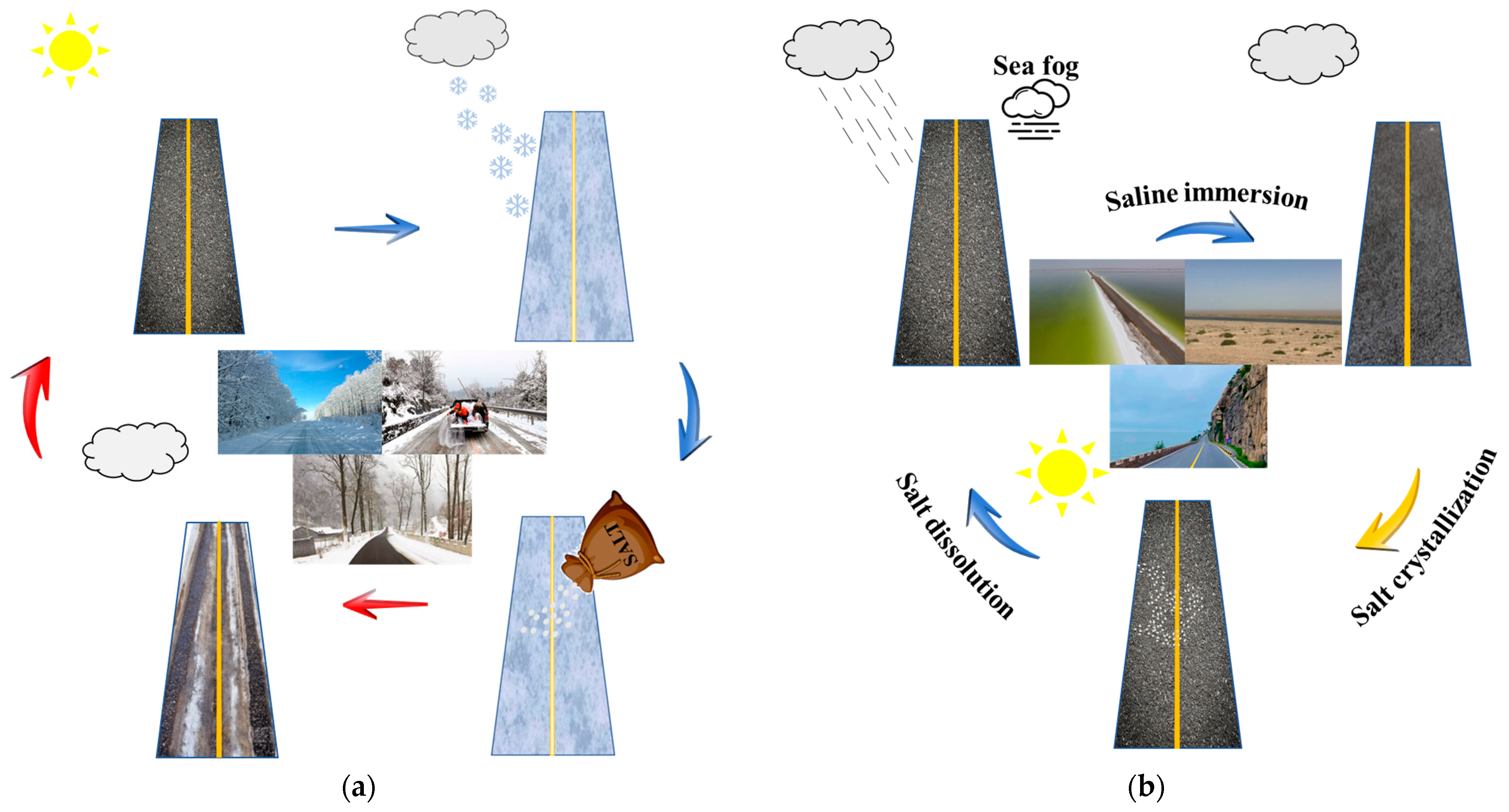
| Salt Ion | Salt | Major Findings | Main Reason | References |
|---|---|---|---|---|
| Cl− | NaCl | Increase in complex modulus and deformation resistance, decrease in phase angle | Sodium ions reacted with light components in the asphalt binder, resulting in a decrease in the proportion of light components | [1,12,56] |
| CaCl2 | Increase in complex modulus and deformation resistance, decrease in phase angle | - | [12] | |
| SO42− | H2SO4 | Increase in softening point and complex modulus, decrease in phase angle | Reaction in thioether and thiol compounds with sulfuric acid solutions to create sulfoxide compounds and disulfides, resulting in an increased proportion of asphaltenes | [56] |
| Na2SO4 | Increase in complex modules and viscosity, decrease in ductility | The plasticity of the asphalt binder was deteriorated by sulfates, and sodium ions reacted with the light components of the asphalt binder, resulting in a decrease in the proportion of light components | [56,58,61] | |
| HCO3−/CO32− | NaHCO3 | Increase in shear strain and decrease in creep recovery rate | - | [2] |
| Na2CO3 | Increase in softening point and complex modulus, decrease in phase angle | Carboxylic acid reacted with sodium carbonate solution to form water-soluble soap compounds, resulting in a decrease in the proportion of light components | [56] | |
| CH3COO− | CH3COONa | Increase in penetration, decrease in viscosity, and lead to asphalt binder softening | Acetate ions contain lipophilic groups (CH3-) and hydrophilic groups (COO-) that soften the asphalt binder | [57,58] |
| Salt additive | MFL | Increase in consistency and viscosity, decrease in penetration, and lead to asphalt binder hardening | Carboxylic acids and phenols in asphalt binder were dissolved and ionized in salt solutions, resulting in a decrease in the proportion of light components | [28] |
| Wavenumber (cm−1) | Representative |
|---|---|
| 2924 | Typical asymmetric stretching vibration of methylene C–H |
| 2852 | Symmetric stretching vibration of methylene C–H |
| 1461 | Scissor vibration of methylene -CH2- |
| 1377 | Umbrella vibration of methyl CH3- |
| 812 and 868 | Stretching vibrations of the benzene ring |
| 724 | Synergistic vibrations of methylene segment (CH2)n (n ≥ 4) |
| 747 | Bending vibrations of aromatic branch chain |
| Near 1600 | Vibration of benzene ring skeleton and hydroxyl group |
| 1030 | Stretching vibrations of the sulfoxide group (S = O) |
| 1700 | Stretching vibrations of the carbonyl group (C = O) |
| 966~698 | Benzene ring substitution area |
| 930 | Stretching vibration of C-O |
| 840 and 780 | Out-of-plane bending vibration of =CH, or stretching vibration of S-O |
| 630 | Asymmetric variable-angle vibration of , or in-plane bending vibration of |
| 966 | Out-of-plane bending vibration of -CH = CH- |
| 745 | Stretching vibrations of C-Cl |
| 1133 | Plan deformation of tertiary alcohol |
| 1617 | Bending vibration of |
| 3467 | Stretching vibration of OH− |
| Indicator Variation | Erosion Modes | Explanation | References |
|---|---|---|---|
| Increased at 2924 cm−1, 2852 cm−1, 1461 cm−1, and 1377 cm−1, and enhanced at 812 cm−1, 868 cm−1, and 724 cm−1 | Saline immersion | The increase in -CH2- and -CH3-, and the increase in the content of aromatic polycyclic compounds and long-chain cycloalkanes. | [1] |
| Varied at 1456 cm−1, 1377 cm−1, and 966~698 cm−1 | Salt storage additive internal doping | The absorption peaks of saturated fractions, aromatic hydrocarbons, and the benzene ring substitution region were affected, resulting in salt aging effects. | [28,90] |
| New absorption peaks appeared at 930 cm−1, 840 cm−1, 780 cm−1, and 630 cm−1, and the peak areas decreased at 1377 cm−1 and 966 cm−1 | Saline immersion | The salts penetrated them and reacted with the asphalt binder, and the content of aromatic and SBS modifiers was reduced. | [54] |
| Increased at 745 cm−1, 1030 cm−1, 1720 cm−1, and 1133 cm−1 | Saline immersion | Asphalt oxidation occurred during the salt erosion, and the element Cl replaced the element H in the asphalt binder, leading to an increase in asphalt binder aging. | [12] |
| Affected the absorption peak near 1600 cm−1 | Saline immersion | The chloride salt exerted a significant impact on the absorption peaks representing the aromatic fraction and the benzene ring substitution region. | [91] |
| New absorption peaks appeared near 1617 cm−1 and at 3467 cm−1, and peak intensity varied at 2925 cm−1 and 1450 cm−1 | Saline immersion | Sodium chloride resulted in enhanced vibration or increased content of groups; OH− in sodium acetate reacted with acid groups in the asphalt binder; sodium sulfate led to reduced bond energy or content of -CH2 | [61] |
| Erosion Environments | Element Content (%) | ||||||
|---|---|---|---|---|---|---|---|
| C | O | S | N | Cl | Na | Ca | |
| Undamaged | 98.6 | 1.0 | 0.4 | - | - | - | - |
| 10% NaCl for 90d | 93.2 | 3.2 | 2.0 | - | 0.7 | 0.9 | - |
| 10% CaCl2 for 90d | 93.8 | 3.0 | 2.2 | - | 0.6 | - | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Zhang, Q.; Liang, J.; Cheng, Y.; Jin, W. Mechanical Performance of Asphalt Materials Under Salt Erosion Environments: A Literature Review. Polymers 2025, 17, 1078. https://doi.org/10.3390/polym17081078
Wang W, Zhang Q, Liang J, Cheng Y, Jin W. Mechanical Performance of Asphalt Materials Under Salt Erosion Environments: A Literature Review. Polymers. 2025; 17(8):1078. https://doi.org/10.3390/polym17081078
Chicago/Turabian StyleWang, Wensheng, Qingyu Zhang, Jiaxiang Liang, Yongchun Cheng, and Weidong Jin. 2025. "Mechanical Performance of Asphalt Materials Under Salt Erosion Environments: A Literature Review" Polymers 17, no. 8: 1078. https://doi.org/10.3390/polym17081078
APA StyleWang, W., Zhang, Q., Liang, J., Cheng, Y., & Jin, W. (2025). Mechanical Performance of Asphalt Materials Under Salt Erosion Environments: A Literature Review. Polymers, 17(8), 1078. https://doi.org/10.3390/polym17081078