Porogenic Solvents in Molecularly Imprinted Polymer Synthesis: A Comprehensive Review of Current Practices and Emerging Trends
Abstract
:1. Introduction
2. Physical Properties of Porogenic Solvents
3. Aprotic Porogenic Solvents
4. Protic Porogenic Solvents
5. Combining Protic with Aprotic Solvents
6. Ionic Liquids and Deep Eutectic Solvents
| Template | DES (HBA:HBD) | Co-Solvent | Reaction Type | Application | IF | Ref. |
|---|---|---|---|---|---|---|
| Levofloxacin Tetraclyne | Betaine–EG (1:2) | water | Condensation and Polymerization | Sensing | 1.4–2.2 * | [161] |
| Thionine | Glucose–Citric Acid (1:1) | water | Electro-polymerization | Sensing | n.d. | [165] |
| Triazines | L-menthol–Formic acid (1:1) | / | Bulk polymerization | Extraction | 2.5 * | [167] |
| Kaempferol | ChCl: 1,4-BD (1:2) | water | Condensation | Extraction | 2.7 | [163] |
| Vanillylmandelic acid Homovanillic acid | ChCl: 1,4-BD (1:2) | water | Condensation | Extraction | 19.2–111.6 | [164] |
| 4,4′-dichlorobenzhydrol | ChCl: 1,4-BD (1:2) | water | Condensation | Extraction | 9.1 | [168] |
| Naproxene | ChCl–1-butylimidazole (1:1) | ACN | Bulk polymerization | Extraction | 1.7 | [169] |
| Organophosphates | ChCl: Glycerol | 70% EtOH | Polymerization | Extraction | 4.5 | [170] |
| Clorprenaline Bambuterol | ChCl:EG (1:2) | / | Condensation | Extraction | ~6 * | [171] |
| Lysozyme BSA | ChCl:EG (1:2) | water | Polymerization | Extraction | n.d. | [172] |
| Quinolones | ChCl:EG (1:2) ChCl: TMAC (1:2) ChCl: TMAB(1:2) | water | Condensation | Extraction | n.d. | [173] |
| Fenbufen | ChCl:EG (1:2) | [BMIM]BF4 | Bulk polymerization | Extraction | 3.9 | [133] |
| Levofloxacin | ChCl:EG (1:2) | / | Bulk polymerization | Extraction | 1.8 | [162] |
| Cetirizine | ChCl:EG (1:6.8) | [BMIM]BF4 and DMF | Metallic pivot radical polymerization | Extraction | 31.5 | [166] |
7. Emerging Porogenic Systems
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| H3BTC | 1,3,5-benzenetricarboxylic acid |
| 1,4-BD | 1,4-butandiol |
| ACN | acetonitrile |
| AG | aminoglutethimide |
| ATR | atrazine |
| CBZ | carbamazepine |
| CTAB | cetrimonium bromide |
| ChCl | choline chloride |
| DES | deep eutectic solvent |
| DMSO | dimethyl sulfoxide |
| EGDMA | ethylene glycol dimethacrylate |
| EG | ethylene glycol |
| HPLC | high-performance liquid chromatography |
| HMA | homovanillic acid |
| HBA | hydrogen bond acceptor |
| HBD | hydrogen bond donor |
| HIR | hydrophilic imprinted resin |
| igG | immunoglobulin G |
| IF | imprinting factor |
| IL | ionic liquid |
| LC/MS | liquid chromatography/mass spectrometry |
| LAG | liquid-assisted grinding |
| LCST | lower critical solution temperature |
| MOG | metal–organic gel |
| MWCNT | mingle-walled carbon nanotubes |
| MIP | molecularly imprinted polymer |
| NIP | non-imprinted polymer |
| NP | nanoparticle |
| NAP | naproxen |
| SDS | sodium dodecylsulfate |
| SPE | solid-phase extraction |
| SY | sunset yellow |
| scCO2 | supercritical CO2 |
| TEOS | tetraethyl orthosilicate |
| THF | tetrahydrofuran |
| TMAB | tetramethylammonium bromide |
| TMAC | tetramethylammonium chloride |
| TCM | traditional Chinese medicine |
| VMA | vanillylmandelic acid |
| VOC | volatile organic compound |
References
- BelBruno, J.J. Molecularly imprinted polymers. Chem. Rev. 2018, 119, 94–119. [Google Scholar] [CrossRef] [PubMed]
- Vasapollo, G.; Sole, R.D.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G. Molecularly imprinted polymers: Present and future prospective. Int. J. Mol. Sci. 2011, 12, 5908–5945. [Google Scholar] [CrossRef] [PubMed]
- Olsson, G.D.; Karlsson, B.C.; Shoravi, S.; Wiklander, J.G.; Nicholls, I.A. Mechanisms underlying molecularly imprinted polymer molecular memory and the role of crosslinker: Resolving debate on the nature of template recognition in phenylalanine anilide imprinted polymers. J. Mol. Recognit. 2012, 25, 69–73. [Google Scholar] [CrossRef]
- Katz, A.; Davis, M.E. Investigations into the mechanisms of molecular recognition with imprinted polymers. Macromolecules 1999, 32, 4113–4121. [Google Scholar] [CrossRef]
- Hu, T.; Chen, R.; Wang, Q.; He, C.; Liu, S. Recent advances and applications of molecularly imprinted polymers in solid-phase extraction for real sample analysis. J. Sep. Sci. 2021, 44, 274–309. [Google Scholar] [CrossRef]
- Saylan, Y.; Kılıç, S.; Denizli, A. Biosensing applications of molecularly imprinted-polymer-based nanomaterials. Processes 2024, 12, 177. [Google Scholar] [CrossRef]
- Mustafa, Y.L.; Keirouz, A.; Leese, H.S. Molecularly imprinted polymers in diagnostics: Accessing analytes in biofluids. J. Mater. Chem. B 2022, 10, 7418–7449. [Google Scholar] [CrossRef]
- Rebelo, P.; Costa-Rama, E.; Seguro, I.; Pacheco, J.G.; Nouws, H.P.; Cordeiro, M.N.D.; Delerue-Matos, C. Molecularly imprinted polymer-based electrochemical sensors for environmental analysis. Biosens. Bioelectron. 2021, 172, 112719. [Google Scholar] [CrossRef]
- Jia, B.; Feng, F.; Wang, X.; Song, Y.; Zhang, F. Recent advances in magnetic molecularly imprinted polymers and their application in the food safety analysis. J. Future Foods 2024, 4, 1–20. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Wang, S. Recent advances in the removal of emerging contaminants from water by novel molecularly imprinted materials in advanced oxidation processes—A review. Sci. Total Environ. 2023, 883, 163702. [Google Scholar] [CrossRef]
- Van Wissen, G.; Lowdon, J.W.; Cleij, T.J.; Diliën, H.; Eersels, K.; van Grinsven, B. Thermal detection of riboflavin in almond milk using molecularly imprinted polymers. Microchem. J. 2025, 212, 113181. [Google Scholar] [CrossRef]
- Frigoli, M.; Lowdon, J.W.; Cleij, T.J.; Diliën, H.; Eersels, K.; van Grinsven, B. Detection of antibiotic sulfamethoxazole residues in milk using a molecularly imprinted polymer-based thermal biosensor. Food Chem. 2025, 476, 143525. [Google Scholar] [CrossRef] [PubMed]
- Bouvarel, T.; Delaunay, N.; Pichon, V. Molecularly imprinted polymers in miniaturized extraction and separation devices. J. Sep. Sci. 2021, 44, 1727–1751. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.C.; Cao, J.X.; Xie, G.M.; Wang, M.W.; Shi, Y.Y.; Yi, Y.; Yang, C.L.; Xiao, Y.H.; Wei, X.L.; Tian, B.M. Study on preparation and process of poly (MMA-St) thermally expandable core-shell microspheres. J. Appl. Polym. Sci. 2021, 138, 49927. [Google Scholar] [CrossRef]
- Gonçalves, L.M. Electropolymerized molecularly imprinted polymers: Perceptions based on recent literature for soon-to-be world-class scientists. Curr. Opin. Electrochem. 2021, 25, 100640. [Google Scholar] [CrossRef]
- Moein, M.M.; Abdel-Rehim, A.; Abdel-Rehim, M. Recent applications of molecularly imprinted sol-gel methodology in sample preparation. Molecules 2019, 24, 2889. [Google Scholar] [CrossRef]
- Frigoli, M.; Lowdon, J.W.; Caldara, M.; Arreguin-Campos, R.; Sewall, J.; Cleij, T.J.; Diliën, H.; Eersels, K.; Van Grinsven, B. Thermal pyocyanin sensor based on molecularly imprinted polymers for the indirect detection of Pseudomonas aeruginosa. ACS Sens. 2023, 8, 353–362. [Google Scholar] [CrossRef]
- Esfandyari-Manesh, M.; Javanbakht, M.; Atyabi, F.; Badiei, A.; Dinarvand, R. Effect of porogenic solvent on the morphology, recognition and release properties of carbamazepine-molecularly imprinted polymer nanospheres. J. Appl. Polym. Sci. 2011, 121, 1118–1126. [Google Scholar] [CrossRef]
- Mansour, F.R.; Waheed, S.; Paull, B.; Maya, F. Porogens and porogen selection in the preparation of porous polymer monoliths. J. Sep. Sci. 2020, 43, 56–69. [Google Scholar] [CrossRef]
- Lakshmi, D.S.; KS, R.; Castro-Muñoz, R.; Tańczyk, M. Emerging trends in porogens toward material fabrication: Recent progresses and challenges. Polymers 2022, 14, 5209. [Google Scholar] [CrossRef]
- Li, X.-R.; Wang, X.-L.; Koseki, H. Study on thermal decomposition characteristics of AIBN. J. Hazard. Mater. 2008, 159, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Rosengren, A.M.; Golker, K.; Karlsson, J.G.; Nicholls, I.A. Dielectric constants are not enough: Principal component analysis of the influence of solvent properties on molecularly imprinted polymer–ligand rebinding. Biosens. Bioelectron. 2009, 25, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Andersson, L.I. Application of molecular imprinting to the development of aqueous buffer and organic solvent based radioligand binding assays for (S)-propranolol. Anal. Chem. 1996, 68, 111–117. [Google Scholar] [CrossRef]
- Jouyban, A.; Soltanpour, S.; Chan, H.-K. A simple relationship between dielectric constant of mixed solvents with solvent composition and temperature. Int. J. Pharm. 2004, 269, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Stefanis, E.; Panayiotou, C. Prediction of Hansen solubility parameters with a new group-contribution method. Int. J. Thermophys. 2008, 29, 568–585. [Google Scholar] [CrossRef]
- Reichardt, C. Solvatochromic dyes as solvent polarity indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Riddick, J.A.; Bunger, W.B.; Sakano, T.K. Organic Solvents: Physical Properties and Methods of Purification, 4th ed.; Wiley-Interscience: New York, NY, USA, 1986. [Google Scholar]
- Dickert, F.L.; Geiger, U.; Lieberzeit, P.; Reutner, U. Solvatochromic betaine dyes as optochemical sensor materials: Detection of polar and non-polar vapors. Sens. Actuators B Chem. 2000, 70, 263–269. [Google Scholar] [CrossRef]
- Carda–Broch, S.; Berthod, A.; Armstrong, D. Solvent properties of the 1-butyl-3-methylimidazolium hexafluorophosphate ionic liquid. Anal. Bioanal. Chem. 2003, 375, 191–199. [Google Scholar] [CrossRef]
- Mirza, N.R.; Nicholas, N.J.; Wu, Y.; Kentish, S.; Stevens, G.W. Estimation of normal boiling temperatures, critical properties, and acentric factors of deep eutectic solvents. J. Chem. Eng. Data 2015, 60, 1844–1854. [Google Scholar] [CrossRef]
- Griffiths, T.R.; Pugh, D.C. Correlations among solvent polarity scales, dielectric constant and dipole moment, and a means to reliable predictions of polarity scale values from cu. Coord. Chem. Rev. 1979, 29, 129–211. [Google Scholar] [CrossRef]
- Hunger, J.; Stoppa, A.; Schrödle, S.; Hefter, G.; Buchner, R. Temperature dependence of the dielectric properties and dynamics of ionic liquids. ChemPhysChem 2009, 10, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Di Noto, V.; Vezzu, K.; Conti, F.; Giffin, G.A.; Lavina, S.; Bertucco, A. Broadband Electric Spectroscopy at High CO2 Pressure: Dipole Moment of CO2 and Relaxation Phenomena of the CO2–Poly (vinyl chloride) System. J. Phys. Chem. B 2011, 115, 9014–9021. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Du, Y.; Xue, Y.; Frisch, H. Solubility parameters. In Physical Properties of Polymers Handbook; Springer: New York, NY, USA, 2007; pp. 289–303. [Google Scholar]
- Mutelet, F.; Butet, V.; Jaubert, J.-N. Application of inverse gas chromatography and regular solution theory for characterization of ionic liquids. Ind. Eng. Chem. Res. 2005, 44, 4120–4127. [Google Scholar] [CrossRef]
- Novaes, F.J.M.; de Faria, D.C.; Ferraz, F.Z.; de Aquino Neto, F.R. Hansen solubility parameters applied to the extraction of phytochemicals. Plants 2023, 12, 3008. [Google Scholar] [CrossRef]
- Williams, L.L.; Rubin, J.B.; Edwards, H. Calculation of Hansen solubility parameter values for a range of pressure and temperature conditions, including the supercritical fluid region. Ind. Eng. Chem. Res. 2004, 43, 4967–4972. [Google Scholar] [CrossRef]
- Del Valle, J.; García Blanco, F.; Catalán, J. Empirical parameters for solvent acidity, basicity, dipolarity, and polarizability of the ionic liquids [BMIM][BF4] and [BMIM][PF6]. J. Phys. Chem. B 2015, 119, 4683–4692. [Google Scholar] [CrossRef]
- Omar, K.A.; Sadeghi, R. Physicochemical properties of deep eutectic solvents: A review. J. Mol. Liq. 2022, 360, 119524. [Google Scholar] [CrossRef]
- Gao, F.; Chang, H.; Li, J.; Wang, R.; Gu, Y. Replacing polar aprotic solvents with water in organic synthesis. Curr. Opin. Green Sustain. Chem. 2023, 40, 100774. [Google Scholar] [CrossRef]
- Rossini, E.; Knapp, E.W. Proton solvation in protic and aprotic solvents. J. Comput. Chem. 2016, 37, 1082–1091. [Google Scholar] [CrossRef]
- Yan, H.; Row, K.H. Characteristic and synthetic approach of molecularly imprinted polymer. Int. J. Mol. Sci. 2006, 7, 155–178. [Google Scholar] [CrossRef]
- Wu, L.; Zhu, K.; Zhao, M.; Li, Y. Theoretical and experimental study of nicotinamide molecularly imprinted polymers with different porogens. Anal. Chim. Acta 2005, 549, 39–44. [Google Scholar] [CrossRef]
- Farrington, K.; Regan, F. Investigation of the nature of MIP recognition: The development and characterisation of a MIP for Ibuprofen. Biosens. Bioelectron. 2007, 22, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Yuan, X.; Zhang, J.; Wang, B.; Sun, X.; Chen, X.; Zhao, L. Dummy-surface molecularly imprinted polymers as a sorbent of micro-solid-phase extraction combined with dispersive liquid–liquid microextraction for determination of five 2-phenylpropionic acid NSAIDs in aquatic environmental samples. Anal. Bioanal. Chem. 2018, 410, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Gadzała-Kopciuch, R.; Ričanyová, J.; Buszewski, B. Isolation and detection of steroids from human urine by molecularly imprinted solid-phase extraction and liquid chromatography. J. Chromatogr. B 2009, 877, 1177–1184. [Google Scholar] [CrossRef]
- Pap, T.M.; Horváth, V.; Tolokán, A.; Horvai, G.; Sellergren, B. Effect of solvents on the selectivity of terbutylazine imprinted polymer sorbents used in solid-phase extraction. J. Chromatogr. A 2002, 973, 1–12. [Google Scholar] [CrossRef]
- Malosse, L.; Buvat, P.; Adès, D.; Siove, A. Detection of degradation products of chemical warfare agents by highly porous molecularly imprinted microspheres. Analyst 2008, 133, 588–595. [Google Scholar] [CrossRef]
- Mirzaei, M.; Najafabadi, S.A.H.; Abdouss, M.; Azodi-Deilami, S.; Asadi, E.; Hosseini, M.R.M.; Piramoon, M. Preparation and utilization of microporous molecularly imprinted polymer for sustained release of tetracycline. J. Appl. Polym. Sci. 2013, 128, 1557–1562. [Google Scholar] [CrossRef]
- Székely, G.; Bandarra, J.; Heggie, W.; Ferreira, F.C.; Sellergren, B. Design, preparation and characterization of novel molecularly imprinted polymers for removal of potentially genotoxic 1, 3-diisopropylurea from API solutions. Sep. Purif. Technol. 2012, 86, 190–198. [Google Scholar] [CrossRef]
- Buszewski, B.; Ričanyová, J.; Gadzała-Kopciuch, R.; Szumski, M. Supramolecular recognition of estrogens via molecularly imprinted polymers. Anal. Bioanal. Chem. 2010, 397, 2977–2986. [Google Scholar] [CrossRef]
- Marroquin-Garcia, R.; van Wissen, G.; Cleij, T.J.; Eersels, K.; van Grinsven, B.; Diliën, H. Single-use dye displacement colorimetry assay based on molecularly imprinted polymers: Towards fast and on-site detection of xylazine in alcoholic beverages. Food Control 2024, 161, 110403. [Google Scholar] [CrossRef]
- Lowdon, J.W.; Eersels, K.; Arreguin-Campos, R.; Caldara, M.; Heidt, B.; Rogosic, R.; Jimenez-Monroy, K.L.; Cleij, T.J.; Diliën, H.; van Grinsven, B. A molecularly imprinted polymer-based dye displacement assay for the rapid visual detection of amphetamine in urine. Molecules 2020, 25, 5222. [Google Scholar] [CrossRef] [PubMed]
- Frigoli, M.; Lowdon, J.W.; Donetti, N.; Crapnell, R.D.; Banks, C.E.; Cleij, T.J.; Diliën, H.; Eersels, K.; van Grinsven, B. Electrochemical Detection of Pseudomonas aeruginosa Quorum Sensing Molecule (S)-N-Butyryl Homoserine Lactone Using Molecularly Imprinted Polymers. ACS Omega 2024, 9, 36411–36420. [Google Scholar] [CrossRef] [PubMed]
- Panjan, P.; Monasterio, R.P.; Carrasco-Pancorbo, A.; Fernandez-Gutierrez, A.; Sesay, A.M.; Fernandez-Sanchez, J.F. Development of a folic acid molecularly imprinted polymer and its evaluation as a sorbent for dispersive solid-phase extraction by liquid chromatography coupled to mass spectrometry. J. Chromatogr. A 2018, 1576, 26–33. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, J.; Molinelli, A.; Nolan, K.; Smyth, M.; Mizaikoff, B. Anatomy of a successful imprint: Analysing the recognition mechanisms of a molecularly imprinted polymer for quercetin. Biosens. Bioelectron. 2006, 21, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Meier, F.; Schott, B.; Riedel, D.; Mizaikoff, B. Computational and experimental study on the influence of the porogen on the selectivity of 4-nitrophenol molecularly imprinted polymers. Anal. Chim. Acta 2012, 744, 68–74. [Google Scholar] [CrossRef]
- Santora, B.P.; Gagné, M.R.; Moloy, K.G.; Radu, N.S. Porogen and cross-linking effects on the surface area, pore volume distribution, and morphology of macroporous polymers obtained by bulk polymerization. Macromolecules 2001, 34, 658–661. [Google Scholar] [CrossRef]
- Pardeshi, S.; Dhodapkar, R.; Kumar, A. Influence of porogens on the specific recognition of molecularly imprinted poly (acrylamide-co-ethylene glycol dimethacrylate). Compos. Interfaces 2014, 21, 13–30. [Google Scholar] [CrossRef]
- Nagy-Szakolczai, A.; Dorkó, Z.; Tóth, B.; Horvai, G. New methods to study the behavior of molecularly imprinted polymers in aprotic solvents. Polymers 2018, 10, 1015. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Bai, R.; Ferlin, F.; Vaccaro, L.; Li, M.; Gu, Y. Replacement strategies for non-green dipolar aprotic solvents. Green Chem. 2020, 22, 6240–6257. [Google Scholar] [CrossRef]
- Amann, A.; de Lacy Costello, B.; Miekisch, W.; Schubert, J.; Buszewski, B.; Pleil, J.; Ratcliffe, N.; Risby, T. The human volatilome: Volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J. Breath Res. 2014, 8, 034001. [Google Scholar] [CrossRef]
- Fan, J.-P.; Tian, Z.-Y.; Tong, S.; Zhang, X.-H.; Xie, Y.-L.; Xu, R.; Qin, Y.; Li, L.; Zhu, J.-H.; Ouyang, X.-K. A novel molecularly imprinted polymer of the specific ionic liquid monomer for selective separation of synephrine from methanol–water media. Food Chem. 2013, 141, 3578–3585. [Google Scholar] [CrossRef] [PubMed]
- Reid, J.E.; Gammons, R.J.; Slattery, J.M.; Walker, A.J.; Shimizu, S. Interactions in water–ionic liquid mixtures: Comparing protic and aprotic systems. J. Phys. Chem. B 2017, 121, 599–609. [Google Scholar] [CrossRef]
- Buback, M.; Hutchinson, R.A.; Lacik, I. Radical polymerization kinetics of water-soluble monomers. Prog. Polym. Sci. 2023, 138, 101645. [Google Scholar] [CrossRef]
- Gromov, V.F.; Bune, E.V.; Teleshov, E.N. Characteristic features of the radical polymerisation of water-soluble monomers. Russ. Chem. Rev. 1994, 63, 507. [Google Scholar] [CrossRef]
- Kuchta, F.-D.; van Herk, A.M.; German, A.L. Propagation kinetics of acrylic and methacrylic acid in water and organic solvents studied by pulsed-laser polymerization. Macromolecules 2000, 33, 3641–3649. [Google Scholar] [CrossRef]
- Zaidi, S.A. Bacterial imprinting methods and their applications: An overview. Crit. Rev. Anal. Chem. 2021, 51, 609–618. [Google Scholar] [CrossRef]
- Zhou, X.; Lai, C.; Huang, D.; Zeng, G.; Chen, L.; Qin, L.; Xu, P.; Cheng, M.; Huang, C.; Zhang, C. Preparation of water-compatible molecularly imprinted thiol-functionalized activated titanium dioxide: Selective adsorption and efficient photodegradation of 2, 4-dinitrophenol in aqueous solution. J. Hazard. Mater. 2018, 346, 113–123. [Google Scholar] [CrossRef]
- Arabi, M.; Ghaedi, M.; Ostovan, A. Development of a lower toxic approach based on green synthesis of water-compatible molecularly imprinted nanoparticles for the extraction of hydrochlorothiazide from human urine. ACS Sustain. Chem. Eng. 2017, 5, 3775–3785. [Google Scholar] [CrossRef]
- Zhou, T.; Ding, L.; Che, G.; Jiang, W.; Sang, L. Recent advances and trends of molecularly imprinted polymers for specific recognition in aqueous matrix: Preparation and application in sample pretreatment. TrAC Trends Anal. Chem. 2019, 114, 11–28. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, B.; Wang, S.; Zhang, W. Recyclable trypsin immobilized magnetic nanoparticles based on hydrophilic polyethylenimine modification and their proteolytic characteristics. Anal. Methods 2018, 10, 459–466. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.; Hu, Y.; Li, G. Microwave heating in preparation of magnetic molecularly imprinted polymer beads for trace triazines analysis in complicated samples. Anal. Chem. 2009, 81, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Ostovan, A.; Ghaedi, M.; Arabi, M.; Yang, Q.; Li, J.; Chen, L. Hydrophilic multitemplate molecularly imprinted biopolymers based on a green synthesis strategy for determination of B-family vitamins. ACS Appl. Mater. Interfaces 2018, 10, 4140–4150. [Google Scholar] [CrossRef]
- Foroughirad, S.; Haddadi-Asl, V.; Khosravi, A.; Salami-Kalajahi, M. Effect of porogenic solvent in synthesis of mesoporous and microporous molecularly imprinted polymer based on magnetic halloysite nanotubes. Mater. Today Commun. 2021, 26, 101780. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Tsalbouris, A.; Kabir, A.; Furton, K.G.; Samanidou, V.F. Synthesis and application of molecularly imprinted polymers using sol–gel matrix imprinting technology for the efficient solid-phase extraction of BPA from water. Microchem. J. 2020, 157, 104965. [Google Scholar] [CrossRef]
- Juan-Díaz, M.; Martínez-Ibáñez, M.; Hernández-Escolano, M.; Cabedo, L.; Izquierdo, R.; Suay, J.; Gurruchaga, M.; Goñi, I. Study of the degradation of hybrid sol–gel coatings in aqueous medium. Prog. Org. Coat. 2014, 77, 1799–1806. [Google Scholar] [CrossRef]
- Hasanah, A.; Suherman, M.; Susanti, I.; Pitaloka, I.; Mustarichie, R. Performance evaluation of atenolol molecular imprinted polymer using two different polymerization and two different porogen. Rasayan J. Chem 2019, 12, 1269–1278. [Google Scholar] [CrossRef]
- Herrera-Chacón, A.; Dinç-Zor, Ş.; Del Valle, M. Integrating molecularly imprinted polymer beads in graphite-epoxy electrodes for the voltammetric biosensing of histamine in wines. Talanta 2020, 208, 120348. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, T.; Li, H.; Liu, H.; Huang, N.; Xu, Y.; Ding, J.; Ding, L.; Li, Y. Preparation of magnetic surface molecularly imprinted polymers for the selective extraction of triazines in environmental water samples. Int. J. Environ. Anal. Chem. 2018, 98, 1049–1062. [Google Scholar] [CrossRef]
- Hu, X.; Xie, L.; Guo, J.; Li, H.; Jiang, X.; Zhang, Y.; Shi, S. Hydrophilic gallic acid–imprinted polymers over magnetic mesoporous silica microspheres with excellent molecular recognition ability in aqueous fruit juices. Food Chem. 2015, 179, 206–212. [Google Scholar] [CrossRef]
- Sun, Y. Molecularly imprinted polymer for 2, 4-dichlorophenoxyacetic acid prepared by a sol-gel method. J. Chem. Sci. 2014, 126, 1005–1011. [Google Scholar] [CrossRef]
- Wang, F.; Ling, B.; Li, Q.; Abouhany, R. Dual roles of 3-aminopropyltriethoxysilane in preparing molecularly imprinted silica particles for specific recognition of target molecules. RSC Adv. 2020, 10, 20368–20373. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, Q.; Wang, X.; Arabi, M.; Peng, H.; Li, J.; Xiong, H.; Chen, L. Facile approach to the synthesis of molecularly imprinted ratiometric fluorescence nanosensor for the visual detection of folic acid. Food Chem. 2020, 319, 126575. [Google Scholar] [CrossRef] [PubMed]
- Kaya, S.I.; Corman, M.E.; Uzun, L.; Ozkan, S.A. Simple preparation of surface molecularly imprinted polymer based on silica particles for trace level assay of bisphenol F. Anal. Bioanal. Chem. 2022, 414, 5793–5803. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-J.; Chen, P.-Y.; Nien, P.-C.; Lin, C.-Y.; Vittal, R.; Ling, T.-R.; Ho, K.-C. Preparation of a novel molecularly imprinted polymer by the sol–gel process for sensing creatinine. Anal. Chim. Acta 2012, 711, 83–90. [Google Scholar] [CrossRef]
- Li, B.; Xu, J.; Hall, A.J.; Haupt, K.; Tse Sum Bui, B. Water-compatible silica sol–gel molecularly imprinted polymer as a potential delivery system for the controlled release of salicylic acid. J. Mol. Recognit. 2014, 27, 559–565. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, J.; Liu, M.; Han, X.; Peng, Y.; Tian, X.; Liu, J.; Zhang, S. Synthesis of molecularly imprinted polymer via emulsion polymerization for application in solanesol separation. Appl. Sci. 2020, 10, 2868. [Google Scholar] [CrossRef]
- Mezhoud, S.; Le Droumaguet, B.; Aimedieu, P.; Monchiet, V.; Bornert, M.; Grande, D. Investigation of morphology associated with biporous polymeric materials obtained by the double porogen templating approach. Colloid. Polym. Sci. 2021, 299, 537–550. [Google Scholar] [CrossRef]
- Azizi, A.; Bottaro, C.S. A critical review of molecularly imprinted polymers for the analysis of organic pollutants in environmental water samples. J. Chromatogr. A 2020, 1614, 460603. [Google Scholar] [CrossRef]
- Pircher, N.; Fischhuber, D.; Carbajal, L.; Strauß, C.; Nedelec, J.M.; Kasper, C.; Rosenau, T.; Liebner, F. Preparation and reinforcement of dual-porous biocompatible cellulose scaffolds for tissue engineering. Macromol. Mater. Eng. 2015, 300, 911–924. [Google Scholar] [CrossRef]
- Lovell, P.A.; Schork, F.J. Fundamentals of emulsion polymerization. Biomacromolecules 2020, 21, 4396–4441. [Google Scholar] [CrossRef]
- Song, Z.; Li, J.; Lu, W.; Li, B.; Yang, G.; Bi, Y.; Arabi, M.; Wang, X.; Ma, J.; Chen, L. Molecularly imprinted polymers based materials and their applications in chromatographic and electrophoretic separations. TrAC Trends Anal. Chem. 2022, 146, 116504. [Google Scholar] [CrossRef]
- Fresco-Cala, B.; Cárdenas, S. Advanced polymeric solids containing nano-and micro-particles prepared via emulsion-based polymerization approaches. A review. Anal. Chim. Acta 2022, 1208, 339669. [Google Scholar] [CrossRef]
- Sun, Y.; Ren, T.; Deng, Z.; Yang, Y.; Zhong, S. Molecularly imprinted polymers fabricated using Janus particle-stabilized Pickering emulsions and charged monomer polymerization. New J. Chem. 2018, 42, 7355–7363. [Google Scholar] [CrossRef]
- Lowdon, J.W.; Diliën, H.; van Grinsven, B.; Eersels, K.; Cleij, T.J. Colorimetric Sensing of Amoxicillin Facilitated by Molecularly Imprinted Polymers. Polymers 2021, 13, 2221. [Google Scholar] [CrossRef] [PubMed]
- El-Aasser, M.; Loncar, F., Jr.; Vanderhoff, J. Swelling of carboxyl-containing copolymer latexes. Macromol. Chem. Phys. 1985, 10, 335–357. [Google Scholar] [CrossRef]
- Díaz-Álvarez, M.; Turiel, E.; Martín-Esteban, A. Recent advances and future trends in molecularly imprinted polymers-based sample preparation. J. Sep. Sci. 2023, 46, 2300157. [Google Scholar] [CrossRef]
- Zeng, H.; Yu, X.; Wan, J.; Cao, X. Rational design and synthesis of molecularly imprinted polymers (MIP) for purifying tylosin by seeded precipitation polymerization. Process Biochem. 2020, 94, 329–339. [Google Scholar] [CrossRef]
- Chen, D.-M.; Fu, Q.; Du, W.; Sun, S.-J.; Huang, P.; Chang, C. Preparation and evaluation of monolithic molecularly imprinted stationary phase for S-naproxen. J. Pharm. Anal. 2011, 1, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, S.; Singh, S.K. Precipitation polymerization: A versatile tool for preparing molecularly imprinted polymer beads for chromatography applications. RSC Adv. 2016, 6, 23525–23536. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, Y.; Yuan, Y.; Zeng, H. Development and characterization of molecularly imprinted polymer microspheres for the selective detection of kaempferol in traditional Chinese medicines. Anal. Methods 2011, 3, 348–355. [Google Scholar] [CrossRef]
- Liang, S.; Wan, J.; Zhu, J.; Cao, X. Effects of porogens on the morphology and enantioselectivity of core–shell molecularly imprinted polymers with ursodeoxycholic acid. Sep. Purif. Technol. 2010, 72, 208–216. [Google Scholar] [CrossRef]
- Chevalier, Y.; Bolzinger, M.-A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surf. Physicochem. Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, W.; Yu, H.; Wang, A. Preparation of porous adsorbent via Pickering emulsion template for water treatment: A review. J. Environ. Sci. 2020, 88, 217–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Z.; Yan, R.; Fu, X.; Wang, G.; Wang, Y.; Li, Z.; Zhang, X.; Hou, J. Facile fabrication of snowman-like magnetic molecularly imprinted polymer microspheres for bisphenol A via one-step Pickering emulsion polymerization. React. Funct. Polym. 2021, 164, 104911. [Google Scholar] [CrossRef]
- Lan, Y.; Corradini, M.; Weiss, R.G.; Raghavan, S.; Rogers, M. To gel or not to gel: Correlating molecular gelation with solvent parameters. Chem. Soc. Rev. 2015, 44, 6035–6058. [Google Scholar] [CrossRef]
- Moein, M.M.; Javanbakht, M.; Karimi, M.; Akbari-Adergani, B.; Abdel-Rehim, M. Three-phase molecularly imprinted sol–gel based hollow fiber liquid-phase microextraction combined with liquid chromatography–tandem mass spectrometry for enrichment and selective determination of a tentative lung cancer biomarker. J. Chromatogr. B 2015, 995, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, T.; Atayi, K. Synthesis of hydrogen phosphate anion-imprinted polymer via emulsion polymerization and its use as the recognition element of graphene/graphite paste potentiometric electrode. Mater. Chem. Phys. 2018, 209, 180–187. [Google Scholar] [CrossRef]
- Zhao, X.; Cui, Y.; Wang, J.; Wang, J. Preparation of fluorescent molecularly imprinted polymers via pickering emulsion interfaces and the application for visual sensing analysis of Listeria Monocytogenes. Polymers 2019, 11, 984. [Google Scholar] [CrossRef]
- Pan, J.; Li, L.; Hang, H.; Wu, R.; Dai, X.; Shi, W.; Yan, Y. Fabrication and evaluation of magnetic/hollow double-shelled imprinted sorbents formed by Pickering emulsion polymerization. Langmuir 2013, 29, 8170–8178. [Google Scholar] [CrossRef]
- Ou, H.; Chen, Q.; Pan, J.; Zhang, Y.; Huang, Y.; Qi, X. Selective removal of erythromycin by magnetic imprinted polymers synthesized from chitosan-stabilized Pickering emulsion. J. Hazard. Mater. 2015, 289, 28–37. [Google Scholar] [CrossRef]
- Sun, Y.; Zhong, S. Molecularly imprinted polymers fabricated via Pickering emulsions stabilized solely by food-grade casein colloidal nanoparticles for selective protein recognition. Anal. Bioanal. Chem. 2018, 410, 3133–3143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dramou, P.; He, H.; Tan, S.; Pham-Huy, C.; Pan, H. Molecularly imprinted stationary phase prepared by reverse micro-emulsion polymerization for selective recognition of gatifloxacin in aqueous media. J. Chromatogr. Sci. 2012, 50, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, H.; Zhang, Q.; Cui, Y.; Wu, Z.; Zheng, R.; Liu, L. Molecularly imprinted polymers prepared by precipitation polymerization and used for inducing crystallization of oleanolic acid in supercritical CO2. Sep. Purif. Technol. 2011, 81, 411–417. [Google Scholar] [CrossRef]
- Spivak, D.A. Optimization, evaluation, and characterization of molecularly imprinted polymers. Adv. Drug Del. Rev. 2005, 57, 1779–1794. [Google Scholar] [CrossRef]
- Lowdon, J.W.; Diliën, H.; Singla, P.; Peeters, M.; Cleij, T.J.; van Grinsven, B.; Eersels, K. MIPs for commercial application in low-cost sensors and assays–An overview of the current status quo. Sens. Actuators B Chem. 2020, 325, 128973. [Google Scholar] [CrossRef] [PubMed]
- Mohsenzadeh, E.; Ratautaite, V.; Brazys, E.; Ramanavicius, S.; Zukauskas, S.; Plausinaitis, D.; Ramanavicius, A. Design of molecularly imprinted polymers (MIP) using computational methods: A review of strategies and approaches. WIREs Comput. Mol. Sci. 2024, 14, e1713. [Google Scholar] [CrossRef]
- Wu, X.; Du, J.; Li, M.; Wu, L.; Han, C.; Su, F. Recent advances in green reagents for molecularly imprinted polymers. RSC Adv. 2018, 8, 311–327. [Google Scholar] [CrossRef]
- Pei, Y.; Zhang, Y.; Ma, J.; Fan, M.; Zhang, S.; Wang, J. Ionic liquids for advanced materials. Mater. Today Nano 2022, 17, 100159. [Google Scholar] [CrossRef]
- Liu, H.; Jin, P.; Zhu, F.; Nie, L.; Qiu, H. A review on the use of ionic liquids in preparation of molecularly imprinted polymers for applications in solid-phase extraction. TrAC Trends Anal. Chem. 2021, 134, 116132. [Google Scholar] [CrossRef]
- Ding, S.; Lyu, Z.; Niu, X.; Zhou, Y.; Liu, D.; Falahati, M.; Du, D.; Lin, Y. Integrating ionic liquids with molecular imprinting technology for biorecognition and biosensing: A review. Biosens. Bioelectron. 2020, 149, 111830. [Google Scholar] [CrossRef]
- Han, X.; Armstrong, D.W. Ionic liquids in separations. Acc. Chem. Res. 2007, 40, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yan, F.; Texter, J. Advanced applications of ionic liquids in polymer science. Prog. Polym. Sci. 2009, 34, 431–448. [Google Scholar] [CrossRef]
- Wang, N.; Cui, B. An overview of ionic liquid-based adsorbents in food analysis. TrAC Trends Anal. Chem. 2022, 146, 116496. [Google Scholar] [CrossRef]
- Zhu, G.; Gao, X.; Wang, X.; Wang, J.; Fan, J. Influence of hydrogen bond accepting ability of anions on the adsorption performance of ionic liquid surface molecularly imprinted polymers. J. Chromatogr. A 2018, 1532, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.-D.; Huang, Y.-P.; Xin, X.-L.; Liu, Z.-S.; Aisa, H.A. Preparation of metallic pivot-based imprinted monolith for polar template. J. Chromatogr. B 2013, 934, 109–116. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, X.-L.; Ma, L.; Shang, P.-P.; Huang, Y.-P.; Liu, Z.-S. Improving affinity of β-cyclodextrin-based molecularly imprinted polymer using room temperature ionic liquid. Eur. Polym. J. 2019, 116, 275–282. [Google Scholar] [CrossRef]
- Deng, W.; Yang, C.; Gong, M.; Zhang, Z.; Li, H. Preparation of rutin imprinted monolith (RIM) by using porogen containing ion liquid [BMIM] PF6 and its molecular recognition. J. Chromatogr. B 2024, 1233, 123986. [Google Scholar] [CrossRef]
- Guo, L.; Deng, Q.; Fang, G.; Gao, W.; Wang, S. Preparation and evaluation of molecularly imprinted ionic liquids polymer as sorbent for on-line solid-phase extraction of chlorsulfuron in environmental water samples. J. Chromatogr. A 2011, 1218, 6271–6277. [Google Scholar] [CrossRef]
- Booker, K.; Holdsworth, C.I.; Doherty, C.M.; Hill, A.J.; Bowyer, M.C.; McCluskey, A. Ionic liquids as porogens for molecularly imprinted polymers: Propranolol, a model study. Org. Biomol. Chem. 2014, 12, 7201–7210. [Google Scholar] [CrossRef]
- Liu, X.-L.; Yao, H.-F.; Chai, M.-H.; He, W.; Huang, Y.-P.; Liu, Z.-S. Green synthesis of carbon nanotubes-reinforced molecularly imprinted polymer composites for drug delivery of fenbufen. AAPS PharmSciTech 2018, 19, 3895–3906. [Google Scholar] [CrossRef]
- Booker, K.; Bowyer, M.C.; Holdsworth, C.I.; McCluskey, A. Efficient preparation and improved sensitivity of molecularly imprinted polymers using room temperature ionic liquids. Chem. Commun. 2006, 16, 1730–1732. [Google Scholar] [CrossRef]
- He, C.; Long, Y.; Pan, J.; Li, K.; Liu, F. Molecularly imprinted silica prepared with immiscible ionic liquid as solvent and porogen for selective recognition of testosterone. Talanta 2008, 74, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.K.; Sun, G.-Y.; Jia, M.; Yang, J.; Liu, Z.-S.; Huang, Y.-P.; Aisa, H.A. Cost-effective imprinting to minimize consumption of template in room-temperature ionic liquid for fast purification of chlorogenic acid from the extract of E. ulmoides leaves. Anal. Bioanal. Chem. 2019, 411, 1261–1271. [Google Scholar] [CrossRef]
- Sun, Y.K.; Jia, M.; Yang, J.; Huang, Y.-P.; Liu, Z.-S.; Aisa, H.A. A strategy of utilizing Zn (II) as metallic pivot in room temperature ionic liquid to prepare molecularly imprinted polymers for compound with intramolecular hydrogen bonds. Anal. Bioanal. Chem. 2018, 410, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Ban, L.; Han, X.; Wang, X.-H.; Huang, Y.-P.; Liu, Z.-S. Carprofen-imprinted monolith prepared by reversible addition–fragmentation chain transfer polymerization in room temperature ionic liquids. Anal. Bioanal. Chem. 2013, 405, 8597–8605. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; He, J.; Cai, G.; Lin, A.; Zheng, W.; Liu, X.; Chen, L.; He, X.; Zhang, Y. Room temperature ionic liquid-mediated molecularly imprinted polymer monolith for the selective recognition of quinolones in pork samples. J. Sep. Sci. 2010, 33, 3786–3793. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.-J.; Yang, J.; Zhao, Y.-X.; Liu, Z.-S.; Aisa, H.A. Preparation of ionic liquid-mediated imprinted monolith for selective capture and purification of corilagin. J. Chromatogr. B 2017, 1041, 98–103. [Google Scholar] [CrossRef]
- Li, X.-J.; Chen, X.-X.; Sun, G.-Y.; Zhao, Y.X.; Liu, Z.-S.; Aisa, H.A. Green synthesis and evaluation of isoquercitrin imprinted polymers for class-selective separation and purification of flavonol glycosides. Anal. Methods 2015, 7, 4717–4724. [Google Scholar] [CrossRef]
- Wu, X.; Wu, L. Molecularly imprinted polymers for the solid-phase extraction of four fluoroquilones from milk and lake water samples. J. Sep. Sci. 2015, 38, 3615–3621. [Google Scholar] [CrossRef]
- Xu, Z.; Fang, G.; Wang, S. Molecularly imprinted solid phase extraction coupled to high-performance liquid chromatography for determination of trace dichlorvos residues in vegetables. Food Chem. 2010, 119, 845–850. [Google Scholar] [CrossRef]
- Wang, X.-H.; Zhang, J.; Peng, C.; Dong, Q.; Huang, Y.-P.; Liu, Z.-S. Comparison of multi-recognition molecularly imprinted polymers for recognition of melamine, cyromazine, triamterene, and trimethoprim. Anal. Bioanal. Chem. 2015, 407, 7145–7155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, W.; Wang, N.; Ni, X.; Wang, H.; Gu, Z.; Wu, X.; Xu, W. Synthesis and Evaluation of Ionic Liquid–Mediated Molecularly Imprinted Polymer for Highly Selective Recognition of Dibutyl Phthalate from Liquor Samples. Adv. Polym. Tech. 2017, 36, 220–229. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Yoo, C.-I.; Ahn, Y.-S. N,N-dimethylformamide. Scand. J. Work Environ. Health 2019, 45, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, E.; Song, S.-H.; Lee, C.-W.; Kwon, J.-T.; Park, E.Y.; Kim, B. Toluene concentrations in the blood and risk of thyroid cancer among residents living near national industrial complexes in South Korea: A population-based cohort study. Environ. Int. 2021, 146, 106304. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; De la Guardia, M.; Andruch, V.; Vilková, M. Deep eutectic solvents vs ionic liquids: Similarities and differences. Microchem. J. 2020, 159, 105539. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Hayyan, A.; Al-Saadi, M.A.; AlNashef, I.M.; Mirghani, M.E.; Saheed, O.K. Are deep eutectic solvents benign or toxic? Chemosphere 2013, 90, 2193–2195. [Google Scholar] [CrossRef]
- Azzouz, A.; Hayyan, M. Techno-economic feasibility analysis: The missing piece in the puzzle of deep eutectic solvents. Sustain. Mater. Technol. 2023, 39, e00795. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Ncube, S.; Nomngongo, P.N.; Pakade, V.E. Molecular imprinting with deep eutectic solvents: Synthesis, applications, their significance, and benefits. J. Mol. Liq. 2022, 362, 119696. [Google Scholar] [CrossRef]
- Zhekenov, T.; Toksanbayev, N.; Kazakbayeva, Z.; Shah, D.; Mjalli, F.S. Formation of type III Deep Eutectic Solvents and effect of water on their intermolecular interactions. Fluid Phase Equilib. 2017, 441, 43–48. [Google Scholar] [CrossRef]
- Abbott, A.P.; Alabdullah, S.S.; Al-Murshedi, A.Y.; Ryder, K.S. Brønsted acidity in deep eutectic solvents and ionic liquids. Faraday Discuss. 2018, 206, 365–377. [Google Scholar] [CrossRef]
- Hayyan, A.; Mjalli, F.S.; AlNashef, I.M.; Al-Wahaibi, T.; Al-Wahaibi, Y.M.; Hashim, M.A. Fruit sugar-based deep eutectic solvents and their physical properties. Thermochim. Acta 2012, 541, 70–75. [Google Scholar] [CrossRef]
- Santana, A.P.; Mora-Vargas, J.A.; Guimaraes, T.G.; Amaral, C.D.; Oliveira, A.; Gonzalez, M.H. Sustainable synthesis of natural deep eutectic solvents (NADES) by different methods. J. Mol. Liq. 2019, 293, 111452. [Google Scholar] [CrossRef]
- Dai, Y.; Van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W. Deep eutectic solvents: A review of fundamentals and applications. Chem. Rev. 2020, 121, 1232–1285. [Google Scholar] [CrossRef] [PubMed]
- Zainal-Abidin, M.H.; Hayyan, M.; Wong, W.F. Hydrophobic deep eutectic solvents: Current progress and future directions. J. Ind. Eng. Chem. 2021, 97, 142–162. [Google Scholar] [CrossRef]
- Fu, N.; Liu, X.; Li, L.; Tang, B.; Row, K.H. Ternary choline chloride/caffeic acid/ethylene glycol deep eutectic solvent as both a monomer and template in a molecularly imprinted polymer. J. Sep. Sci. 2017, 40, 2286–2291. [Google Scholar] [CrossRef]
- Cheng, G.; Chen, N.; Li, Z.; Zhao, K.; Duan, R.; Chen, Z.; Zhu, G. Fabrication of deep eutectic solvent-molecularly imprinted polymer in water: A green strategy for adsorption of bisphenol A. J. Environ. Chem. Eng. 2023, 11, 109651. [Google Scholar] [CrossRef]
- Li, X.; Row, K.H. Purification of antibiotics from the millet extract using hybrid molecularly imprinted polymers based on deep eutectic solvents. RSC Adv. 2017, 7, 16997–17004. [Google Scholar] [CrossRef]
- Meng, J.; Wang, X. Microextraction by Packed Molecularly Imprinted Polymer Combined Ultra-High-Performance Liquid Chromatography for the Determination of Levofloxacin in Human Plasma. J. Chem. 2019, 2019, 4783432. [Google Scholar] [CrossRef]
- Liang, F.; Li, W.; Li, M.; Li, X.; He, J.; Wu, Q. Kaempferol molecularly imprinted polymers: Preparation, characterization and application to the separation of kaempferol from ginkgo leaves. Polym. Int. 2023, 72, 586–596. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, L.; Qiao, F.; Yan, H. Facile and green synthesis of a hydrophilic imprinted resin in a deep eutectic solvent–water medium for the specific molecular recognition of tumor biomarkers in complex biological matrices. Green Chem. 2024, 26, 2000–2010. [Google Scholar] [CrossRef]
- Porfireva, A.; Goida, A.; Evtugyn, V.; Evtugyn, G. Impedimetric sensor based on molecularly imprinted polythionine from deep eutectic solvent for epinephrine determination. Green Anal. Chem. 2024, 9, 100113. [Google Scholar] [CrossRef]
- Wei, Z.-H.; Sun, X.; Mu, L.-N.; Huang, Y.-P.; Liu, Z.-S. Improving affinity of imprinted monolithic polymer prepared in deep eutectic solvent by metallic pivot. J. Chromatogr. A 2019, 1602, 48–55. [Google Scholar] [CrossRef]
- Monnier, A.; Díaz-Álvarez, M.; Turiel, E.; Martín-Esteban, A. Evaluation of deep eutectic solvents in the synthesis of molecularly imprinted fibers for the solid-phase microextraction of triazines in soil samples. Anal. Bioanal. Chem. 2024, 416, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Qiao, F.; Yan, H. A simple and benign protocol for the synthesis of a deep eutectic solvent-based hydrophilic molecularly imprinted resin in water for excellent selective molecular recognition in aqueous phase. Green Chem. 2021, 23, 5179–5188. [Google Scholar] [CrossRef]
- Husin, N.A.; Muhamad, M.; Yahaya, N.; Miskam, M.; Kamal, N.N.S.N.M.; Asman, S.; Raoov, M.; Zain, N.N.M. Application of a new choline-imidazole based deep eutectic solvents in hybrid magnetic molecularly imprinted polymer for efficient and selective removal of naproxen from aqueous samples. Mater. Chem. Phys. 2021, 261, 124228. [Google Scholar] [CrossRef]
- Surapong, N.; Pongpinyo, P.; Santaladchaiyakit, Y.; Burakham, R. A biobased magnetic dual-dummy-template molecularly imprinted polymer using a deep eutectic solvent as a coporogen for highly selective enrichment of organophosphates. Food Chem. 2023, 418, 136045. [Google Scholar] [CrossRef]
- Liang, S.; Yan, H.; Cao, J.; Han, Y.; Shen, S.; Bai, L. Molecularly imprinted phloroglucinol–formaldehyde–melamine resin prepared in a deep eutectic solvent for selective recognition of clorprenaline and bambuterol in urine. Anal. Chim. Acta 2017, 951, 68–77. [Google Scholar] [CrossRef]
- Ma, W.; An, Y.; Row, K.H. Preparation and evaluation of a green solvent-based molecularly imprinted monolithic column for the recognition of proteins by high-performance liquid chromatography. Analyst 2019, 144, 6327–6333. [Google Scholar] [CrossRef]
- Tang, W.; Row, K.H. Fabrication of water-compatible molecularly imprinted resin in a hydrophilic deep eutectic solvent for the determination and purification of quinolones in wastewaters. Polymers 2019, 11, 871. [Google Scholar] [CrossRef]
- Furtado, A.I.; Viveiros, R.; Bonifácio, V.D.; Melo, A.; Casimiro, T. Biomolecular Fishing: Design, Green Synthesis, and Performance of l-Leucine-Molecularly Imprinted Polymers. ACS Omega 2023, 8, 9179–9186. [Google Scholar] [CrossRef] [PubMed]
- Iacob, B.-C.; Bodoki, A.E.; Da Costa Carvalho, D.F.; Serpa Paulino, A.A.; Barbu-Tudoran, L.; Bodoki, E. Unlocking New Avenues: Solid-State Synthesis of Molecularly Imprinted Polymers. Int. J. Mol. Sci. 2024, 25, 5504. [Google Scholar] [CrossRef]
- Furtado, A.I.; Lowdon, J.W.; Eersels, K.; van Grinsven, B.; Cruz, A.; Serpa, J.; Bonifácio, V.D.; Viveiros, R.; Casimiro, T. Mechanosynthesis and thermal bio–sensing of beryllium–based molecularly imprinted polymers. Biosens. Bioelectron. X 2025, 24, 100605. [Google Scholar] [CrossRef]
- Friščić, T.; Mottillo, C.; Titi, H.M. Mechanochemistry for synthesis. Angew. Chem. 2020, 132, 1030–1041. [Google Scholar] [CrossRef]
- Beckman, E.J. Supercritical and near-critical CO2 in green chemical synthesis and processing. J. Supercrit. Fluids 2004, 28, 121–191. [Google Scholar] [CrossRef]
- Lamaoui, A.; Mani, V.; Durmus, C.; Salama, K.N.; Amine, A. Molecularly imprinted polymers: A closer look at the template removal and analyte binding. Biosens. Bioelectron. 2023, 243, 115774. [Google Scholar] [CrossRef]
- Furtado, A.I.; Bonifácio, V.D.; Viveiros, R.; Casimiro, T. Design of Molecularly Imprinted Polymers Using Supercritical Carbon Dioxide Technology. Molecules 2024, 29, 926. [Google Scholar] [CrossRef]
- Duarte, A.R.C.; Casimiro, T.; Aguiar-Ricardo, A.; Simplício, A.L.; Duarte, C.M. Supercritical fluid polymerisation and impregnation of molecularly imprinted polymers for drug delivery. J. Supercrit. Fluids 2006, 39, 102–106. [Google Scholar] [CrossRef]
- Viveiros, R.; Lopes, M.I.; Heggie, W.; Casimiro, T. Green approach on the development of lock-and-key polymers for API purification. Chem. Eng. J. 2017, 308, 229–239. [Google Scholar] [CrossRef]
- Székely, G.; Fritz, E.; Bandarra, J.; Heggie, W.; Sellergren, B. Removal of potentially genotoxic acetamide and arylsulfonate impurities from crude drugs by molecular imprinting. J. Chromatogr. A 2012, 1240, 52–58. [Google Scholar] [CrossRef]
- Byun, H.-S.; Chun, D.; Shim, W.-G. Separation and recognition characteristics by MIP manufacture using supercritical CO2 technology. J. Ind. Eng. Chem. 2021, 97, 356–367. [Google Scholar] [CrossRef]
- Caldara, M.; Lowdon, J.W.; Rogosic, R.; Arreguin-Campos, R.; Jimenez-Monroy, K.L.; Heidt, B.; Tschulik, K.; Cleij, T.J.; Diliën, H.; Eersels, K. Thermal detection of glucose in urine using a molecularly imprinted polymer as a recognition element. ACS Sens. 2021, 6, 4515–4525. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Q.-M.; Tian, L.-L.; Yang, C.; Yu, S.-H.; Yang, C. Research progress of the molecularly imprinted cryogel. Chin. J. Anal. Chem. 2015, 43, 1777–1784. [Google Scholar] [CrossRef]
- Memic, A.; Colombani, T.; Eggermont, L.J.; Rezaeeyazdi, M.; Steingold, J.; Rogers, Z.J.; Navare, K.J.; Mohammed, H.S.; Bencherif, S.A. Latest advances in cryogel technology for biomedical applications. Adv. Ther. 2019, 2, 1800114. [Google Scholar] [CrossRef]
- Bakhshpour, M.; Göktürk, I.; Bereli, N.; Denizli, A. Molecularly imprinted cryogel cartridges for the selective recognition of tyrosine. Biotechnol. Prog. 2020, 36, e3006. [Google Scholar] [CrossRef] [PubMed]
- Kartal, F.; Denizli, A. Molecularly imprinted cryogel beads for cholesterol removal from milk samples. Colloids Surf. B. Biointerfaces 2020, 190, 110860. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, G.; Fu, C. Synthesis and characteristics of tyrosine imprinted beads via suspension polymerization. React. Funct. Polym. 2003, 56, 167–173. [Google Scholar] [CrossRef]
- Perçin, I.; Idil, N.; Denizli, A. Molecularly imprinted poly (N-isopropylacrylamide) thermosensitive based cryogel for immunoglobulin G purification. Process Biochem. 2019, 80, 181–189. [Google Scholar] [CrossRef]
- Wardani, N.I.; Kangkamano, T.; Wannapob, R.; Kanatharana, P.; Thavarungkul, P.; Limbut, W. Electrochemical sensor based on molecularly imprinted polymer cryogel and multiwalled carbon nanotubes for direct insulin detection. Talanta 2023, 254, 124137. [Google Scholar] [CrossRef]
- Ma, L.; Tang, L.; Li, R.-S.; Huang, Y.-P.; Liu, Z.-S. Water-compatible molecularly imprinted polymers prepared using metal–organic gel as porogen. RSC Adv. 2015, 5, 84601–84609. [Google Scholar] [CrossRef]
- Liu, G.; Li, S.; Shi, C.; Huo, M.; Lin, Y. Progress in research and application of metal–organic gels: A review. Nanomaterials 2023, 13, 1178. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Gao, H.; Yan, G.; Gao, H.; Chen, M. Metal–Organic Gel-Modulated Synthesis of Hierarchically Porous Molecularly Imprinted Polymers for Efficient Removal of Sildenafil from Water. ACS Omega 2021, 6, 7478–7486. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chai, M.-H.; Yao, H.-F.; Huang, Y.-P.; Liu, Z.-S. Molecularly imprinted polymers doped with carbon nanotube with aid of metal-organic gel for drug delivery systems. Pharm. Res. 2020, 37, 193. [Google Scholar] [CrossRef] [PubMed]

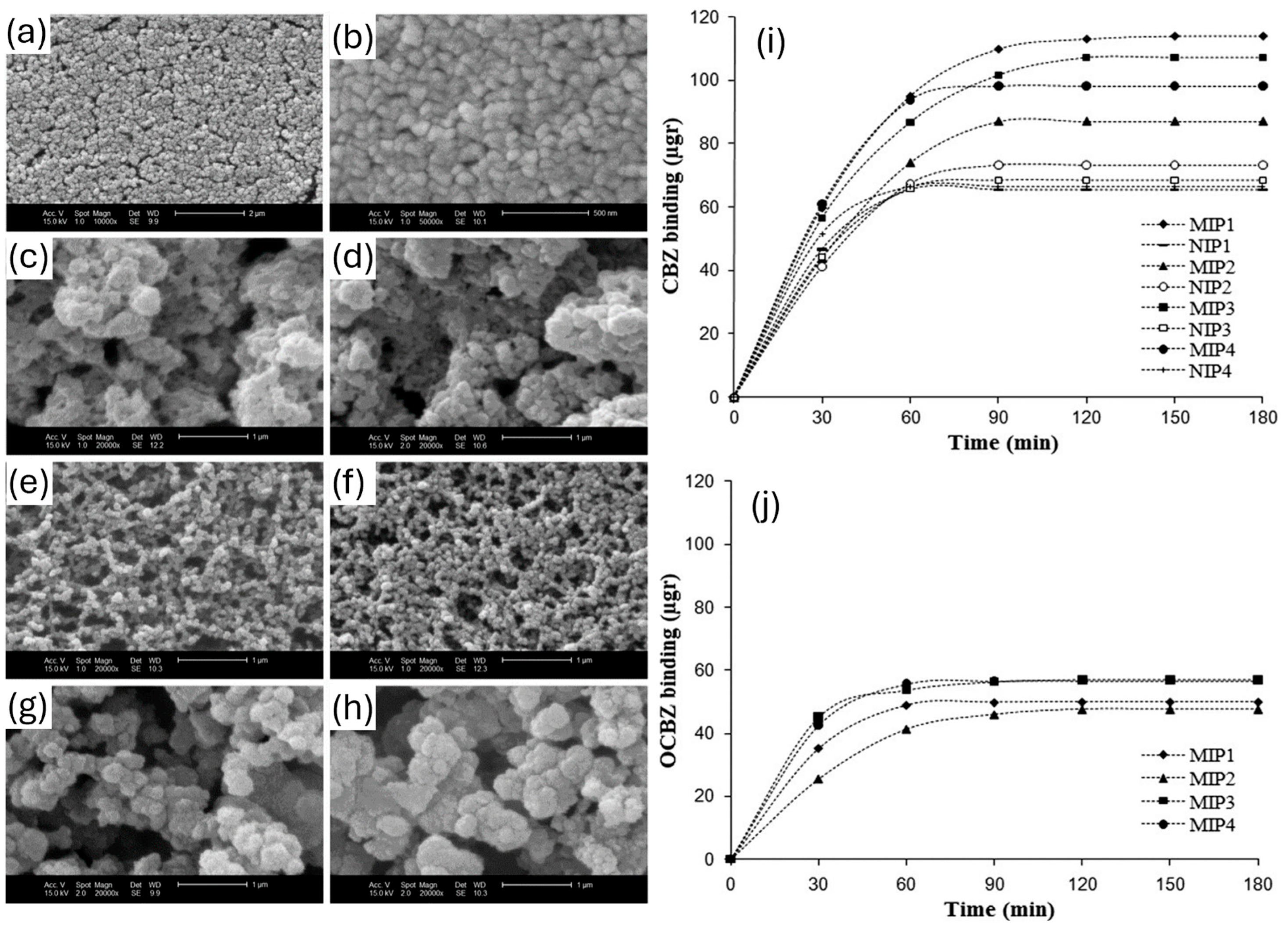


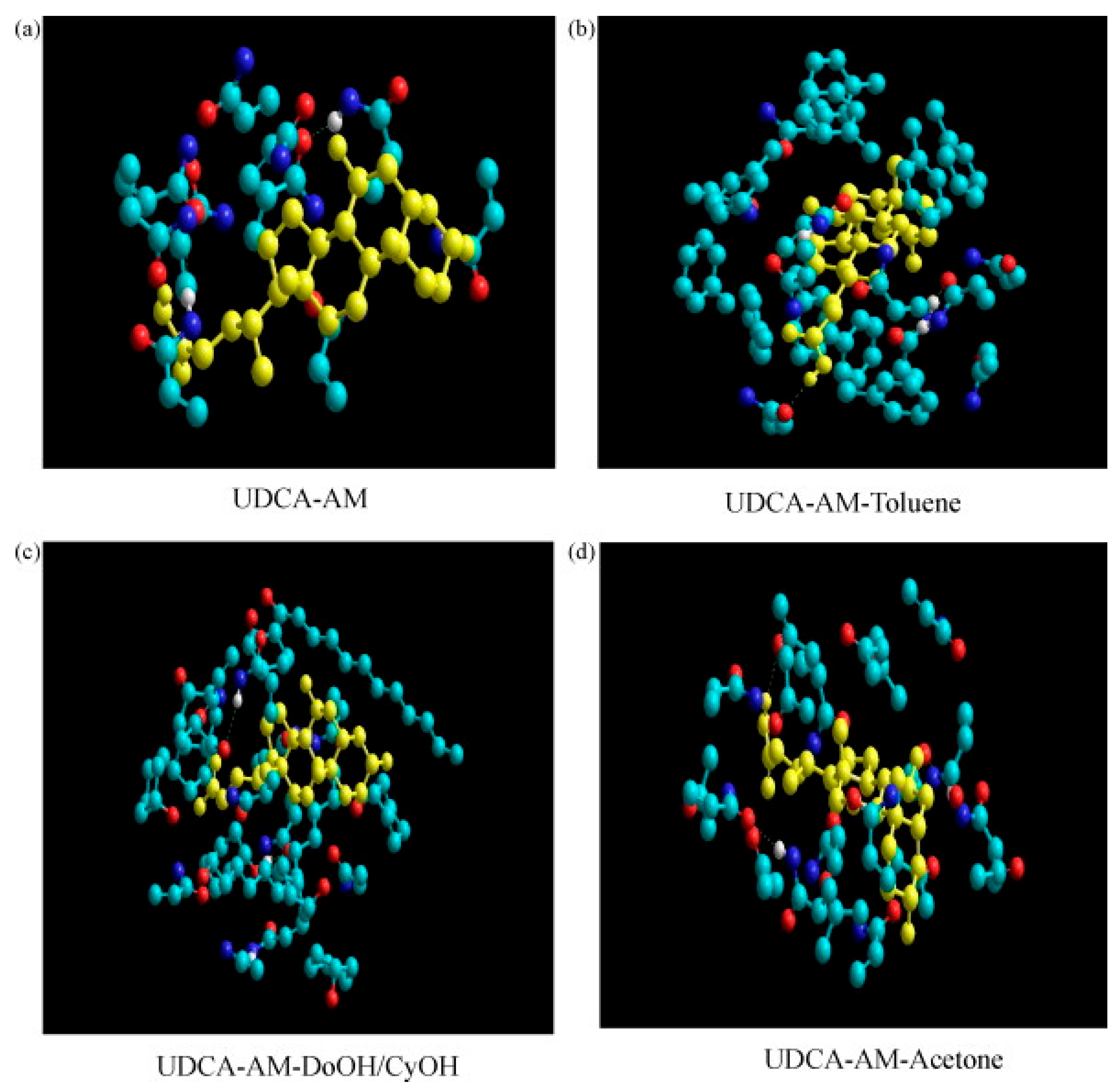



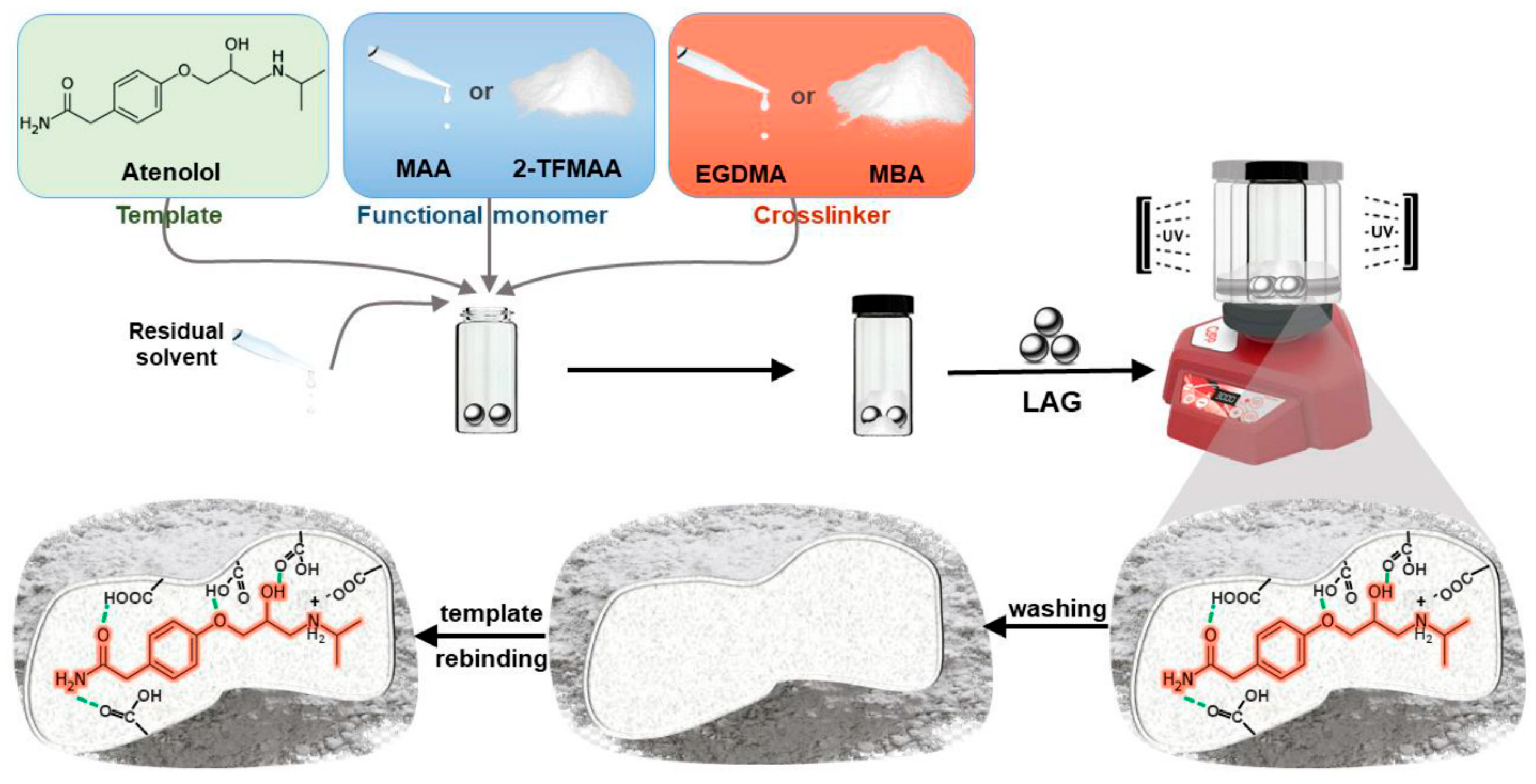


| Solvent | bp [°C] | Dielectric Constant | Hansen Solubility Parameter [MPa1/2] | Polarity ET (30) [kcal/mol] | ||
|---|---|---|---|---|---|---|
| δd | δp | δH | ||||
| Acetone | 56.3 | 21 | 15.5 | 10.4 | 7.0 | 42.2 |
| ACN | 81.6 | 37.5 | 15.3 | 18 | 6.1 | 45.6 |
| Chloroform | 61.2 | 4.8 | 17.8 | 3.1 | 5.7 | 39.1 |
| DCM | 39.8 | 8.9 | 18.2 | 6.3 | 6.1 | 40.7 |
| DMF | 153 | 38.3 | 16.8 | 11.5 | 10.2 | 43.2 |
| DMSO | 189 | 46.4 | 18.4 | 16.4 | 10.2 | 45.1 |
| THF | 66 | 7.6 | 16.8 | 5.7 | 8 | 37.4 |
| Toluene | 111 | 2.4 | 18 | 1.4 | 2 | 33.9 |
| EtOH | 78.3 | 34.6 | 15.8 | 8.8 | 19.4 | 51.9 |
| MeOH | 64.7 | 33.6 | 15.1 | 12.3 | 22.3 | 55.4 |
| Water | 100 | 80.4 | 15.5 | 16 | 42.4 | 63.1 |
| [BMIM][PF6] | 180 * | 16.1 | 17.9 | 9.8 | 8.1 | 49.0–53.2 |
| EG:ChCl (1:2) | 439 | - | 15.9 | 5.3 | 19.5 | 57.3 |
| scCO2 | - | 1.03–1.6 | 15.6 | 5.2 | 5.8 | 34.5 |
| Target | Porogen | Polymerization Approach | Application | IF | Ref. |
|---|---|---|---|---|---|
| Nicotinamide | ACN Chloroform Toluene | Monolithic bulk free-radical | Solid-phase extraction | n.d. | [44] |
| Ibuprofen | DMF | Monolithic bulk free-radical | Solid-phase extraction | ~8 * | [45] |
| 2-phenylproponic acids | DMF | Precipitation | Solid-phase extraction | 1.3–3.3 | [46] |
| Progesterone, testosterone | Toluene Chloroform ACN | Monolithic bulk free-radical | Solid-phase extraction | 2.4–3.1 | [47] |
| Terbutylazine | Toluene | Monolithic bulk free-radical | Solid-phase extraction | n.d. | [48] |
| Pinacolyl methylphosphonate | Toluene and ACN | Precipitation | Sensing | ~1.3 * | [49] |
| Tetracycline | Chloroform and ACN | Precipitation | Drug release | 5.1 | [50] |
| Diisopropylurea | Dichloromethane | Monolithic bulk free-radical | Solid-phase extraction | ~2 * | [51] |
| Estradiol | Toluene | Monolithic bulk free-radical | Solid-phase extraction | 4.8 | [52] |
| Xylazine | Toluene and chloroform (3:1) | Monolithic bulk free-radical | Sensing | 2.0 | [53] |
| Pyocyanin | Chloroform | Monolithic bulk free-radical | Sensing | 1.6 | [17] |
| Amphetamine | DMSO | Monolithic bulk free-radical | Sensing | 4.4 | [54] |
| (S)-N-Butyryl homoserine lactone | DMSO | Monolithic bulk free-radical | Sensing | 2.8 | [55] |
| Folic acid | DMSO–ACN (5:3) | Monolithic bulk free-radical | Solid-phase extraction | 4.0 | [56] |
| Quercetin | Acetone | Monolithic bulk free-radical | Solid-phase extraction | 8.2 | [57] |
| Template | Porogen | Approach | Application | Additive | IF | Reference |
|---|---|---|---|---|---|---|
| Triazines | Water | Radical polymerization | Solid-phase extraction | 5% toluene | 2.7–4.7 | [74] |
| Sunset Yellow | Water | Radical polymerization | Solid-phase extraction | - | 1.33 | [76] |
| Atenolol | Butanol or propanol | Bulk or precipitation polymerization | Solid-phase extraction | - | 4.2 and 11.7 | [79] |
| Histamine | EtOH | Precipitation polymerization | Sensing | - | 2.3 | [80] |
| Synephrine | Methanol/water (4:1) | Precipitation polymerization | Solid-phase extraction | - | ~2 * | [64] |
| Triazines | EtOH/water (9:1) | Radical polymerization on silica particle | Solid-phase extraction | Poly-vinylpyrrolidone | n.d. | [81] |
| Gallic acid | Water | basic polymerization | Solid-phase extraction | Phosphate buffer | 1.7 | [82] |
| B-vitamins | Water/acetic acid (99/1) | Condensation | Solid-phase extraction | 1 M NaOH | ~3–4 * | [75] |
| Bisphenol A | Acidified isopropanol | Sol–gel approach | Solid-phase extraction | 1 M NH4OH | 6.6 | [77] |
| 2,4-Dichlorophenoxy-acetic acid | EtOH/water (10:3) | Sol–gel approach | Solid-phase extraction | Conc. HCl | 1.5 | [83] |
| Hydrochlorothiazide | Water | Sol–gel approach | Analyte monitoring | CTAB and NH4OH | ~4.5 * | [71] |
| 1-naphthyl phosphate | Water/EtOH (5:3) | Sol–gel approach | Solid-phase extraction | - | 32.2 | [84] |
| Folic Acid | Water | Sol–gel approach | Sensing | NH4OH | 2.2 | [85] |
| Bisphenol F | Water | Sol–gel approach on electrode | Sensing | CTAB and NH3 | ~6 * | [86] |
| Creatinine | Water | Sol–gel Approach | Sensing | 1 M HCl and Al3Cl3 | 2.4 | [87] |
| Salicylic acid | EtOH/water (4:1) | Sol–gel approach | Drug release | 0.1 M HCl | 9.0 | [88] |
| Template | Porogen | Approach | Additive | Application | IF | Particle Size | Ref. |
|---|---|---|---|---|---|---|---|
| (S)-Naproxen | n-dodecanol/toluene | Bulk | - | Enantiomeric Separation | n.d. | n.d. | [101] |
| Ursodeoxycholic acid | Toluene/water Acetone/water DoOH/CyOH/water | Emulsion | SDS | Extraction | ~2.5 * | 250 nm | [104] |
| Amoxicillin | Water/DMSO | Emulsion | SDS | Sensing | 45.6 | 8–10 µm | [97] |
| Phosphate anion | Water/chloroform | Emulsion | CTAB | Sensing | n.d. | n.d. | [110] |
| Bovine hemoglobin | Water/toluene | Pickering emulsion | Hb-coated Janus hydroxyapatite NPs | Extraction | 4.0 | 50 µm | [96] |
| Listeria Monocytogenes | Water/DMA | Pickering emulsion | N-Acrylchitosan-Quantum Dot | Sensing | 4.6 | 200 µm | [111] |
| λ-cyhalothrin | Water/hexadecane | Pickering emulsion | Attapulgite particles | Extraction | 1.7 | 50 µm | [112] |
| Bisphenol A | Water/toluene | Pickering emulsion | Fe3O4 NPs | Environmental monitoring | 1.7 | 100 µm | [107] |
| Erythromycin | Water/toluene | Pickering emulsion | Chitosan NPs and Hydrophobic Fe3O4 | Extraction | 1.3 | 53 µm | [113] |
| Bovine hemoglobin | Water/n-hexane/corn oil | Pickering emulsion | Colloidal casein NPs | Protein Purification | 4.1 | 300 nm | [114] |
| Gatifloxacin | Cyclohexane/water | Reverse micro-emulsion | Span 60 | Extraction | 2.0 | n.d. | [115] |
| Kaempferol | ACN/methanol (4:1) | Precipitation | - | Extraction | 5.0 | 8 µm | [103] |
| Oleanolic acid | Chloroform/methanol (3:1) | Precipitation | - | Extraction | 4.8 | 20 µm | [116] |
| Huppuric acid | ACN/water | Sol–gel approach | Trifluoro-acetic acid | Extraction | 5.1 | n.d. | [109] |
| Template | IL | Co-Solvent | Reaction Type | Application | IF | Ref. |
|---|---|---|---|---|---|---|
| Aesculin | [BMIM]BF4 | DMSO | Cyclodextrin–bulk polymerization | Drug release | 2.4 | [129] |
| Rutin | [BMIM]PF6 | DMF and ACN | Bulk polymerization | Extraction/Separation | 4.8 | [130] |
| Testosterone | [BMIM]BF4 | Aq. HCl | Condensation/sol–gel approach | Extraction/Separation | 13.9 | [135] |
| Chlorogenic Acid | [BMIM]BF4 | DMSO | Bulk polymerization | Extraction/separation | 9.7 | [136] |
| Chicoric Acid | [BMIM]BF4 | DMSO | Metallic-pivot bulk polymerization | Extraction/Separation | 24.8 | [137] |
| Carprofen | [BMIM]BF4 | DMF and DMSO | RAFT polymerization | Extraction/Separation | 1.8 | [138] |
| Norfloxacin | [BMIM]BF4 | DMF and DMSO | Bulk polymerization | Extraction/Separation | 3.4 | [139] |
| Corigalin | [BMIM]BF4 | DMF and DMSO | Bulk polymerization | Extraction/Separation | 9.0 | [140] |
| Isoquercitrin | [BMIM]BF4 | DMF and DMSO | Bulk polymerization | Extraction/Separation | 3.0 | [141] |
| Fluoroquilones | [BMIM]BF4 | DMSO and CHCl3 | Molecular Crowding Polymerization | Sensing | 3.2 | [142] |
| Dichlorvos | [BMIM]PF6 | ACN and toluene | Bulk polymerization | Sensing | 1.6 | [143] |
| Melamine Triamterene Cyromazine Trimethoprim | [BMIM]BF4 | MeOH/water | Bulk polymerization | Sensing | 2.1–3.9 | [144] |
| Dibutyl Phtalate | [BMIM]BF4 | CHCl3 | Bulk polymerization | Sensing | 2.0 | [145] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Wissen, G.; Lowdon, J.W.; Cleij, T.J.; Eersels, K.; van Grinsven, B. Porogenic Solvents in Molecularly Imprinted Polymer Synthesis: A Comprehensive Review of Current Practices and Emerging Trends. Polymers 2025, 17, 1057. https://doi.org/10.3390/polym17081057
van Wissen G, Lowdon JW, Cleij TJ, Eersels K, van Grinsven B. Porogenic Solvents in Molecularly Imprinted Polymer Synthesis: A Comprehensive Review of Current Practices and Emerging Trends. Polymers. 2025; 17(8):1057. https://doi.org/10.3390/polym17081057
Chicago/Turabian Stylevan Wissen, Gil, Joseph W. Lowdon, Thomas J. Cleij, Kasper Eersels, and Bart van Grinsven. 2025. "Porogenic Solvents in Molecularly Imprinted Polymer Synthesis: A Comprehensive Review of Current Practices and Emerging Trends" Polymers 17, no. 8: 1057. https://doi.org/10.3390/polym17081057
APA Stylevan Wissen, G., Lowdon, J. W., Cleij, T. J., Eersels, K., & van Grinsven, B. (2025). Porogenic Solvents in Molecularly Imprinted Polymer Synthesis: A Comprehensive Review of Current Practices and Emerging Trends. Polymers, 17(8), 1057. https://doi.org/10.3390/polym17081057







