Porogenic Solvents in Molecularly Imprinted Polymer Synthesis: A Comprehensive Review of Current Practices and Emerging Trends
Abstract
1. Introduction
2. Physical Properties of Porogenic Solvents
3. Aprotic Porogenic Solvents
4. Protic Porogenic Solvents
5. Combining Protic with Aprotic Solvents
6. Ionic Liquids and Deep Eutectic Solvents
| Template | DES (HBA:HBD) | Co-Solvent | Reaction Type | Application | IF | Ref. |
|---|---|---|---|---|---|---|
| Levofloxacin Tetraclyne | Betaine–EG (1:2) | water | Condensation and Polymerization | Sensing | 1.4–2.2 * | [161] |
| Thionine | Glucose–Citric Acid (1:1) | water | Electro-polymerization | Sensing | n.d. | [165] |
| Triazines | L-menthol–Formic acid (1:1) | / | Bulk polymerization | Extraction | 2.5 * | [167] |
| Kaempferol | ChCl: 1,4-BD (1:2) | water | Condensation | Extraction | 2.7 | [163] |
| Vanillylmandelic acid Homovanillic acid | ChCl: 1,4-BD (1:2) | water | Condensation | Extraction | 19.2–111.6 | [164] |
| 4,4′-dichlorobenzhydrol | ChCl: 1,4-BD (1:2) | water | Condensation | Extraction | 9.1 | [168] |
| Naproxene | ChCl–1-butylimidazole (1:1) | ACN | Bulk polymerization | Extraction | 1.7 | [169] |
| Organophosphates | ChCl: Glycerol | 70% EtOH | Polymerization | Extraction | 4.5 | [170] |
| Clorprenaline Bambuterol | ChCl:EG (1:2) | / | Condensation | Extraction | ~6 * | [171] |
| Lysozyme BSA | ChCl:EG (1:2) | water | Polymerization | Extraction | n.d. | [172] |
| Quinolones | ChCl:EG (1:2) ChCl: TMAC (1:2) ChCl: TMAB(1:2) | water | Condensation | Extraction | n.d. | [173] |
| Fenbufen | ChCl:EG (1:2) | [BMIM]BF4 | Bulk polymerization | Extraction | 3.9 | [133] |
| Levofloxacin | ChCl:EG (1:2) | / | Bulk polymerization | Extraction | 1.8 | [162] |
| Cetirizine | ChCl:EG (1:6.8) | [BMIM]BF4 and DMF | Metallic pivot radical polymerization | Extraction | 31.5 | [166] |
7. Emerging Porogenic Systems
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| H3BTC | 1,3,5-benzenetricarboxylic acid |
| 1,4-BD | 1,4-butandiol |
| ACN | acetonitrile |
| AG | aminoglutethimide |
| ATR | atrazine |
| CBZ | carbamazepine |
| CTAB | cetrimonium bromide |
| ChCl | choline chloride |
| DES | deep eutectic solvent |
| DMSO | dimethyl sulfoxide |
| EGDMA | ethylene glycol dimethacrylate |
| EG | ethylene glycol |
| HPLC | high-performance liquid chromatography |
| HMA | homovanillic acid |
| HBA | hydrogen bond acceptor |
| HBD | hydrogen bond donor |
| HIR | hydrophilic imprinted resin |
| igG | immunoglobulin G |
| IF | imprinting factor |
| IL | ionic liquid |
| LC/MS | liquid chromatography/mass spectrometry |
| LAG | liquid-assisted grinding |
| LCST | lower critical solution temperature |
| MOG | metal–organic gel |
| MWCNT | mingle-walled carbon nanotubes |
| MIP | molecularly imprinted polymer |
| NIP | non-imprinted polymer |
| NP | nanoparticle |
| NAP | naproxen |
| SDS | sodium dodecylsulfate |
| SPE | solid-phase extraction |
| SY | sunset yellow |
| scCO2 | supercritical CO2 |
| TEOS | tetraethyl orthosilicate |
| THF | tetrahydrofuran |
| TMAB | tetramethylammonium bromide |
| TMAC | tetramethylammonium chloride |
| TCM | traditional Chinese medicine |
| VMA | vanillylmandelic acid |
| VOC | volatile organic compound |
References
- BelBruno, J.J. Molecularly imprinted polymers. Chem. Rev. 2018, 119, 94–119. [Google Scholar] [CrossRef] [PubMed]
- Vasapollo, G.; Sole, R.D.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G. Molecularly imprinted polymers: Present and future prospective. Int. J. Mol. Sci. 2011, 12, 5908–5945. [Google Scholar] [CrossRef] [PubMed]
- Olsson, G.D.; Karlsson, B.C.; Shoravi, S.; Wiklander, J.G.; Nicholls, I.A. Mechanisms underlying molecularly imprinted polymer molecular memory and the role of crosslinker: Resolving debate on the nature of template recognition in phenylalanine anilide imprinted polymers. J. Mol. Recognit. 2012, 25, 69–73. [Google Scholar] [CrossRef]
- Katz, A.; Davis, M.E. Investigations into the mechanisms of molecular recognition with imprinted polymers. Macromolecules 1999, 32, 4113–4121. [Google Scholar] [CrossRef]
- Hu, T.; Chen, R.; Wang, Q.; He, C.; Liu, S. Recent advances and applications of molecularly imprinted polymers in solid-phase extraction for real sample analysis. J. Sep. Sci. 2021, 44, 274–309. [Google Scholar] [CrossRef]
- Saylan, Y.; Kılıç, S.; Denizli, A. Biosensing applications of molecularly imprinted-polymer-based nanomaterials. Processes 2024, 12, 177. [Google Scholar] [CrossRef]
- Mustafa, Y.L.; Keirouz, A.; Leese, H.S. Molecularly imprinted polymers in diagnostics: Accessing analytes in biofluids. J. Mater. Chem. B 2022, 10, 7418–7449. [Google Scholar] [CrossRef]
- Rebelo, P.; Costa-Rama, E.; Seguro, I.; Pacheco, J.G.; Nouws, H.P.; Cordeiro, M.N.D.; Delerue-Matos, C. Molecularly imprinted polymer-based electrochemical sensors for environmental analysis. Biosens. Bioelectron. 2021, 172, 112719. [Google Scholar] [CrossRef]
- Jia, B.; Feng, F.; Wang, X.; Song, Y.; Zhang, F. Recent advances in magnetic molecularly imprinted polymers and their application in the food safety analysis. J. Future Foods 2024, 4, 1–20. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Wang, S. Recent advances in the removal of emerging contaminants from water by novel molecularly imprinted materials in advanced oxidation processes—A review. Sci. Total Environ. 2023, 883, 163702. [Google Scholar] [CrossRef]
- Van Wissen, G.; Lowdon, J.W.; Cleij, T.J.; Diliën, H.; Eersels, K.; van Grinsven, B. Thermal detection of riboflavin in almond milk using molecularly imprinted polymers. Microchem. J. 2025, 212, 113181. [Google Scholar] [CrossRef]
- Frigoli, M.; Lowdon, J.W.; Cleij, T.J.; Diliën, H.; Eersels, K.; van Grinsven, B. Detection of antibiotic sulfamethoxazole residues in milk using a molecularly imprinted polymer-based thermal biosensor. Food Chem. 2025, 476, 143525. [Google Scholar] [CrossRef] [PubMed]
- Bouvarel, T.; Delaunay, N.; Pichon, V. Molecularly imprinted polymers in miniaturized extraction and separation devices. J. Sep. Sci. 2021, 44, 1727–1751. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.C.; Cao, J.X.; Xie, G.M.; Wang, M.W.; Shi, Y.Y.; Yi, Y.; Yang, C.L.; Xiao, Y.H.; Wei, X.L.; Tian, B.M. Study on preparation and process of poly (MMA-St) thermally expandable core-shell microspheres. J. Appl. Polym. Sci. 2021, 138, 49927. [Google Scholar] [CrossRef]
- Gonçalves, L.M. Electropolymerized molecularly imprinted polymers: Perceptions based on recent literature for soon-to-be world-class scientists. Curr. Opin. Electrochem. 2021, 25, 100640. [Google Scholar] [CrossRef]
- Moein, M.M.; Abdel-Rehim, A.; Abdel-Rehim, M. Recent applications of molecularly imprinted sol-gel methodology in sample preparation. Molecules 2019, 24, 2889. [Google Scholar] [CrossRef]
- Frigoli, M.; Lowdon, J.W.; Caldara, M.; Arreguin-Campos, R.; Sewall, J.; Cleij, T.J.; Diliën, H.; Eersels, K.; Van Grinsven, B. Thermal pyocyanin sensor based on molecularly imprinted polymers for the indirect detection of Pseudomonas aeruginosa. ACS Sens. 2023, 8, 353–362. [Google Scholar] [CrossRef]
- Esfandyari-Manesh, M.; Javanbakht, M.; Atyabi, F.; Badiei, A.; Dinarvand, R. Effect of porogenic solvent on the morphology, recognition and release properties of carbamazepine-molecularly imprinted polymer nanospheres. J. Appl. Polym. Sci. 2011, 121, 1118–1126. [Google Scholar] [CrossRef]
- Mansour, F.R.; Waheed, S.; Paull, B.; Maya, F. Porogens and porogen selection in the preparation of porous polymer monoliths. J. Sep. Sci. 2020, 43, 56–69. [Google Scholar] [CrossRef]
- Lakshmi, D.S.; KS, R.; Castro-Muñoz, R.; Tańczyk, M. Emerging trends in porogens toward material fabrication: Recent progresses and challenges. Polymers 2022, 14, 5209. [Google Scholar] [CrossRef]
- Li, X.-R.; Wang, X.-L.; Koseki, H. Study on thermal decomposition characteristics of AIBN. J. Hazard. Mater. 2008, 159, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Rosengren, A.M.; Golker, K.; Karlsson, J.G.; Nicholls, I.A. Dielectric constants are not enough: Principal component analysis of the influence of solvent properties on molecularly imprinted polymer–ligand rebinding. Biosens. Bioelectron. 2009, 25, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Andersson, L.I. Application of molecular imprinting to the development of aqueous buffer and organic solvent based radioligand binding assays for (S)-propranolol. Anal. Chem. 1996, 68, 111–117. [Google Scholar] [CrossRef]
- Jouyban, A.; Soltanpour, S.; Chan, H.-K. A simple relationship between dielectric constant of mixed solvents with solvent composition and temperature. Int. J. Pharm. 2004, 269, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Stefanis, E.; Panayiotou, C. Prediction of Hansen solubility parameters with a new group-contribution method. Int. J. Thermophys. 2008, 29, 568–585. [Google Scholar] [CrossRef]
- Reichardt, C. Solvatochromic dyes as solvent polarity indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Riddick, J.A.; Bunger, W.B.; Sakano, T.K. Organic Solvents: Physical Properties and Methods of Purification, 4th ed.; Wiley-Interscience: New York, NY, USA, 1986. [Google Scholar]
- Dickert, F.L.; Geiger, U.; Lieberzeit, P.; Reutner, U. Solvatochromic betaine dyes as optochemical sensor materials: Detection of polar and non-polar vapors. Sens. Actuators B Chem. 2000, 70, 263–269. [Google Scholar] [CrossRef]
- Carda–Broch, S.; Berthod, A.; Armstrong, D. Solvent properties of the 1-butyl-3-methylimidazolium hexafluorophosphate ionic liquid. Anal. Bioanal. Chem. 2003, 375, 191–199. [Google Scholar] [CrossRef]
- Mirza, N.R.; Nicholas, N.J.; Wu, Y.; Kentish, S.; Stevens, G.W. Estimation of normal boiling temperatures, critical properties, and acentric factors of deep eutectic solvents. J. Chem. Eng. Data 2015, 60, 1844–1854. [Google Scholar] [CrossRef]
- Griffiths, T.R.; Pugh, D.C. Correlations among solvent polarity scales, dielectric constant and dipole moment, and a means to reliable predictions of polarity scale values from cu. Coord. Chem. Rev. 1979, 29, 129–211. [Google Scholar] [CrossRef]
- Hunger, J.; Stoppa, A.; Schrödle, S.; Hefter, G.; Buchner, R. Temperature dependence of the dielectric properties and dynamics of ionic liquids. ChemPhysChem 2009, 10, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Di Noto, V.; Vezzu, K.; Conti, F.; Giffin, G.A.; Lavina, S.; Bertucco, A. Broadband Electric Spectroscopy at High CO2 Pressure: Dipole Moment of CO2 and Relaxation Phenomena of the CO2–Poly (vinyl chloride) System. J. Phys. Chem. B 2011, 115, 9014–9021. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Du, Y.; Xue, Y.; Frisch, H. Solubility parameters. In Physical Properties of Polymers Handbook; Springer: New York, NY, USA, 2007; pp. 289–303. [Google Scholar]
- Mutelet, F.; Butet, V.; Jaubert, J.-N. Application of inverse gas chromatography and regular solution theory for characterization of ionic liquids. Ind. Eng. Chem. Res. 2005, 44, 4120–4127. [Google Scholar] [CrossRef]
- Novaes, F.J.M.; de Faria, D.C.; Ferraz, F.Z.; de Aquino Neto, F.R. Hansen solubility parameters applied to the extraction of phytochemicals. Plants 2023, 12, 3008. [Google Scholar] [CrossRef]
- Williams, L.L.; Rubin, J.B.; Edwards, H. Calculation of Hansen solubility parameter values for a range of pressure and temperature conditions, including the supercritical fluid region. Ind. Eng. Chem. Res. 2004, 43, 4967–4972. [Google Scholar] [CrossRef]
- Del Valle, J.; García Blanco, F.; Catalán, J. Empirical parameters for solvent acidity, basicity, dipolarity, and polarizability of the ionic liquids [BMIM][BF4] and [BMIM][PF6]. J. Phys. Chem. B 2015, 119, 4683–4692. [Google Scholar] [CrossRef]
- Omar, K.A.; Sadeghi, R. Physicochemical properties of deep eutectic solvents: A review. J. Mol. Liq. 2022, 360, 119524. [Google Scholar] [CrossRef]
- Gao, F.; Chang, H.; Li, J.; Wang, R.; Gu, Y. Replacing polar aprotic solvents with water in organic synthesis. Curr. Opin. Green Sustain. Chem. 2023, 40, 100774. [Google Scholar] [CrossRef]
- Rossini, E.; Knapp, E.W. Proton solvation in protic and aprotic solvents. J. Comput. Chem. 2016, 37, 1082–1091. [Google Scholar] [CrossRef]
- Yan, H.; Row, K.H. Characteristic and synthetic approach of molecularly imprinted polymer. Int. J. Mol. Sci. 2006, 7, 155–178. [Google Scholar] [CrossRef]
- Wu, L.; Zhu, K.; Zhao, M.; Li, Y. Theoretical and experimental study of nicotinamide molecularly imprinted polymers with different porogens. Anal. Chim. Acta 2005, 549, 39–44. [Google Scholar] [CrossRef]
- Farrington, K.; Regan, F. Investigation of the nature of MIP recognition: The development and characterisation of a MIP for Ibuprofen. Biosens. Bioelectron. 2007, 22, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Yuan, X.; Zhang, J.; Wang, B.; Sun, X.; Chen, X.; Zhao, L. Dummy-surface molecularly imprinted polymers as a sorbent of micro-solid-phase extraction combined with dispersive liquid–liquid microextraction for determination of five 2-phenylpropionic acid NSAIDs in aquatic environmental samples. Anal. Bioanal. Chem. 2018, 410, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Gadzała-Kopciuch, R.; Ričanyová, J.; Buszewski, B. Isolation and detection of steroids from human urine by molecularly imprinted solid-phase extraction and liquid chromatography. J. Chromatogr. B 2009, 877, 1177–1184. [Google Scholar] [CrossRef]
- Pap, T.M.; Horváth, V.; Tolokán, A.; Horvai, G.; Sellergren, B. Effect of solvents on the selectivity of terbutylazine imprinted polymer sorbents used in solid-phase extraction. J. Chromatogr. A 2002, 973, 1–12. [Google Scholar] [CrossRef]
- Malosse, L.; Buvat, P.; Adès, D.; Siove, A. Detection of degradation products of chemical warfare agents by highly porous molecularly imprinted microspheres. Analyst 2008, 133, 588–595. [Google Scholar] [CrossRef]
- Mirzaei, M.; Najafabadi, S.A.H.; Abdouss, M.; Azodi-Deilami, S.; Asadi, E.; Hosseini, M.R.M.; Piramoon, M. Preparation and utilization of microporous molecularly imprinted polymer for sustained release of tetracycline. J. Appl. Polym. Sci. 2013, 128, 1557–1562. [Google Scholar] [CrossRef]
- Székely, G.; Bandarra, J.; Heggie, W.; Ferreira, F.C.; Sellergren, B. Design, preparation and characterization of novel molecularly imprinted polymers for removal of potentially genotoxic 1, 3-diisopropylurea from API solutions. Sep. Purif. Technol. 2012, 86, 190–198. [Google Scholar] [CrossRef]
- Buszewski, B.; Ričanyová, J.; Gadzała-Kopciuch, R.; Szumski, M. Supramolecular recognition of estrogens via molecularly imprinted polymers. Anal. Bioanal. Chem. 2010, 397, 2977–2986. [Google Scholar] [CrossRef]
- Marroquin-Garcia, R.; van Wissen, G.; Cleij, T.J.; Eersels, K.; van Grinsven, B.; Diliën, H. Single-use dye displacement colorimetry assay based on molecularly imprinted polymers: Towards fast and on-site detection of xylazine in alcoholic beverages. Food Control 2024, 161, 110403. [Google Scholar] [CrossRef]
- Lowdon, J.W.; Eersels, K.; Arreguin-Campos, R.; Caldara, M.; Heidt, B.; Rogosic, R.; Jimenez-Monroy, K.L.; Cleij, T.J.; Diliën, H.; van Grinsven, B. A molecularly imprinted polymer-based dye displacement assay for the rapid visual detection of amphetamine in urine. Molecules 2020, 25, 5222. [Google Scholar] [CrossRef] [PubMed]
- Frigoli, M.; Lowdon, J.W.; Donetti, N.; Crapnell, R.D.; Banks, C.E.; Cleij, T.J.; Diliën, H.; Eersels, K.; van Grinsven, B. Electrochemical Detection of Pseudomonas aeruginosa Quorum Sensing Molecule (S)-N-Butyryl Homoserine Lactone Using Molecularly Imprinted Polymers. ACS Omega 2024, 9, 36411–36420. [Google Scholar] [CrossRef] [PubMed]
- Panjan, P.; Monasterio, R.P.; Carrasco-Pancorbo, A.; Fernandez-Gutierrez, A.; Sesay, A.M.; Fernandez-Sanchez, J.F. Development of a folic acid molecularly imprinted polymer and its evaluation as a sorbent for dispersive solid-phase extraction by liquid chromatography coupled to mass spectrometry. J. Chromatogr. A 2018, 1576, 26–33. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, J.; Molinelli, A.; Nolan, K.; Smyth, M.; Mizaikoff, B. Anatomy of a successful imprint: Analysing the recognition mechanisms of a molecularly imprinted polymer for quercetin. Biosens. Bioelectron. 2006, 21, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Meier, F.; Schott, B.; Riedel, D.; Mizaikoff, B. Computational and experimental study on the influence of the porogen on the selectivity of 4-nitrophenol molecularly imprinted polymers. Anal. Chim. Acta 2012, 744, 68–74. [Google Scholar] [CrossRef]
- Santora, B.P.; Gagné, M.R.; Moloy, K.G.; Radu, N.S. Porogen and cross-linking effects on the surface area, pore volume distribution, and morphology of macroporous polymers obtained by bulk polymerization. Macromolecules 2001, 34, 658–661. [Google Scholar] [CrossRef]
- Pardeshi, S.; Dhodapkar, R.; Kumar, A. Influence of porogens on the specific recognition of molecularly imprinted poly (acrylamide-co-ethylene glycol dimethacrylate). Compos. Interfaces 2014, 21, 13–30. [Google Scholar] [CrossRef]
- Nagy-Szakolczai, A.; Dorkó, Z.; Tóth, B.; Horvai, G. New methods to study the behavior of molecularly imprinted polymers in aprotic solvents. Polymers 2018, 10, 1015. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Bai, R.; Ferlin, F.; Vaccaro, L.; Li, M.; Gu, Y. Replacement strategies for non-green dipolar aprotic solvents. Green Chem. 2020, 22, 6240–6257. [Google Scholar] [CrossRef]
- Amann, A.; de Lacy Costello, B.; Miekisch, W.; Schubert, J.; Buszewski, B.; Pleil, J.; Ratcliffe, N.; Risby, T. The human volatilome: Volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J. Breath Res. 2014, 8, 034001. [Google Scholar] [CrossRef]
- Fan, J.-P.; Tian, Z.-Y.; Tong, S.; Zhang, X.-H.; Xie, Y.-L.; Xu, R.; Qin, Y.; Li, L.; Zhu, J.-H.; Ouyang, X.-K. A novel molecularly imprinted polymer of the specific ionic liquid monomer for selective separation of synephrine from methanol–water media. Food Chem. 2013, 141, 3578–3585. [Google Scholar] [CrossRef] [PubMed]
- Reid, J.E.; Gammons, R.J.; Slattery, J.M.; Walker, A.J.; Shimizu, S. Interactions in water–ionic liquid mixtures: Comparing protic and aprotic systems. J. Phys. Chem. B 2017, 121, 599–609. [Google Scholar] [CrossRef]
- Buback, M.; Hutchinson, R.A.; Lacik, I. Radical polymerization kinetics of water-soluble monomers. Prog. Polym. Sci. 2023, 138, 101645. [Google Scholar] [CrossRef]
- Gromov, V.F.; Bune, E.V.; Teleshov, E.N. Characteristic features of the radical polymerisation of water-soluble monomers. Russ. Chem. Rev. 1994, 63, 507. [Google Scholar] [CrossRef]
- Kuchta, F.-D.; van Herk, A.M.; German, A.L. Propagation kinetics of acrylic and methacrylic acid in water and organic solvents studied by pulsed-laser polymerization. Macromolecules 2000, 33, 3641–3649. [Google Scholar] [CrossRef]
- Zaidi, S.A. Bacterial imprinting methods and their applications: An overview. Crit. Rev. Anal. Chem. 2021, 51, 609–618. [Google Scholar] [CrossRef]
- Zhou, X.; Lai, C.; Huang, D.; Zeng, G.; Chen, L.; Qin, L.; Xu, P.; Cheng, M.; Huang, C.; Zhang, C. Preparation of water-compatible molecularly imprinted thiol-functionalized activated titanium dioxide: Selective adsorption and efficient photodegradation of 2, 4-dinitrophenol in aqueous solution. J. Hazard. Mater. 2018, 346, 113–123. [Google Scholar] [CrossRef]
- Arabi, M.; Ghaedi, M.; Ostovan, A. Development of a lower toxic approach based on green synthesis of water-compatible molecularly imprinted nanoparticles for the extraction of hydrochlorothiazide from human urine. ACS Sustain. Chem. Eng. 2017, 5, 3775–3785. [Google Scholar] [CrossRef]
- Zhou, T.; Ding, L.; Che, G.; Jiang, W.; Sang, L. Recent advances and trends of molecularly imprinted polymers for specific recognition in aqueous matrix: Preparation and application in sample pretreatment. TrAC Trends Anal. Chem. 2019, 114, 11–28. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, B.; Wang, S.; Zhang, W. Recyclable trypsin immobilized magnetic nanoparticles based on hydrophilic polyethylenimine modification and their proteolytic characteristics. Anal. Methods 2018, 10, 459–466. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.; Hu, Y.; Li, G. Microwave heating in preparation of magnetic molecularly imprinted polymer beads for trace triazines analysis in complicated samples. Anal. Chem. 2009, 81, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Ostovan, A.; Ghaedi, M.; Arabi, M.; Yang, Q.; Li, J.; Chen, L. Hydrophilic multitemplate molecularly imprinted biopolymers based on a green synthesis strategy for determination of B-family vitamins. ACS Appl. Mater. Interfaces 2018, 10, 4140–4150. [Google Scholar] [CrossRef]
- Foroughirad, S.; Haddadi-Asl, V.; Khosravi, A.; Salami-Kalajahi, M. Effect of porogenic solvent in synthesis of mesoporous and microporous molecularly imprinted polymer based on magnetic halloysite nanotubes. Mater. Today Commun. 2021, 26, 101780. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Tsalbouris, A.; Kabir, A.; Furton, K.G.; Samanidou, V.F. Synthesis and application of molecularly imprinted polymers using sol–gel matrix imprinting technology for the efficient solid-phase extraction of BPA from water. Microchem. J. 2020, 157, 104965. [Google Scholar] [CrossRef]
- Juan-Díaz, M.; Martínez-Ibáñez, M.; Hernández-Escolano, M.; Cabedo, L.; Izquierdo, R.; Suay, J.; Gurruchaga, M.; Goñi, I. Study of the degradation of hybrid sol–gel coatings in aqueous medium. Prog. Org. Coat. 2014, 77, 1799–1806. [Google Scholar] [CrossRef]
- Hasanah, A.; Suherman, M.; Susanti, I.; Pitaloka, I.; Mustarichie, R. Performance evaluation of atenolol molecular imprinted polymer using two different polymerization and two different porogen. Rasayan J. Chem 2019, 12, 1269–1278. [Google Scholar] [CrossRef]
- Herrera-Chacón, A.; Dinç-Zor, Ş.; Del Valle, M. Integrating molecularly imprinted polymer beads in graphite-epoxy electrodes for the voltammetric biosensing of histamine in wines. Talanta 2020, 208, 120348. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, T.; Li, H.; Liu, H.; Huang, N.; Xu, Y.; Ding, J.; Ding, L.; Li, Y. Preparation of magnetic surface molecularly imprinted polymers for the selective extraction of triazines in environmental water samples. Int. J. Environ. Anal. Chem. 2018, 98, 1049–1062. [Google Scholar] [CrossRef]
- Hu, X.; Xie, L.; Guo, J.; Li, H.; Jiang, X.; Zhang, Y.; Shi, S. Hydrophilic gallic acid–imprinted polymers over magnetic mesoporous silica microspheres with excellent molecular recognition ability in aqueous fruit juices. Food Chem. 2015, 179, 206–212. [Google Scholar] [CrossRef]
- Sun, Y. Molecularly imprinted polymer for 2, 4-dichlorophenoxyacetic acid prepared by a sol-gel method. J. Chem. Sci. 2014, 126, 1005–1011. [Google Scholar] [CrossRef]
- Wang, F.; Ling, B.; Li, Q.; Abouhany, R. Dual roles of 3-aminopropyltriethoxysilane in preparing molecularly imprinted silica particles for specific recognition of target molecules. RSC Adv. 2020, 10, 20368–20373. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, Q.; Wang, X.; Arabi, M.; Peng, H.; Li, J.; Xiong, H.; Chen, L. Facile approach to the synthesis of molecularly imprinted ratiometric fluorescence nanosensor for the visual detection of folic acid. Food Chem. 2020, 319, 126575. [Google Scholar] [CrossRef] [PubMed]
- Kaya, S.I.; Corman, M.E.; Uzun, L.; Ozkan, S.A. Simple preparation of surface molecularly imprinted polymer based on silica particles for trace level assay of bisphenol F. Anal. Bioanal. Chem. 2022, 414, 5793–5803. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-J.; Chen, P.-Y.; Nien, P.-C.; Lin, C.-Y.; Vittal, R.; Ling, T.-R.; Ho, K.-C. Preparation of a novel molecularly imprinted polymer by the sol–gel process for sensing creatinine. Anal. Chim. Acta 2012, 711, 83–90. [Google Scholar] [CrossRef]
- Li, B.; Xu, J.; Hall, A.J.; Haupt, K.; Tse Sum Bui, B. Water-compatible silica sol–gel molecularly imprinted polymer as a potential delivery system for the controlled release of salicylic acid. J. Mol. Recognit. 2014, 27, 559–565. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, J.; Liu, M.; Han, X.; Peng, Y.; Tian, X.; Liu, J.; Zhang, S. Synthesis of molecularly imprinted polymer via emulsion polymerization for application in solanesol separation. Appl. Sci. 2020, 10, 2868. [Google Scholar] [CrossRef]
- Mezhoud, S.; Le Droumaguet, B.; Aimedieu, P.; Monchiet, V.; Bornert, M.; Grande, D. Investigation of morphology associated with biporous polymeric materials obtained by the double porogen templating approach. Colloid. Polym. Sci. 2021, 299, 537–550. [Google Scholar] [CrossRef]
- Azizi, A.; Bottaro, C.S. A critical review of molecularly imprinted polymers for the analysis of organic pollutants in environmental water samples. J. Chromatogr. A 2020, 1614, 460603. [Google Scholar] [CrossRef]
- Pircher, N.; Fischhuber, D.; Carbajal, L.; Strauß, C.; Nedelec, J.M.; Kasper, C.; Rosenau, T.; Liebner, F. Preparation and reinforcement of dual-porous biocompatible cellulose scaffolds for tissue engineering. Macromol. Mater. Eng. 2015, 300, 911–924. [Google Scholar] [CrossRef]
- Lovell, P.A.; Schork, F.J. Fundamentals of emulsion polymerization. Biomacromolecules 2020, 21, 4396–4441. [Google Scholar] [CrossRef]
- Song, Z.; Li, J.; Lu, W.; Li, B.; Yang, G.; Bi, Y.; Arabi, M.; Wang, X.; Ma, J.; Chen, L. Molecularly imprinted polymers based materials and their applications in chromatographic and electrophoretic separations. TrAC Trends Anal. Chem. 2022, 146, 116504. [Google Scholar] [CrossRef]
- Fresco-Cala, B.; Cárdenas, S. Advanced polymeric solids containing nano-and micro-particles prepared via emulsion-based polymerization approaches. A review. Anal. Chim. Acta 2022, 1208, 339669. [Google Scholar] [CrossRef]
- Sun, Y.; Ren, T.; Deng, Z.; Yang, Y.; Zhong, S. Molecularly imprinted polymers fabricated using Janus particle-stabilized Pickering emulsions and charged monomer polymerization. New J. Chem. 2018, 42, 7355–7363. [Google Scholar] [CrossRef]
- Lowdon, J.W.; Diliën, H.; van Grinsven, B.; Eersels, K.; Cleij, T.J. Colorimetric Sensing of Amoxicillin Facilitated by Molecularly Imprinted Polymers. Polymers 2021, 13, 2221. [Google Scholar] [CrossRef] [PubMed]
- El-Aasser, M.; Loncar, F., Jr.; Vanderhoff, J. Swelling of carboxyl-containing copolymer latexes. Macromol. Chem. Phys. 1985, 10, 335–357. [Google Scholar] [CrossRef]
- Díaz-Álvarez, M.; Turiel, E.; Martín-Esteban, A. Recent advances and future trends in molecularly imprinted polymers-based sample preparation. J. Sep. Sci. 2023, 46, 2300157. [Google Scholar] [CrossRef]
- Zeng, H.; Yu, X.; Wan, J.; Cao, X. Rational design and synthesis of molecularly imprinted polymers (MIP) for purifying tylosin by seeded precipitation polymerization. Process Biochem. 2020, 94, 329–339. [Google Scholar] [CrossRef]
- Chen, D.-M.; Fu, Q.; Du, W.; Sun, S.-J.; Huang, P.; Chang, C. Preparation and evaluation of monolithic molecularly imprinted stationary phase for S-naproxen. J. Pharm. Anal. 2011, 1, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, S.; Singh, S.K. Precipitation polymerization: A versatile tool for preparing molecularly imprinted polymer beads for chromatography applications. RSC Adv. 2016, 6, 23525–23536. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, Y.; Yuan, Y.; Zeng, H. Development and characterization of molecularly imprinted polymer microspheres for the selective detection of kaempferol in traditional Chinese medicines. Anal. Methods 2011, 3, 348–355. [Google Scholar] [CrossRef]
- Liang, S.; Wan, J.; Zhu, J.; Cao, X. Effects of porogens on the morphology and enantioselectivity of core–shell molecularly imprinted polymers with ursodeoxycholic acid. Sep. Purif. Technol. 2010, 72, 208–216. [Google Scholar] [CrossRef]
- Chevalier, Y.; Bolzinger, M.-A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surf. Physicochem. Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, W.; Yu, H.; Wang, A. Preparation of porous adsorbent via Pickering emulsion template for water treatment: A review. J. Environ. Sci. 2020, 88, 217–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Z.; Yan, R.; Fu, X.; Wang, G.; Wang, Y.; Li, Z.; Zhang, X.; Hou, J. Facile fabrication of snowman-like magnetic molecularly imprinted polymer microspheres for bisphenol A via one-step Pickering emulsion polymerization. React. Funct. Polym. 2021, 164, 104911. [Google Scholar] [CrossRef]
- Lan, Y.; Corradini, M.; Weiss, R.G.; Raghavan, S.; Rogers, M. To gel or not to gel: Correlating molecular gelation with solvent parameters. Chem. Soc. Rev. 2015, 44, 6035–6058. [Google Scholar] [CrossRef]
- Moein, M.M.; Javanbakht, M.; Karimi, M.; Akbari-Adergani, B.; Abdel-Rehim, M. Three-phase molecularly imprinted sol–gel based hollow fiber liquid-phase microextraction combined with liquid chromatography–tandem mass spectrometry for enrichment and selective determination of a tentative lung cancer biomarker. J. Chromatogr. B 2015, 995, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, T.; Atayi, K. Synthesis of hydrogen phosphate anion-imprinted polymer via emulsion polymerization and its use as the recognition element of graphene/graphite paste potentiometric electrode. Mater. Chem. Phys. 2018, 209, 180–187. [Google Scholar] [CrossRef]
- Zhao, X.; Cui, Y.; Wang, J.; Wang, J. Preparation of fluorescent molecularly imprinted polymers via pickering emulsion interfaces and the application for visual sensing analysis of Listeria Monocytogenes. Polymers 2019, 11, 984. [Google Scholar] [CrossRef]
- Pan, J.; Li, L.; Hang, H.; Wu, R.; Dai, X.; Shi, W.; Yan, Y. Fabrication and evaluation of magnetic/hollow double-shelled imprinted sorbents formed by Pickering emulsion polymerization. Langmuir 2013, 29, 8170–8178. [Google Scholar] [CrossRef]
- Ou, H.; Chen, Q.; Pan, J.; Zhang, Y.; Huang, Y.; Qi, X. Selective removal of erythromycin by magnetic imprinted polymers synthesized from chitosan-stabilized Pickering emulsion. J. Hazard. Mater. 2015, 289, 28–37. [Google Scholar] [CrossRef]
- Sun, Y.; Zhong, S. Molecularly imprinted polymers fabricated via Pickering emulsions stabilized solely by food-grade casein colloidal nanoparticles for selective protein recognition. Anal. Bioanal. Chem. 2018, 410, 3133–3143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dramou, P.; He, H.; Tan, S.; Pham-Huy, C.; Pan, H. Molecularly imprinted stationary phase prepared by reverse micro-emulsion polymerization for selective recognition of gatifloxacin in aqueous media. J. Chromatogr. Sci. 2012, 50, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, H.; Zhang, Q.; Cui, Y.; Wu, Z.; Zheng, R.; Liu, L. Molecularly imprinted polymers prepared by precipitation polymerization and used for inducing crystallization of oleanolic acid in supercritical CO2. Sep. Purif. Technol. 2011, 81, 411–417. [Google Scholar] [CrossRef]
- Spivak, D.A. Optimization, evaluation, and characterization of molecularly imprinted polymers. Adv. Drug Del. Rev. 2005, 57, 1779–1794. [Google Scholar] [CrossRef]
- Lowdon, J.W.; Diliën, H.; Singla, P.; Peeters, M.; Cleij, T.J.; van Grinsven, B.; Eersels, K. MIPs for commercial application in low-cost sensors and assays–An overview of the current status quo. Sens. Actuators B Chem. 2020, 325, 128973. [Google Scholar] [CrossRef] [PubMed]
- Mohsenzadeh, E.; Ratautaite, V.; Brazys, E.; Ramanavicius, S.; Zukauskas, S.; Plausinaitis, D.; Ramanavicius, A. Design of molecularly imprinted polymers (MIP) using computational methods: A review of strategies and approaches. WIREs Comput. Mol. Sci. 2024, 14, e1713. [Google Scholar] [CrossRef]
- Wu, X.; Du, J.; Li, M.; Wu, L.; Han, C.; Su, F. Recent advances in green reagents for molecularly imprinted polymers. RSC Adv. 2018, 8, 311–327. [Google Scholar] [CrossRef]
- Pei, Y.; Zhang, Y.; Ma, J.; Fan, M.; Zhang, S.; Wang, J. Ionic liquids for advanced materials. Mater. Today Nano 2022, 17, 100159. [Google Scholar] [CrossRef]
- Liu, H.; Jin, P.; Zhu, F.; Nie, L.; Qiu, H. A review on the use of ionic liquids in preparation of molecularly imprinted polymers for applications in solid-phase extraction. TrAC Trends Anal. Chem. 2021, 134, 116132. [Google Scholar] [CrossRef]
- Ding, S.; Lyu, Z.; Niu, X.; Zhou, Y.; Liu, D.; Falahati, M.; Du, D.; Lin, Y. Integrating ionic liquids with molecular imprinting technology for biorecognition and biosensing: A review. Biosens. Bioelectron. 2020, 149, 111830. [Google Scholar] [CrossRef]
- Han, X.; Armstrong, D.W. Ionic liquids in separations. Acc. Chem. Res. 2007, 40, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yan, F.; Texter, J. Advanced applications of ionic liquids in polymer science. Prog. Polym. Sci. 2009, 34, 431–448. [Google Scholar] [CrossRef]
- Wang, N.; Cui, B. An overview of ionic liquid-based adsorbents in food analysis. TrAC Trends Anal. Chem. 2022, 146, 116496. [Google Scholar] [CrossRef]
- Zhu, G.; Gao, X.; Wang, X.; Wang, J.; Fan, J. Influence of hydrogen bond accepting ability of anions on the adsorption performance of ionic liquid surface molecularly imprinted polymers. J. Chromatogr. A 2018, 1532, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.-D.; Huang, Y.-P.; Xin, X.-L.; Liu, Z.-S.; Aisa, H.A. Preparation of metallic pivot-based imprinted monolith for polar template. J. Chromatogr. B 2013, 934, 109–116. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, X.-L.; Ma, L.; Shang, P.-P.; Huang, Y.-P.; Liu, Z.-S. Improving affinity of β-cyclodextrin-based molecularly imprinted polymer using room temperature ionic liquid. Eur. Polym. J. 2019, 116, 275–282. [Google Scholar] [CrossRef]
- Deng, W.; Yang, C.; Gong, M.; Zhang, Z.; Li, H. Preparation of rutin imprinted monolith (RIM) by using porogen containing ion liquid [BMIM] PF6 and its molecular recognition. J. Chromatogr. B 2024, 1233, 123986. [Google Scholar] [CrossRef]
- Guo, L.; Deng, Q.; Fang, G.; Gao, W.; Wang, S. Preparation and evaluation of molecularly imprinted ionic liquids polymer as sorbent for on-line solid-phase extraction of chlorsulfuron in environmental water samples. J. Chromatogr. A 2011, 1218, 6271–6277. [Google Scholar] [CrossRef]
- Booker, K.; Holdsworth, C.I.; Doherty, C.M.; Hill, A.J.; Bowyer, M.C.; McCluskey, A. Ionic liquids as porogens for molecularly imprinted polymers: Propranolol, a model study. Org. Biomol. Chem. 2014, 12, 7201–7210. [Google Scholar] [CrossRef]
- Liu, X.-L.; Yao, H.-F.; Chai, M.-H.; He, W.; Huang, Y.-P.; Liu, Z.-S. Green synthesis of carbon nanotubes-reinforced molecularly imprinted polymer composites for drug delivery of fenbufen. AAPS PharmSciTech 2018, 19, 3895–3906. [Google Scholar] [CrossRef]
- Booker, K.; Bowyer, M.C.; Holdsworth, C.I.; McCluskey, A. Efficient preparation and improved sensitivity of molecularly imprinted polymers using room temperature ionic liquids. Chem. Commun. 2006, 16, 1730–1732. [Google Scholar] [CrossRef]
- He, C.; Long, Y.; Pan, J.; Li, K.; Liu, F. Molecularly imprinted silica prepared with immiscible ionic liquid as solvent and porogen for selective recognition of testosterone. Talanta 2008, 74, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.K.; Sun, G.-Y.; Jia, M.; Yang, J.; Liu, Z.-S.; Huang, Y.-P.; Aisa, H.A. Cost-effective imprinting to minimize consumption of template in room-temperature ionic liquid for fast purification of chlorogenic acid from the extract of E. ulmoides leaves. Anal. Bioanal. Chem. 2019, 411, 1261–1271. [Google Scholar] [CrossRef]
- Sun, Y.K.; Jia, M.; Yang, J.; Huang, Y.-P.; Liu, Z.-S.; Aisa, H.A. A strategy of utilizing Zn (II) as metallic pivot in room temperature ionic liquid to prepare molecularly imprinted polymers for compound with intramolecular hydrogen bonds. Anal. Bioanal. Chem. 2018, 410, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Ban, L.; Han, X.; Wang, X.-H.; Huang, Y.-P.; Liu, Z.-S. Carprofen-imprinted monolith prepared by reversible addition–fragmentation chain transfer polymerization in room temperature ionic liquids. Anal. Bioanal. Chem. 2013, 405, 8597–8605. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; He, J.; Cai, G.; Lin, A.; Zheng, W.; Liu, X.; Chen, L.; He, X.; Zhang, Y. Room temperature ionic liquid-mediated molecularly imprinted polymer monolith for the selective recognition of quinolones in pork samples. J. Sep. Sci. 2010, 33, 3786–3793. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.-J.; Yang, J.; Zhao, Y.-X.; Liu, Z.-S.; Aisa, H.A. Preparation of ionic liquid-mediated imprinted monolith for selective capture and purification of corilagin. J. Chromatogr. B 2017, 1041, 98–103. [Google Scholar] [CrossRef]
- Li, X.-J.; Chen, X.-X.; Sun, G.-Y.; Zhao, Y.X.; Liu, Z.-S.; Aisa, H.A. Green synthesis and evaluation of isoquercitrin imprinted polymers for class-selective separation and purification of flavonol glycosides. Anal. Methods 2015, 7, 4717–4724. [Google Scholar] [CrossRef]
- Wu, X.; Wu, L. Molecularly imprinted polymers for the solid-phase extraction of four fluoroquilones from milk and lake water samples. J. Sep. Sci. 2015, 38, 3615–3621. [Google Scholar] [CrossRef]
- Xu, Z.; Fang, G.; Wang, S. Molecularly imprinted solid phase extraction coupled to high-performance liquid chromatography for determination of trace dichlorvos residues in vegetables. Food Chem. 2010, 119, 845–850. [Google Scholar] [CrossRef]
- Wang, X.-H.; Zhang, J.; Peng, C.; Dong, Q.; Huang, Y.-P.; Liu, Z.-S. Comparison of multi-recognition molecularly imprinted polymers for recognition of melamine, cyromazine, triamterene, and trimethoprim. Anal. Bioanal. Chem. 2015, 407, 7145–7155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, W.; Wang, N.; Ni, X.; Wang, H.; Gu, Z.; Wu, X.; Xu, W. Synthesis and Evaluation of Ionic Liquid–Mediated Molecularly Imprinted Polymer for Highly Selective Recognition of Dibutyl Phthalate from Liquor Samples. Adv. Polym. Tech. 2017, 36, 220–229. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Yoo, C.-I.; Ahn, Y.-S. N,N-dimethylformamide. Scand. J. Work Environ. Health 2019, 45, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, E.; Song, S.-H.; Lee, C.-W.; Kwon, J.-T.; Park, E.Y.; Kim, B. Toluene concentrations in the blood and risk of thyroid cancer among residents living near national industrial complexes in South Korea: A population-based cohort study. Environ. Int. 2021, 146, 106304. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; De la Guardia, M.; Andruch, V.; Vilková, M. Deep eutectic solvents vs ionic liquids: Similarities and differences. Microchem. J. 2020, 159, 105539. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Hayyan, A.; Al-Saadi, M.A.; AlNashef, I.M.; Mirghani, M.E.; Saheed, O.K. Are deep eutectic solvents benign or toxic? Chemosphere 2013, 90, 2193–2195. [Google Scholar] [CrossRef]
- Azzouz, A.; Hayyan, M. Techno-economic feasibility analysis: The missing piece in the puzzle of deep eutectic solvents. Sustain. Mater. Technol. 2023, 39, e00795. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Ncube, S.; Nomngongo, P.N.; Pakade, V.E. Molecular imprinting with deep eutectic solvents: Synthesis, applications, their significance, and benefits. J. Mol. Liq. 2022, 362, 119696. [Google Scholar] [CrossRef]
- Zhekenov, T.; Toksanbayev, N.; Kazakbayeva, Z.; Shah, D.; Mjalli, F.S. Formation of type III Deep Eutectic Solvents and effect of water on their intermolecular interactions. Fluid Phase Equilib. 2017, 441, 43–48. [Google Scholar] [CrossRef]
- Abbott, A.P.; Alabdullah, S.S.; Al-Murshedi, A.Y.; Ryder, K.S. Brønsted acidity in deep eutectic solvents and ionic liquids. Faraday Discuss. 2018, 206, 365–377. [Google Scholar] [CrossRef]
- Hayyan, A.; Mjalli, F.S.; AlNashef, I.M.; Al-Wahaibi, T.; Al-Wahaibi, Y.M.; Hashim, M.A. Fruit sugar-based deep eutectic solvents and their physical properties. Thermochim. Acta 2012, 541, 70–75. [Google Scholar] [CrossRef]
- Santana, A.P.; Mora-Vargas, J.A.; Guimaraes, T.G.; Amaral, C.D.; Oliveira, A.; Gonzalez, M.H. Sustainable synthesis of natural deep eutectic solvents (NADES) by different methods. J. Mol. Liq. 2019, 293, 111452. [Google Scholar] [CrossRef]
- Dai, Y.; Van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W. Deep eutectic solvents: A review of fundamentals and applications. Chem. Rev. 2020, 121, 1232–1285. [Google Scholar] [CrossRef] [PubMed]
- Zainal-Abidin, M.H.; Hayyan, M.; Wong, W.F. Hydrophobic deep eutectic solvents: Current progress and future directions. J. Ind. Eng. Chem. 2021, 97, 142–162. [Google Scholar] [CrossRef]
- Fu, N.; Liu, X.; Li, L.; Tang, B.; Row, K.H. Ternary choline chloride/caffeic acid/ethylene glycol deep eutectic solvent as both a monomer and template in a molecularly imprinted polymer. J. Sep. Sci. 2017, 40, 2286–2291. [Google Scholar] [CrossRef]
- Cheng, G.; Chen, N.; Li, Z.; Zhao, K.; Duan, R.; Chen, Z.; Zhu, G. Fabrication of deep eutectic solvent-molecularly imprinted polymer in water: A green strategy for adsorption of bisphenol A. J. Environ. Chem. Eng. 2023, 11, 109651. [Google Scholar] [CrossRef]
- Li, X.; Row, K.H. Purification of antibiotics from the millet extract using hybrid molecularly imprinted polymers based on deep eutectic solvents. RSC Adv. 2017, 7, 16997–17004. [Google Scholar] [CrossRef]
- Meng, J.; Wang, X. Microextraction by Packed Molecularly Imprinted Polymer Combined Ultra-High-Performance Liquid Chromatography for the Determination of Levofloxacin in Human Plasma. J. Chem. 2019, 2019, 4783432. [Google Scholar] [CrossRef]
- Liang, F.; Li, W.; Li, M.; Li, X.; He, J.; Wu, Q. Kaempferol molecularly imprinted polymers: Preparation, characterization and application to the separation of kaempferol from ginkgo leaves. Polym. Int. 2023, 72, 586–596. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, L.; Qiao, F.; Yan, H. Facile and green synthesis of a hydrophilic imprinted resin in a deep eutectic solvent–water medium for the specific molecular recognition of tumor biomarkers in complex biological matrices. Green Chem. 2024, 26, 2000–2010. [Google Scholar] [CrossRef]
- Porfireva, A.; Goida, A.; Evtugyn, V.; Evtugyn, G. Impedimetric sensor based on molecularly imprinted polythionine from deep eutectic solvent for epinephrine determination. Green Anal. Chem. 2024, 9, 100113. [Google Scholar] [CrossRef]
- Wei, Z.-H.; Sun, X.; Mu, L.-N.; Huang, Y.-P.; Liu, Z.-S. Improving affinity of imprinted monolithic polymer prepared in deep eutectic solvent by metallic pivot. J. Chromatogr. A 2019, 1602, 48–55. [Google Scholar] [CrossRef]
- Monnier, A.; Díaz-Álvarez, M.; Turiel, E.; Martín-Esteban, A. Evaluation of deep eutectic solvents in the synthesis of molecularly imprinted fibers for the solid-phase microextraction of triazines in soil samples. Anal. Bioanal. Chem. 2024, 416, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Qiao, F.; Yan, H. A simple and benign protocol for the synthesis of a deep eutectic solvent-based hydrophilic molecularly imprinted resin in water for excellent selective molecular recognition in aqueous phase. Green Chem. 2021, 23, 5179–5188. [Google Scholar] [CrossRef]
- Husin, N.A.; Muhamad, M.; Yahaya, N.; Miskam, M.; Kamal, N.N.S.N.M.; Asman, S.; Raoov, M.; Zain, N.N.M. Application of a new choline-imidazole based deep eutectic solvents in hybrid magnetic molecularly imprinted polymer for efficient and selective removal of naproxen from aqueous samples. Mater. Chem. Phys. 2021, 261, 124228. [Google Scholar] [CrossRef]
- Surapong, N.; Pongpinyo, P.; Santaladchaiyakit, Y.; Burakham, R. A biobased magnetic dual-dummy-template molecularly imprinted polymer using a deep eutectic solvent as a coporogen for highly selective enrichment of organophosphates. Food Chem. 2023, 418, 136045. [Google Scholar] [CrossRef]
- Liang, S.; Yan, H.; Cao, J.; Han, Y.; Shen, S.; Bai, L. Molecularly imprinted phloroglucinol–formaldehyde–melamine resin prepared in a deep eutectic solvent for selective recognition of clorprenaline and bambuterol in urine. Anal. Chim. Acta 2017, 951, 68–77. [Google Scholar] [CrossRef]
- Ma, W.; An, Y.; Row, K.H. Preparation and evaluation of a green solvent-based molecularly imprinted monolithic column for the recognition of proteins by high-performance liquid chromatography. Analyst 2019, 144, 6327–6333. [Google Scholar] [CrossRef]
- Tang, W.; Row, K.H. Fabrication of water-compatible molecularly imprinted resin in a hydrophilic deep eutectic solvent for the determination and purification of quinolones in wastewaters. Polymers 2019, 11, 871. [Google Scholar] [CrossRef]
- Furtado, A.I.; Viveiros, R.; Bonifácio, V.D.; Melo, A.; Casimiro, T. Biomolecular Fishing: Design, Green Synthesis, and Performance of l-Leucine-Molecularly Imprinted Polymers. ACS Omega 2023, 8, 9179–9186. [Google Scholar] [CrossRef] [PubMed]
- Iacob, B.-C.; Bodoki, A.E.; Da Costa Carvalho, D.F.; Serpa Paulino, A.A.; Barbu-Tudoran, L.; Bodoki, E. Unlocking New Avenues: Solid-State Synthesis of Molecularly Imprinted Polymers. Int. J. Mol. Sci. 2024, 25, 5504. [Google Scholar] [CrossRef]
- Furtado, A.I.; Lowdon, J.W.; Eersels, K.; van Grinsven, B.; Cruz, A.; Serpa, J.; Bonifácio, V.D.; Viveiros, R.; Casimiro, T. Mechanosynthesis and thermal bio–sensing of beryllium–based molecularly imprinted polymers. Biosens. Bioelectron. X 2025, 24, 100605. [Google Scholar] [CrossRef]
- Friščić, T.; Mottillo, C.; Titi, H.M. Mechanochemistry for synthesis. Angew. Chem. 2020, 132, 1030–1041. [Google Scholar] [CrossRef]
- Beckman, E.J. Supercritical and near-critical CO2 in green chemical synthesis and processing. J. Supercrit. Fluids 2004, 28, 121–191. [Google Scholar] [CrossRef]
- Lamaoui, A.; Mani, V.; Durmus, C.; Salama, K.N.; Amine, A. Molecularly imprinted polymers: A closer look at the template removal and analyte binding. Biosens. Bioelectron. 2023, 243, 115774. [Google Scholar] [CrossRef]
- Furtado, A.I.; Bonifácio, V.D.; Viveiros, R.; Casimiro, T. Design of Molecularly Imprinted Polymers Using Supercritical Carbon Dioxide Technology. Molecules 2024, 29, 926. [Google Scholar] [CrossRef]
- Duarte, A.R.C.; Casimiro, T.; Aguiar-Ricardo, A.; Simplício, A.L.; Duarte, C.M. Supercritical fluid polymerisation and impregnation of molecularly imprinted polymers for drug delivery. J. Supercrit. Fluids 2006, 39, 102–106. [Google Scholar] [CrossRef]
- Viveiros, R.; Lopes, M.I.; Heggie, W.; Casimiro, T. Green approach on the development of lock-and-key polymers for API purification. Chem. Eng. J. 2017, 308, 229–239. [Google Scholar] [CrossRef]
- Székely, G.; Fritz, E.; Bandarra, J.; Heggie, W.; Sellergren, B. Removal of potentially genotoxic acetamide and arylsulfonate impurities from crude drugs by molecular imprinting. J. Chromatogr. A 2012, 1240, 52–58. [Google Scholar] [CrossRef]
- Byun, H.-S.; Chun, D.; Shim, W.-G. Separation and recognition characteristics by MIP manufacture using supercritical CO2 technology. J. Ind. Eng. Chem. 2021, 97, 356–367. [Google Scholar] [CrossRef]
- Caldara, M.; Lowdon, J.W.; Rogosic, R.; Arreguin-Campos, R.; Jimenez-Monroy, K.L.; Heidt, B.; Tschulik, K.; Cleij, T.J.; Diliën, H.; Eersels, K. Thermal detection of glucose in urine using a molecularly imprinted polymer as a recognition element. ACS Sens. 2021, 6, 4515–4525. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Q.-M.; Tian, L.-L.; Yang, C.; Yu, S.-H.; Yang, C. Research progress of the molecularly imprinted cryogel. Chin. J. Anal. Chem. 2015, 43, 1777–1784. [Google Scholar] [CrossRef]
- Memic, A.; Colombani, T.; Eggermont, L.J.; Rezaeeyazdi, M.; Steingold, J.; Rogers, Z.J.; Navare, K.J.; Mohammed, H.S.; Bencherif, S.A. Latest advances in cryogel technology for biomedical applications. Adv. Ther. 2019, 2, 1800114. [Google Scholar] [CrossRef]
- Bakhshpour, M.; Göktürk, I.; Bereli, N.; Denizli, A. Molecularly imprinted cryogel cartridges for the selective recognition of tyrosine. Biotechnol. Prog. 2020, 36, e3006. [Google Scholar] [CrossRef] [PubMed]
- Kartal, F.; Denizli, A. Molecularly imprinted cryogel beads for cholesterol removal from milk samples. Colloids Surf. B. Biointerfaces 2020, 190, 110860. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, G.; Fu, C. Synthesis and characteristics of tyrosine imprinted beads via suspension polymerization. React. Funct. Polym. 2003, 56, 167–173. [Google Scholar] [CrossRef]
- Perçin, I.; Idil, N.; Denizli, A. Molecularly imprinted poly (N-isopropylacrylamide) thermosensitive based cryogel for immunoglobulin G purification. Process Biochem. 2019, 80, 181–189. [Google Scholar] [CrossRef]
- Wardani, N.I.; Kangkamano, T.; Wannapob, R.; Kanatharana, P.; Thavarungkul, P.; Limbut, W. Electrochemical sensor based on molecularly imprinted polymer cryogel and multiwalled carbon nanotubes for direct insulin detection. Talanta 2023, 254, 124137. [Google Scholar] [CrossRef]
- Ma, L.; Tang, L.; Li, R.-S.; Huang, Y.-P.; Liu, Z.-S. Water-compatible molecularly imprinted polymers prepared using metal–organic gel as porogen. RSC Adv. 2015, 5, 84601–84609. [Google Scholar] [CrossRef]
- Liu, G.; Li, S.; Shi, C.; Huo, M.; Lin, Y. Progress in research and application of metal–organic gels: A review. Nanomaterials 2023, 13, 1178. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Gao, H.; Yan, G.; Gao, H.; Chen, M. Metal–Organic Gel-Modulated Synthesis of Hierarchically Porous Molecularly Imprinted Polymers for Efficient Removal of Sildenafil from Water. ACS Omega 2021, 6, 7478–7486. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chai, M.-H.; Yao, H.-F.; Huang, Y.-P.; Liu, Z.-S. Molecularly imprinted polymers doped with carbon nanotube with aid of metal-organic gel for drug delivery systems. Pharm. Res. 2020, 37, 193. [Google Scholar] [CrossRef] [PubMed]

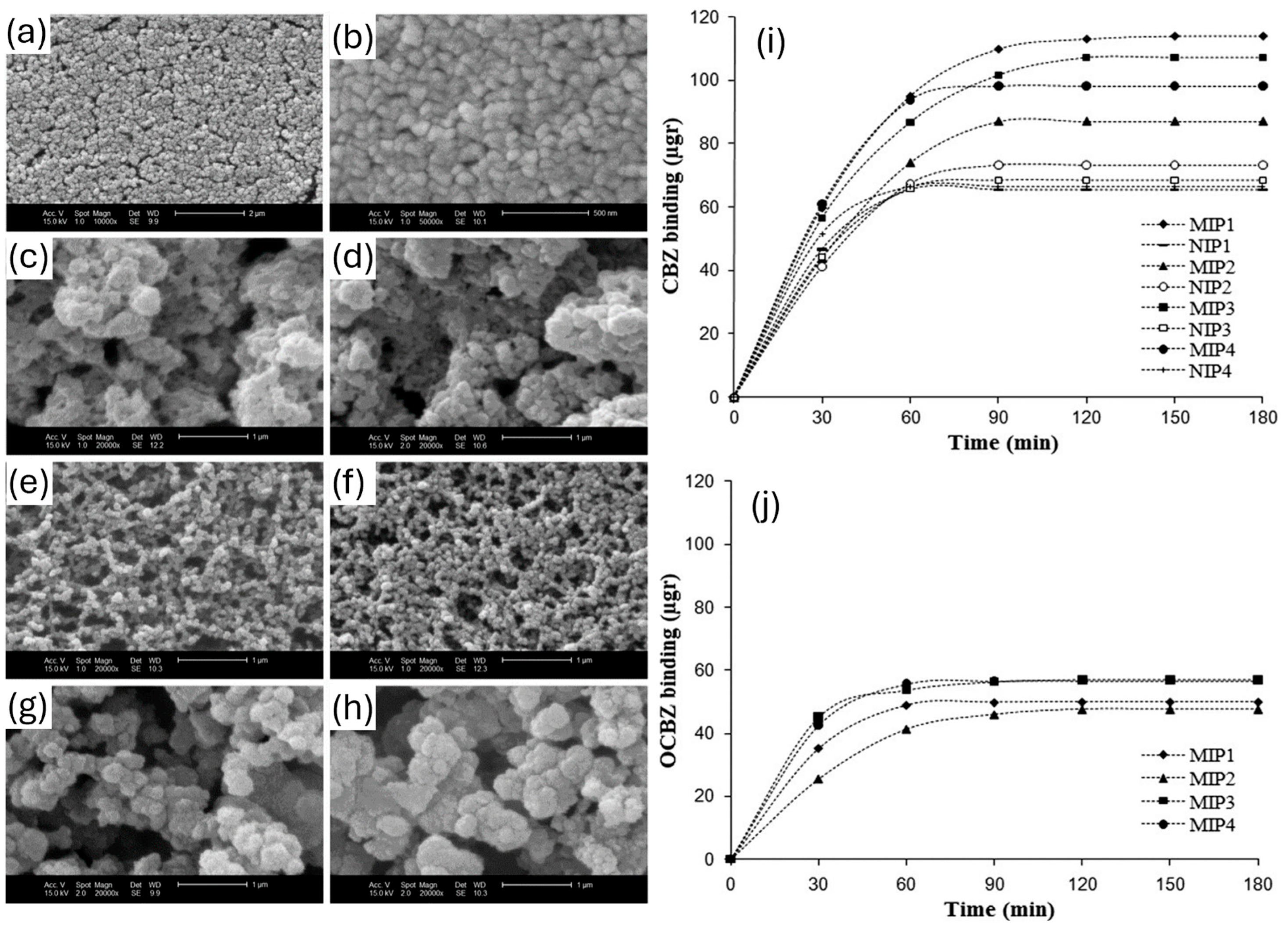


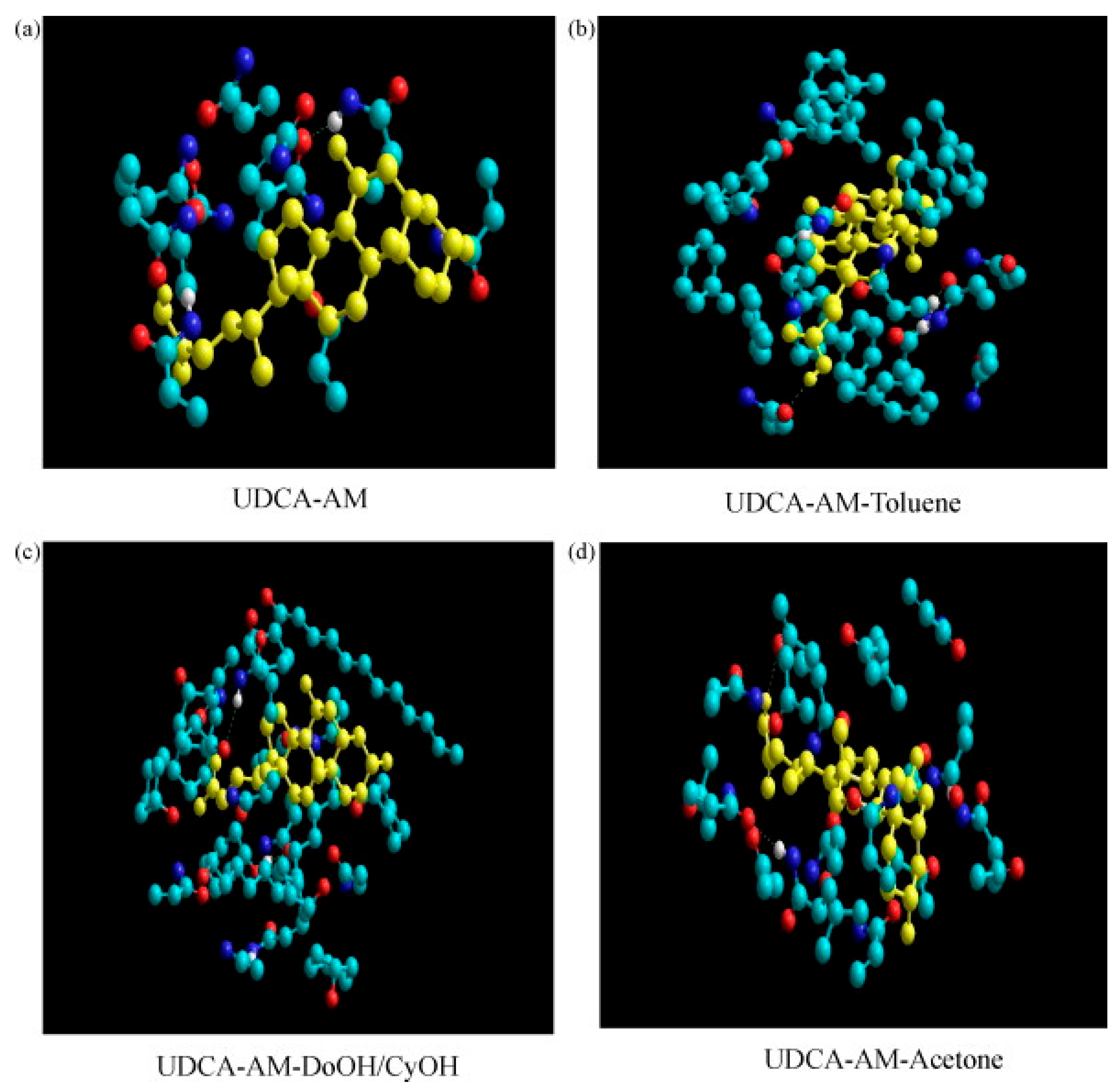



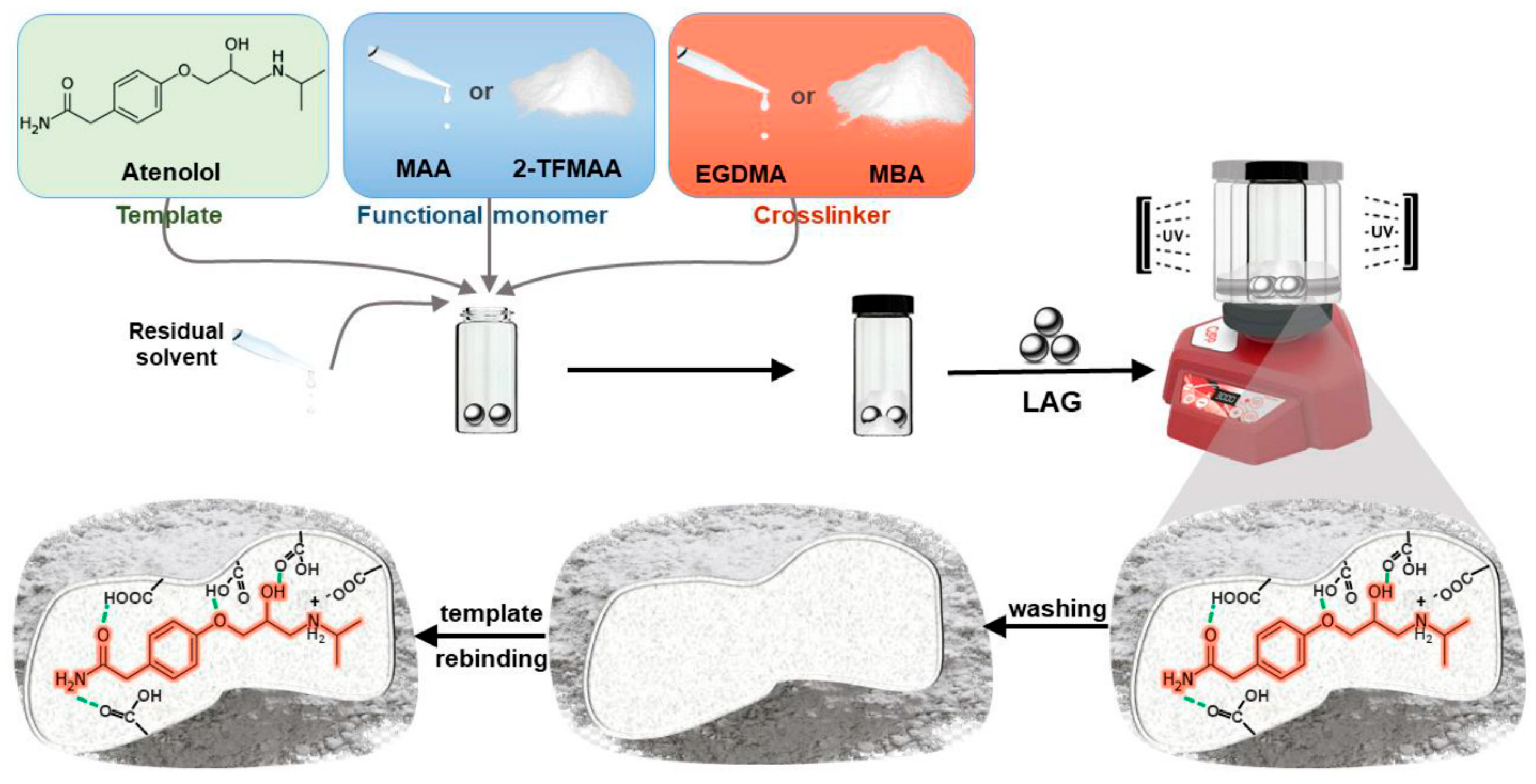


| Solvent | bp [°C] | Dielectric Constant | Hansen Solubility Parameter [MPa1/2] | Polarity ET (30) [kcal/mol] | ||
|---|---|---|---|---|---|---|
| δd | δp | δH | ||||
| Acetone | 56.3 | 21 | 15.5 | 10.4 | 7.0 | 42.2 |
| ACN | 81.6 | 37.5 | 15.3 | 18 | 6.1 | 45.6 |
| Chloroform | 61.2 | 4.8 | 17.8 | 3.1 | 5.7 | 39.1 |
| DCM | 39.8 | 8.9 | 18.2 | 6.3 | 6.1 | 40.7 |
| DMF | 153 | 38.3 | 16.8 | 11.5 | 10.2 | 43.2 |
| DMSO | 189 | 46.4 | 18.4 | 16.4 | 10.2 | 45.1 |
| THF | 66 | 7.6 | 16.8 | 5.7 | 8 | 37.4 |
| Toluene | 111 | 2.4 | 18 | 1.4 | 2 | 33.9 |
| EtOH | 78.3 | 34.6 | 15.8 | 8.8 | 19.4 | 51.9 |
| MeOH | 64.7 | 33.6 | 15.1 | 12.3 | 22.3 | 55.4 |
| Water | 100 | 80.4 | 15.5 | 16 | 42.4 | 63.1 |
| [BMIM][PF6] | 180 * | 16.1 | 17.9 | 9.8 | 8.1 | 49.0–53.2 |
| EG:ChCl (1:2) | 439 | - | 15.9 | 5.3 | 19.5 | 57.3 |
| scCO2 | - | 1.03–1.6 | 15.6 | 5.2 | 5.8 | 34.5 |
| Target | Porogen | Polymerization Approach | Application | IF | Ref. |
|---|---|---|---|---|---|
| Nicotinamide | ACN Chloroform Toluene | Monolithic bulk free-radical | Solid-phase extraction | n.d. | [44] |
| Ibuprofen | DMF | Monolithic bulk free-radical | Solid-phase extraction | ~8 * | [45] |
| 2-phenylproponic acids | DMF | Precipitation | Solid-phase extraction | 1.3–3.3 | [46] |
| Progesterone, testosterone | Toluene Chloroform ACN | Monolithic bulk free-radical | Solid-phase extraction | 2.4–3.1 | [47] |
| Terbutylazine | Toluene | Monolithic bulk free-radical | Solid-phase extraction | n.d. | [48] |
| Pinacolyl methylphosphonate | Toluene and ACN | Precipitation | Sensing | ~1.3 * | [49] |
| Tetracycline | Chloroform and ACN | Precipitation | Drug release | 5.1 | [50] |
| Diisopropylurea | Dichloromethane | Monolithic bulk free-radical | Solid-phase extraction | ~2 * | [51] |
| Estradiol | Toluene | Monolithic bulk free-radical | Solid-phase extraction | 4.8 | [52] |
| Xylazine | Toluene and chloroform (3:1) | Monolithic bulk free-radical | Sensing | 2.0 | [53] |
| Pyocyanin | Chloroform | Monolithic bulk free-radical | Sensing | 1.6 | [17] |
| Amphetamine | DMSO | Monolithic bulk free-radical | Sensing | 4.4 | [54] |
| (S)-N-Butyryl homoserine lactone | DMSO | Monolithic bulk free-radical | Sensing | 2.8 | [55] |
| Folic acid | DMSO–ACN (5:3) | Monolithic bulk free-radical | Solid-phase extraction | 4.0 | [56] |
| Quercetin | Acetone | Monolithic bulk free-radical | Solid-phase extraction | 8.2 | [57] |
| Template | Porogen | Approach | Application | Additive | IF | Reference |
|---|---|---|---|---|---|---|
| Triazines | Water | Radical polymerization | Solid-phase extraction | 5% toluene | 2.7–4.7 | [74] |
| Sunset Yellow | Water | Radical polymerization | Solid-phase extraction | - | 1.33 | [76] |
| Atenolol | Butanol or propanol | Bulk or precipitation polymerization | Solid-phase extraction | - | 4.2 and 11.7 | [79] |
| Histamine | EtOH | Precipitation polymerization | Sensing | - | 2.3 | [80] |
| Synephrine | Methanol/water (4:1) | Precipitation polymerization | Solid-phase extraction | - | ~2 * | [64] |
| Triazines | EtOH/water (9:1) | Radical polymerization on silica particle | Solid-phase extraction | Poly-vinylpyrrolidone | n.d. | [81] |
| Gallic acid | Water | basic polymerization | Solid-phase extraction | Phosphate buffer | 1.7 | [82] |
| B-vitamins | Water/acetic acid (99/1) | Condensation | Solid-phase extraction | 1 M NaOH | ~3–4 * | [75] |
| Bisphenol A | Acidified isopropanol | Sol–gel approach | Solid-phase extraction | 1 M NH4OH | 6.6 | [77] |
| 2,4-Dichlorophenoxy-acetic acid | EtOH/water (10:3) | Sol–gel approach | Solid-phase extraction | Conc. HCl | 1.5 | [83] |
| Hydrochlorothiazide | Water | Sol–gel approach | Analyte monitoring | CTAB and NH4OH | ~4.5 * | [71] |
| 1-naphthyl phosphate | Water/EtOH (5:3) | Sol–gel approach | Solid-phase extraction | - | 32.2 | [84] |
| Folic Acid | Water | Sol–gel approach | Sensing | NH4OH | 2.2 | [85] |
| Bisphenol F | Water | Sol–gel approach on electrode | Sensing | CTAB and NH3 | ~6 * | [86] |
| Creatinine | Water | Sol–gel Approach | Sensing | 1 M HCl and Al3Cl3 | 2.4 | [87] |
| Salicylic acid | EtOH/water (4:1) | Sol–gel approach | Drug release | 0.1 M HCl | 9.0 | [88] |
| Template | Porogen | Approach | Additive | Application | IF | Particle Size | Ref. |
|---|---|---|---|---|---|---|---|
| (S)-Naproxen | n-dodecanol/toluene | Bulk | - | Enantiomeric Separation | n.d. | n.d. | [101] |
| Ursodeoxycholic acid | Toluene/water Acetone/water DoOH/CyOH/water | Emulsion | SDS | Extraction | ~2.5 * | 250 nm | [104] |
| Amoxicillin | Water/DMSO | Emulsion | SDS | Sensing | 45.6 | 8–10 µm | [97] |
| Phosphate anion | Water/chloroform | Emulsion | CTAB | Sensing | n.d. | n.d. | [110] |
| Bovine hemoglobin | Water/toluene | Pickering emulsion | Hb-coated Janus hydroxyapatite NPs | Extraction | 4.0 | 50 µm | [96] |
| Listeria Monocytogenes | Water/DMA | Pickering emulsion | N-Acrylchitosan-Quantum Dot | Sensing | 4.6 | 200 µm | [111] |
| λ-cyhalothrin | Water/hexadecane | Pickering emulsion | Attapulgite particles | Extraction | 1.7 | 50 µm | [112] |
| Bisphenol A | Water/toluene | Pickering emulsion | Fe3O4 NPs | Environmental monitoring | 1.7 | 100 µm | [107] |
| Erythromycin | Water/toluene | Pickering emulsion | Chitosan NPs and Hydrophobic Fe3O4 | Extraction | 1.3 | 53 µm | [113] |
| Bovine hemoglobin | Water/n-hexane/corn oil | Pickering emulsion | Colloidal casein NPs | Protein Purification | 4.1 | 300 nm | [114] |
| Gatifloxacin | Cyclohexane/water | Reverse micro-emulsion | Span 60 | Extraction | 2.0 | n.d. | [115] |
| Kaempferol | ACN/methanol (4:1) | Precipitation | - | Extraction | 5.0 | 8 µm | [103] |
| Oleanolic acid | Chloroform/methanol (3:1) | Precipitation | - | Extraction | 4.8 | 20 µm | [116] |
| Huppuric acid | ACN/water | Sol–gel approach | Trifluoro-acetic acid | Extraction | 5.1 | n.d. | [109] |
| Template | IL | Co-Solvent | Reaction Type | Application | IF | Ref. |
|---|---|---|---|---|---|---|
| Aesculin | [BMIM]BF4 | DMSO | Cyclodextrin–bulk polymerization | Drug release | 2.4 | [129] |
| Rutin | [BMIM]PF6 | DMF and ACN | Bulk polymerization | Extraction/Separation | 4.8 | [130] |
| Testosterone | [BMIM]BF4 | Aq. HCl | Condensation/sol–gel approach | Extraction/Separation | 13.9 | [135] |
| Chlorogenic Acid | [BMIM]BF4 | DMSO | Bulk polymerization | Extraction/separation | 9.7 | [136] |
| Chicoric Acid | [BMIM]BF4 | DMSO | Metallic-pivot bulk polymerization | Extraction/Separation | 24.8 | [137] |
| Carprofen | [BMIM]BF4 | DMF and DMSO | RAFT polymerization | Extraction/Separation | 1.8 | [138] |
| Norfloxacin | [BMIM]BF4 | DMF and DMSO | Bulk polymerization | Extraction/Separation | 3.4 | [139] |
| Corigalin | [BMIM]BF4 | DMF and DMSO | Bulk polymerization | Extraction/Separation | 9.0 | [140] |
| Isoquercitrin | [BMIM]BF4 | DMF and DMSO | Bulk polymerization | Extraction/Separation | 3.0 | [141] |
| Fluoroquilones | [BMIM]BF4 | DMSO and CHCl3 | Molecular Crowding Polymerization | Sensing | 3.2 | [142] |
| Dichlorvos | [BMIM]PF6 | ACN and toluene | Bulk polymerization | Sensing | 1.6 | [143] |
| Melamine Triamterene Cyromazine Trimethoprim | [BMIM]BF4 | MeOH/water | Bulk polymerization | Sensing | 2.1–3.9 | [144] |
| Dibutyl Phtalate | [BMIM]BF4 | CHCl3 | Bulk polymerization | Sensing | 2.0 | [145] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Wissen, G.; Lowdon, J.W.; Cleij, T.J.; Eersels, K.; van Grinsven, B. Porogenic Solvents in Molecularly Imprinted Polymer Synthesis: A Comprehensive Review of Current Practices and Emerging Trends. Polymers 2025, 17, 1057. https://doi.org/10.3390/polym17081057
van Wissen G, Lowdon JW, Cleij TJ, Eersels K, van Grinsven B. Porogenic Solvents in Molecularly Imprinted Polymer Synthesis: A Comprehensive Review of Current Practices and Emerging Trends. Polymers. 2025; 17(8):1057. https://doi.org/10.3390/polym17081057
Chicago/Turabian Stylevan Wissen, Gil, Joseph W. Lowdon, Thomas J. Cleij, Kasper Eersels, and Bart van Grinsven. 2025. "Porogenic Solvents in Molecularly Imprinted Polymer Synthesis: A Comprehensive Review of Current Practices and Emerging Trends" Polymers 17, no. 8: 1057. https://doi.org/10.3390/polym17081057
APA Stylevan Wissen, G., Lowdon, J. W., Cleij, T. J., Eersels, K., & van Grinsven, B. (2025). Porogenic Solvents in Molecularly Imprinted Polymer Synthesis: A Comprehensive Review of Current Practices and Emerging Trends. Polymers, 17(8), 1057. https://doi.org/10.3390/polym17081057







