Coordination of Mg2+ with Chitosan for Enhanced Triboelectric Performance
Abstract
1. Introduction
2. Materials and Methods
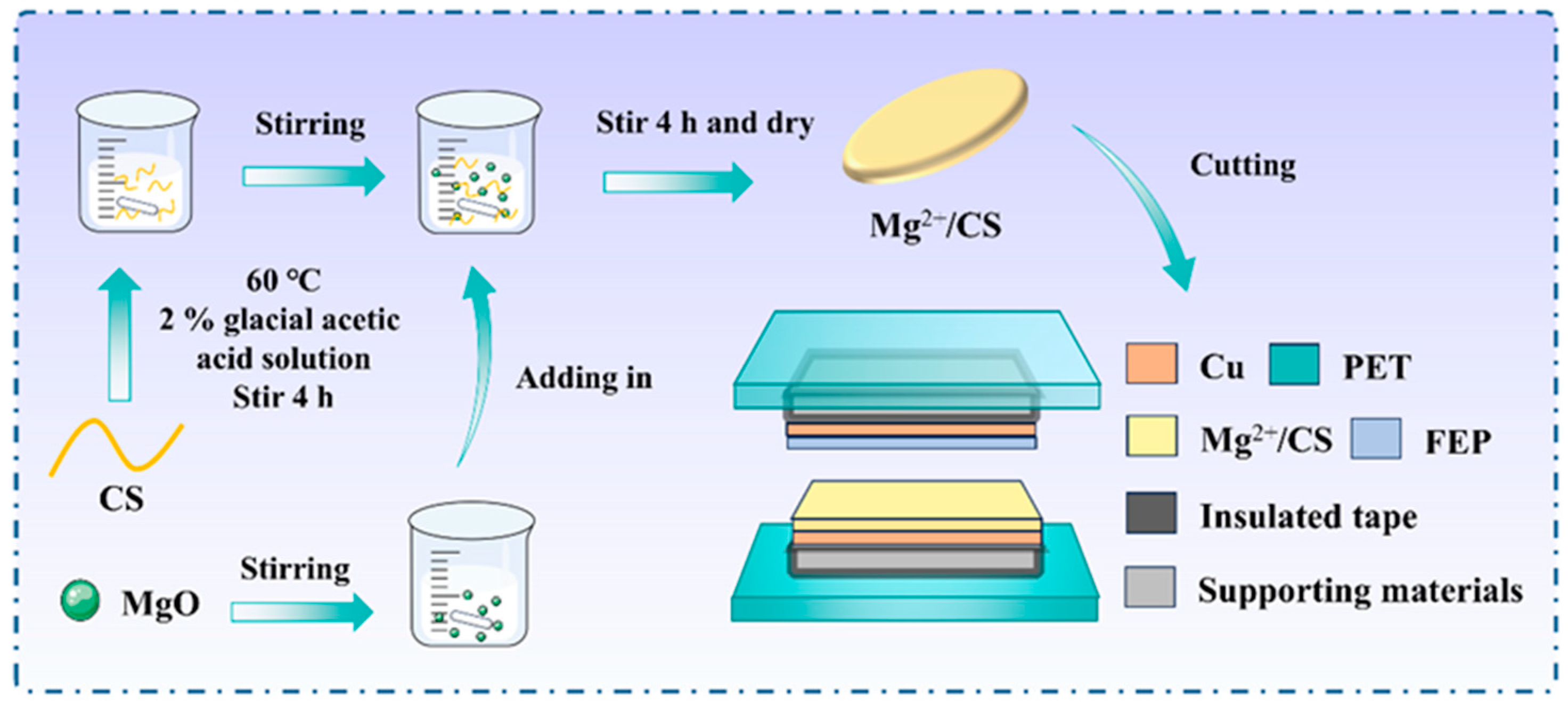
2.1. The Preparation of CS/Mg2+
2.2. The Fabrication of Mg2+/CS−TENG
2.3. Characterization and Measurement
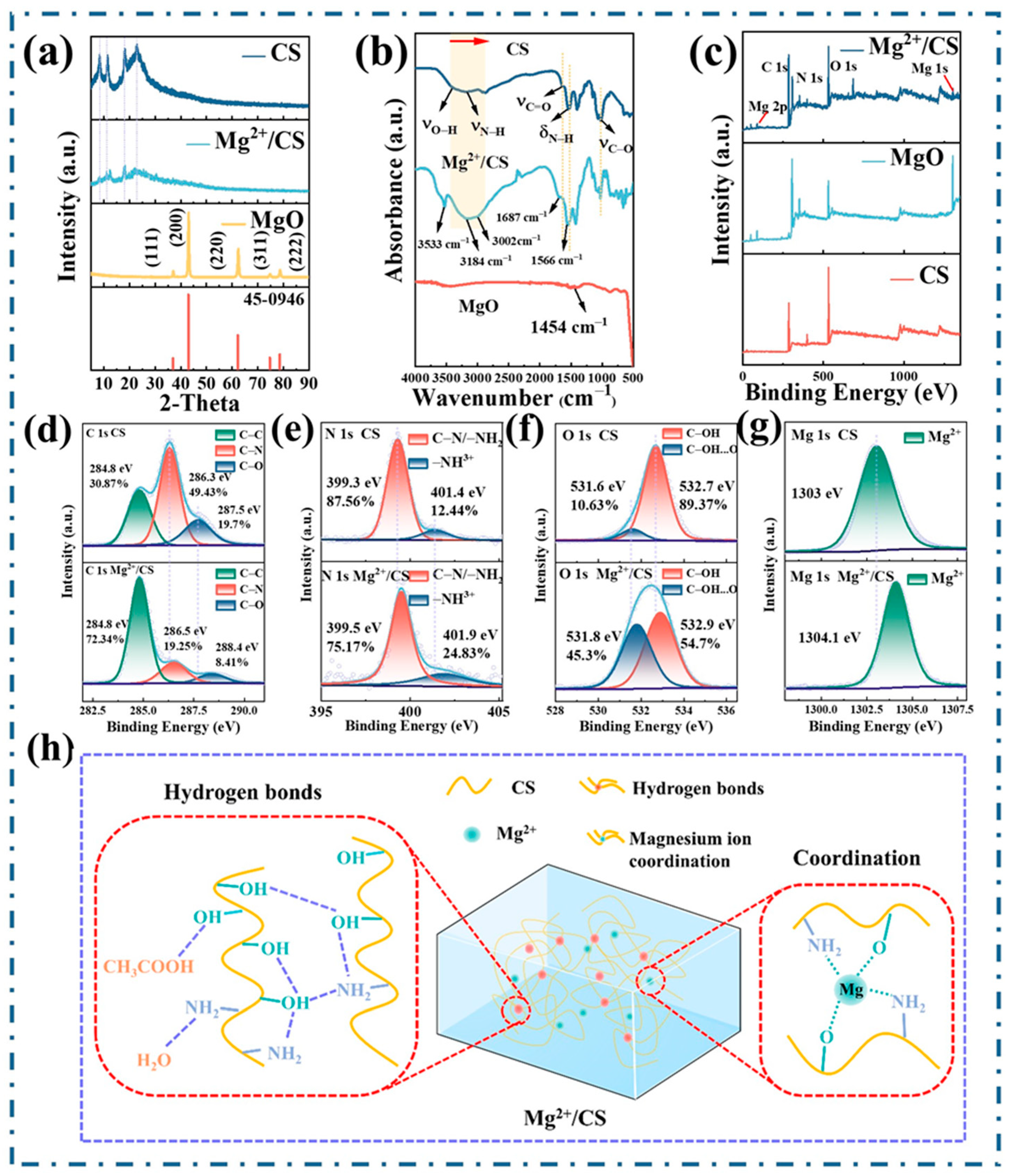
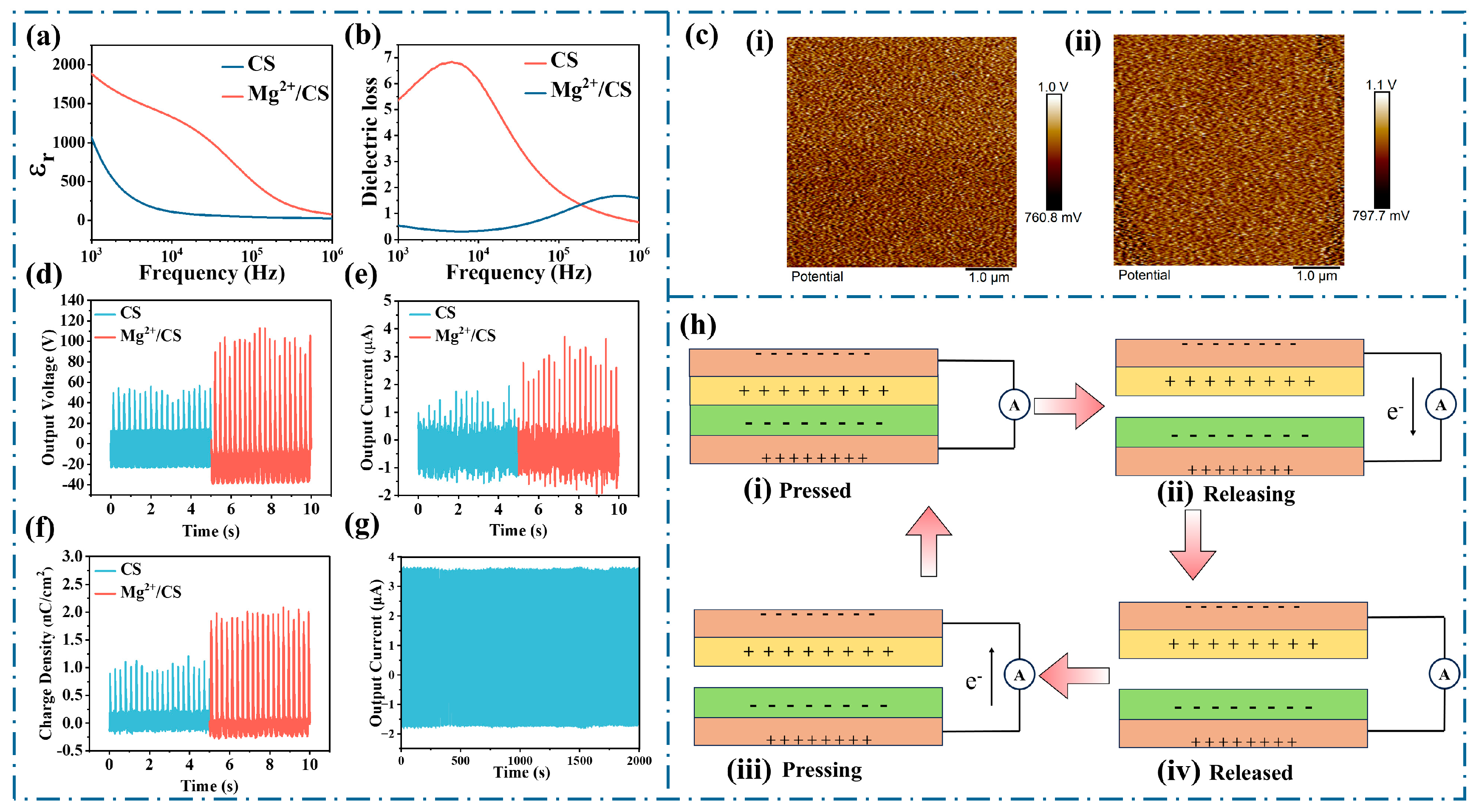
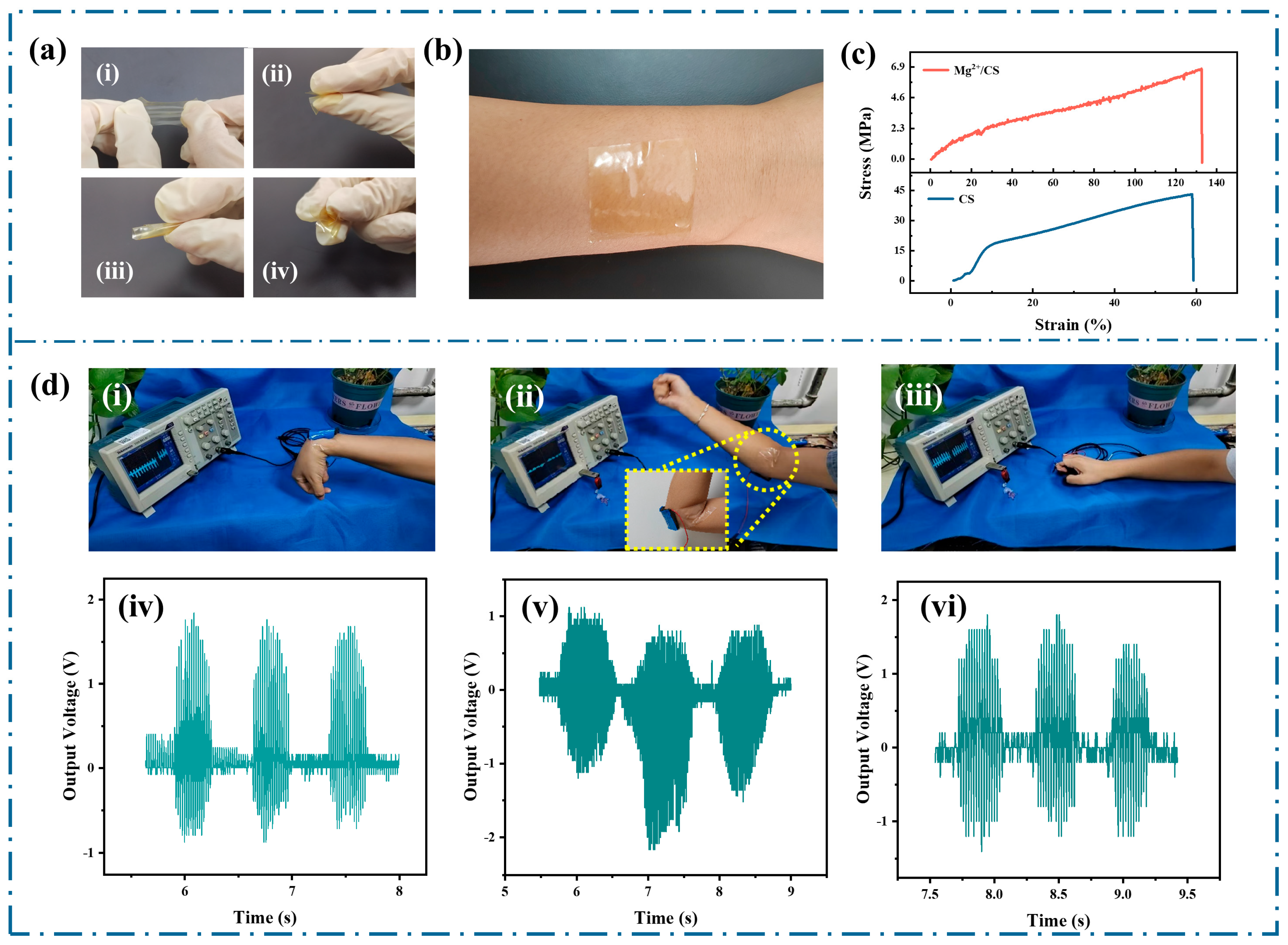
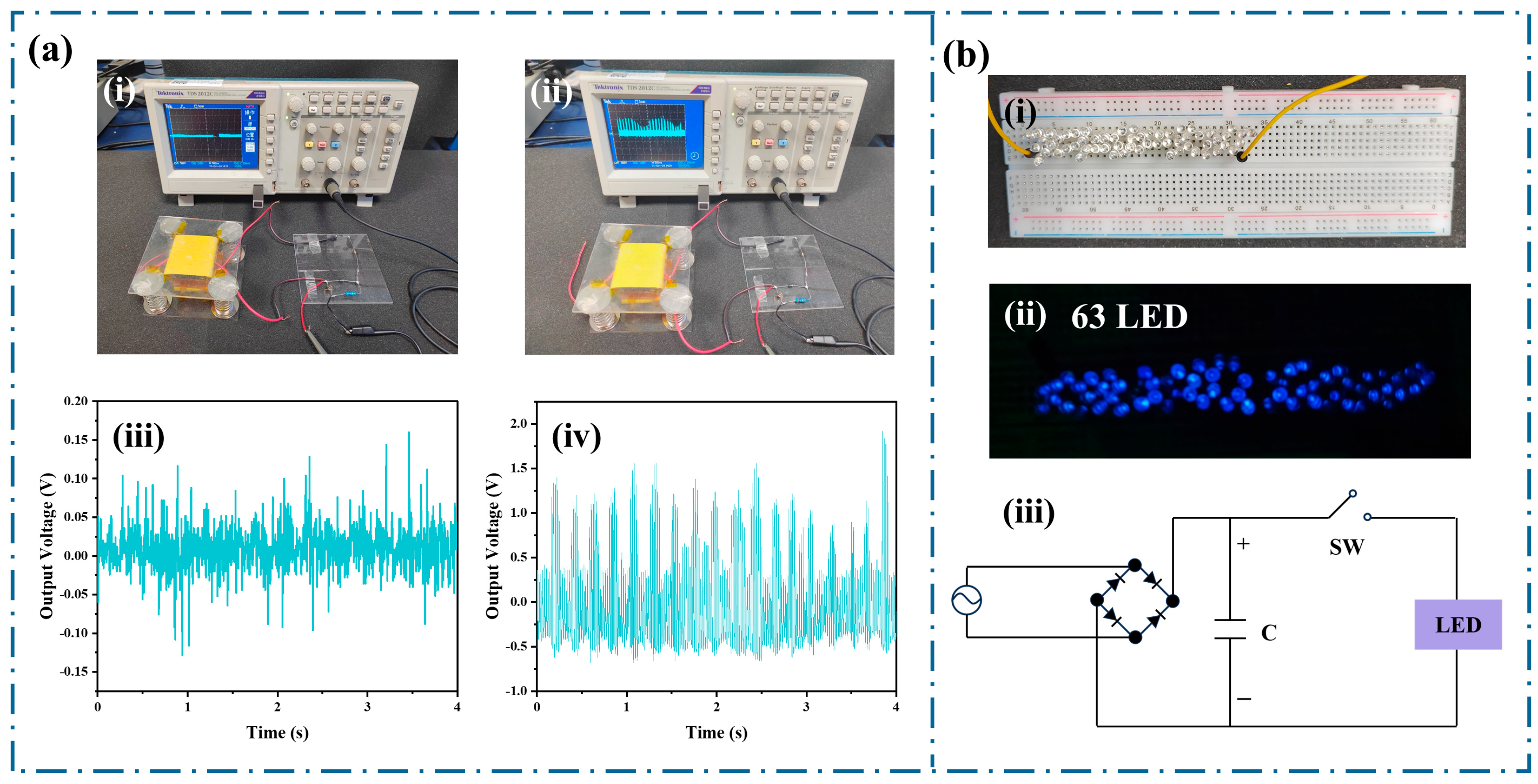
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Z.; Sun, M.; Zhang, X.; Lu, X.; Zeng, Y.; Shang, J.; Zhang, Y.; Zhang, Q.; Xie, K.; Zou, Y. Research on the mapping relationship between dynamic characteristics and electrical output performance of contact-separation triboelectric nanogenerator. Mater. Today Commun. 2025, 43, 111551. [Google Scholar] [CrossRef]
- Li, R.; Lv, S.; Du, Y. An enclosed solid-liquid triboelectric nanogenerator based on Janus-type TPU nanofibers. Mater. Today Commun. 2024, 41, 110262. [Google Scholar] [CrossRef]
- Luo, Q.; Li, Y.; Ji, J.; Xiao, K.; Deng, J.; Zhang, J.; Hu, X.; Hou, Q.; Wang, Y.; Zeng, R.; et al. Enhancing power density in D-mode GaN HEMT direct-current triboelectric nanogenerators through ICP-etched surface engineering. Mater. Today Commun. 2024, 41, 111128. [Google Scholar] [CrossRef]
- Li, X.; Hu, N.; Fan, Q.; Sun, M.; Hu, T.; Ni, Z. High-performance triboelectric nanogenerator based on natural silk fibroin and microstructured polytetrafluoroethylene for self-powered electronics and wearable sensing. Mater. Today Commun. 2024, 38, 108418. [Google Scholar] [CrossRef]
- Sun, J.; Choi, H.; Cha, S.; Ahn, D.; Choi, M.; Park, S.; Cho, Y.; Lee, J.; Park, T.-E.; Park, J.-J. Highly Enhanced Triboelectric Performance from Increased Dielectric Constant Induced by Ionic and Interfacial Polarization for Chitosan Based Multi-Modal Sensing System. Adv. Funct. Mater. 2022, 32, 2109139. [Google Scholar] [CrossRef]
- Yu, S.; Xu, X.; Feng, J.; Liu, M.; Hu, K. Chitosan and chitosan coating nanoparticles for the treatment of brain disease. Int. J. Pharm. 2019, 560, 282–293. [Google Scholar] [CrossRef]
- Sivanesan, I.; Gopal, J.; Muthu, M.; Shin, J.; Mari, S.; Oh, J. Green Synthesized Chitosan/Chitosan Nanoforms/Nanocomposites for Drug Delivery Applications. Polymers 2021, 13, 2256. [Google Scholar] [CrossRef] [PubMed]
- Zaiki, Y.; Iskandar, A.; Wong, W. Functionalized chitosan for cancer nano drug delivery. Biotechnol. Adv. 2023, 67, 108200. [Google Scholar] [CrossRef]
- Irmukhametova, G.; Al Azzam, K.M.; Mun, G.A.; Bekbayeva, L.; Dinara, Z.; Yermukhambetova, B.B.; Nechipurenko, S.V.; Efremov, S.A.; Negim, E.-S.; Samy, M. Obtaining and Characterization of Biodegradable Polymer Blends Based on Polyvinyl Alcohol, Starch, and Chitosan. Polymers 2025, 17, 479. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, L.; Zheng, K.; Gao, Q.; Feng, W.; Hu, S.; Yue, M.; Shan, X. Microelectronic printed chitosan/chondroitin sulfate/ZnO flexible and environmentally friendly triboelectric nanogenerator. J. Colloid Interface Sci. 2024, 669, 275–282. [Google Scholar] [CrossRef]
- Hu, N.; Wang, X.; Yang, J.; Liu, M.; Chen, J.; Yu, X.; Zhu, W.; Zhang, M. Waterproof Triboelectric Nanogenerators Based on Ag Nanoparticle/Chitosan Composites for Transmitting Morse Code. ACS Appl. Nano Mater. 2023, 6, 19777–19785. [Google Scholar] [CrossRef]
- Pongampai, S.; Charoonsuk, T.; Pinpru, N.; Pulphol, P.; Vittayakorn, W.; Pakawanit, P.; Vittayakorn, N. Triboelectric-piezoelectric hybrid nanogenerator based on BaTiO3-Nanorods/Chitosan enhanced output performance with self-charge-pumping system. Compos. Part B Eng. 2021, 208, 108602. [Google Scholar] [CrossRef]
- Hu, N.; Wang, X.; Yang, J.; Chen, J.; Yu, X.; Zhu, W.; Zhang, M. Triboelectric nanogenerators based on degradable TiN/chitosan films for monitoring human movement. J. Mater. Sci. Mater. Electron. 2024, 35, 2022. [Google Scholar] [CrossRef]
- Charoonsuk, T.; Supansomboon, S.; Pakawanit, P.; Vittayakorn, W.; Pongampai, S.; Woramongkolchai, S.; Vittayakorn, N. Simple enhanced charge density of chitosan film by the embedded ion method for the flexible triboelectric nanogenerator. Carbohydr. Polym. 2022, 297, 120070. [Google Scholar] [CrossRef]
- Wang, L.; Daoud, W.A. Hybrid conductive hydrogels for washable human motion energy harvester and self-powered temperature-stress dual sensor. Nano Energy 2019, 66, 104080. [Google Scholar] [CrossRef]
- Chaturvedi, E.; Roy, P. Tailoring Ionic Salt in Chitosan-Activated Carbon-Based Triboelectric Nanogenerators for Enhanced Mechanical Energy Harvesting. ACS Appl. Energy Mater. 2024, 7, 9735–9745. [Google Scholar] [CrossRef]
- Kundu, S.; Solomatin, N.; Kauffmann, Y.; Kraytsberg, A.; Ein-Eli, Y. Revealing and excluding the root cause of the electronic conductivity in Mg-ion MgSc2Se4 solid electrolyte. Appl. Mater. Today 2021, 23, 100998. [Google Scholar] [CrossRef]
- Rösch, B.; Gentner, T.X.; Eyselein, J.; Langer, J.; Elsen, H.; Harder, S. Strongly reducing magnesium(0) complexes. Nature 2021, 592, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Ismail, F.; Abdellah, A.; Sudheeshkumar, V.; Rakhsha, A.; Chen, W.; Chen, N.; Higgins, D.C. Atomically Isolated Nickel–Nitrogen–Carbon Electrocatalysts Derived by the Utilization of Mg2+ ions as Spacers in Bimetallic Ni/Mg–Metal–Organic Framework Precursors for Boosting the Electroreduction of CO2. ACS Appl. Energy Mater. 2022, 5, 9408–9417. [Google Scholar] [CrossRef]
- Rauf, S.; Hanif, M.B.; Mushtaq, N.; Tayyab, Z.; Ali, N.; Shah, M.A.K.Y.; Motola, M.; Saleem, A.; Asghar, M.I.; Iqbal, R.; et al. Modulating the Energy Band Structure of the Mg-Doped Sr0.5Pr0.5Fe0.2Mg0.2Ti0.6O3−δ Electrolyte with Boosted Ionic Conductivity and Electrochemical Performance for Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2022, 14, 43067–43084. [Google Scholar] [CrossRef]
- Shaikh, N.S.; Mali, S.S.; Patil, J.V.; Mujawar, A.I.; Shaikh, J.S.; Pathan, S.C.; Praserthdam, S.; Hong, C.K.; Kanjanaboos, P. Mg2+ ion-powered hybrid supercapacitor with β-MnO2 as a cathode and α-Fe2O3 as an anode. J. Energy Storage 2022, 50, 104525. [Google Scholar] [CrossRef]
- Samuels, R.J. Solid state characterization of the structure of chitosan films. J. Polym. Sci. 1981, 19, 1081–1105. [Google Scholar] [CrossRef]
- Wang, B.; Xiong, X.; Ren, H.; Huang, Z. Preparation of MgO nanocrystals and catalytic mechanism on phenol ozonation. RSC Adv. 2017, 7, 43464–43473. [Google Scholar] [CrossRef]
- Cui, L.; Gao, S.; Song, X.; Huang, L.; Dong, H.; Liu, J.; Chen, F.; Yu, S. Preparation and characterization of chitosan membranes. RSC Adv. 2018, 8, 28433–28439. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Gao, S.; Yang, Z.; Li, Y.; Chen, W.; Wu, B.; Wu, W. Engineered and Laser-Processed Chitosan Biopolymers for Sustainable and Biodegradable Triboelectric Power Generation. Adv. Mater. 2018, 30, 1706267. [Google Scholar] [CrossRef]
- Essien, E.R.; Atasie, V.N.; Oyebanji, T.O.; Nwude, D.O. Biomimetic synthesis of magnesium oxide nanoparticles using Chromolaena odorata (L.) leaf extract. Chem. Pap. 2020, 74, 2101–2109. [Google Scholar] [CrossRef]
- Li, S.; Wu, L.; Liu, Q.; Zhu, M.; Li, Z.; Wang, C.; Jiang, X.; Li, J. Uncovering the Dominant Role of an Extended Asymmetric Four-Coordinated Water Network in the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2023, 145, 26711–26719. [Google Scholar] [CrossRef]
- Scatena, L.F.; Brown, M.G.; Richmond, G.L. Water at Hydrophobic Surfaces: Weak Hydrogen Bonding and Strong Orientation Effects. Science 2001, 292, 908–912. [Google Scholar] [CrossRef]
- Fang, Z.; Lou, W.; Zhang, W.; Guan, X.; He, J.; Lin, J. Modulating crystallinity and dielectric constant of chitosan film for triboelectric polarity shift and performance enhancement in triboelectric nanogenerators. Nano Energy 2023, 117, 108923. [Google Scholar] [CrossRef]
- Yin, Q.; Chen, Y.; Zhou, M.; Jiang, X.; Wu, J.; Sun, Y. Synthesis and photophysical properties of deuteration of pirfenidone. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 204, 88–98. [Google Scholar] [CrossRef]
- Hernández, R.B.; Franco, A.P.; Yola, O.R.; López-Delgado, A.; Felcman, J.; Recio, M.A.L.; Mercê, A.L.R. Coordination study of chitosan and Fe3+. J. Mol. Struct. 2008, 877, 89–99. [Google Scholar] [CrossRef]
- Amaral, I.F.; Granja, P.L. Chemical modification of chitosan by phosphorylation: An XPS, FT-IR and SEM study. J. Biomater. Sci. Polym. Ed. 2012, 16, 1575–1593. [Google Scholar] [CrossRef]
- Luo, W.; Liu, K.; Luo, T.; Fu, J.; Zhang, H.; Ma, C.; Chan, T.-S.; Kao, C.-W.; Lin, Z.; Chai, L.; et al. Promoting C–F Bond Activation for Perfluorinated Compounds Decomposition via Atomically Synergistic Lewis and Brønsted Acid Sites. J. Am. Chem. Soc. 2025, 147, 7391–7399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Huang, H.; Wang, X.; Cai, K.; Chen, J.; Xu, Y.; Yu, F.; Nie, S.; Wang, S.; Liu, X. Anti-moisture, anti-bacterial cellulosic triboelectric materials enabled by hydroxyl coordination effect. Nano Energy 2024, 124, 109472. [Google Scholar] [CrossRef]
- Wang, B.; Jiang, K.; Zhang, Z.; Xie, Z.; Fang, T.; Wang, X.; Liu, K.; Chen, Y.; Liu, M.; Jia, Y.; et al. Heterojunction polarization enhancement and shielding for AlGaN-based solar-blind ultraviolet avalanche detectors. Opt. Lett. 2024, 49, 3279–3282. [Google Scholar] [CrossRef]
- Lv, X.; Liang, S.; Sun, F.; Zhang, J.; Li, Q.; Ge, Y.; Li, F.; Jing, B.; Li, L. Study of the rheological properties and solution microstructure of progressively cross-linked aqueous gels. J. Appl. Polym. Sci. 2021, 138, 50964. [Google Scholar] [CrossRef]
- Del Bene, J.E.; Alkorta, I.; Elguero, J. Ab Initio Study of Ternary Complexes X:(HCNH)+:Z with X, Z = NCH, CNH, FH, ClH, and FCl: Diminutive Cooperative Effects on Structures, Binding Energies, and Spin–Spin Coupling Constants Across Hydrogen Bonds. J. Phys. Chem. A 2011, 115, 12677–12687. [Google Scholar] [CrossRef]
- Li, D.; Li, C.; Liu, Y. Effects of solvent polarity and hydrogen bonding on coumarin 500. J. Mol. Liq. 2016, 218, 670–675. [Google Scholar] [CrossRef]
- Torii, H. Extended Nature of the Molecular Dipole of Hydrogen-Bonded Water. J. Phys. Chem. A 2013, 117, 2044–2051. [Google Scholar] [CrossRef]
- Zheng, W.; Li, Z.; Chen, K.; Liu, S.; Chi, Z.; Xu, J.; Zhang, Y. Temperature-Resistant Intrinsic High Dielectric Constant Polyimides: More Flexibility of the Dipoles, Larger Permittivity of the Materials. Molecules 2022, 27, 6337. [Google Scholar] [CrossRef]
- Qian, G.; Hu, M.; Zhang, S.; Wang, M.; Chen, C.; Yao, J. Synthesis of Superheat-Resistant Polyimides with Enhanced Dielectric Constant by Introduction of Cu(ΙΙ)-Coordination. Polymers 2020, 12, 442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.; Shao, Y.; Luo, C.; Ma, H.-Z.; Yu, H.; Liu, X.; Yin, B.; Wu, J.-L.; Yang, M.-B. Preparation of a high-performance chitosan-based triboelectric nanogenerator by regulating the surface microstructure and dielectric constant. J. Mater. Chem. C 2023, 11, 260–268. [Google Scholar] [CrossRef]
- Maamoun, A.A.; Esawi, A.M.K.; Mahmoud, A.A.; Naeim, D.M.; Arafa, M. Waste-to-energy: Repurposing flexible polyurethane waste for triboelectric nanogenerator applications. Appl. Energy 2025, 377, 124579. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, Z.; Cui, X.; Zeng, P.; Li, F.; Liu, X.; Feng, G.; Liu, M. Construction of Charge Transport Channels at the NiOx/Perovskite Interface through Moderate Dipoles toward Highly Efficient Inverted Solar Cells. ACS Appl. Mater. Interfaces 2022, 14, 13431–13439. [Google Scholar] [CrossRef] [PubMed]
- Sriphan, S.; Vittayakorn, N. Facile roughness fabrications and their roughness effects on electrical outputs of the triboelectric nanogenerator. Smart Mater. Struct. 2018, 27, 105026. [Google Scholar] [CrossRef]
- Kim, J.-N.; Lee, J.; Go, T.W.; Rajabi-Abhari, A.; Mahato, M.; Park, J.Y.; Lee, H. Skin-attachable and biofriendly chitosan-diatom triboelectric nanogenerator. Nano Energy 2020, 75, 104904. [Google Scholar] [CrossRef]
- Panda, S.; Hajra, S.; Kim, H.-G.; Achary, P.G.R.; Pakawanit, P.; Yang, Y.; Mishra, Y.K.; Kim, H.J. Sustainable Solutions for Oral Health Monitoring: Biowaste-Derived Triboelectric Nanogenerator. ACS Appl. Mater. Interfaces 2023, 15, 36096–36106. [Google Scholar] [CrossRef]
- Cheedarala, R.K.; Song, J.I. Moderately Transparent Chitosan-PVA Blended Membrane for Strong Mechanical Stiffness and as a Robust Bio-Material Energy Harvester Through Contact-Separation Mode TENG. Front. Nanotechnol. 2021, 3, 667453. [Google Scholar] [CrossRef]

| Main Material | Friction Material | S (cm) | Triboelectric Property | Test Conditions | |||
|---|---|---|---|---|---|---|---|
| VOC | ISC | ||||||
| chitosan-diatom [46] | FEP | 3 × 4 | 150 V | ↓ | 1.0 μA | ↑ | 8 N |
| CS/AgNWs [42] | PVDF | 2 × 2 | 47.9 V | ↑ | 4.1 mA | ↓ | 3 Hz |
| Chitosan/chondroitin Sulfate/ZnO [10] | FEP | 2.5 × 4 | 105 V | ↑ | 3.3 μA | ↑ | 30 N, 1 Hz |
| CS/PVA [47] | Rice paper | 2.5 × 2.5 | 20 V | ↑ | 200 nA | ↑ | 5 N 1 Hz |
| CS/PVA [48] | PVDF | 3 × 3 | 20.8 V | ↑ | 878 nA | ↑ | 5 Hz |
| Mg2+/CS (our work) | FEP | 2.5 × 3.5 | 113.1 V | 3.7 μA | 10 N | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Wang, L.; Zheng, K.; Hu, S.; Zhang, X.; Mu, Z. Coordination of Mg2+ with Chitosan for Enhanced Triboelectric Performance. Polymers 2025, 17, 1001. https://doi.org/10.3390/polym17081001
He J, Wang L, Zheng K, Hu S, Zhang X, Mu Z. Coordination of Mg2+ with Chitosan for Enhanced Triboelectric Performance. Polymers. 2025; 17(8):1001. https://doi.org/10.3390/polym17081001
Chicago/Turabian StyleHe, Jingjia, Lili Wang, Kaiyuan Zheng, Shoukang Hu, Xueke Zhang, and Ziyu Mu. 2025. "Coordination of Mg2+ with Chitosan for Enhanced Triboelectric Performance" Polymers 17, no. 8: 1001. https://doi.org/10.3390/polym17081001
APA StyleHe, J., Wang, L., Zheng, K., Hu, S., Zhang, X., & Mu, Z. (2025). Coordination of Mg2+ with Chitosan for Enhanced Triboelectric Performance. Polymers, 17(8), 1001. https://doi.org/10.3390/polym17081001






