High Thermoelectric Performance of Flexible and Free-Standing Composite Films Enabled by 3D Inorganic Ag2Se Conductive Networks Filled with Organic PVDF
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
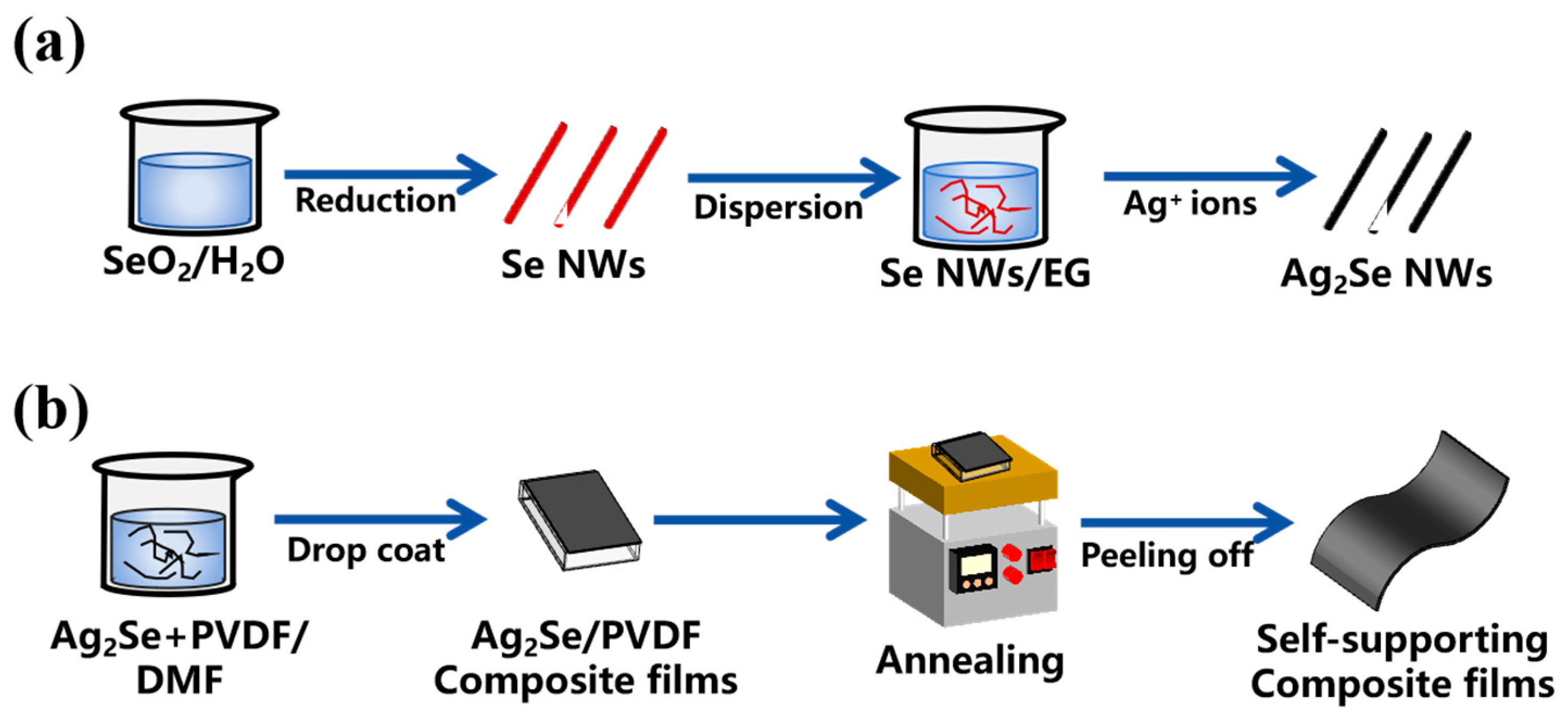
2.2. Synthesis of Ag2Se NWs
2.3. Fabrication of Ag2Se/PVDF Free-Standing Flexible TE Films
2.4. Materials Characterization
2.5. Thermoelectric Property and Flexibility Testing
2.6. Performance Evaluation of the Flexible Thermoelectric Device (FTD)
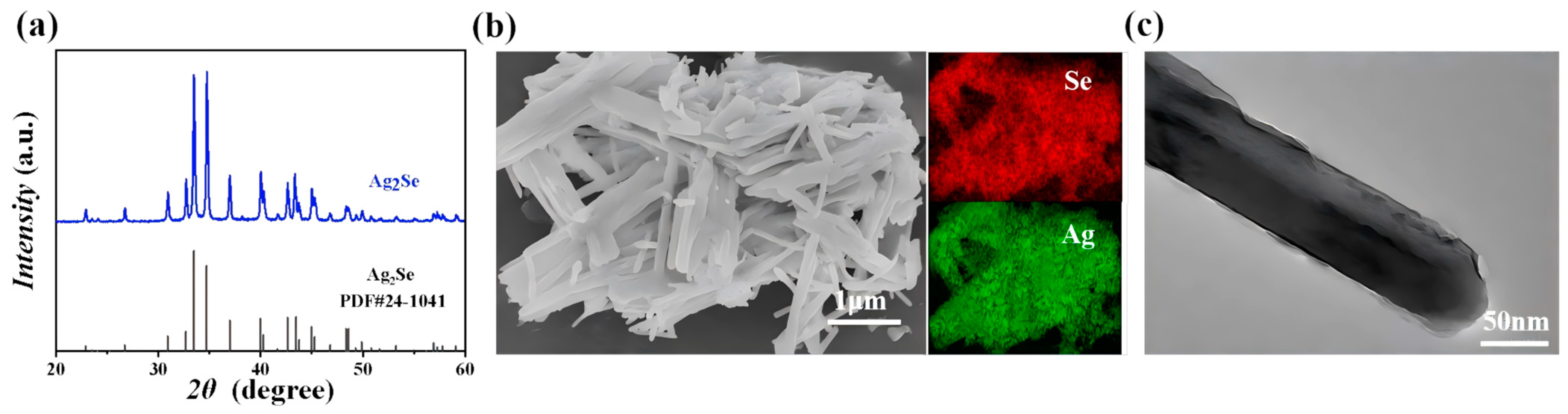
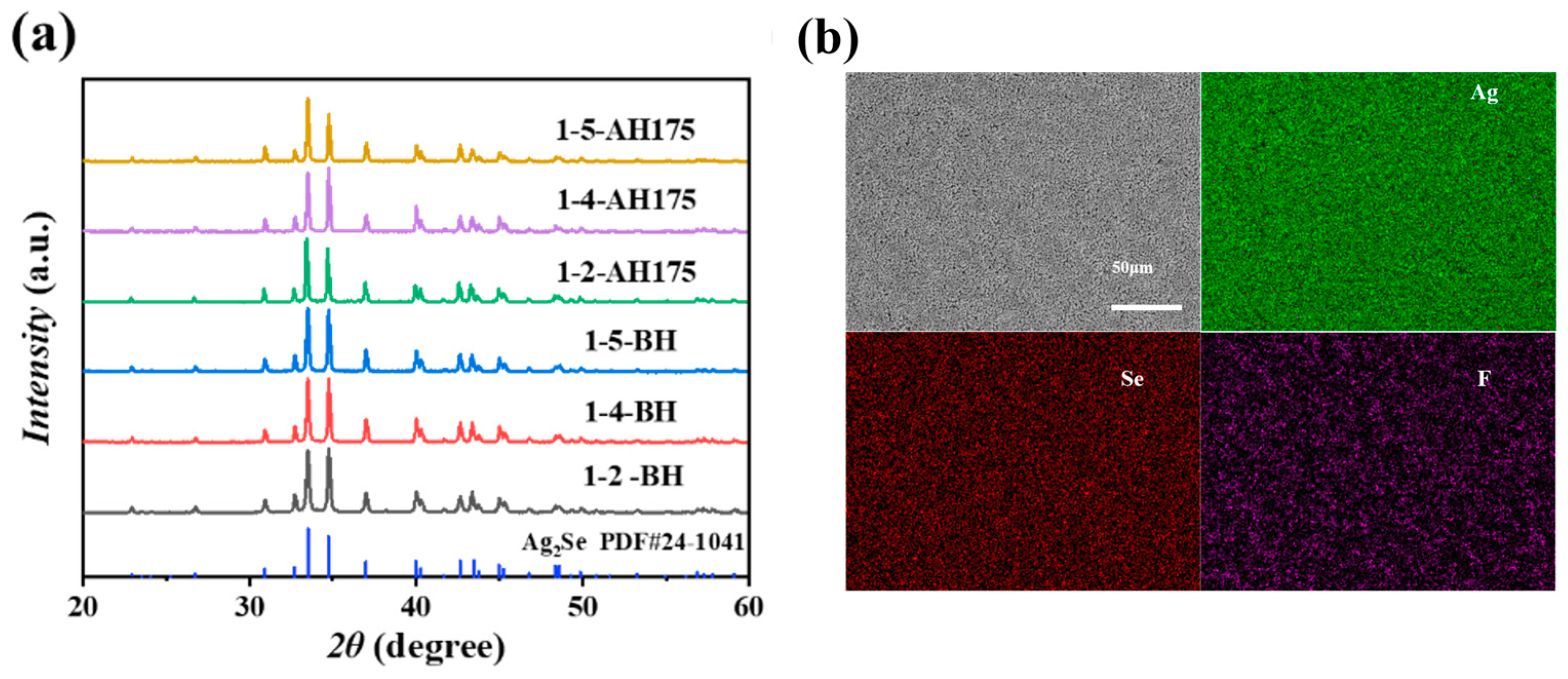
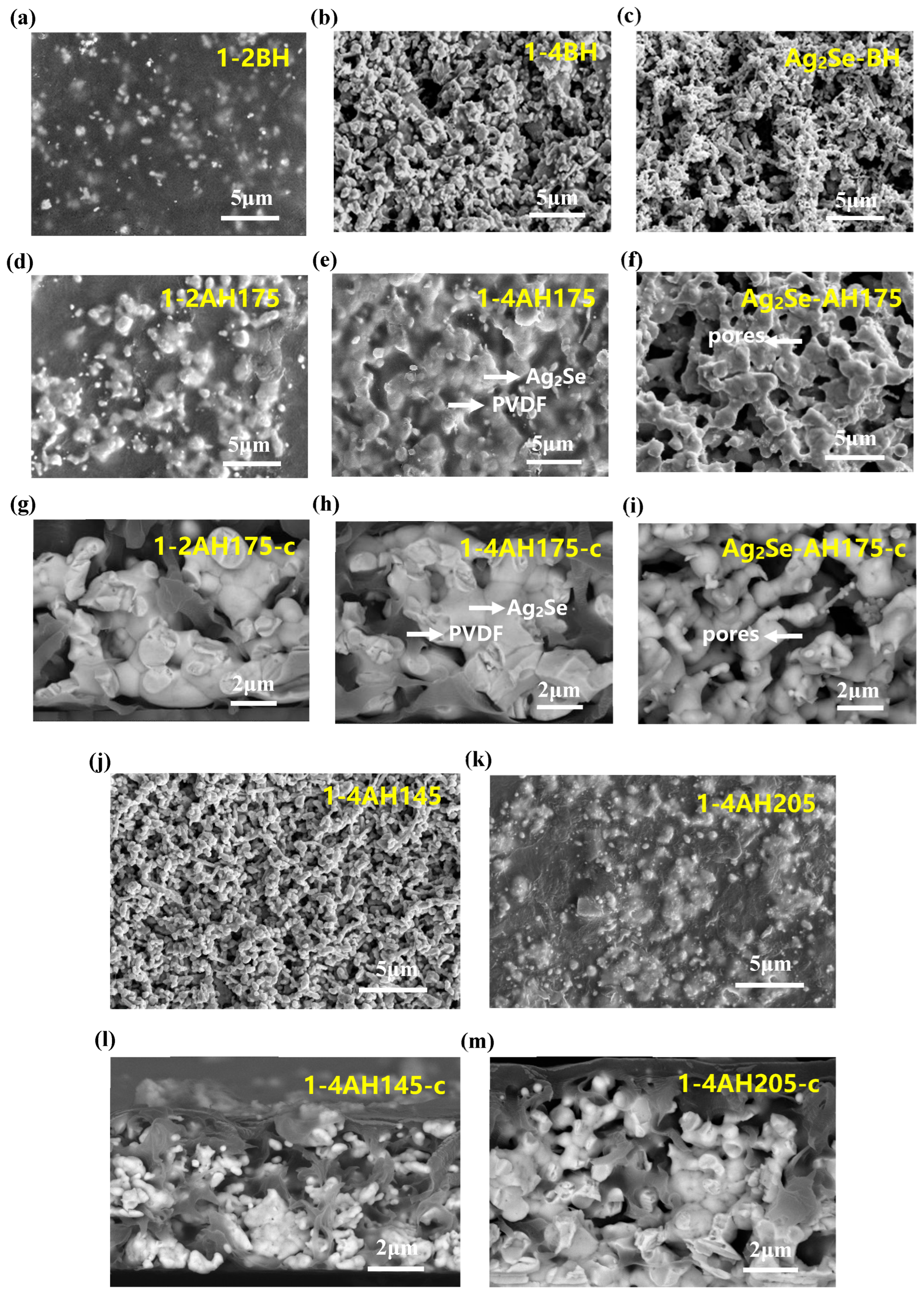
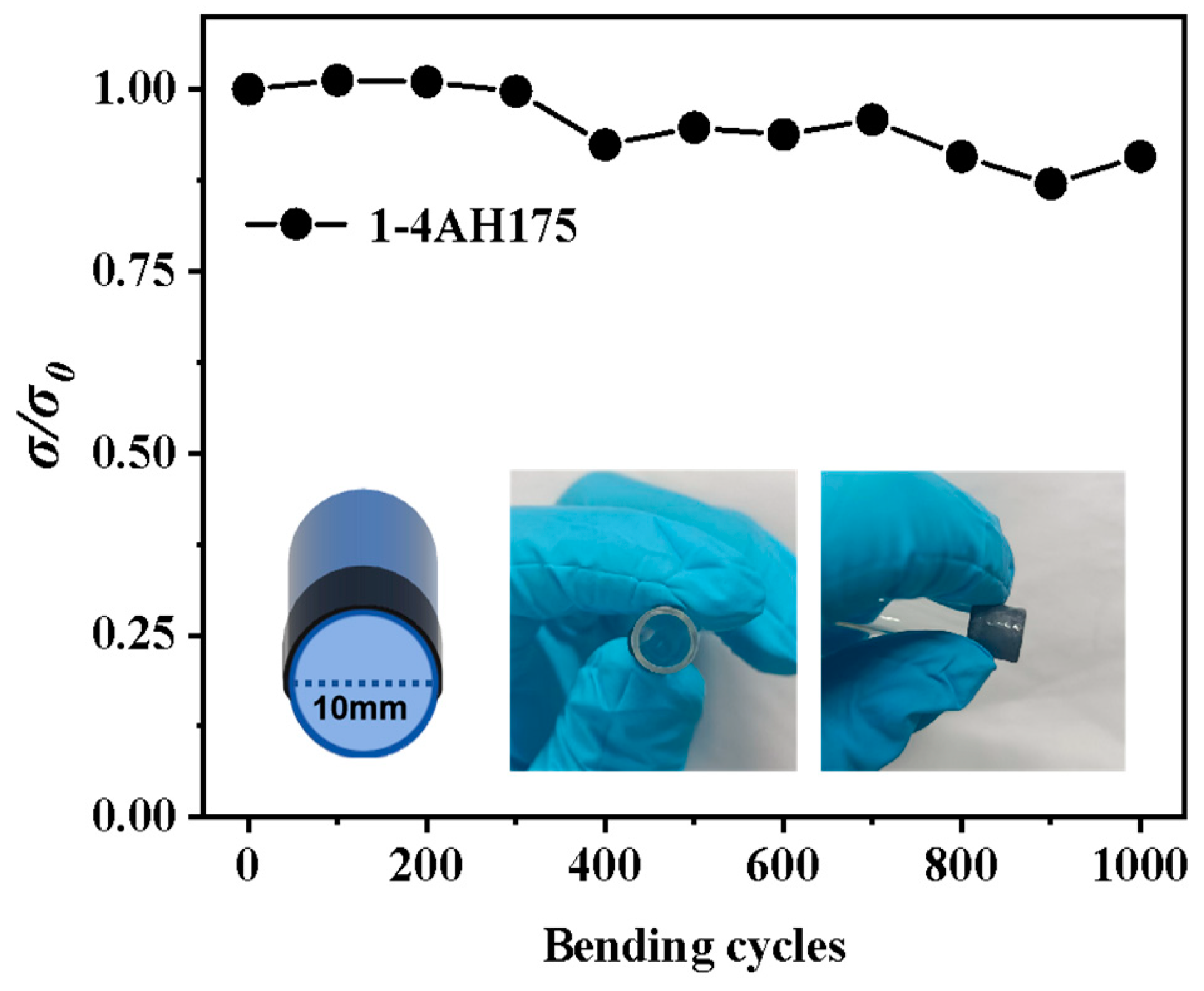
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mao, J.; Liu, Z.; Zhou, J.; Zhu, H.; Zhang, Q.; Chen, G.; Ren, Z. Advances in thermoelectrics. Adv. Phys. 2018, 67, 69–147. [Google Scholar] [CrossRef]
- Liang, J.; Liu, J.; Qiu, P.; Ming, C.; Zhou, Z.; Gao, Z.; Zhao, K.; Chen, L.; Shi, X. Modulation of the morphotropic phase boundary for high-performance ductile thermoelectric materials. Nat. Commun. 2023, 14, 8442. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, S.; Qiu, P.; Peng, L.; Wei, T.-R.; Zhang, Z.; Shi, X.; Chen, L. Flexible thermoelectrics based on ductile semiconductors. Science 2022, 377, 854–858. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-L.; Wang, L.; Lyu, W.; Cao, T.; Chen, W.; Hu, B.; Chen, Z.-G. Advancing flexible thermoelectrics for integrated electronics. Chem. Soc. Rev. 2024, 53, 9254–9305. [Google Scholar] [CrossRef] [PubMed]
- Beretta, D.; Neophytou, N.; Hodges, J.M.; Kanatzidis, M.G.; Narducci, D.; Martin-Gonzalez, M.; Beekman, M.; Balke, B.; Cerretti, G.; Tremel, W.; et al. Thermoelectrics: From history, a window to the future. Mater. Sci. Eng. R Rep. 2019, 138, 100501. [Google Scholar] [CrossRef]
- Tian, Y.; Ren, G.-K.; Wei, Z.; Zheng, Z.; Deng, S.; Ma, L.; Li, Y.; Zhou, Z.; Chen, X.; Shi, Y.; et al. Advances of thermoelectric power generation for room temperature: Applications, devices, materials and beyond. Renew. Energy 2024, 226, 120443. [Google Scholar] [CrossRef]
- Yan, Q.; Kanatzidis, M.G. High-performance thermoelectrics and challenges for practical devices. Nat. Mater. 2022, 21, 503–513. [Google Scholar] [CrossRef]
- Abbasi, M.S.; Sultana, R.; Ahmed, I.; Adnan, M.; Shah, U.A.; Irshad, M.S.; Vu, H.N.; Do, L.T.; Thi Vu, H.H.; Pham, T.-D.; et al. Contemporary advances in organic thermoelectric materials: Fundamentals, properties, optimization strategies, and applications. Renew. Sustain. Energy Rev. 2024, 200, 114579. [Google Scholar] [CrossRef]
- Deng, L.; Liu, Y.; Zhang, Y.; Wang, S.; Gao, P. Organic Thermoelectric Materials: Niche Harvester of Thermal Energy. Adv. Funct. Mater. 2023, 33, 2210770. [Google Scholar] [CrossRef]
- Prunet, G.; Pawula, F.; Fleury, G.; Cloutet, E.; Robinson, A.J.; Hadziioannou, G.; Pakdel, A. A review on conductive polymers and their hybrids for flexible and wearable thermoelectric applications. Mater. Today Phys. 2021, 18, 100402. [Google Scholar] [CrossRef]
- Gharahcheshmeh, M.H.; Dautel, B.; Chowdhury, K. Enhanced Carrier Mobility and Thermoelectric Performance by Nanostructure Engineering of PEDOT Thin Films Fabricated via the OCVD Method Using SbCl5 Oxidant. Adv. Funct. Mater. 2025, 35, 2418331. [Google Scholar] [CrossRef]
- Bao, Y.; Sun, Y.; Jiao, F.; Hu, W. Recent Advances in Multicomponent Organic Composite Thermoelectric Materials. Adv. Electron. Mater. 2023, 9, 2201310. [Google Scholar] [CrossRef]
- Jin, H.; Li, J.; Iocozzia, J.; Zeng, X.; Wei, P.-C.; Yang, C.; Li, N.; Liu, Z.; He, J.H.; Zhu, T.; et al. Hybrid Organic–Inorganic Thermoelectric Materials and Devices. Angew. Chem. Int. Ed. 2019, 58, 15206–15226. [Google Scholar] [CrossRef]
- Lin, M.-H.; Hong, S.-H.; Ding, J.-F.; Liu, C.-L. Organic Porous Materials and Their Nanohybrids for Next-Generation Thermoelectric Application. ACS Appl. Mater. Interfaces 2024, 16, 67116–67133. [Google Scholar] [CrossRef]
- Soleimani, Z.; Zoras, S.; Ceranic, B.; Shahzad, S.; Cui, Y. A review on recent developments of thermoelectric materials for room-temperature applications. Sustain. Energy Technol. Assess. 2020, 37, 100604. [Google Scholar]
- Wang, J.; Liu, L.; Wu, F.; Liu, Z.; Fan, Z.; Chen, L.; Chen, Y. Recent Developments of n-Type Organic Thermoelectric Materials: Influence of Structure Modification on Molecule Arrangement and Solution Processing. ChemSusChem 2022, 15, e202102420. [Google Scholar]
- Wu, H.; Shi, X.-l.; Duan, J.; Liu, Q.; Chen, Z.-G. Advances in Ag2Se-based thermoelectrics from materials to applications. Energy Environ. Sci. 2023, 16, 1870–1906. [Google Scholar] [CrossRef]
- Wei, T.-R.; Qiu, P.; Zhao, K.; Shi, X.; Chen, L. Ag2Q-Based (Q = S, Se, Te) Silver Chalcogenide Thermoelectric Materials. Adv. Mater. 2023, 35, 2110236. [Google Scholar]
- Perez-Taborda, J.A.; Caballero-Calero, O.; Vera-Londono, L.; Briones, F.; Martin-Gonzalez, M. High Thermoelectric zT in n-Type Silver Selenide films at Room Temperature. Adv. Energy Mater. 2018, 8, 1702024. [Google Scholar]
- Luo, Y.; Hou, S.; Liu, Y.; Sun, X.; Tang, Z.; Yu, F.; Mao, J.; Zhang, Q.; Cao, F. Enhanced Thermoelectric Performance in Flexible Sulfur-Alloyed Ag2Se Thin Films. ACS Appl. Mater. Interfaces 2024, 16, 36620–36627. [Google Scholar] [CrossRef]
- Hu, Q.-X.; Liu, W.-D.; Zhang, L.; Gao, H.; Wang, D.-Z.; Wu, T.; Shi, X.-L.; Li, M.; Liu, Q.-F.; Yang, Y.-L.; et al. Carrier Separation Boosts Thermoelectric Performance of Flexible n-Type Ag2Se-Based Films. Adv. Energy Mater. 2024, 14, 2401890. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Wang, Z.; Wei, P.; Zhao, W.; Chen, L.; Cai, K. High thermoelectric performance of flexible Ag/Ag2Se composite film on nylon for low-grade energy harvesting. J. Mater. Sci. Technol. 2024, 179, 79–85. [Google Scholar] [CrossRef]
- Ding, Y.; Qiu, Y.; Cai, K.; Yao, Q.; Chen, S.; Chen, L.; He, J. High performance n-type Ag2Se film on nylon membrane for flexible thermoelectric power generator. Nat. Commun. 2019, 10, 841. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Zhang, X.; Guan, L.; Jin, S.; Jia, J.; Wen, J.; Zeng, W. In situ Synthesized Staggered-Layer-Boosted Flexible Ag2Se and Cu2Se Thin Films for Wearable Thermoelectric Power Generators. Adv. Funct. Mater. 2024, 2419392. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Li, J.; Wu, C.; Liu, Y.; Wei, P.; Zhao, W.; Cai, K. Screen printing Ag2Se/carbon nanocomposite films for flexible thermoelectric applications. Carbon 2024, 229, 119480. [Google Scholar] [CrossRef]
- Aghayari, S. PVDF composite nanofibers applications. Heliyon 2022, 8, e11620. [Google Scholar] [CrossRef]
- Park, D.; Kim, M.; Kim, J. High-performance PANI-coated Ag2Se nanowire and PVDF thermoelectric composite film for flexible energy harvesting. J. Alloys Compd. 2021, 884, 161098. [Google Scholar] [CrossRef]
- Xu, Q.; Qu, S.; Ming, C.; Qiu, P.; Yao, Q.; Zhu, C.; Wei, T.-R.; He, J.; Shi, X.; Chen, L. Conformal organic–inorganic semiconductor composites for flexible thermoelectrics. Energy Environ. Sci. 2020, 13, 511–518. [Google Scholar] [CrossRef]
- Dun, C.; Hewitt, C.A.; Huang, H.; Xu, J.; Zhou, C.; Huang, W.; Cui, Y.; Zhou, W.; Jiang, Q.; Carroll, D.L. Flexible n-type thermoelectric films based on Cu-doped Bi2Se3 nanoplate and Polyvinylidene Fluoride composite with decoupled Seebeck coefficient and electrical conductivity. Nano Energy 2015, 18, 306–314. [Google Scholar] [CrossRef]
- Chen, Y.; He, M.; Liu, B.; Bazan, G.C.; Zhou, J.; Liang, Z. Bendable n-Type Metallic Nanocomposites with Large Thermoelectric Power Factor. Adv. Mater. 2017, 29, 1604752. [Google Scholar] [CrossRef]
- Park, D.; Ju, H.; Kim, J. Enhanced thermoelectric properties of flexible N-type Ag2Se nanowire/polyvinylidene fluoride composite films synthesized via solution mixing. J. Ind. Eng. Chem. 2021, 93, 333–338. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Z.; Sun, C.; Deng, H.; Fu, Q. Biomimetic Approach to Facilitate the High Filler Content in Free-Standing and Flexible Thermoelectric Polymer Composite Films Based on PVDF and Ag2Se Nanowires. ACS Appl. Mater. Interfaces 2020, 12, 51506–51516. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Ming, C.; Qiu, P.; Xu, K.; Xu, Q.; Yao, Q.; Lu, P.; Zeng, H.; Shi, X.; Chen, L. High-performance n-type Ta4SiTe4/polyvinylidene fluoride (PVDF)/graphdiyne organic–inorganic flexible thermoelectric composites. Energy Environ. Sci. 2021, 14, 6586–6594. [Google Scholar] [CrossRef]
- Lu, Y.; Qiu, Y.; Cai, K.; Li, X.; Gao, M.; Jiang, C.; He, J. Ultrahigh performance PEDOT/Ag2Se/CuAgSe composite film for wearable thermoelectric power generators. Mater. Today Phys. 2020, 14, 100223. [Google Scholar] [CrossRef]
- Gelbstein, Y. Thermoelectric power and structural properties in two-phase Sn/SnTe alloys. J. Appl. Phys. 2009, 105, 023713. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, Y.; Yan, Z.; Zhang, Z.; Zhang, Y.; Shi, P.; Xue, C. Screen-Printed Flexible Thermoelectric Device Based on Hybrid Silver Selenide/PVP Composite Films. Nanomaterials 2021, 11, 2042. [Google Scholar] [CrossRef]
- Chen, Z.-P.; Gao, C.-Y.; Li, Y.; Li, H.-P.; Fan, X.-H.; Yang, L.-M. Investigation into thermoelectric performance of a flexible n-type nanocomposite film from the AgxSe nanotube composited with PPy. J. Alloys Compd. 2024, 988, 174297. [Google Scholar] [CrossRef]
- Zhou, C.; Dun, C.; Ge, B.; Wang, K.; Shi, Z.; Liu, G.; Carroll, D.L.; Qiao, G. Highly robust and flexible n-type thermoelectric film based on Ag2Te nanoshuttle/polyvinylidene fluoride hybrids. Nanoscale 2018, 10, 14830–14834. [Google Scholar] [CrossRef]
- Hewitt, C.A.; Kaiser, A.B.; Craps, M.; Czerw, R.; Roth, S.; Carroll, D.L. Temperature dependent thermoelectric properties of freestanding few layer graphene/polyvinylidene fluoride composite thin films. Synth. Met. 2013, 165, 56–59. [Google Scholar] [CrossRef]
- Zhou, C.; Dun, C.; Wang, Q.; Wang, K.; Shi, Z.; Carroll, D.L.; Liu, G.; Qiao, G. Nanowires as Building Blocks to Fabricate Flexible Thermoelectric Fabric: The Case of Copper Telluride Nanowires. ACS Appl. Mater. Interfaces 2015, 7, 21015–21020. [Google Scholar]
- Palaporn, D.; Mongkolthanaruk, W.; Faungnawakij, K.; Kurosaki, K.; Pinitsoontorn, S. Flexible Thermoelectric Paper and Its Thermoelectric Generator from Bacterial Cellulose/Ag2Se Nanocomposites. ACS Appl. Energy Mater. 2022, 5, 3489–3501. [Google Scholar]
- Shin, D.E.; Alluri, N.R.; Park, K.-I. Flexible Thermoelectric Energy Harvester with Stacked Structure of Thermoelectric Composite Films Made of PVDF and Bi2Te3-Based Particles. ACS Appl. Energy Mater. 2024, 7, 8288–8293. [Google Scholar]
- Niu, J.; Chen, T.; Liang, G.; Ma, H.; Zhang, X.; Fan, P.; Zheng, Z. In-situ growth of high room temperature thermoelectric performance Ag2Se thin films. Mater. Lett. 2022, 312, 131662. [Google Scholar] [CrossRef]
- Zang, J.; Ma, Y.; Zhao, Y.; Guo, R.; Liu, Y.; Liu, D.; Xue, C. Effect of post-annealing treatment on the thermoelectric properties of Ag2Se flexible thin film prepared by magnetron sputtering method. Results Phys. 2023, 45, 106222. [Google Scholar] [CrossRef]
- Jindal, S.; Singh, S.; Saini, G.S.S.; Tripathi, S.K. Optimization of thermoelectric power factor of (013)-oriented Ag2Se films via thermal annealing. Mater. Res. Bull. 2022, 145, 111525. [Google Scholar]


| Films | S (μV K−1) | σ (S cm−1) | PF (μW m−1 K−2) | Pd (W m−2) | Refs. |
|---|---|---|---|---|---|
| PVP/Ag2Se | −58.5 | ~ | 4.3 (390 K) | 0.08 (40 K) | [36] |
| PPy/Ag2Se | ~ | 590.9 | 282.8 | ~ | [37] |
| PVDF/PANI-coated Ag2Se | ~ | ~ | 196.6 | 2.33 (30 K) | [27] |
| PVDF/Ag2Se | −95.9 | 205.52 | 189.02 | 0.0098 (30 K) | [32] |
| PVDF/Ag2Se | ~ | ~ | 180.6 (400 K) | ~ | [31] |
| Ni/PVDF | −20.6 | ~ | 200 | ~ | [30] |
| Ag2Te/PVDF | −60 | 86 | 30.9 | ~ | [38] |
| Cu-doped Bi2Se3/PVDF | −84 | 146 | 103 | ~ | [29] |
| TaSiTe4/GDY/PVDF | ~ | 113 | 489 | 6 (35 K) | [33] |
| FLG/PVDF | 18.3 | 20.05 | 0.52 | ~ | [39] |
| Cu1.75Te NWs/PVDF | 9.6 | 2490 | 23 | ~ | [40] |
| BC/Ag2Se | −171 | 132 | 386 | 0.0047 (25 K) | [41] |
| Bi0.5Sb1.5Te3 (BST)/PVDF Bi0.5Te2.7Se0.3 (BTS)/PVDF | 112 −96 | 0.74 0.50 | 0.94 0.47 | 0.00025 (20 K) | [42] |
| This work | −138.4 | 255.2 | 488.8 | 1.75 (30 K) 3.19 (40 K) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Hu, Y.; Hu, Y.; Xiao, X.; Yao, Q. High Thermoelectric Performance of Flexible and Free-Standing Composite Films Enabled by 3D Inorganic Ag2Se Conductive Networks Filled with Organic PVDF. Polymers 2025, 17, 972. https://doi.org/10.3390/polym17070972
Xu Z, Hu Y, Hu Y, Xiao X, Yao Q. High Thermoelectric Performance of Flexible and Free-Standing Composite Films Enabled by 3D Inorganic Ag2Se Conductive Networks Filled with Organic PVDF. Polymers. 2025; 17(7):972. https://doi.org/10.3390/polym17070972
Chicago/Turabian StyleXu, Zishuo, Yuejuan Hu, Yuchen Hu, Xianfeng Xiao, and Qin Yao. 2025. "High Thermoelectric Performance of Flexible and Free-Standing Composite Films Enabled by 3D Inorganic Ag2Se Conductive Networks Filled with Organic PVDF" Polymers 17, no. 7: 972. https://doi.org/10.3390/polym17070972
APA StyleXu, Z., Hu, Y., Hu, Y., Xiao, X., & Yao, Q. (2025). High Thermoelectric Performance of Flexible and Free-Standing Composite Films Enabled by 3D Inorganic Ag2Se Conductive Networks Filled with Organic PVDF. Polymers, 17(7), 972. https://doi.org/10.3390/polym17070972






