Targeting Injectable Hydrogels: The Role of Diphenylalanine Peptide Derivative in the Gelation Dynamics of Pluronic® F127 †
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
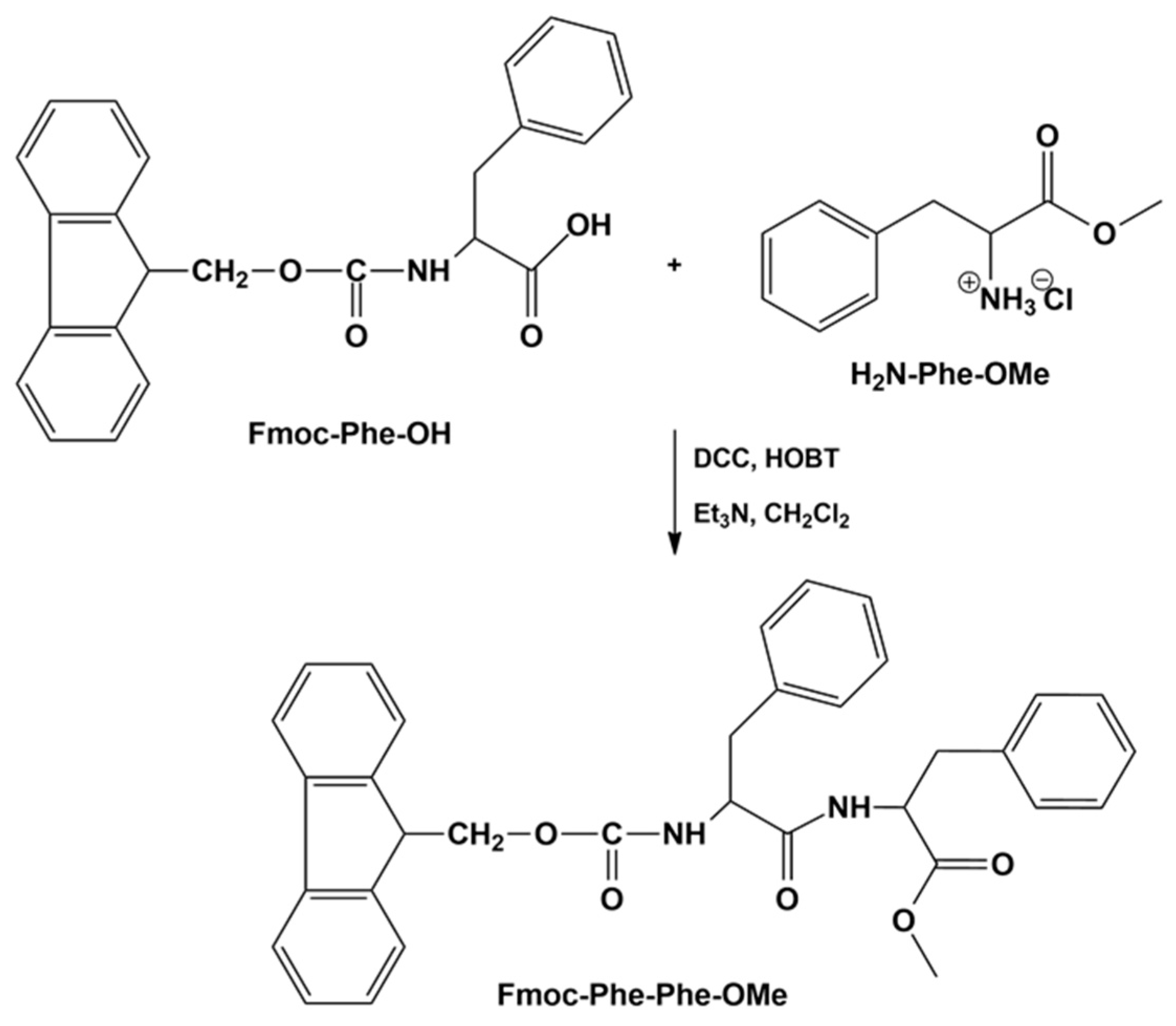
2.2.1. Syntheses of Phenylalanine Methyl Ester and Fmoc-Phenylalanyl-Phenylalanine Methyl Ester
2.2.2. UV–Vis Spectroscopy
2.2.3. Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC)
2.2.4. 1H and 13C-NMR Spectroscopy
2.2.5. MALDI-ToF/ToF Mass Spectrometry
2.2.6. Rheological Measurements
3. Results and Discussion
3.1. Characterization of Phenylalanine Methyl Ester (Phe-Ome)
3.2. Separation of Fmoc-Phenylalanyl-Phenylalanine Methyl Ester (Fmoc-Phe-Phe-Ome)
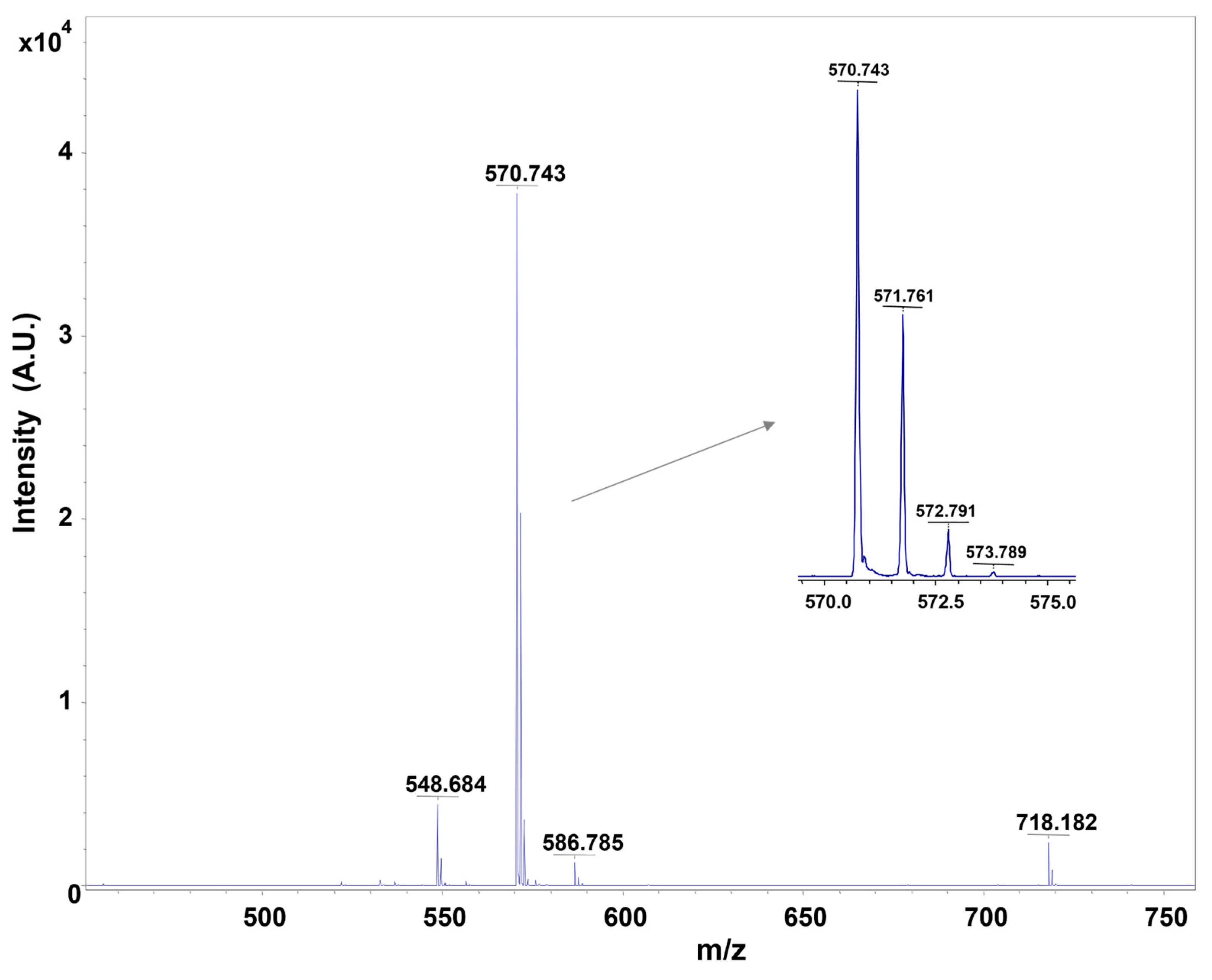
3.3. Characterization of Fmoc-Phe-Phe-Ome by Mass Spectrometry
3.4. Rheological Investigation
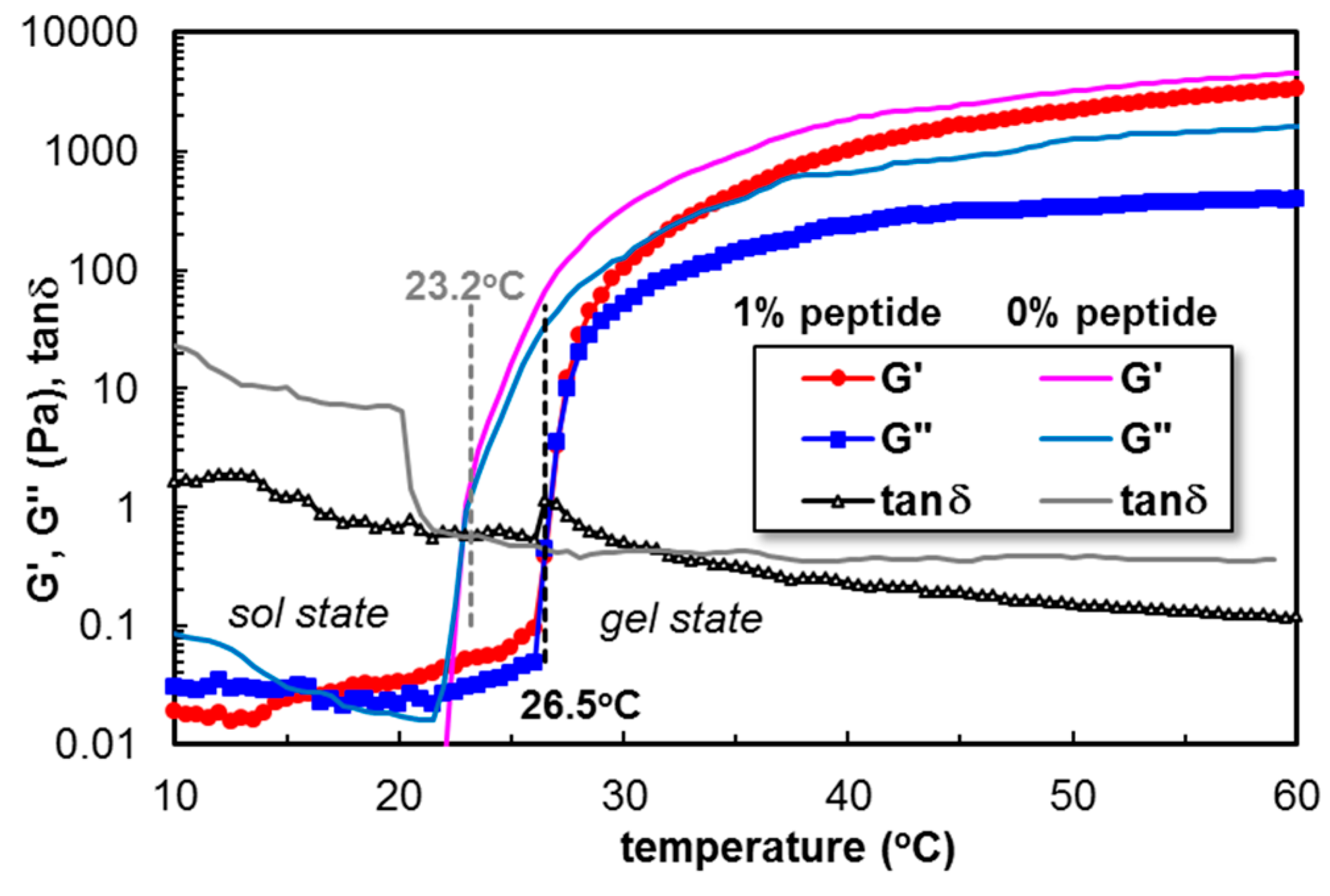
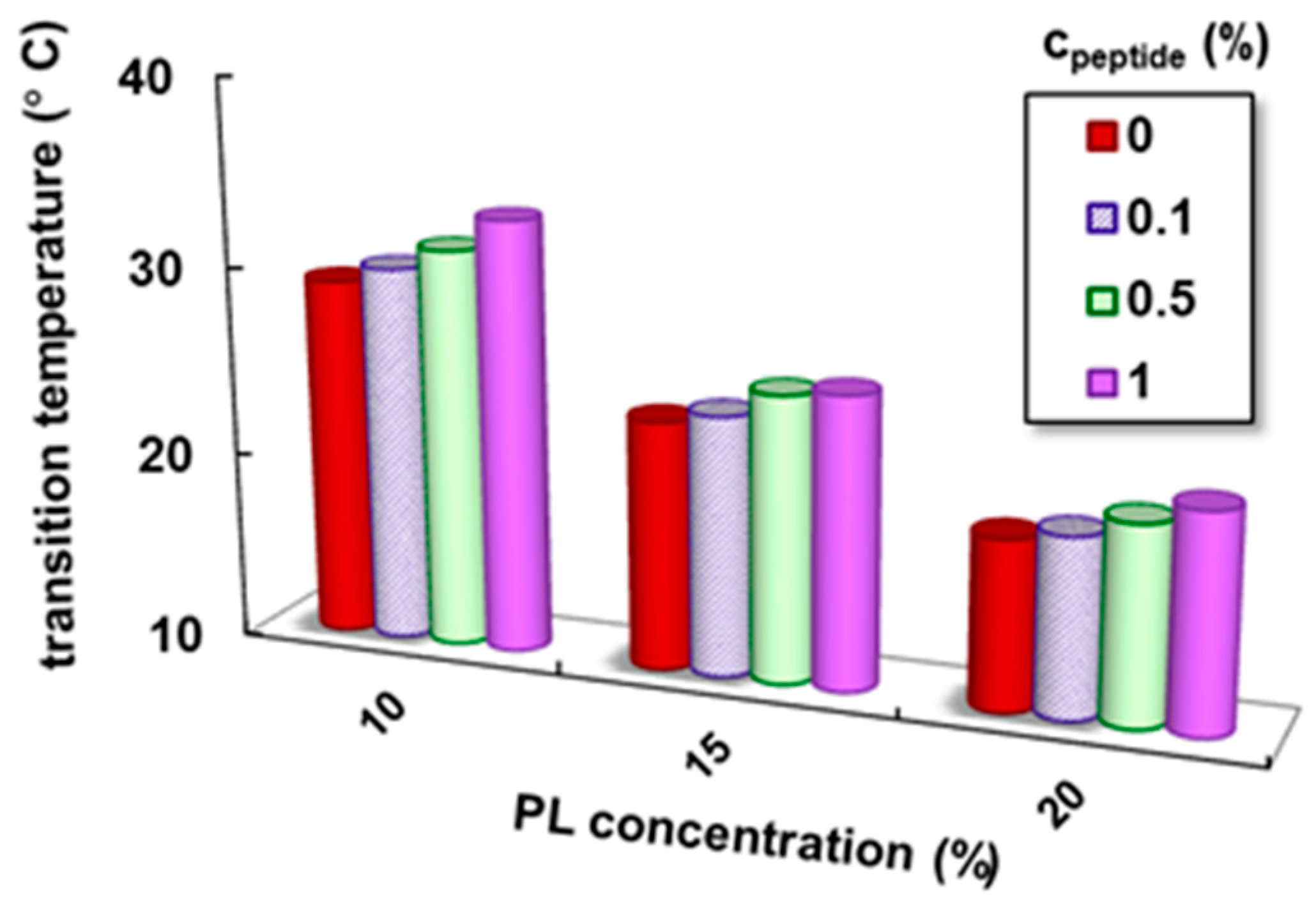
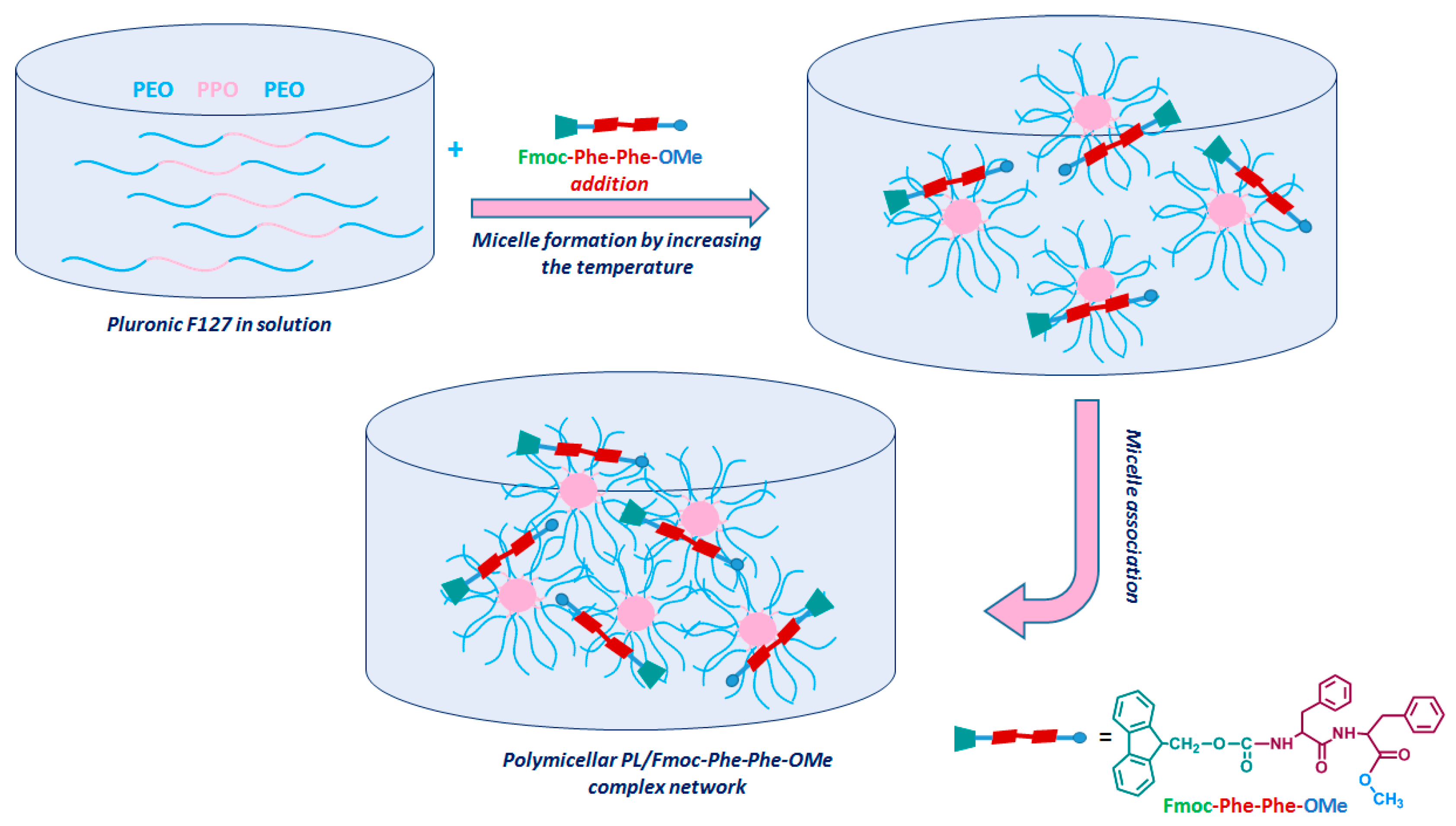
3.4.1. The Influence of Fmoc-Phe-Phe-Ome on the PL Gelation Induced by the Temperature Increase
3.4.2. The Network Formation at Physiological Temperature
3.4.3. The Shear Flow Behavior
3.4.4. Self-Healing Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mu, R.; Zhu, D.; Abdulmalik, S.; Wijekoon, S.; Wei, G.; Kumbar, S.G. Stimuli-responsive peptide assemblies: Design, self-assembly, modulation, and biomedical applications. Bioact. Mater. 2024, 35, 181–207. [Google Scholar] [PubMed]
- Xiang, Y.X.; Liu, C.; Ma, S.A.; Wang, X.T.; Zhu, L.Y.; Bao, C.Y. Stimuli-Responsive Peptide Self-Assembly to Construct Hydrogels with Actuation and Shape Memory Behaviors. Adv. Funct. Mater. 2023, 33, 2300416. [Google Scholar]
- Wang, L.; Gong, C.C.; Yuan, X.Z.; Wei, G. Controlling the self-assembly of biomolecules into functional nanomaterials through internal interactions and external stimulations: A review. Nanomaterials 2019, 9, 285. [Google Scholar] [CrossRef]
- Chronopoulou, L.; Sennato, S.; Bordi, F.; Giannella, D.; Di Nitto, A.; Barbetta, A.; Dentini, M.; Togna, A.R.; Togna, G.I.; Moschinic, S.; et al. Designing unconventional Fmoc-peptide-based biomaterials: Structure and related properties. Soft Matter 2014, 10, 1944–1952. [Google Scholar] [CrossRef]
- Mahler, A.; Reches, M.; Rechter, M.; Cohen, S.; Gazit, E. Rigid, Self-assembled hydrogel composed of a modified aromatic dipeptide. Adv. Mater. 2006, 18, 1365–1370. [Google Scholar]
- Veloso, S.R.S.; Rosa, M.; Diaferia, C.; Fernandes, C. A review on the rheological properties of single amino acids and short dipeptide gels. Gels 2024, 10, 507. [Google Scholar]
- Jerschabek, V.; Fidelis, C.; Kemesies, M.; Volmer, J.; Hashemi-Haeri, H.; Hinderberger, D. Composite hydrogels of phenylalanine dipeptides with trivalent metal cations. ChemRxiv 2025, in press. [CrossRef]
- Diaferia, C.; Rosa, E.; Morelli, G.; Accardo, A. Fmoc-Diphenylalanine hydrogels: Optimization of preparation methods and structural insights. Pharmaceuticals 2022, 15, 1048. [Google Scholar] [CrossRef]
- Celik, E.; Bayram, C.; Akçapınar, R.; Türk, M.; Denkbas, E.B. The effect of calcium chloride concentration on alginate/Fmoc-diphenylalanine hydrogel networks. Mater. Sci. Eng. C 2016, 66, 221–229. [Google Scholar]
- Hassan, M.M.; Martin, A.D.; Thordarson, P. Macromolecular crowding and hydrophobic effects on Fmoc-diphenylalanine hydrogel formation in PEG:water mixtures. J. Mater. Chem. B 2015, 3, 9269–9276. [Google Scholar]
- Gallo, E.; Diaferia, C.; Rosa, E.; Smaldone, G.; Morelli, G.; Accardo, A. Peptide-based hydrogels and nanogels for delivery of doxorubicin. Int. J. Nanomed. 2021, 16, 1617. [Google Scholar] [CrossRef]
- Najafi, H.; Abolmaali, S.S.; Heidari, R.; Valizadeh, H.; Jafari, M.; Tamaddon, A.M.; Azarpira, N. Nitric oxide releasing nanofibrous Fmoc-dipeptide hydrogels for amelioration of renal ischemia/reperfusion injury. J. Control. Release 2021, 337, 1–13. [Google Scholar] [CrossRef]
- Jayawarna, V.; Ali, M.; Jowitt, T.A.; Miller, A.F.; Saiani, A.; Gough, J.E.; Ulijn, R.V. Nanostructured hydrogels for three-dimensional cell culture through self-assembly of fluorenylmethoxycarbonyl–dipeptides. Adv. Mater. 2006, 18, 611–614. [Google Scholar] [CrossRef]
- Ghosh, M.; Halperin-Sternfeld, M.; Grinberg, I.; Adler-Abramovich, L. Injectable alginate-peptide composite hydrogel as a scaffold for bone tissue regeneration. Nanomaterials 2019, 9, 497. [Google Scholar] [CrossRef] [PubMed]
- Field, E.H.; Ratcliffe, J.; Johnson, C.J.; Binger, K.J.; Reynolds, N.P. Self-healing, 3D printed bioinks from self-assembled peptide and alginate hybrid hydrogels. Biomater. Adv. 2025, 169, 214145. [Google Scholar] [CrossRef]
- Yan, X.; He, Q.; Wang, K.; Duan, L.; Cui, Y.; Li, J. Transition of cationic dipeptide nanotubes into vesicles and oligonucleotide delivery. Angew. Chem. Int. Ed. 2007, 46, 2431–2434. [Google Scholar] [CrossRef]
- Han, W.; Yuan, Y.; Li, H.; Fu, Z.; Wang, M.; Guan, S.; Wang, L. Design and anti-tumor activity of self-loaded nanocarriers of siRNA. Colloids Surf. B 2019, 183, 110385. [Google Scholar] [CrossRef]
- Han, W.; Meng, F.; Gan, H.; Guo, F.; Ke, J.; Wang, L. Targeting self-assembled F127-peptide polymer with pH sensitivity for release of anticancer drugs. RSC Adv. 2021, 11, 1461–1471. [Google Scholar] [CrossRef]
- Gallo, E.; Diaferia, C.; Smaldone, G.; Rosa, E.; Pecoraro, G.; Morelli, G.; Accardo, A. Fmoc-FF hydrogels and nanogels for improved and selective delivery of dexamethasone in leukemic cells and diagnostic applications. Sci. Rep. 2024, 14, 9940. [Google Scholar] [CrossRef]
- Hopkins, C.C.; de Bruyn, J.R. Gelation and long-time relaxation of aqueous solutions of Pluronic F127. J. Rheol. 2019, 63, 191–201. [Google Scholar] [CrossRef]
- Jalaal, M.; Cottrell, G.; Balmforth, N.; Stoeber, B. On the rheology of Pluronic F127 aqueous solutions. J. Rheol. 2017, 61, 139–146. [Google Scholar]
- Suman, K.; Sourav, S.; Josh, Y.M. Rheological signatures of gel–glass transition and a revised phase diagram of an aqueous triblock copolymer solution of Pluronic F127. Phys. Fluids 2021, 33, 073610. [Google Scholar]
- Lupu, A.; Bercea, M.; Avadanei, M.; Gradinaru, L.M.; Nita, L.E.; Gradinaru, V.R. Temperature sensitive Pluronic F127-based gels incorporating natural therapeutic agents. Macromol. Mat. Eng. 2024, 2400341. [Google Scholar] [CrossRef]
- Wang, Q.; Zuo, Z.; Cheung, C.K.C.; Leung, S.S.Y. Updates on thermosensitive hydrogel for nasal, ocular and cutaneous delivery. Int. J. Pharm. 2019, 559, 86–101. [Google Scholar]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A review of Poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar]
- Lupu, A.; Rosca, I.; Gradinaru, V.R.; Bercea, M. Temperature induced gelation and antimicrobial properties of Pluronic F127 based systems. Polymers 2023, 15, 355. [Google Scholar] [CrossRef]
- Constantin, M.; Cosman, B.; Bercea, M.; Ailiesei, G.L.; Fundueanu, G. Thermosensitive Poloxamer-graft-carboxymethyl pullulan: A potential injectable hydrogel for drug delivery. Polymers 2021, 13, 3025. [Google Scholar] [CrossRef]
- Ur-Rehman, T.; Tavelin, S.; Gröbner, G. Chitosan in situ gelation for improved drug loading and retention in Poloxamer 407 gels. Int. J. Pharm. 2011, 409, 19–29. [Google Scholar]
- Lupu, A.; Gradinaru, L.M.; Rusu, D.; Bercea, M. Self-healing of Pluronic®F127 hydrogels in the presence of various polysaccharides. Gels 2023, 9, 719. [Google Scholar] [CrossRef]
- Popescu, I.; Constantin, M.; Bercea, M.; Cosman, B.; Suflet, D.M.; Fundueanu, G. Poloxamer/carboxymethyl pullulan aqueous systems—Miscibility and thermogelation studies using viscometry, rheology and dynamic light scattering. Polymers 2023, 15, 1909. [Google Scholar] [CrossRef]
- Zhang, N.; Xia, Y.; Zou, Y.; Yang, W.; Zhang, J.; Zhong, Z.; Meng, F. ATN-161 Peptide functionalized reversibly cross-linked polymersomes mediate targeted doxorubicin delivery into melanoma-bearing C57BL/6 mice. Mol. Pharm. 2017, 14, 2538–2547. [Google Scholar]
- Li, J.; Sha, Y. A convenient synthesis of amino acid methyl esters. Molecules 2008, 13, 1111–1119. [Google Scholar] [CrossRef]
- Cormanich, R.A.; Ducati, L.C.; Tormena, C.F.; Rittner, R. Phenylalanine and tyrosine methyl ester intramolecular interactions and conformational analysis by 1H NMR and infrared spectroscopies and theoretical calculations. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 123, 482–489. [Google Scholar]
- Suckau, D.; Resemann, A.; Schuerenberg, M.; Hufnagel, P.; Franzen, J.; Holle, A. A novel MALDI LIFT-ToF/ToF mass spectrometer for proteomics. Anal. Bioanal. Chem. 2003, 376, 952–965. [Google Scholar]
- Maux, D.; Enjalbal, C.; Martinez, J.; Aubagnac, J.-L.; Combarieu, R. Static secondary ion mass spectrometry to monitor solid-phase peptide synthesis. J. Am. Soc. Mass Spectrom. 2001, 12, 1099–1105. [Google Scholar]
- Wu, H.; Zhang, X.; Wang, Z.; Chen, X.; Li, Y.; Fang, J.; Zheng, S.; Zhang, L.; Li, C.; Hao, L. Preparation, properties and in vitro osteogensis of self-reinforcing injectable hydrogel. Eur. J. Pharm. Sci. 2024, 192, 106617. [Google Scholar] [PubMed]
- Bercea, M. Rheology as a tool for fine-tuning the properties of printable bioinspired gels. Molecules 2023, 28, 2766. [Google Scholar] [CrossRef]
- Nath, D.; Ralhan, J.; Joseph, J.P.; Miglani, C.; Pal, A. Thermoresponsive injectable hydrogel to mimic the heat- and strain-stiffening behavior of biopolymers toward muscle cell proliferation. Biomacromolecules 2024, 25, 853–863. [Google Scholar]
- Gradinaru, L.M.; Bercea, M.; Lupu, A.; Gradinaru, V.R. Development of polyurethane/peptide-based carriers with self-healing properties. Polymers 2023, 15, 1697. [Google Scholar] [CrossRef] [PubMed]
- Lupu, A.; Gradinaru, L.M.; Balan-Porcarasu, M.; Darie-Ion, L.; Petre, B.A.; Gradinaru, V.R. The hidden power of a novel collagen octapeptide: Unveiling its antioxidant and cofactors releasing capacity from polyurethane based systems. React. Funct. Polym. 2025, 207, 106131. [Google Scholar]
- Martin, A.D.; Robinson, A.B.; Mason, A.F.; Wojciechowski, J.P.; Thordarson, P. Exceptionally strong hydrogels through self-assembly of an indole-capped dipeptide. Chem. Commun. 2014, 50, 15541–15544. [Google Scholar]
- Salay, L.C.; Prazeres, E.A.; Huachaca, N.S.M.; Lemos, M.; Piccoli, J.P.; Sanches, P.R.S.; Cilli, E.M.; Santos, R.S.; Feitosa, E. Molecular interactions between Pluronic F127 and the peptide tritrpticin in aqueous solution. Colloid Polym. Sci. 2018, 296, 809–817. [Google Scholar] [CrossRef]
- Bertsch, P.; Diba, M.; Mooney, D.J.; Leeuwenburgh, S.C.G. Self-healing injectable hydrogels for tissue regeneration. Chem. Rev. 2023, 123, 834–873. [Google Scholar]
- Zhao, X.; Chen, X.; Yuk, H.; Lin, S.; Liu, X.; Parada, G. Soft materials by design: Unconventional polymer networks give extreme properties. Chem. Rev. 2021, 121, 4309–4372. [Google Scholar]
- Pathan, N.; Shende, P. Strategic conceptualization and potential of self-healing polymers in biomedical field. Mater. Sci. Eng. C 2021, 125, 112099. [Google Scholar]
- Ye, E.; Chee, P.L.; Prasad, A.; Fang, X.; Owh, C.; Yeo, V.J.J.; Loh, X.J. Supramolecular soft biomaterials for biomedical applications. Mater. Today 2014, 17, 194–202. [Google Scholar] [CrossRef]
- Argudo, P.G.; Contreras-Montoya, R.; de Cienfuegos, L.A.; Cuerva, J.M.; Cano, M.; Alba-Molina, D.; Martín-Romero, M.T.; Camacho, L.; Giner-Casares, J.J. Unravelling the 2D self-assembly of Fmoc-dipeptides at fluid interfaces. Soft Matter 2018, 14, 9343–9350. [Google Scholar]
- Ryan, D.M.; Nilsson, B.L. Self-assembled amino acids and dipeptides as noncovalent hydrogels for tissue engineering. Polym. Chem. 2012, 3, 18–33. [Google Scholar] [CrossRef]
- Rajbhandary, A.; Nilsson, B.L. Investigating the effects of peptoid substitutions in self-assembly of Fmoc-diphenylalanine derivatives. Biopolymers 2017, 108, e22994. [Google Scholar]
- Smith, A.M.; Williams, R.J.; Tang, C.; Coppo, P.; Collins, R.F.; Turner, M.L.; Saiani, A.; Ulijn, R.V. Fmoc-diphenylalanine self assembles to a hydrogel via a novel architecture based on π-π interlocked β-sheets. Adv. Mater. 2008, 20, 37–41. [Google Scholar]
- Diaferia, C.; Ghosh, M.; Sibillano, T.; Gallo, E.; Stornaiuolo, M.; Giannini, C.; Morelli, G.; Adler-Abramovich, L.; Accardo, A. Fmoc-FF and hexapeptides-based multicomponent hydrogels as scaffold materials. Soft Matter. 2019, 15, 487–496. [Google Scholar] [CrossRef]
- Jarak, I.; Varela, C.L.; da Silva, E.T.; Roleira, F.F.M.; Veiga, F.; Figueiras, A. Pluronic-based nanovehicles: Recent advances in anticancer therapeutic applications. Eur. J. Med. Chem. 2020, 206, 112526. [Google Scholar]
- Coulter, S.; Pentlavalli, S.; An, Y.; Vora, L.K.; Cross, E.; Moore, J.; Sun, H.; Schweins, R.; McCarthy, H.; Laverty, G. In situ forming, enzyme-responsive peptoid-peptide hydrogels: An advanced long-acting injectable drug delivery system. J. Am. Chem. Soc. 2024, 146, 21401–21416. [Google Scholar] [CrossRef]



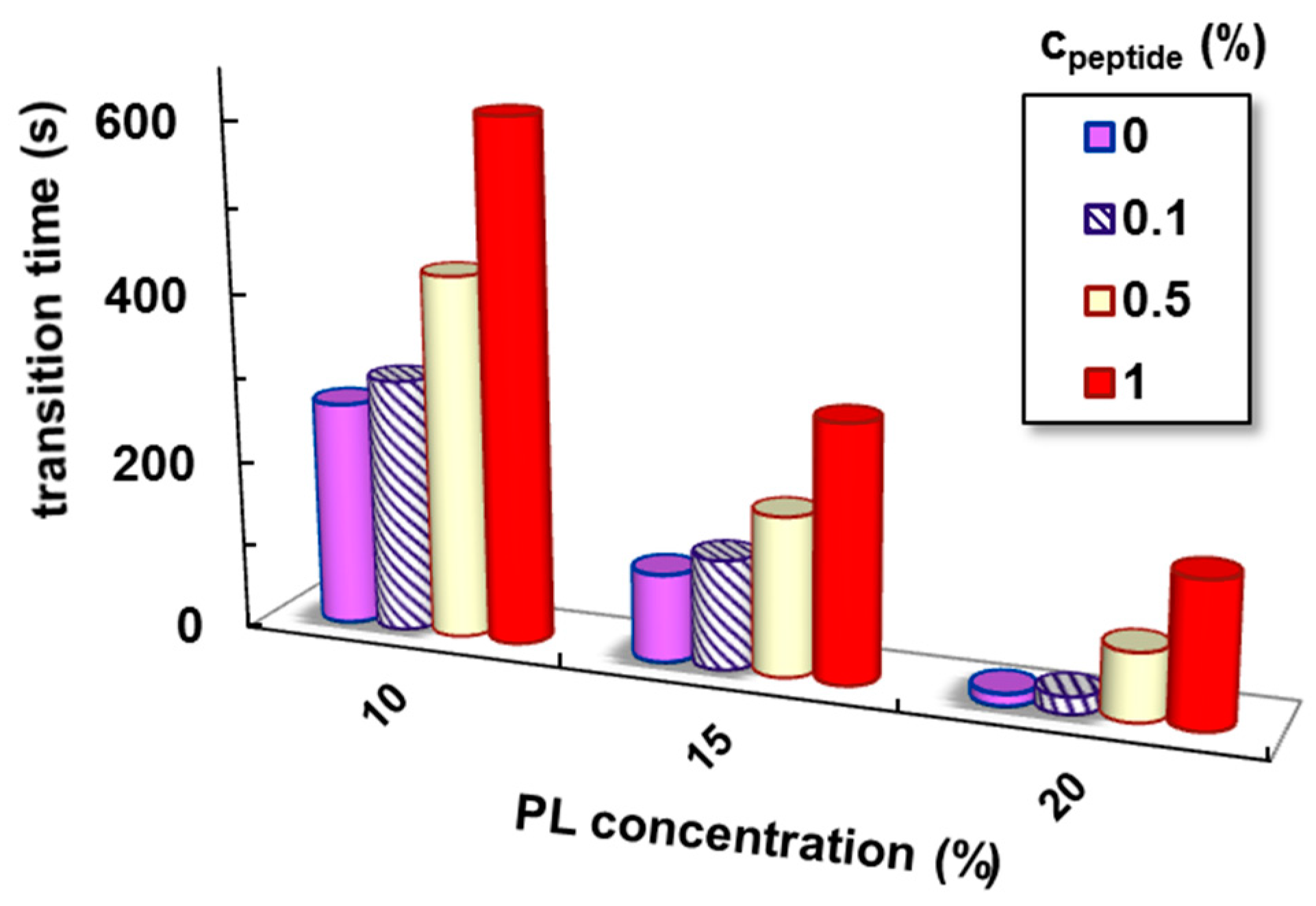
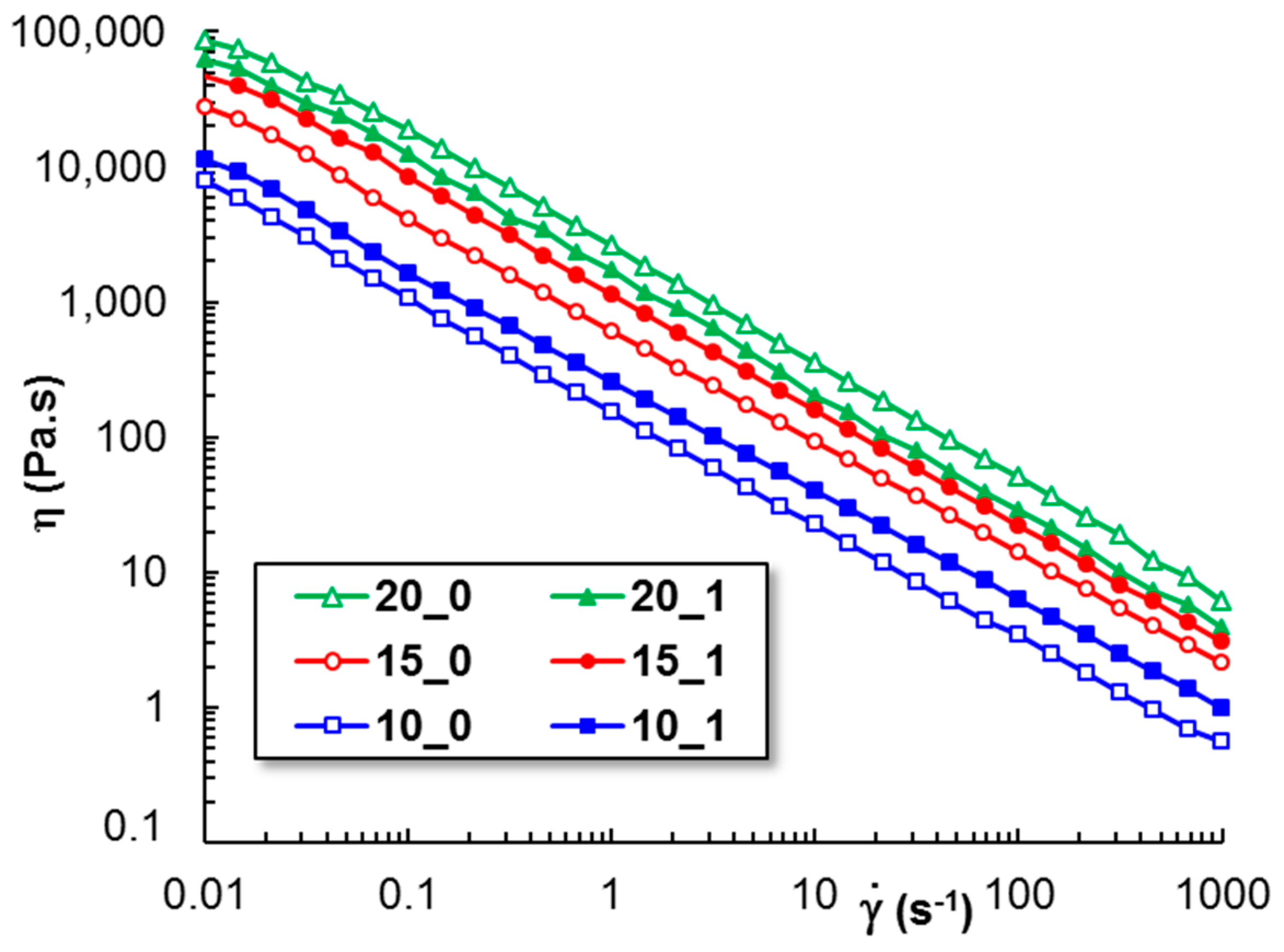

| cPL (%) | cpeptide (%) | Tsol–gel (°C) | Sol–Gel Transition Time (s) | G′ * (Pa) | G″ * (Pa) | Tanδ * | γL * (%) | τo * (Pa) | η100 ** (Pa·s) |
|---|---|---|---|---|---|---|---|---|---|
| 10 | 0 | 29.1 | 266 | 2795 | 269 | 0.0963 | 15.7 | 63.0 | 3.4 |
| 0.1 | 30.0 | 300 | 2880 | 291 | 0.1011 | 15.2 | 64.3 | 3.5 | |
| 0.5 | 31.2 | 429 | 3081 | 402 | 0.1305 | 15.9 | 66.1 | 4.8 | |
| 1 | 33.0 | 615 | 3359 | 521 | 0.1551 | 16.5 | 68.5 | 6.3 | |
| 15 | 0 | 23.2 | 104 | 8040 | 1200 | 0.1493 | 20.7 | 123 | 14.1 |
| 0.1 | 23.8 | 129 | 8930 | 1371 | 0.1535 | 20.6 | 125 | 14.6 | |
| 0.5 | 25.3 | 188 | 10,100 | 1780 | 0.1762 | 25.4 | 137 | 16.9 | |
| 1 | 26.5 | 302 | 11,550 | 2281 | 0.1964 | 32.9 | 149 | 21.9 | |
| 20 | 0 | 19.0 | 14 | 14,900 | 2940 | 0.1973 | 66.3 | 404 | 50.9 |
| 0.1 | 19.5 | 20 | 14,946 | 3030 | 0.2027 | 67.4 | 399 | 43.2 | |
| 0.5 | 20.5 | 81 | 15,455 | 3021 | 0.1955 | 65.5 | 367 | 32.8 | |
| 1 | 21.5 | 173 | 16,050 | 2912 | 0.1814 | 63.2 | 352 | 28.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gradinaru, V.R.; Bercea, M.; Gradinaru, L.M.; Puiu, A.; Lupu, A.; Petre, B.A. Targeting Injectable Hydrogels: The Role of Diphenylalanine Peptide Derivative in the Gelation Dynamics of Pluronic® F127. Polymers 2025, 17, 930. https://doi.org/10.3390/polym17070930
Gradinaru VR, Bercea M, Gradinaru LM, Puiu A, Lupu A, Petre BA. Targeting Injectable Hydrogels: The Role of Diphenylalanine Peptide Derivative in the Gelation Dynamics of Pluronic® F127. Polymers. 2025; 17(7):930. https://doi.org/10.3390/polym17070930
Chicago/Turabian StyleGradinaru, Vasile Robert, Maria Bercea, Luiza Madalina Gradinaru, Alexandru Puiu, Alexandra Lupu, and Brindusa Alina Petre. 2025. "Targeting Injectable Hydrogels: The Role of Diphenylalanine Peptide Derivative in the Gelation Dynamics of Pluronic® F127" Polymers 17, no. 7: 930. https://doi.org/10.3390/polym17070930
APA StyleGradinaru, V. R., Bercea, M., Gradinaru, L. M., Puiu, A., Lupu, A., & Petre, B. A. (2025). Targeting Injectable Hydrogels: The Role of Diphenylalanine Peptide Derivative in the Gelation Dynamics of Pluronic® F127. Polymers, 17(7), 930. https://doi.org/10.3390/polym17070930










