A Phosphate-Modified Aqueous Acrylic–Alkyd Resin for Protective Technology to Prevent Corrosion of Iron Substrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
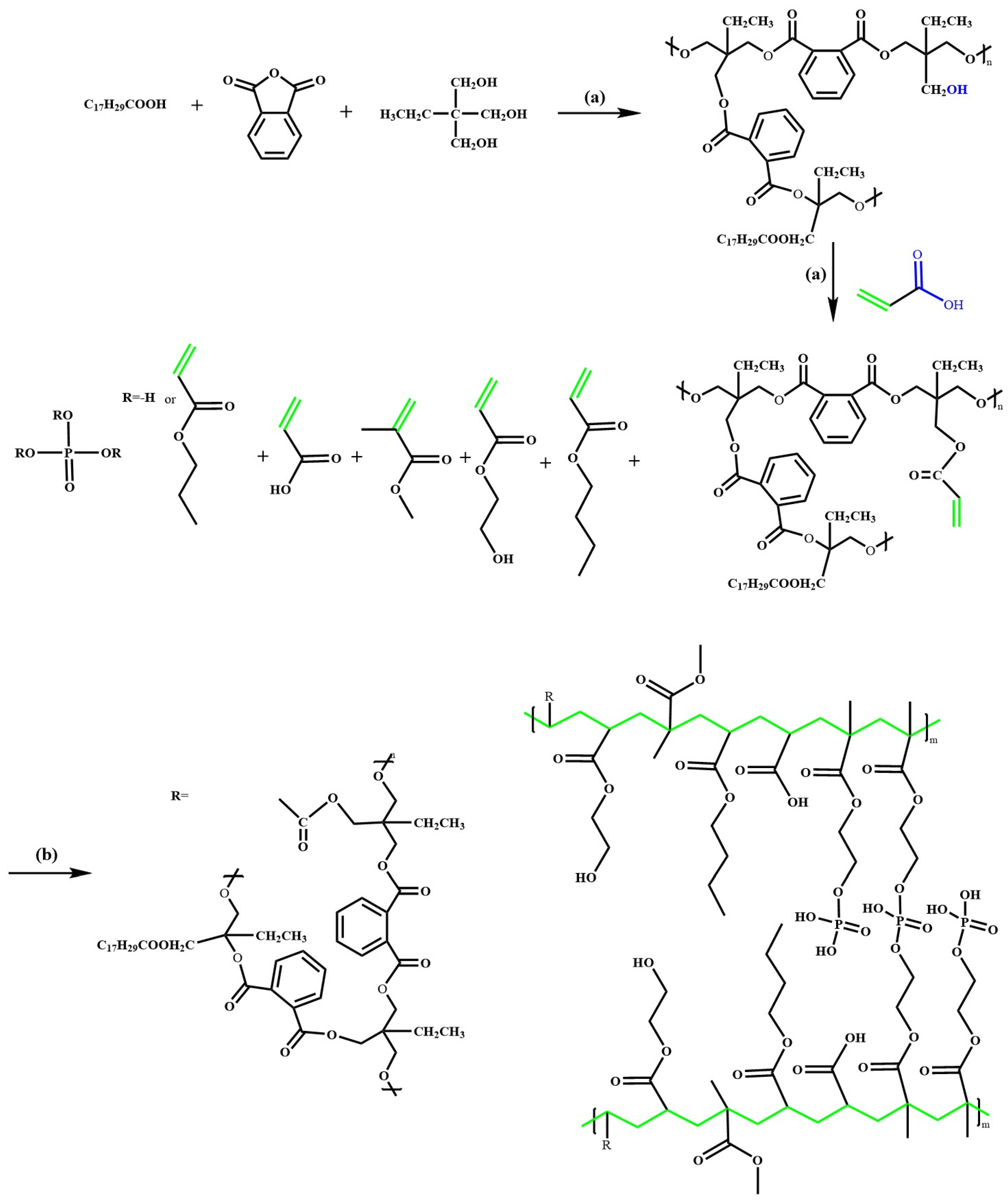
2.2. Alkyd Resin Formulation
2.3. Synthesis of HEMAP-Modified Acrylic–Alkyd Resin Copolymers
2.4. Process of Preparing Resin Film
2.5. Formulation of Resin Paint Film
2.6. Characterization
2.7. Water Resistance Test of Resin Adhesive Film
2.8. Thermal Stability and Dynamic Thermo-Mechanical Analysis Tests of Resin Adhesive Films
2.9. Electrochemical and Salt Spray Testing of Paint Samples
3. Results
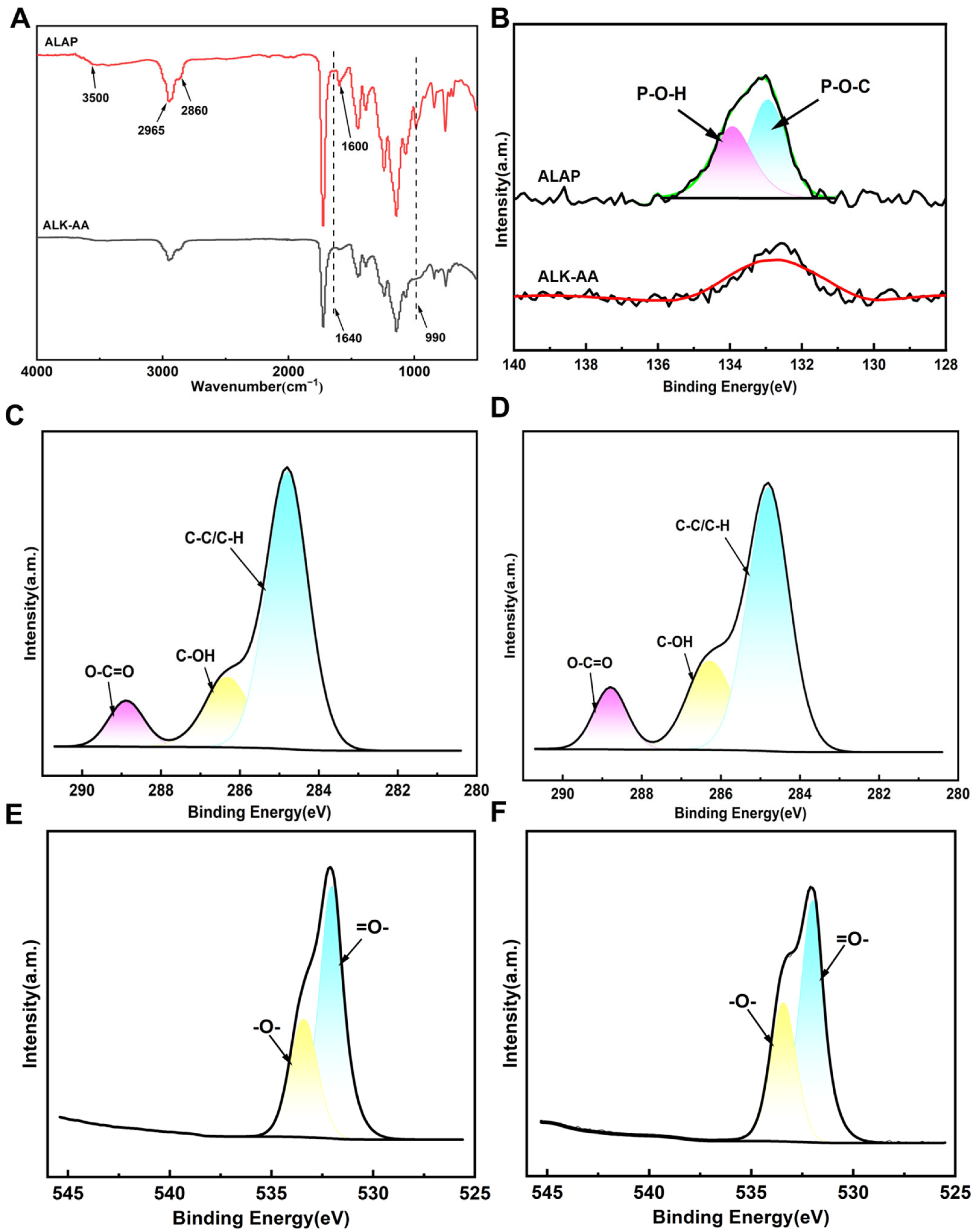
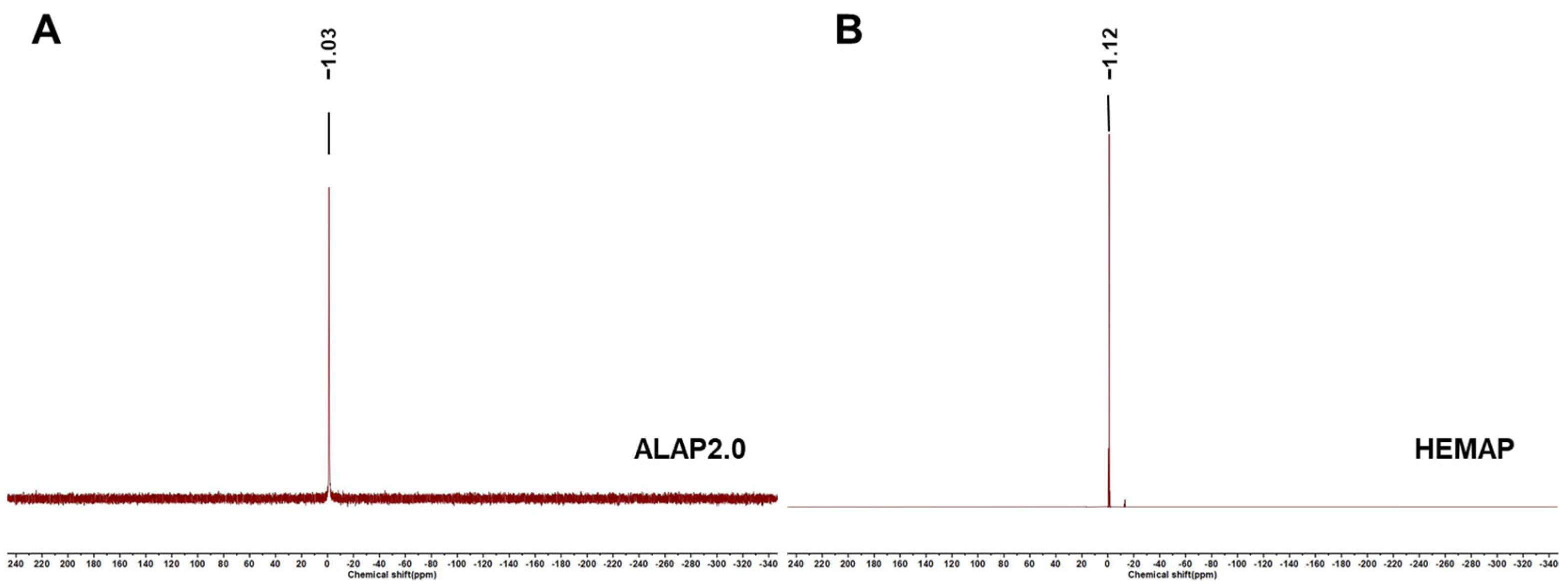
3.1. Substance and Chemical Composition of the Resin Film
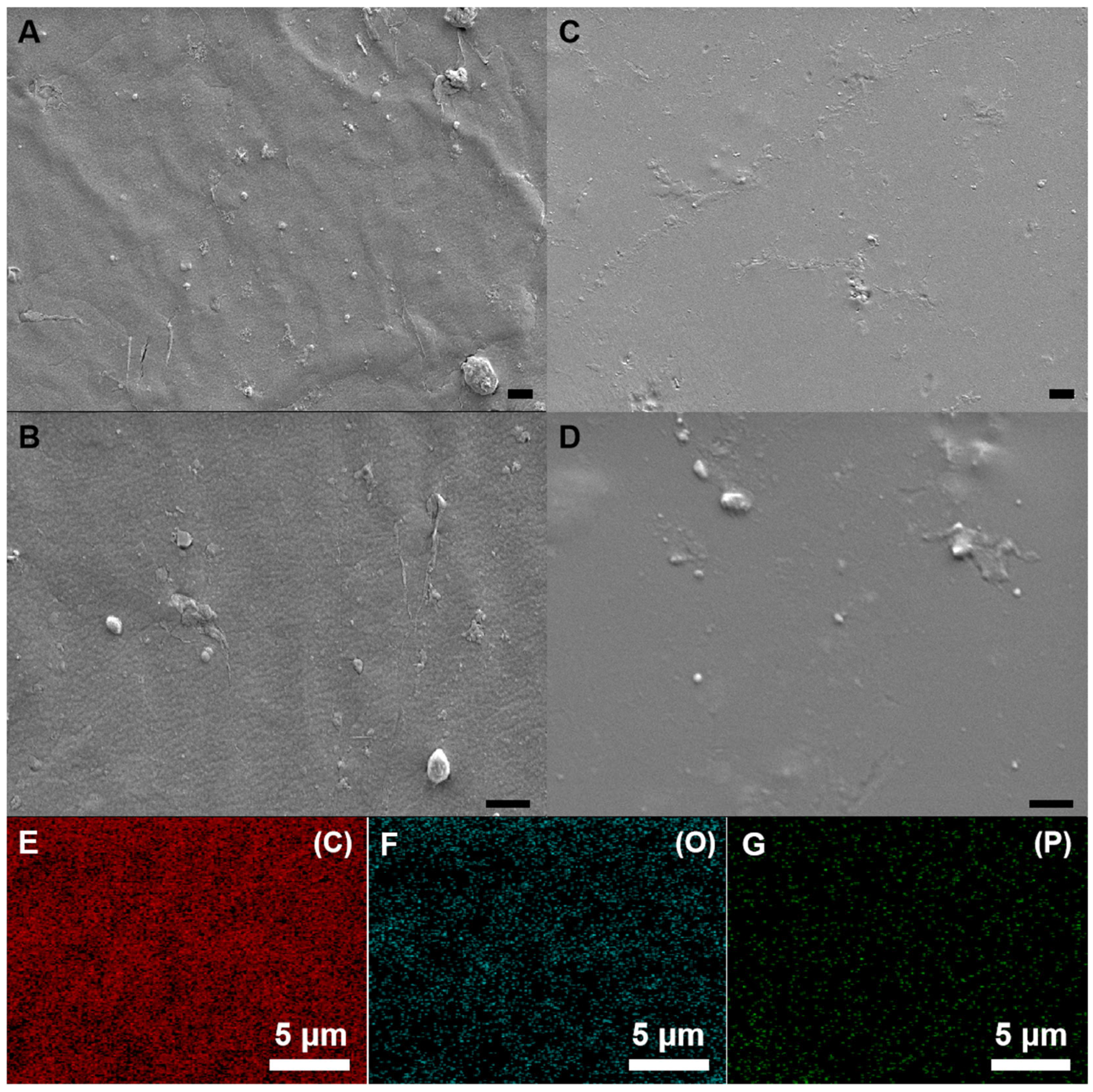
3.2. Microscopic Morphology of Resins
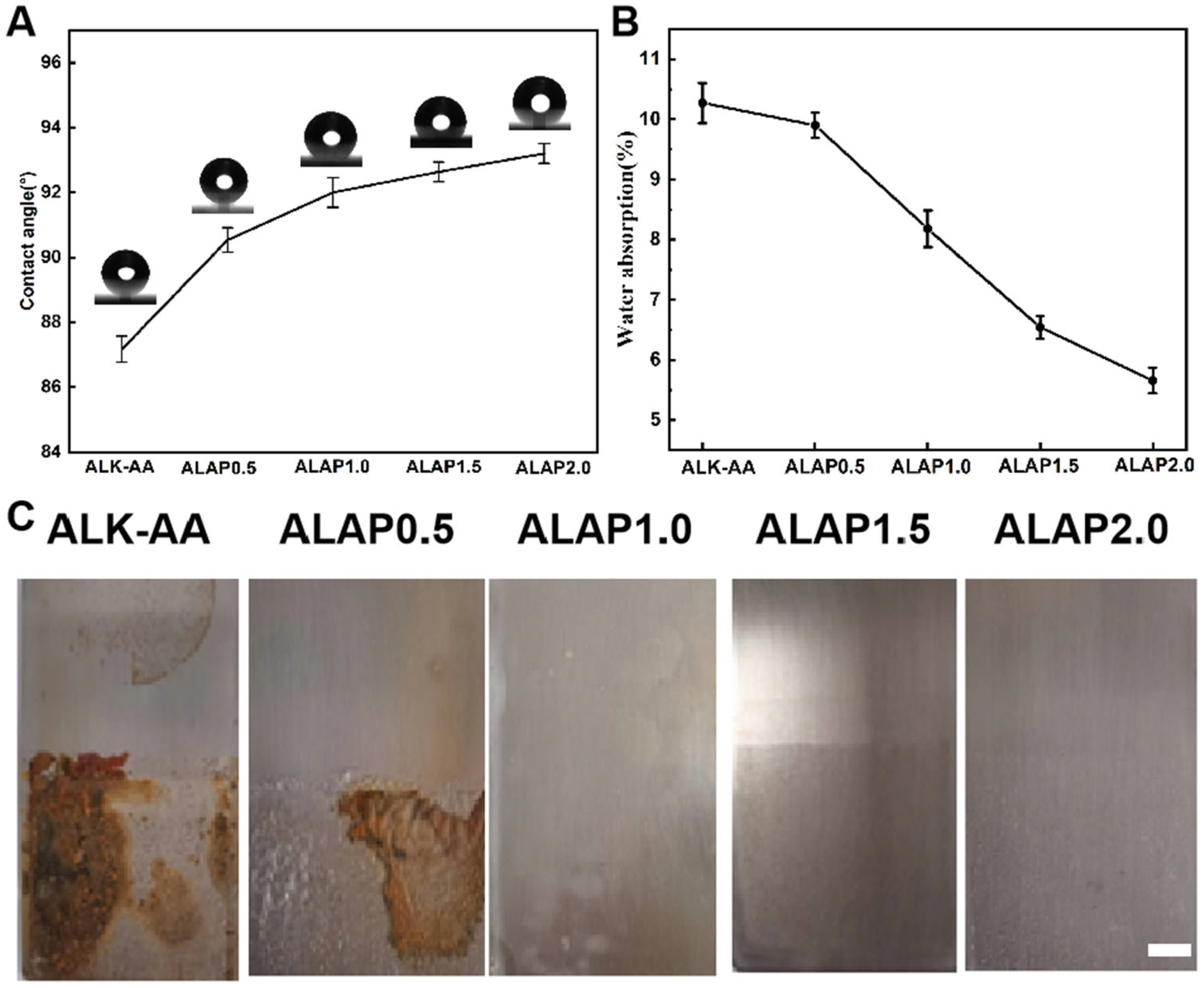
3.3. Thin Film Properties of Samples
3.4. Water-Resistance Analysis
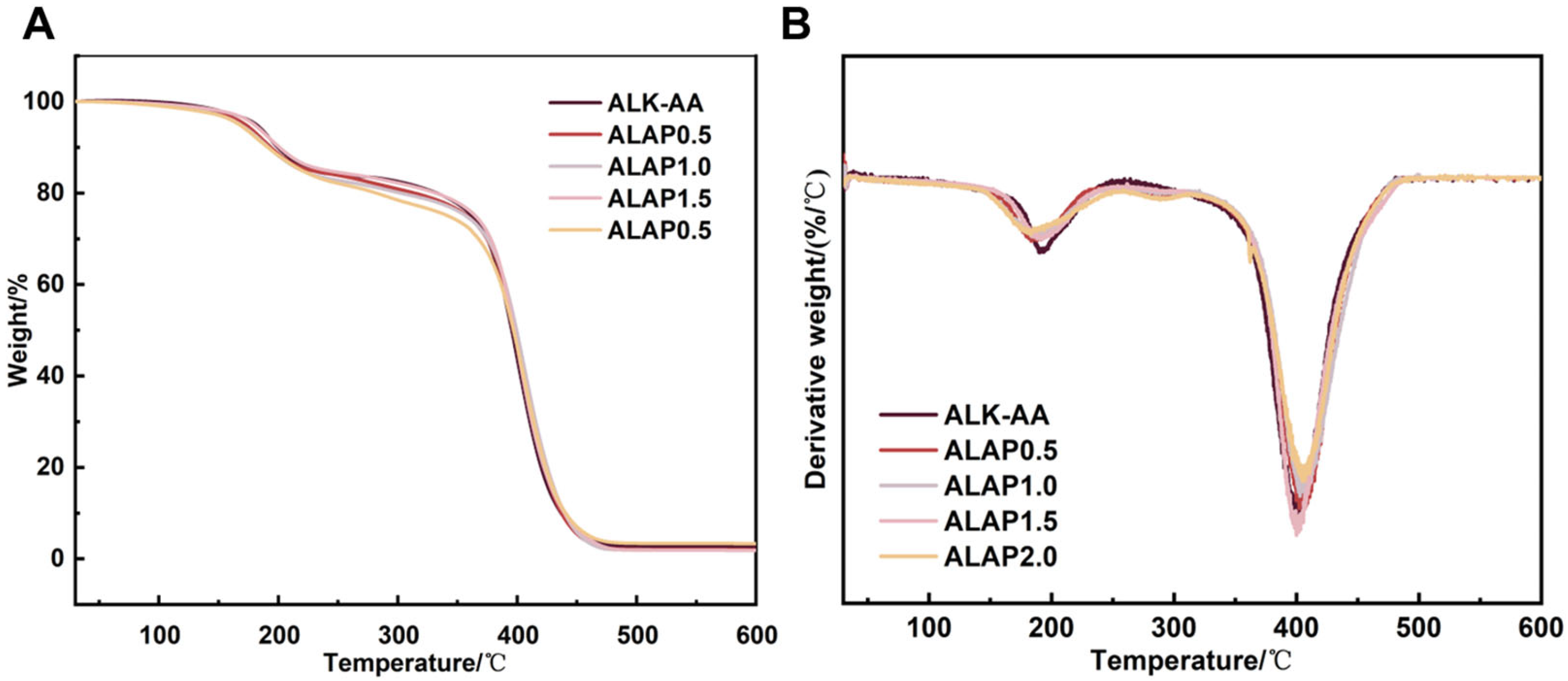
3.5. Thermal Stability of Resins
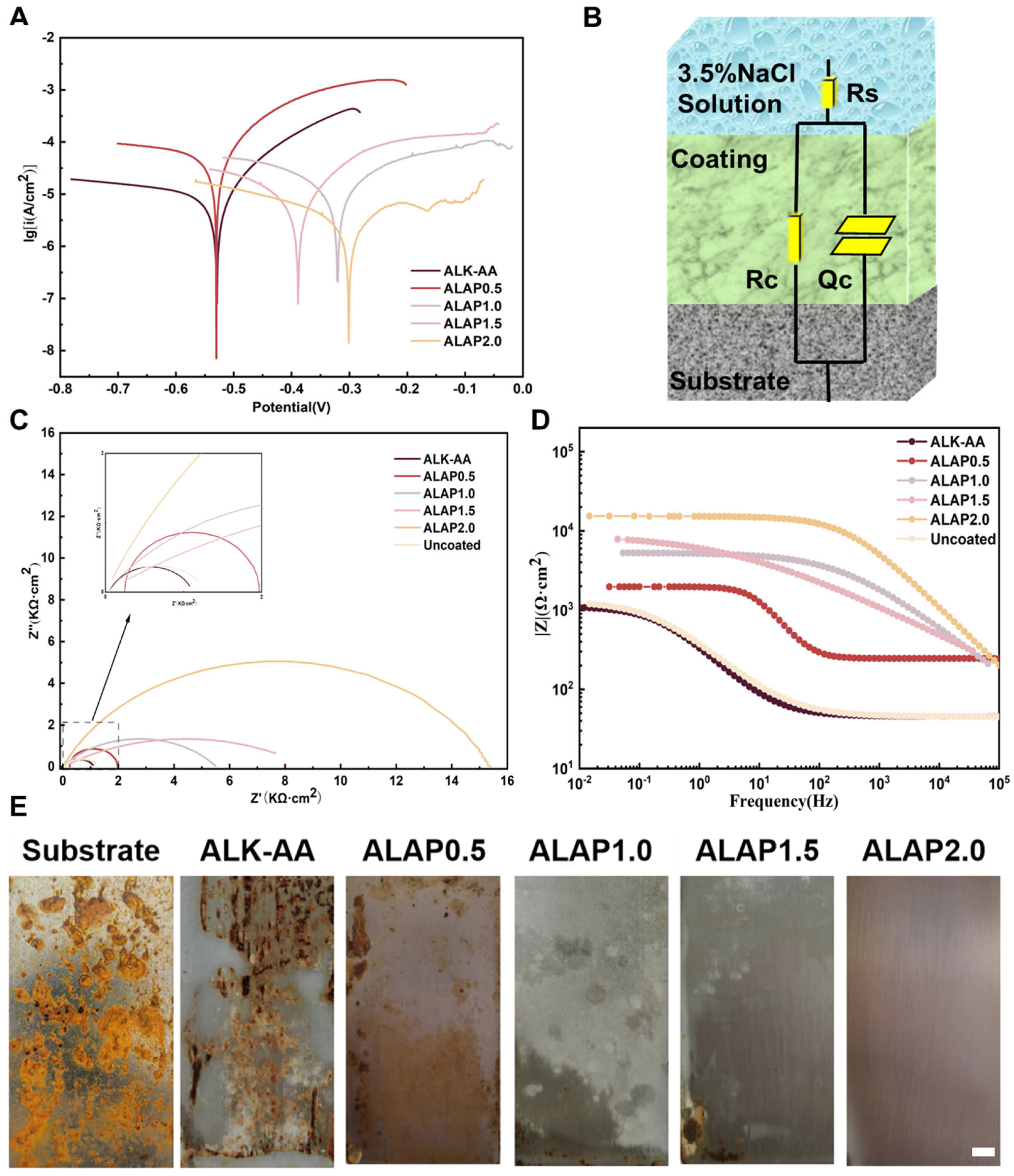
3.6. Analysis of Corrosion Resistance of Resins
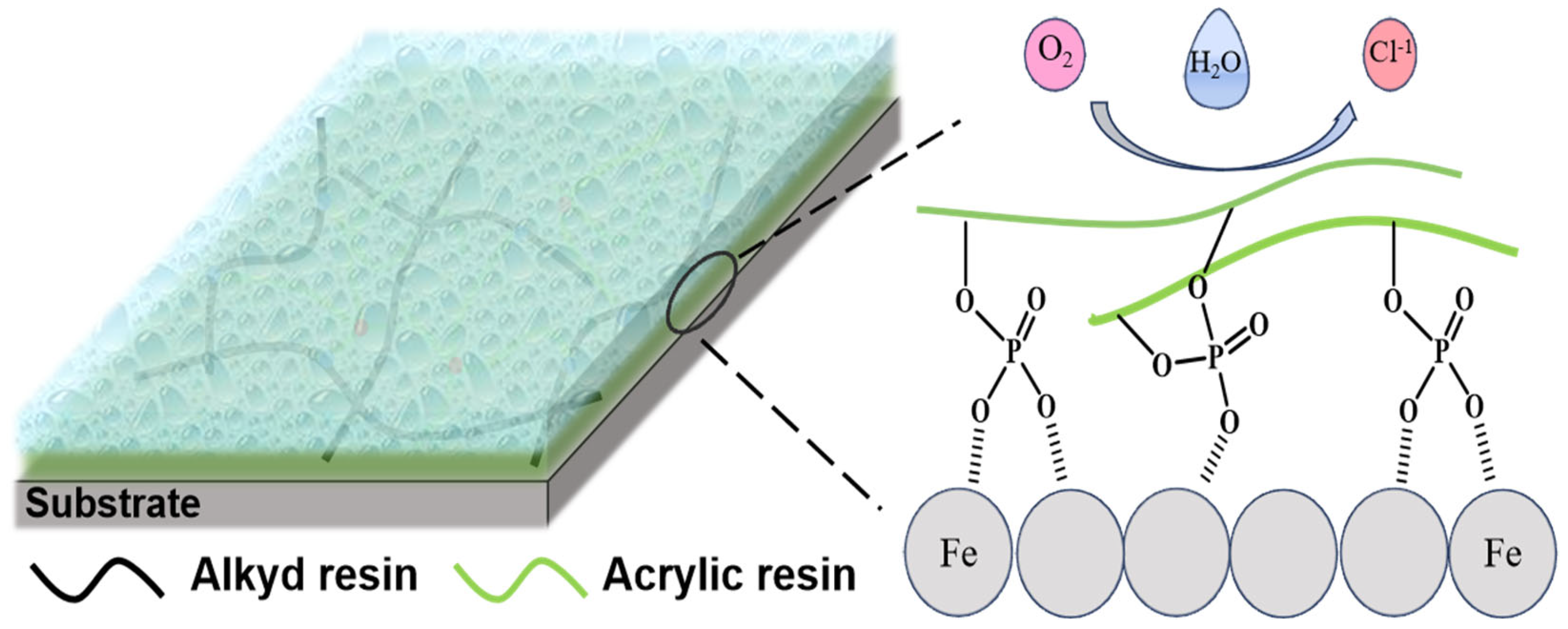
3.7. Resin Anti-Corrosion Mechanism Investigation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Yan, W.; Zhao, X. Research on two-dimensional layered materials for metal corrosion protection: Advances and challenges. Prog. Org. Coat. 2024, 194, 108589. [Google Scholar] [CrossRef]
- Qiang, Y.; Ran, B.; Li, M. GO-functionalized MXene towards superior anti-corrosion coating. J. Colloid Interface Sci. 2023, 642, 595–603. [Google Scholar] [PubMed]
- Pengpeng, L.; Xue, F.; Xin, L. Anticorrosion Coating with Heterogeneous Assembly of Nanofillers Modulated by a Magnetic Field. ACS Appl. Mater. Interfaces 2023, 15, 7538–7551. [Google Scholar] [PubMed]
- Zhou, C.; Pan, M.; Li, S. Metal organic frameworks (MOFs) as multifunctional nanoplatform for anticorrosion surfaces and coatings. Adv. Colloid Interface Sci. 2022, 305, 102707. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, J.; Zhong, J. Thin Nacre-Biomimetic Coating with Super-Anticorrosion Performance. ACS Nano 2018, 12, 10189–10200. [Google Scholar] [CrossRef]
- Su, Y.; Qiu, S.; Yang, D. Active anti-corrosion of epoxy coating by nitrite ions intercalated MgAl LDH. J. Hazard. Mater. 2020, 391, 122215. [Google Scholar]
- Li, X.; Xue, Z.; Sun, W. Bio-inspired self-healing MXene/polyurethane coating with superior active/passive anticorrosion performance for Mg alloy. Chem. Eng. J. 2023, 454, 140187. [Google Scholar]
- Shen, R.; Long, M.; Lei, C. Anticorrosive waterborne polyurethane coatings derived from castor oil and renewable diols. Chem. Eng. J. 2022, 433, 134470. [Google Scholar]
- Ge, X.; Fan, W.; Tang, H. Anticorrosion performance of an eco-friendly coating system including an epoxy tie primer with aluminum tripolyphosphates and a polyurethane topcoat for marine aluminum alloy. Prog. Org. Coat. 2023, 174, 107294. [Google Scholar]
- Deyab, M.A.; Ouarsal, R.; Al-Sabagh, A.M. Enhancement of corrosion protection performance of epoxy coating by introducing new hydrogenphosphate compound. Prog. Org. Coat. 2017, 107, 37–42. [Google Scholar]
- Yan, Z.; Wang, S.; Bi, J. Strengthening waterborne acrylic resin modified with trimethylolpropane triacrylate and compositing with carbon nanotubes for enhanced anticorrosion. Adv. Compos. Hybrid Mater. 2022, 5, 2116–2130. [Google Scholar] [CrossRef]
- Wang, X.; Tebyetekerwa, M.; Chen, Y. Improving the performance of acrylic-epoxy ester hybrid coatings with phosphate monomers. Polym. Chem. 2024, 15, 2265–2276. [Google Scholar]
- Tarasova, N.; Zanin, A.; Krivoborodov, E. The New Approach to the Preparation of Polyacrylamide-Based Hydrogels: Initiation of Polymerization of Acrylamide with 1,3-Dimethylimidazolium (Phosphonooxy-)Oligosulphanide Under Drying Aqueous Solutions. Polymers 2021, 13, 1806. [Google Scholar] [CrossRef] [PubMed]
- Tarasova, N.; Krivoborodov, E.; Zanin, A. Formation of Hydrogels Based on a Copolymer of N-Vinyl-2-pyrrolidone and Glycidyl Methacrylate in the Presence of the Reaction Product of 1,3-Dimethylmidazolium Dimethylphosphate and Elemental Sulfur. Gels 2022, 8, 136. [Google Scholar] [CrossRef]
- Selim, M.S.; Yang, H.; Li, Y. Ceramic hyperbranched alkyd/γ-Al2O3 nanorods composite as a surface coating. Prog. Org. Coat. 2018, 120, 217–227. [Google Scholar] [CrossRef]
- Elmourabit, M.; Zarki, Y.; Arfoy, B. Corrosion protection of mild steel by an alkyd resin coating reinforced with calcium zinc phosphate derived phosphate sludge as green inhibitive pigment. Emergent Mater. 2024, 1–19. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.-H.; Meng, X.-Z. A novel cerium organic network modified graphene oxide prepared multifunctional waterborne epoxy-based coating with excellent mechanical and passive/active anti-corrosion properties. Chem. Eng. J. 2023, 465, 142997. [Google Scholar] [CrossRef]
- Rahman, O.U.; Bhat, S.I.; Yu, H. Hyperbranched Soya Alkyd Nanocomposite: A Sustainable Feedstock-Based Anticorrosive Nanocomposite Coatings. ACS Sustain. Chem. Eng. 2017, 5, 9725–9734. [Google Scholar] [CrossRef]
- Cui, M.; Chen, X.; Mei, S. Bioinspired polydopamine nanosheets for the enhancemen in anti-corrosion performance of water-borne epoxy coatings. Chem. Eng. J. 2023, 471, 144760. [Google Scholar] [CrossRef]
- Zhou, H.; Lu, Q.; Su, Z. Study on anticorrosive and self-healing properties of acrylic resin modified with nitrogen-doped carbon quantum dots. Chem. Eng. J. 2024, 494, 153165. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Fei, G. Comparison study on chelated and non-chelated titanate functionalized graphene nanosheets for enhancement of waterborne alkyd anticorrosion coating. Prog. Org. Coat. 2021, 150, 105961. [Google Scholar] [CrossRef]
- Fan, X.; Xia, Y.; Wu, S. Covalently immobilization of modified graphene oxide with waterborne hydroxyl acrylic resin for anticorrosive reinforcement of its coatings. Prog. Org. Coat. 2022, 163, 106685. [Google Scholar] [CrossRef]
- Jiang, W.; Jin, X.; Li, H. Modification of nano-hybrid silicon acrylic resin with anticorrosion and hydrophobic properties. Polym. Test. 2020, 82, 106287. [Google Scholar] [CrossRef]
- Bao, Y.; Fu, R.; Liu, Y. Polyacrylate modified alkyd hybrid latex with high anti-corrosion performance synthesized via solvent-free and emulsifier-free method. Sustain. Chem. Pharm. 2024, 39, 101535. [Google Scholar] [CrossRef]
- Ma, X.; Yun, H.; Wu, N. Superior Water-Resistant Poly(2-hydroxyethyl methacrylate phosphate) Flame Retardant and a Transparent, Flame-Retardant, and Biodegradable Poly(lactide) Blend Film. ACS Appl. Polym. Mater. 2021, 3, 1314–1323. [Google Scholar] [CrossRef]
- Puyadena, M.; Etxeberria, I.; Martin, L. Polyurethane/acrylic hybrid dispersions containing phosphorus reactive flame retardants as transparent coatings for wood. Prog. Org. Coat. 2022, 170, 107005. [Google Scholar] [CrossRef]
- Shi, H.; Han, E.-H.; Liu, F. Protection of 2024-T3 aluminium alloy by corrosion resistant phytic acid conversion coating. Appl. Surf. Sci. 2013, 280, 325–331. [Google Scholar] [CrossRef]
- Khadom, A.A.; Farhan, S.N. Corrosion inhibition of steel in phosphoric acid. Corros. Rev. 2018, 36, 267–280. [Google Scholar] [CrossRef]
- Gao, X.; Li, W.; Yan, R. Effect of zinc ion on the microstructure and electrochemical behavior of phytic acid based conversion coatings on Q235 steels. Surf. Coat. Technol. 2017, 325, 248–256. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Liu, J. Phytic acid-based adhesion promoter for UV-curable coating: High performance, low cost, and eco-friendliness. Prog. Org. Coat. 2022, 167, 106834. [Google Scholar] [CrossRef]
- Yu, H.-C.; Zhang, Y.-T.; Wang, M.-J. Dispersion of Poly(urea-formaldehyde)-Based Microcapsules for Self-Healing and Anticorrosion Applications. Langmuir 2019, 35, 7871–7878. [Google Scholar] [PubMed]
- Nóvoa, X.R.; Pérez, C. The use of smart coatings for metal corrosion control. Curr. Opin. Electrochem. 2023, 40, 101324. [Google Scholar]
- Chaudhari, T.; Rajagopalan, N.; Dam-Johansen, K. Lignin Phosphate: A Biobased Substitute for Zinc Phosphate in Corrosion-Inhibiting Coatings. ACS Sustain. Chem. Eng. 2024, 12, 7813–7830. [Google Scholar]
- GB/T 1727-2021; General Methods for Preparation of Coating Films. National Standards of the People’s Republic of China: Beijing, China, 2021.
- GB/T 29088-2012; Corrosion of Metals and Alloys—Electrochemical Potentiokinetic Reactivation Measurement Using the Double Loop Method. National Standards of the People’s Republic of China: Beijing, China, 2012.
- Irfan, M.; Bhat, S.I.; Ahmad, S. Reduced Graphene Oxide Reinforced Waterborne Soy Alkyd Nanocomposites: Formulation, Characterization, and Corrosion Inhibition Analysis. ACS Sustain. Chem. Eng. 2018, 6, 14820–14830. [Google Scholar]
- Liang, B.; Li, R.; Zhang, C. Synthesis and characterization of a novel tri-functional bio-based methacrylate prepolymer from castor oil and its application in UV-curable coatings. Ind. Crops Prod. 2019, 135, 170–178. [Google Scholar]
- Yan, L.; Xu, Z.; Deng, N. Synthesis of organophosphate-functionalized graphene oxide for enhancing the flame retardancy and smoke suppression properties of transparent fire-retardant coatings. Polym. Degrad. Stab. 2020, 172, 109064. [Google Scholar]
- Zhang, J.; Sun, Z.; Zhu, H. Novel triphosphorylation polyurethane nanoparticles for blood-contacting biomaterials’ coating. J. Mater. Chem. B 2016, 4, 1116–1121. [Google Scholar] [CrossRef]
- Yekta, S.; Sadeghi, M.; Babanezhad, E. Synthesis of CaWO4 nanoparticles and its application for the adsorption-degradation of organophosphorus cyanophos. J. Water Process Eng. 2016, 14, 19–27. [Google Scholar] [CrossRef]
- Deng, Y.; Xia, L.; Song, G.L.; Zhao, Y.; Zhang, Y.; Xu, Y.; Zheng, D. Development of a curcumin-based antifouling and anticorrosion sustainable polybenzoxazine resin composite coating. Compos. Part B Eng. 2021, 225, 109263. [Google Scholar] [CrossRef]
- Wei, J.; Li, B.; Jing, L. Efficient Protection of Mg Alloy Enabled by Combination of A Conventional Anti-Corrosion Coating and A Superamphiphobic Coating. Chem. Eng. J. 2020, 390, 124562. [Google Scholar]
- Zou, Y.; Wang, Y.; Xu, S. Superhydrophobic double-layer coating for efficient heat dissipation and corrosion protection. Chem. Eng. J. 2019, 362, 638–649. [Google Scholar]
- Ding, J.-H.; Zhao, H.-R.; Zheng, Y. A long-term anticorrsive coating through graphene passivation. Carbon 2018, 138, 197–206. [Google Scholar]
- Fu, X.; Du, W.; Dou, H. Nanofiber Composite Coating with Self-Healing and Active Anticorrosive Performances. ACS Appl. Mater. Interfaces 2021, 13, 57880–57892. [Google Scholar] [PubMed]
- Ren, G.; Zheng, W.; Qiao, Z. Thermally conductive superhydrophobic composite coatings with anti-corrosion property. Prog. Org. Coat. 2025, 198, 108913. [Google Scholar]
- Zhao, X.; Liang, Z.; Huang, Y. Influence of phytic acid on flame retardancy and adhesion performance enhancement of poly (vinyl alcohol) hydrogel coating to wood substrate. Prog. Org. Coat. 2021, 161, 106453. [Google Scholar]
| Sample | Mw (g/mol) | Mn (g/mol) | PI |
|---|---|---|---|
| ALK-AA | 96,427 | 42,302 | 2.279 |
| ALAP0.5 | 116,147 | 55,028 | 2.111 |
| ALAP1.0 | 126,170 | 63,986 | 1.972 |
| ALAP1.5 | 139,856 | 75,975 | 1.841 |
| ALAP2.5 | 157,601 | 96,274 | 1.637 |
| Sample | Adhesion | Pencil Hardness | Dry-to-Touch Time (min) | Dry-Hard Time (h) |
|---|---|---|---|---|
| ALK-AA | 0 | HB | <30 | <2 |
| ALAP0.5 | 0 | HB | <30 | <2 |
| ALAP1.0 | 0 | H | <30 | <2 |
| ALAP1.5 | 0 | H | <30 | <2 |
| Sample | RS, ohm | Qc, farad | Rc, ohm | X2 |
|---|---|---|---|---|
| Uncoated | 45.14 | 7.054 × 10−4 | 1268 | 4.07 × 10−3 |
| ALK-AA | 45.33 | 7.128 × 10−4 | 1082 | 3.86 × 10−3 |
| ALAP0.5 | 245.8 | 1.117 × 10−5 | 1726 | 4.77 × 10−3 |
| ALAP1.0 | 1.037 × 10−4 | 2.02 × 10−6 | 5527 | 1.08 × 10−3 |
| ALAP1.5 | 1.299 × 10−3 | 2.184 × 10−5 | 8826 | 2.47 × 10−3 |
| ALAP2.0 | 1.891 × 10−6 | 2.505 × 10−7 | 1.537 × 104 | 1.93 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, C.; He, W.; Sun, S.; Du, W.; Zhao, B. A Phosphate-Modified Aqueous Acrylic–Alkyd Resin for Protective Technology to Prevent Corrosion of Iron Substrates. Polymers 2025, 17, 847. https://doi.org/10.3390/polym17070847
Jiao C, He W, Sun S, Du W, Zhao B. A Phosphate-Modified Aqueous Acrylic–Alkyd Resin for Protective Technology to Prevent Corrosion of Iron Substrates. Polymers. 2025; 17(7):847. https://doi.org/10.3390/polym17070847
Chicago/Turabian StyleJiao, Chenglong, Wei He, Shixiong Sun, Wenhao Du, and Benbo Zhao. 2025. "A Phosphate-Modified Aqueous Acrylic–Alkyd Resin for Protective Technology to Prevent Corrosion of Iron Substrates" Polymers 17, no. 7: 847. https://doi.org/10.3390/polym17070847
APA StyleJiao, C., He, W., Sun, S., Du, W., & Zhao, B. (2025). A Phosphate-Modified Aqueous Acrylic–Alkyd Resin for Protective Technology to Prevent Corrosion of Iron Substrates. Polymers, 17(7), 847. https://doi.org/10.3390/polym17070847






