Performance Properties of Epoxy Resin Modified with Few-Layer Graphene Obtained by the Method of Self-Propagating High-Temperature Synthesis
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. FLG from Biopolymers
3.2. Epoxy Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FLG | Few layers graphene |
| GNS | Graphene nanostructure |
| GNP | Graphene nanoplates |
| GO | Graphene oxide |
| rGO | Reduced graphene oxide |
References
- Jin, F.L.; Li, X.; Park, S.J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Mohan, P.A. Critical review: The modification, properties, and applications of epoxy resins. Polym.-Plast. Technol. Eng. 2013, 52, 107–125. [Google Scholar] [CrossRef]
- Fekiač, J.J.; Krbata, M.; Kohutiar, M.; Janík, R.; Kakošová, L.; Breznická, A.; Eckert, M.; Mikuš, P. Comprehensive Review: Optimization of Epoxy Composites, Mechanical Properties, & Technological Trends. Polymers 2025, 17, 271. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Cao, Q.; Geng, X.; Wang, H.; Wang, P.; Liu, A.; Lan, Y.; Peng, Q. A Review of Current Development of Graphene Mechanics. Crystals 2018, 8, 357. [Google Scholar] [CrossRef]
- Hsissou, R.; Seghiri, R.; Benzekri, Z.; Hilali, M.; Rafik, M.; Elharfi, A. Polymer composite materials: A comprehensive review. Compos. Struct. 2021, 262, 113640. [Google Scholar] [CrossRef]
- Mirzapour, M.; Cousin, P.; Robert, M.; Benmokrane, B. Dispersion Characteristics, the Mechanical, Thermal Stability, and Durability Properties of Epoxy Nanocomposites Reinforced with Carbon Nanotubes, Graphene, or Graphene Oxide. Polymers 2024, 16, 1836. [Google Scholar] [CrossRef]
- Aradhana, R.; Mohanty, S.; Nayak, S.K. Comparison of mechanical, electrical and thermal properties in graphene oxide and reduced graphene oxide filled epoxy nanocomposite adhesives. Polymer 2018, 141, 109–123. [Google Scholar] [CrossRef]
- Zafeiropoulou, K.; Kostagiannakopoulou, C.; Geitona, A.; Tsilimigkra, X.; Sotiriadis, G.; Kostopoulos, V. On the Multi-Functional Behavior of Graphene-Based Nano-Reinforced Polymers. Materials 2021, 14, 5828. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, G.; Qi, J.; Lu, S.; Li, Y. Effect of lubricant functional groups on tribological properties of epoxy/graphene composites. J. Appl. Polym. Sci. 2024, 141, e55923. [Google Scholar] [CrossRef]
- Prolongo, S.; Jiménez-Suárez, A.; Moriche, R.; Ureña, A. Graphene nanoplatelets thickness and lateral size influence on the morphology and behavior of epoxy composites. Eur. Polym. J. 2014, 53, 292–301. [Google Scholar] [CrossRef]
- Wu, W.; Yang, H.; Liu, Y.; Yang, S.; Yang, P. Effect of defects on heat transfer at the graphene/epoxy interface. Int. Commun. Heat Mass. Transf. 2022, 131, 105846. [Google Scholar] [CrossRef]
- Osman, A.; Elhakeem, A.; Kaytbay, S.; Ahmed, A. A comprehensive review on the thermal, electrical, and mechanical properties of graphene-based multi-functional epoxy composites. Adv. Compos. Hybrid Mater. 2022, 5, 547–605. [Google Scholar] [CrossRef]
- Zhou, M.H.; Yin, G.Z.; Prolongo, S.G.; Wang, D.Y. Recent progress on multifunctional thermally conductive epoxy composite. Polymers 2023, 15, 2818. [Google Scholar] [CrossRef]
- Atif, R.; Shyha, I.; Inam, F. Mechanical, thermal, and electrical properties of graphene-epoxy nanocomposites—A review. Polymers 2016, 8, 281. [Google Scholar] [CrossRef]
- Akter, M.; Ozdemir, H.; Bilisik, K. Epoxy/Graphene Nanoplatelet (GNP) Nanocomposites: An Experimental Study on Tensile, Compressive, and Thermal Properties. Polymers 2024, 16, 1483. [Google Scholar] [CrossRef]
- Kong, W.; Kum, H.; Bae, S.H.; Shim, J.; Kim, H.; Kong, L.; Meng, Y.; Wang, K.; Kim, C.; Kim, J. Path towards graphene commercialization from lab to market. Nat. Nanotechnol. 2019, 14, 927–938. [Google Scholar] [CrossRef]
- Gutiérrez-Cruz, A.; Ruiz-Hernández, A.R.; Vega-Clemente, J.F.; Luna-Gazcón, D.G.; Campos-Delgado, J. A review of top-down and bottom-up synthesis methods for the production of graphene, graphene oxide and reduced graphene oxide. J. Mater. Sci. 2022, 57, 14543–14578. [Google Scholar] [CrossRef]
- Santhiran, A.; Iyngaran, P.; Abiman, P.; Kuganathan, N. Graphene synthesis and its recent advances in applications—A review. C 2021, 7, 76. [Google Scholar] [CrossRef]
- Merzhanov, A.G. The chemistry of self-propagating high-temperature synthesis. J. Mater. Chem. 2004, 14, 1779–1786. [Google Scholar] [CrossRef]
- Merzhanov, A.G.; Sharivker, S.Y. Self-propagating high-temperature synthesis of carbides, nitrides, and borides. In Materials Science of Carbides, Nitrides and Borides; Springer: Dordrecht, The Netherlands, 1999; pp. 205–222. [Google Scholar]
- Wang, L.; Wei, B.; Dong, P.; Miao, Q.; Liu, Z.; Xu, F.; Wu, J.; Lou, J.; Vajtai, R.; Fei, W. Large-scale synthesis of few-layer graphene from magnesium and different carbon sources and its application in dye-sensitized solar cells. Mater. Des. 2016, 92, 462–470. [Google Scholar] [CrossRef]
- Voznyakovskii, A.; Vozniakovskii, A.; Kidalov, S. New Way of Synthesis of Few-Layer Graphene Nanosheets by the Self Propagating High-Temperature Synthesis Method from Biopolymers. Nanomaterials 2022, 12, 657. [Google Scholar] [CrossRef] [PubMed]
- ISO/TS 80004-13:2024; Nanotechnologies—Vocabulary—Part 13: Graphene and Other Two-Dimensional (2D) Materials. ISO: Geneva, Switzerland, 2024.
- Voznyakovskii, A.; Neverovskaya, A.; Vozniakovskii, A.; Kidalov, S. A Quantitative Chemical Method for Determining the Surface Concentration of Stone–Wales Defects for 1D and 2D Carbon Nanomaterials. Nanomaterials 2022, 12, 883. [Google Scholar] [CrossRef] [PubMed]
- Kidalov, S.; Voznyakovskii, A.; Vozniakovskii, A.; Titova, S.; Auchynnikau, Y. The Effect of Few-Layer Graphene on the Complex of Hardness, Strength, and Thermo Physical Properties of Polymer Composite Materials Produced by Digital Light Processing (DLP) 3D Printing. Materials 2023, 16, 1157. [Google Scholar] [CrossRef]
- Dideykin, A.; Aleksenskiy, A.; Kirilenko, D.; Brunkov, P.; Goncharov, V.; Baidakova, M.; Sakseev, D.; Vul, A.Y. Monolayer graphene from graphite oxide. Diam. Relat. Mater. 2011, 20, 105–108. [Google Scholar] [CrossRef]
- ISO 178:2019; Plastics—Determination of Flexural Properties. ISO: Geneva, Switzerland, 2019.
- ISO 527-2:2012; Plastics—Determination of Tensile Properties. ISO: Geneva, Switzerland, 2012.
- ISO 604:2002; Plastics—Determination of Compressive Properties. ISO: Geneva, Switzerland, 2002.
- Maggira, M.; Deliyanni, E.A.; Samanidou, V.F. Synthesis of graphene oxide based sponges and their study as sorbents for sample preparation of cow milk prior to HPLC determination of sulfonamides. Molecules 2019, 24, 2086. [Google Scholar] [CrossRef]
- Díez-Betriu, X.; Álvarez-García, S.; Botas, C.; Álvarez, P.; Sánchez-Marcos, J.; Prieto, C.; Menéndez, R.; de Andrés, A. Raman spectroscopy for the study of reduction mechanisms and optimization of conductivity in graphene oxide thin films. J. Mater. Chem. C 2013, 1, 6905–6912. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef]
- Tucureanu, V.; Matei, A.; Avram, A.M. FTIR Spectroscopy for Carbon Family Study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef]
- Vozniakovskii, A.; Kidalov, S.; Otvalko, J.; Neverovskaia, A.Y. Characteristics and mechanical properties of composites based on nitrile butadiene rubber using graphene nanoplatelets. J. Compos. Mater. 2020, 54, 3351–3364. [Google Scholar] [CrossRef]
- Teng, C.C.; Ma, C.C.M.; Lu, C.H.; Yang, S.Y.; Lee, S.H.; Hsiao, M.C.; Yen, M.Y.; Chiou, K.C.; Lee, T.M. Thermal conductivity and structure of non-covalent functionalized graphene/epoxy composites. Carbon 2011, 49, 5107–5116. [Google Scholar] [CrossRef]
- Wang, F.; Drzal, L.T.; Qin, Y.; Huang, Z. Mechanical properties and thermal conductivity of graphene nanoplatelet/epoxy composites. J. Mater. Sci. 2015, 50, 1082–1093. [Google Scholar] [CrossRef]
- Fu, Y.X.; He, Z.X.; Mo, D.C.; Lu, S.S. Thermal conductivity enhancement of epoxy adhesive using graphene sheets as additives. Int. J. Therm. Sci. 2014, 86, 276–283. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Cheng, Y.; Pei, Q.X.; Wang, C.M.; Xiang, Y. Thermal conductivity of defective graphene. Phys. Lett. A 2012, 376, 3668–3672. [Google Scholar] [CrossRef]
- Tang, L.C.; Wan, Y.J.; Yan, D.; Pei, Y.B.; Zhao, L.; Li, Y.B.; Wu, L.B.; Jiang, J.X.; Lai, G.Q. The effect of graphene dispersion on the mechanical properties of graphene/epoxy composites. Carbon 2013, 60, 16–27. [Google Scholar] [CrossRef]
- Prolongo, M.G.; Salom, C.; Arribas, C.; Sánchez-Cabezudo, M.; Masegosa, R.M.; Prolongo, S.G. Influence of graphene nanoplatelets on curing and mechanical properties of graphene/epoxy nanocomposites. J. Therm. Anal. Calorim. 2015, 125, 629–636. [Google Scholar] [CrossRef]
- He, Y.; Fan, X.; Huang, Y.; Liu, C.; Zhao, Z.; Zhu, M. Experimental and theoretical evaluations on the parallel-aligned graphene oxide hybrid epoxy composite coating toward wear resistance. Carbon 2023, 217, 118629. [Google Scholar] [CrossRef]
- Littmann, W.E.; Widner, R.L. Propagation of Contact Fatigue From Surface and Subsurface Origins. J. Basic Eng. 1966, 88, 624–635. [Google Scholar] [CrossRef]


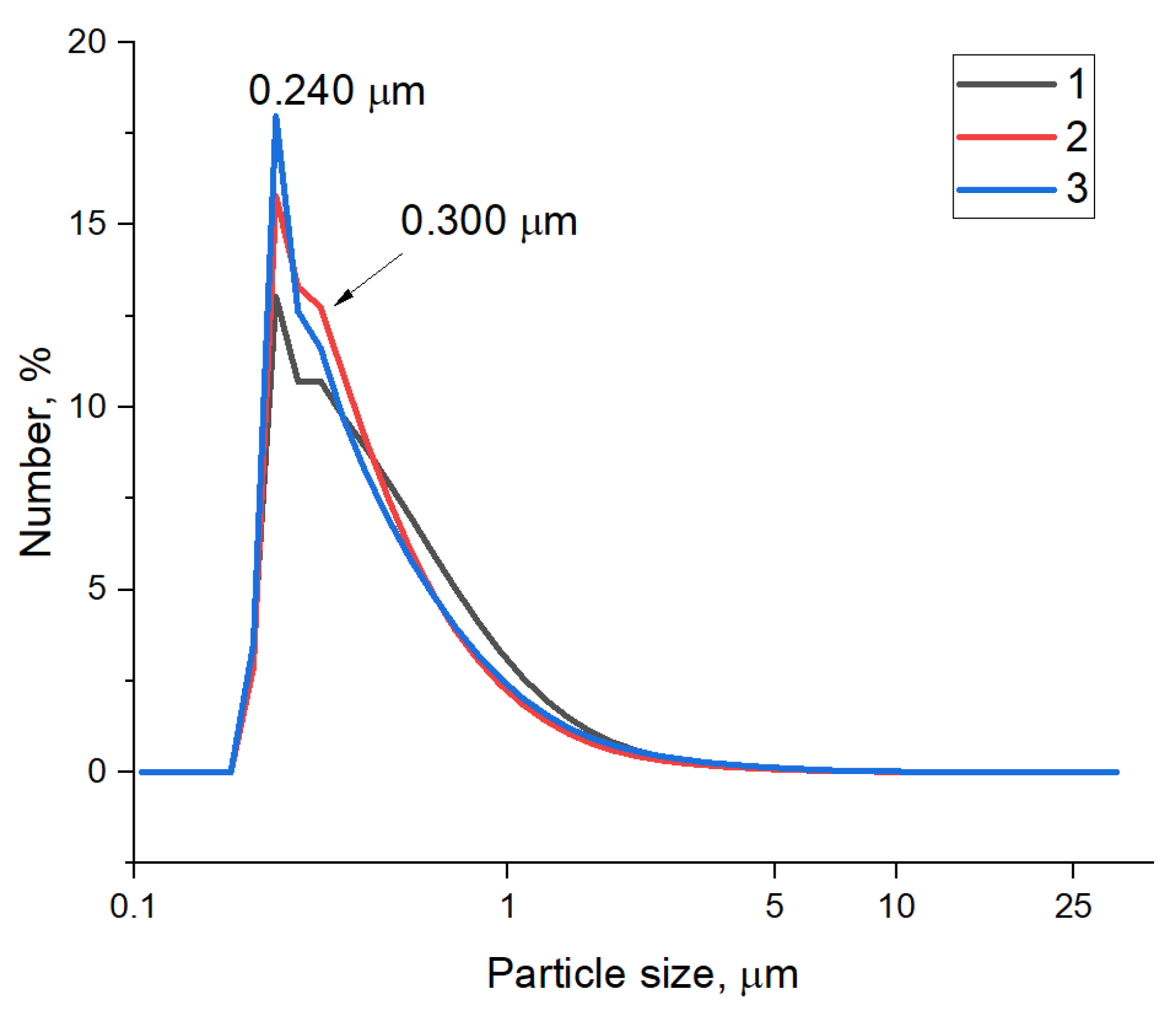


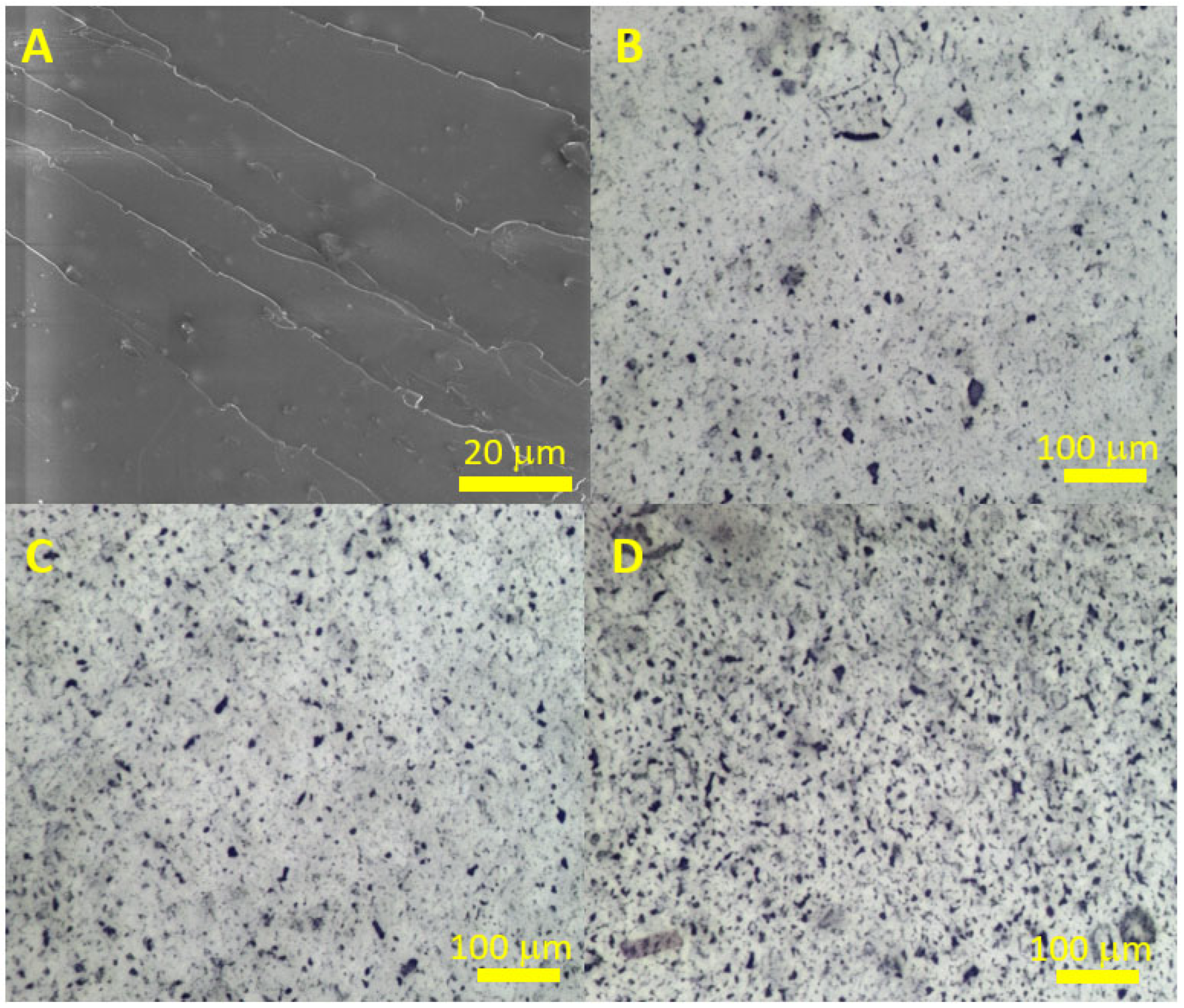


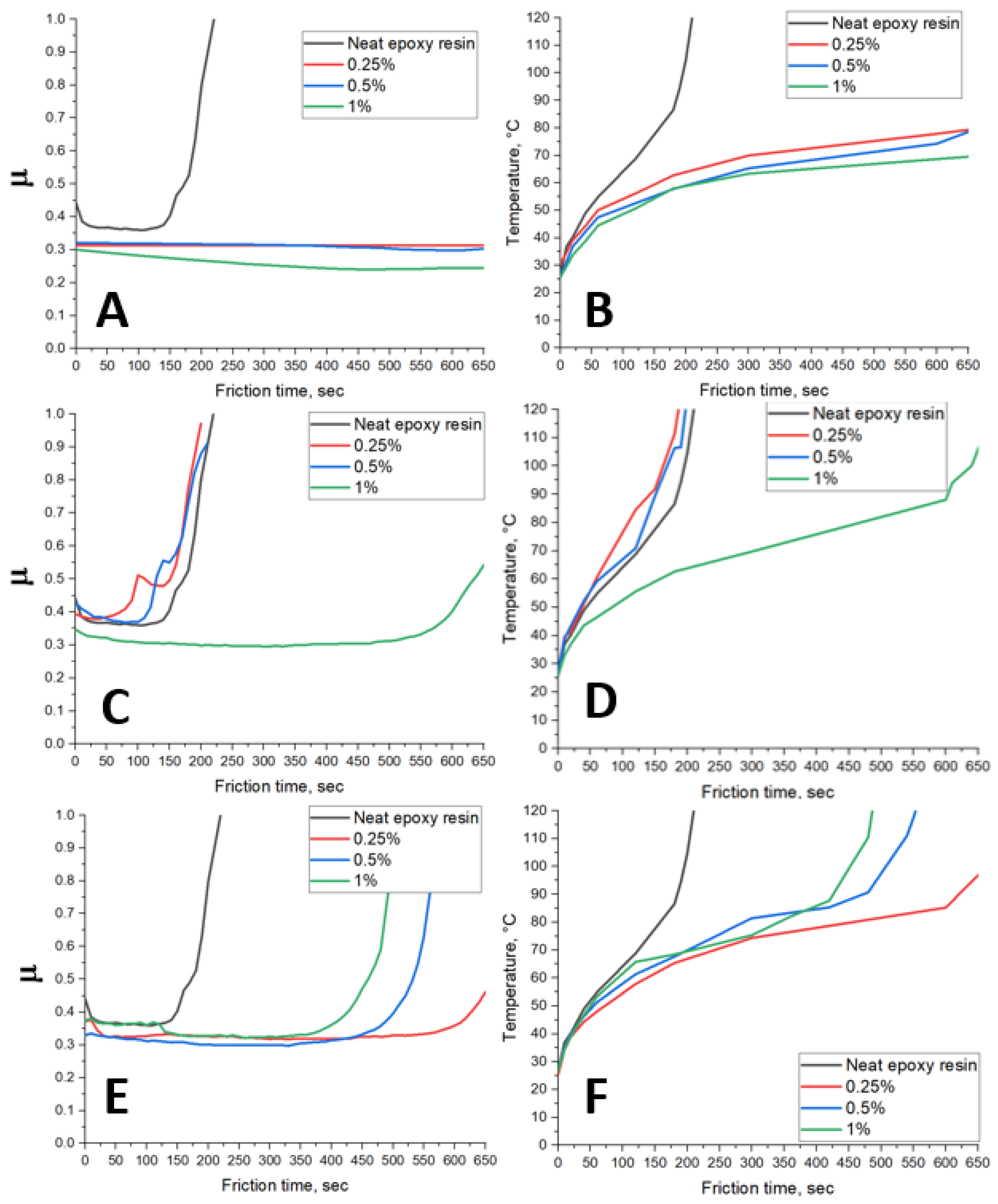
| Sample | C, at. % ± 1% | O, at. % ± 1% | N, at. % ± 1% |
|---|---|---|---|
| FLG from cellulose | 84 | 6 | 10 |
| FLG from glucose | 78 | 11 | 11 |
| FLG from lignin | 89 | 7 | 4 |
| Sample | BET Sample, m2/g ± 6% | Vpore, mL/g ± 6% | Vmicro, mL/g ± 6% |
|---|---|---|---|
| FLG from cellulose | 340 | 0.225 | 0.12 |
| FLG from glucose | 75 | 0.109 | 0 |
| FLG from lignin | 238 | 0.188 | 0.05 |
| Characteristic | FLG from Lignin | rGO |
|---|---|---|
| Tensile strength | −15% (0.5 wt.%) | +10% (0.5 wt.%) |
| Compressive strength | +40% (0.5 wt.%) | +15% (0.5 wt.%) |
| Bending strength | +15% (0.5 wt.%) | +7% (0.5 wt.%) |
| Thermal conductivity | +40% (0.5 wt.%) | +12% (0.5 wt.%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podlozhnyuk, N.; Vozniakovskii, A.; Kidalov, S.; Voznyakovskii, A. Performance Properties of Epoxy Resin Modified with Few-Layer Graphene Obtained by the Method of Self-Propagating High-Temperature Synthesis. Polymers 2025, 17, 812. https://doi.org/10.3390/polym17060812
Podlozhnyuk N, Vozniakovskii A, Kidalov S, Voznyakovskii A. Performance Properties of Epoxy Resin Modified with Few-Layer Graphene Obtained by the Method of Self-Propagating High-Temperature Synthesis. Polymers. 2025; 17(6):812. https://doi.org/10.3390/polym17060812
Chicago/Turabian StylePodlozhnyuk, Nikita, Aleksei Vozniakovskii, Sergey Kidalov, and Alexander Voznyakovskii. 2025. "Performance Properties of Epoxy Resin Modified with Few-Layer Graphene Obtained by the Method of Self-Propagating High-Temperature Synthesis" Polymers 17, no. 6: 812. https://doi.org/10.3390/polym17060812
APA StylePodlozhnyuk, N., Vozniakovskii, A., Kidalov, S., & Voznyakovskii, A. (2025). Performance Properties of Epoxy Resin Modified with Few-Layer Graphene Obtained by the Method of Self-Propagating High-Temperature Synthesis. Polymers, 17(6), 812. https://doi.org/10.3390/polym17060812










