Recent Advances in Silk Fibroin-Based Composites for Bone Repair Applications: A Review
Abstract
1. Introduction
2. Overview of Bone Tissue
2.1. Classification of Bone Tissue
2.2. Bone Tissue Composition and Structure
2.3. Bone Tissue Healing Mechanisms and Repair Materials
3. Characteristics of Silk Fibroin Biomaterials
3.1. Composition and Structure of Silk Fibroin
3.2. The Characteristics of Silk Fibroin
3.2.1. Biocompatibility
3.2.2. Degradability
3.3. Response to Osteogenic Signaling
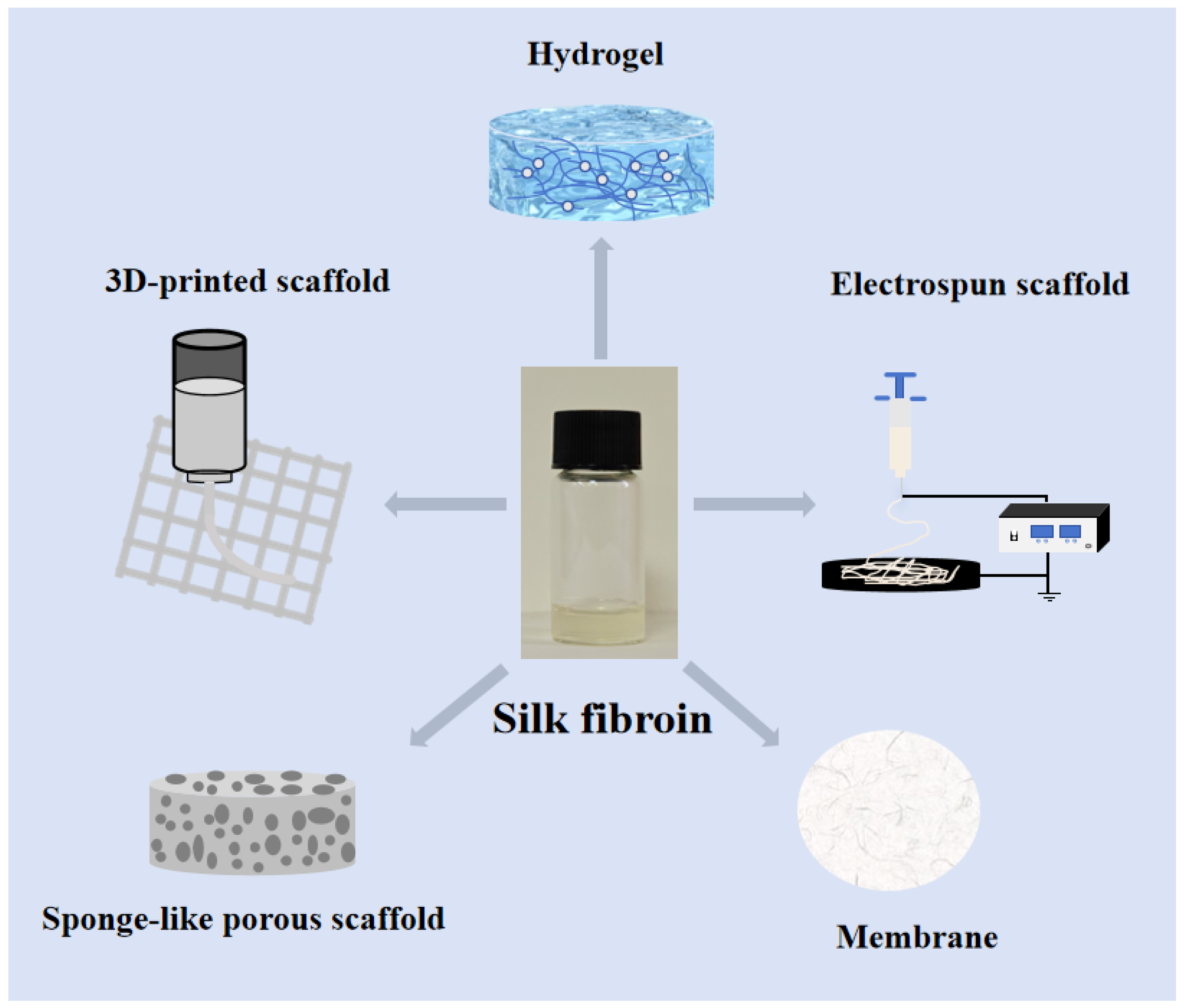
4. Current Status of Silk Protein Applications in Bone Repair
4.1. Silk Fibroin Scaffold Bone Repair Materials
4.1.1. Silk Fibroin Sponge-like Porous Scaffold Bone Repair
4.1.2. 3D-Printed Silk Fibroin Scaffold
4.1.3. Electrospun Silk Fibroin Scaffold
4.1.4. Silk Fibroin Hydrogel Bone Repair Material
4.1.5. Silk Fibroin Membrane Bone Repair Material
5. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Colón, C.J.P.; Molina-Vicenty, I.L.; Frontera-Rodríguez, M.; García-Ferré, A.; Rivera, B.P.; Cintrón-Vélez, G.; Frontera-Rodríguez, S. Muscle and Bone Mass Loss in the Elderly Population: Advances in diagnosis and treatment. J. Biomed. 2018, 3, 40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, W.; Schnitzler, V.; Tancret, F.; Bouler, J.-M. Calcium phosphate cements for bone substitution: Chemistry, handling and mechanical properties. Acta Biomater. 2014, 10, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Inj. Int. J. Care Inj. 2005, 36, 20–27. [Google Scholar] [CrossRef]
- Bajaj, A.K.; Wongworawat, A.A.; Punjabi, A. Management of alveolar clefts. J. Craniofac. Surg. 2003, 14, 840–846. [Google Scholar] [CrossRef]
- Reilly, D.T.; Burstein, A.H. The elastic and ultimate properties of compact bone tissue. J. Biomech. 1975, 8, 393–405. [Google Scholar] [CrossRef]
- Doblaré, M.; Garcıa, J.M.; Gómez, M.J. Modelling bone tissue fracture and healing: A review. Eng. Fract. Mech. 2004, 71, 1809–1840. [Google Scholar] [CrossRef]
- Marrella, A.; Lee, T.Y.; Lee, D.H.; Karuthedom, S.; Syla, D.; Chawla, A.; Khademhosseini, A.; Jang, H.L. Engineering vascularized and innervated bone biomaterials for improved skeletal tissue regeneration. Mater. Today 2018, 21, 362–376. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, T.; Chen, M.; Yao, K.; Huang, X.; Zhang, B.; Li, Y.; Liu, J.; Wang, Y.; Zhao, Z. Bone physiological microenvironment and healing mechanism: Basis for future bone-tissue engineering scaffolds. Bioact. Mater. 2021, 6, 4110–4140. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, D.; Wang, T. Hierarchical structures of bone and bioinspired bone tissue engineering. Small 2016, 12, 4611–4632. [Google Scholar] [CrossRef]
- Aerssens, J.; Boonen, S.; Lowet, G.; Dequeker, J. Interspecies differences in bone composition, density, and quality: Potential implications for in vivo bone research. Endocrinology 1998, 139, 663–670. [Google Scholar] [CrossRef]
- Bilgiç, E.; Boyacıoğlu, Ö.; Gizer, M.; Korkusuz, P.; Korkusuz, F. Architecture of bone tissue and its adaptation to pathological conditions. In Comparative Kinesiology of the Human Body; Academic Press: Cambridge, MA, USA, 2020; pp. 71–90. [Google Scholar]
- Hu, K.; Olsen, B.R. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone 2016, 91, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Malda, J.; Klein, T.J.; Upton, Z. The roles of hypoxia in the In vitro engineering of tissues. Tissue Eng. 2007, 13, 2153–2162. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.M. Overview of the fracture healing cascade. Injury 2005, 36 (Suppl. S3), S5–S7. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, S.; Miramini, S.; Edwards, G.; Rotne, R.; Xu, J.; Ebeling, P.; Zhang, L. The investigation of bone fracture healing under intramembranous and endochondral ossification. Bone Rep. 2021, 14, 100740. [Google Scholar] [CrossRef]
- Thompson, E.M.; Matsiko, A.; Farrell, E.; Kelly, D.J.; O’Brien, F.J. Recapitulating endochondral ossification: A promising route to in vivo bone regeneration. J. Tissue Eng. Regen. Med. 2015, 9, 889–902. [Google Scholar] [CrossRef]
- Garg, P.; Mazur, M.M.; Buck, A.C.; Wandtke, M.E.; Liu, J.; Ebraheim, N.A. Prospective review of mesenchymal stem cells differentiation into osteoblasts. Orthop. Surg. 2017, 9, 13–19. [Google Scholar] [CrossRef]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef]
- Huang, G.; Li, F.; Zhao, X.; Ma, Y.; Li, Y.; Lin, M.; Jin, G.; Lu, T.J.; Genin, G.M.; Xu, F. Functional and biomimetic materials for engineering of the three-dimensional cell microenvironment. Chem. Rev. 2017, 117, 12764–12850. [Google Scholar] [CrossRef]
- Han, F.; Zhu, C.; Guo, Q.; Yang, H.; Li, B. Cellular modulation by the elasticity of biomaterials. J. Mater. Chem. B 2016, 4, 9–26. [Google Scholar] [CrossRef]
- Inoue, S.; Tanaka, K.; Arisaka, F.; Kimura, S.; Ohtomo, K.; Mizuno, S. Silk fibroin of Bombyx mori is secreted, assembling a high molecular mass elementary unit consisting of H-chain, L-chain, and P25, with a 6:6:1 molar ratio. J. Biol. Chem. 2000, 275, 40517–40528. [Google Scholar] [CrossRef]
- Koh, L.D.; Cheng, Y.; Teng, C.P.; Khin, Y.W.; Loh, X.J.; Tee, S.Y.; Low, M.; Ye, E.; Yu, H.D.; Zhang, Y.W.; et al. Structures, mechanical properties and applications of silk fibroin materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Kweon, H.Y.; Park, Y.H. Structural and conformational changes of regenerated Antheraea pernyi silk fibroin films treated with methanol solution. J. Appl. Polym. Sci. 1999, 73, 2887–2894. [Google Scholar] [CrossRef]
- Minoura, N.; Aiba, S.; Higuchi, M.; Gotoh, Y.; Tsukada, M.; Imai, Y. Attachment and growth of fibroblast cells on silk fibroin. Biochem. Biophys. Res. Commun. 1995, 208, 511–516. [Google Scholar] [CrossRef]
- Padol, A.; Jayakumar, K.; Shridhar, N.; Swamy, N.; Swamy, N.; Mohan, K. Safety evaluation of silk protein film (a novel wound healing agent) in terms of acute dermal toxicity, acute dermal irritation and skin sensitization. Toxicol. Int. 2011, 18, 17–21. [Google Scholar] [CrossRef]
- Wharram, S.E.; Zhang, X.; Kaplan, D.L.; McCarthy, S.P. Electrospun Silk Material Systems for Wound Healing. Macromol. Biosci. 2010, 10, 246–257. [Google Scholar] [CrossRef]
- Sakabe, H.; Ito, H.; Miyamoto, T.; Noishiki, Y.; Ha, W.S. In vivo blood compatibility of regenerated silk fibroin. Sen’i Gakkaishi 1989, 45, 487–490. [Google Scholar] [CrossRef]
- Wang, H.J.; Di, L.; Ren, Q.S.; Wang, J.Y. Applications and degradation of proteins used as tissue engineering materials. Materials 2009, 2, 613–635. [Google Scholar] [CrossRef]
- You, R.; Xu, Y.; Liu, Y.; Li, X.; Li, M. Comparison of the in vitro and in vivo degradations of silk fibroin scaffolds from mulberry and nonmulberry silkworms. Biomed. Mater. 2014, 10, 015003. [Google Scholar] [CrossRef]
- Mandal, B.B.; Kundu, S.C. Cell proliferation and migration in silk fibroin 3D scaffolds. Biomaterials 2009, 30, 2956–2965. [Google Scholar] [CrossRef]
- Mauney, J.R.; Nguyen, T.; Gillen, K.; Kirker-Head, C.; Gimble, J.M.; Kaplan, D.L. Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials 2007, 28, 5280–5290. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Gil, E.S.; Shi, H.; Kim, H.J.; Lee, K.; Kaplan, D.L. Relationships between degradability of silk scaffolds and osteogenesis. Biomaterials 2010, 31, 6162–6172. [Google Scholar] [CrossRef] [PubMed]
- Langenbach, F.; Handschel, J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res. Ther. 2013, 4, 117. [Google Scholar] [CrossRef] [PubMed]
- Hilton, M.J.; Tu, X.; Wu, X.; Bai, S.; Zhao, H.; Kobayashi, T.; Kronenberg, H.M.; Teitelbaum, S.L.; Ross, F.P.; Kopan, R.; et al. Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat. Med. 2008, 14, 306–314. [Google Scholar] [CrossRef]
- Jung, S.R.; Song, N.J.; Yang, D.K.; Cho, Y.J.; Kim, B.J.; Hong, J.W.; Yun, U.J.; Jo, D.G.; Lee, Y.M.; Choi, S.Y.; et al. Silk proteins stimulate osteoblast differentiation by suppressing the Notch signaling pathway in mesenchymal stem cells. Nutr. Res. 2013, 33, 162–170. [Google Scholar] [CrossRef]
- Cheng, N.; Dai, J.; Cheng, X.; Li, S.; Miron, R.J.; Wu, T.; Chen, W.; Zhang, Y.; Shi, B. Porous CaP/silk composite scaffolds to repair femur defects in an osteoporotic model. J. Mater. Sci. Mater. Med. 2013, 24, 1963–1975. [Google Scholar] [CrossRef]
- Feng, X.; Xu, P.; Shen, T.; Zhang, Y.; Ye, J.; Gao, C. Influence of pore architectures of silk fibroin/collagen composite scaffolds on the regeneration of osteochondral defects in vivo. J. Mater. Chem. B 2020, 8, 391–405. [Google Scholar] [CrossRef]
- Song, J.; Kim, J.; Woo, H.-M.; Yoon, B.; Park, H.; Park, C.; Kang, B.-J. Repair of rabbit radial bone defects using bone morphogenetic protein-2 combined with 3D porous silk fibroin/β-tricalcium phosphate hybrid scaffolds. J. Biomater. Sci. Polym. Ed. 2018, 29, 716–729. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Tomkins, M.; Fajardo, R.; Meinel, L.; Snyder, B.; Wade, K.; Chen, J.; Vunjak-Novakovic, G.; Kaplan, D.L. Porous silk fibroin 3-D scaffolds for delivery of bone morphogenetic protein-2 in vitro and in vivo. J. Biomed. Mater. Res. Part A: Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2006, 78, 324–334. [Google Scholar] [CrossRef]
- Rameshbabu, A.P.; Bankoti, K.; Datta, S.; Subramani, E.; Apoorva, A.; Ghosh, P.; Jana, S.; Manchikanti, P.; Roy, S.; Chaudhury, K.; et al. Bioinspired 3D porous human placental derived extracellular matrix/silk fibroin sponges for accelerated bone regeneration. Mater. Sci. Eng. C 2020, 113, 110990. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Sun, X.; Zhan, C.; Li, Z.; Qiu, L.; Luo, R.; Liu, H.; Sun, X.; Li, R.; et al. Silk fibroin/collagen/hydroxyapatite scaffolds obtained by 3D printing technology and loaded with recombinant human erythropoietin in the reconstruction of alveolar bone defects. ACS Biomater. Sci. Eng. 2022, 8, 5245–5256. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Sun, M.; Hu, X.; Ren, B.; Cheng, J.; Li, C.; Duan, X.; Fu, X.; Zhang, J.; Chen, H.; et al. Structurally and functionally optimized silk-fibroin–gelatin scaffold using 3D printing to repair cartilage injury in vitro and in vivo. Adv. Mater. 2017, 29, 1701089. [Google Scholar] [CrossRef]
- Patel, D.K.; Dutta, S.D.; Hexiu, J.; Ganguly, K.; Lim, K.-T. 3D-printable chitosan/silk fibroin/cellulose nanoparticle scaffolds for bone regeneration via M2 macrophage polarization. Carbohydr. Polym. 2022, 281, 119077. [Google Scholar] [CrossRef] [PubMed]
- Bojedla, S.S.R.; Kattimani, V.; Alwala, A.M.; Nikzad, M.; Masood, S.H.; Riza, S.; Pati, F. Augmented repair and regeneration of critical size rabbit calvaria defects with 3D printed silk fibroin microfibers reinforced pcl composite scaffolds. Biomed. Mater. Devices 2023, 1, 942–955. [Google Scholar] [CrossRef]
- Yeon, Y.K.; Park, H.S.; Lee, J.M.; Lee, J.S.; Lee, Y.J.; Sultan, M.T.; Seo, Y.B.; Lee, O.J.; Kim, S.H.; Park, C.H. New concept of 3D printed bone clip (polylactic acid/hydroxyapatite/silk composite) for internal fixation of bone fractures. J. Biomater. Sci. Polym. Ed. 2018, 29, 894–906. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, M.; Xie, Q.; Sun, H.; Huang, Y.; Zhang, D.; Yu, Z.; Bi, X.; Chen, J.; Wang, J.; et al. Electrospun silk fibroin/poly (lactide-co-ε-caprolactone) nanofibrous scaffolds for bone regeneration. Int. J. Nanomed. 2016, 11, 1483–1500. [Google Scholar]
- Yang, S.Y.; Hwang, T.H.; Che, L.; Oh, J.S.; Ha, Y.; Ryu, W. Membrane-reinforced three-dimensional electrospun silk fibroin scaffolds for bone tissue engineering. Biomed. Mater. 2015, 10, 035011. [Google Scholar] [CrossRef]
- Wu, J.; Wang, S.; Zheng, Z.; Li, J. Fabrication of Biologically Inspired Electrospun Colagen/Silk fibroin/bioactive glass composited nanofibrous scaffold to accelerate the treatment efficiency of bone repair. Regen. Ther. 2022, 21, 122–138. [Google Scholar] [CrossRef]
- Ko, E.; Lee, J.S.; Kim, H.; Yang, S.Y.; Yang, D.; Yang, K.; Lee, J.; Shin, J.; Yang, H.S.; Ryu, W.; et al. Electrospun silk fibroin nanofibrous scaffolds with two-stage hydroxyapatite functionalization for enhancing the osteogenic differentiation of human adipose-derived mesenchymal stem cells. ACS Appl. Mater. Interfaces 2017, 10, 7614–7625. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, A.; Liu, Y.; Jiao, D.; Zeng, D.; Wang, X.; Cao, L.; Jiang, X. Enhanced bone regeneration of the silk fibroin electrospun scaffolds through the modification of the graphene oxide functionalized by BMP-2 peptide. Int. J. Nanomed. 2019, 14, 733–751. [Google Scholar] [CrossRef]
- Yan, Y.; Cheng, B.; Chen, K.; Cui, W.; Qi, J.; Li, X.; Deng, L. Enhanced osteogenesis of bone marrow-derived mesenchymal stem cells by a functionalized silk fibroin hydrogel for bone defect repair. Adv. Healthc. Mater. 2019, 8, 1801043. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zheng, K.; Huang, X.; Liu, J.; Liu, H.; Boccaccini, A.R.; Wan, Y.; Guo, X.; Shao, Z. Thermally triggered injectable chitosan/silk fibroin/bioactive glass nanoparticle hydrogels for in-situ bone formation in rat calvarial bone defects. Acta Biomater. 2019, 91, 60–71. [Google Scholar] [CrossRef]
- Zou, Y.P.; Liang, H.F.; Wang, B.; Zhang, Q.C.; Su, D.H.; Lu, S.Y.; Zhang, Q.Y.; Wu, T.; Xiao, L.; Xiao, Y.; et al. Precipitation-based silk fibroin fast gelling, highly adhesive, and magnetic nanocomposite hydrogel for repair of irregular bone defects. Adv. Funct. Mater. 2023, 33, 2302442. [Google Scholar] [CrossRef]
- Wang, R.; He, X.; Chen, Z.; Su, S.; Bai, J.; Liu, H.; Zhou, F. A nanoparticle reinforced microporous methacrylated silk fibroin hydrogel to promote bone regeneration. Biomater. Sci. 2024, 12, 2121–2135. [Google Scholar] [CrossRef]
- Hu, Z.C.; Lu, J.Q.; Zhang, T.W.; Liang, H.F.; Yuan, H.; Su, D.H.; Ding, W.; Lian, R.X.; Ge, Y.X.; Liang, B.; et al. Piezoresistive MXene/Silk fibroin nanocomposite hydrogel for accelerating bone regeneration by Re-establishing electrical microenvironment. Bioact. Mater. 2023, 22, 1–17. [Google Scholar] [CrossRef]
- Cai, Y.; Guo, J.; Chen, C.; Yao, C.; Chung, S.-M.; Yao, J.; Lee, I.-S.; Kong, X. Silk fibroin membrane used for guided bone tissue regeneration. Mater. Sci. Eng. C 2017, 70, 148–154. [Google Scholar] [CrossRef]
- Lee, S.W.; Um, I.C.; Kim, S.G.; Cha, M.S. Evaluation of bone formation and membrane degradation in guided bone regeneration using a 4-hexylresorcinol-incorporated silk fabric membrane. Maxillofac. Plast. Reconstr. Surg. 2015, 37, 1–5. [Google Scholar] [CrossRef]
- Seok, H.; Kim, S.G.; Kweon, H.; Jo, Y.Y.; Lee, K.G.; Kang, T.Y.; Chae, W.S.; Min, S.K.; Ahn, J.H.; Park, J.W.; et al. Comparison of different concentrations of tetracycline-loaded silk fibroin membranes on the guided bone regeneration in the rat calvarial defect model. Tissue Eng. Regen. Med. 2014, 11, 476–482. [Google Scholar] [CrossRef]
- Luo, D.; Yao, C.; Zhang, R.; Zhao, R.; Iqbal, M.Z.; Mushtaq, A.; Lee, I.S.; Kong, X. Silk fibroin/collagen blended membrane fabricated via a green papermaking method for potential guided bone regeneration application: In vitro and in vivo evaluation. ACS Biomater. Sci. Eng. 2021, 7, 5788–5797. [Google Scholar] [CrossRef]
- Lee, S.W.; Park, Y.T.; Kim, S.G.; Kweon, H.; Jo, Y.Y.; Lee, H.S. The effects of tetracycline-loaded silk fibroin membrane on guided bone regeneration in a rabbit calvarial defect model. Maxillofac. Plast. Reconstr. Surg. 2012, 34, 293–298. [Google Scholar]
- Uebersax, L.; Apfel, T.; Nuss, K.M.; Vogt, R.; Kim, H.Y.; Meinel, L.; Kaplan, D.L.; Auer, J.A.; Merkle, H.P.; von Rechenberg, B. Biocompatibility and osteoconduction of macroporous silk fibroin implants in cortical defects in sheep. Eur. J. Pharm. Biopharm. 2013, 85, 107–118. [Google Scholar] [CrossRef]
- Lv, Q.; Feng, Q.L. Preparation of 3-D regenerated fibroin scaffolds with freeze drying method and freeze drying/foaming technique. J. Mater. Sci. Mater. Med. 2006, 17, 1349–1356. [Google Scholar] [CrossRef]
- Song, J.-H.; Kim, J.-H.; Park, S.; Kang, W.; Kim, H.-W.; Kim, H.-E.; Jang, J.-H. Signalling responses of osteoblast cells to hydroxyapatite: The activation of ERK and SOX9. J. Bone Miner. Metab. 2008, 26, 138–142. [Google Scholar] [CrossRef]
- Liu, H.; Peng, H.; Wu, Y.; Zhang, C.; Cai, Y.; Xu, G.; Li, Q.; Chen, X.; Ji, J.; Zhang, Y.; et al. The promotion of bone regeneration by nanofibrous hydroxyapatite/chitosan scaffolds by effects on integrin-BMP/Smad signaling pathway in BMSCs. Biomaterials 2013, 34, 4404–4417. [Google Scholar] [CrossRef]
- Chen, B.; Lin, T.; Yang, X.; Li, Y.; Xie, D.; Zheng, W.; Cui, H.; Deng, W.; Tan, X. Low-magnitude, high-frequency vibration promotes the adhesion and the osteogenic differentiation of bone marrow-derived mesenchymal stem cells cultured on a hydroxyapatite-coated surface: The direct role of Wnt/β-catenin signaling pathway activation. Int. J. Mol. Med. 2016, 38, 1531–1540. [Google Scholar] [CrossRef]
- You, X.; Shen, Y.; Yu, W.; He, Y. Enhancement of tendon-bone healing following rotator cuff repair using hydroxyapatite with TGFβ1. Mol. Med. Rep. 2018, 17, 4981–4988. [Google Scholar] [CrossRef]
- Xia, L.; Lin, K.; Jiang, X.; Xu, Y.; Zhang, M.; Chang, J.; Zhang, Z. Enhanced osteogenesis through nano-structured surface design of macroporous hydroxyapatite bioceramic scaffolds via activation of ERK and p38 MAPK signaling pathways. J. Mater. Chem. B 2013, 1, 5403–5416. [Google Scholar] [CrossRef]
- Jiang, J.; Hao, W.; Li, Y.; Yao, J.; Shao, Z.; Li, H.; Yang, J.; Chen, S. Hydroxyapatite/regenerated silk fibroin scaffold-enhanced osteoinductivity and osteoconductivity of bone marrow-derived mesenchymal stromal cells. Biotechnol. Lett. 2013, 35, 1349–1350. [Google Scholar] [CrossRef]
- Bhumiratana, S.; Grayson, W.L.; Castaneda, A.; Rockwood, D.N.; Gil, E.S.; Kaplan, D.L.; Vunjak-Novakovic, G. Nucleation and growth of mineralized bone matrix on silk-hydroxyapatite composite scaffolds. Biomaterials 2011, 32, 2812–2820. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; She, H.; Wang, R.; Bai, F.; Xiang, B. A silk fibroin/chitosan/nanohydroxyapatite biomimetic bone scaffold combined with autologous concentrated growth factor promotes the proliferation and osteogenic differentiation of BMSCs and repair of critical bone defects. Regen. Ther. 2022, 21, 307–321. [Google Scholar] [CrossRef]
- Li, Q.; Xu, S.; Feng, Q.; Dai, Q.; Yao, L.; Zhang, Y.; Gao, H.; Dong, H.; Chen, D.; Cao, X. 3D printed silk-gelatin hydrogel scaffold with different porous structure and cell seeding strategy for cartilage regeneration. Bioact. Mater. 2021, 6, 3396–3410. [Google Scholar] [CrossRef]
- Huiwen, W.; Shuai, L.; Jia, X.; Shihao, D.; Kun, W.; Runhuai, Y.; Haisheng, Q.; Jun, L. 3D-printed nanohydroxyapatite/methylacrylylated silk fibroin scaffold for repairing rat skull defects. J. Biol. Eng. 2024, 18, 22. [Google Scholar] [CrossRef]
- Min, B.M.; Lee, G.; Kim, S.H.; Nam, Y.S.; Lee, T.S.; Park, W.H. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials 2004, 25, 1289–1297. [Google Scholar] [CrossRef]
- Park, S.Y.; Ki, C.S.; Park, Y.H.; Jung, H.M.; Woo, K.M.; Kim, H.J. Electrospun Silk Fibroin Scaffolds with Macropores for Bone Regeneration: An In Vitro and In Vivo Study. Tissue Eng. Part A 2010, 16, 1271–1279. [Google Scholar] [CrossRef]
- Xiang, J.; Li, Y.; Ren, M.; He, P.; Liu, F.; Jing, Z.; Li, Y.; Zhang, H.; Ji, P.; Yang, S. Sandwich-like nanocomposite electrospun silk fibroin membrane to promote osteogenesis and antibacterial activities. Appl. Mater. Today 2022, 26, 101273. [Google Scholar] [CrossRef]
- Numata, K.; Yamazaki, S.; Katashima, T.; Chuah, J.A.; Naga, N.; Sakai, T. Silk-Pectin Hydrogel with Superior Mechanical Properties, Biodegradability, and Biocompatibility. Macromol. Biosci. 2014, 14, 799–806. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Chakraborty, S.; Kundu, S.C. Freeze-gelled silk fibroin protein scaffolds for potential applications in soft tissue engineering. Int. J. Biol. Macromol. 2011, 49, 260–267. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Wang, S.; Zhao, J.; Xu, L.; Zhu, C.; Zeng, D.; Chen, J.; Zhang, Z.; Kaplan, D.L.; et al. The use of injectable sonication-induced silk hydrogel for VEGF(165) and BMP-2 delivery for elevation of the maxillary sinus floor. Biomaterials 2011, 32, 9415–9424. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, M.; Jiang, F.; Yin, S.; Lin, S.; Yang, G.; Lu, Y.; Zhang, W.; Jiang, X. Marginal sealing around integral bilayer scaffolds for repairing osteochondral defects based on photocurable silk hydrogels. Bioact. Mater. 2021, 6, 3976–3986. [Google Scholar] [CrossRef]
- Murphy, A.R.; St John, P.; Kaplan, D.L. Modification of silk fibroin using diazonium coupling chemistry and the effects on hMSC proliferation and differentiation. Biomaterials 2008, 29, 2829–2838. [Google Scholar] [CrossRef]
- Song, J.Y.; Kim, S.G.; Lee, J.W.; Chae, W.S.; Kweon, H.; Jo, Y.Y.; Lee, K.G.; Lee, Y.C.; Choi, J.Y.; Kim, J.Y. Accelerated healing with the use of a silk fibroin membrane for the guided bone regeneration technique. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2011, 112, e26–e33. [Google Scholar] [CrossRef] [PubMed]
- Reizabal, A.; Brito-Pereira, R.; Fernandes, M.; Castro, N.; Correia, V.; Ribeiro, C.; Costa, C.; Perez, L.; Vilas, J.; Lanceros-Méndez, S. Silk fibroin magnetoactive nanocomposite films and membranes for dynamic bone tissue engineering strategies. Materialia 2020, 12, 100709. [Google Scholar] [CrossRef]
| Types of Silk Fibroin-Based Composite Scaffolds | Main Components of Silk Fibroin Composite Scaffolds | Animal Models | Ref |
|---|---|---|---|
| sponge-like porous scaffold | Silk fibroin, CaP | Rat femur | [37] |
| sponge-like porous scaffold | Silk fibroin, collagen | Rabbit cartilage | [38] |
| sponge-like porous scaffold | Silk fibroin, β-tricalcium phosphate, Bone morphogenetic protein-2 | Rabbit left radius | [39] |
| sponge-like porous scaffold | Silk fibroin, Bone morphogenetic protein-2 | Mouse skull | [40] |
| sponge-like porous scaffold | placental-derived extracellular matrix | Rabbit tibia | [41] |
| 3D printing | Silk fibroin, Silk fibroin, Collagen, Hydroxyapatite, Recombinant human erythropoietin | Rabbit alveolar bone | [42] |
| 3D printing | Silk fibroin, Gelatin | Rabbit articular cartilage | [43] |
| 3D printing | Silk fibroin, Cellulose, Chitosan | Rat skull | [44] |
| 3D printing | Silk fibroin, Polycaprolactone | Rabbit skull | [45] |
| 3D printing | Silk fibroin, Polylactic acid, Hydroxyapatite | Rat femur | [46] |
| Electrospun | Silk fibroin, Lactide-co-ε-caprolactone, Human adipose-derived stem cells | Rat femur | [47] |
| Electrospun | Silk fibroin, Hydroxyapatite, BMP-2 | Rat skull | [48] |
| Electrospun | Silk fibroin, Bioactive glass, Collagen | Rat tibia | [49] |
| Electrospun | Silk fibroin, Hydroxyapatite, Polydopamine | Mouse skull | [50] |
| Electrospun | Silk fibroin, Graphene oxide, BMP-2 | Rat skull | [51] |
| Hydrogel | Silk fibroin, NapFFRGD | Mouse skull | [52] |
| Hydrogel | Silk fibroin, Bioactive glass, Chitosan | Rat skull | [53] |
| Hydrogel | Silk fibroin, Tannic Acid, Fe3O4 nanoparticles | Rat skull | [54] |
| Hydrogel | Silk fibroin, LPONITE | Rat skull | [55] |
| Hydrogel | Silk fibroin, MXene | Rat skull | [56] |
| Membrane | Silk fibroin | Rabbit skull | [57] |
| Membrane | Silk fibroin, 4-hexylresorcinol | Rabbit skull | [58] |
| Membrane | Silk fibroin, Tetracycline | Rat skull | [59] |
| Membrane | Silk Fibroin, Collagen | Subcutaneous in rats | [60] |
| Membrane | Silk Fibroin, Tetracycline | Rabbit skull | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Zhang, Q.; Xu, X.; Liu, Z.; Cheng, G.; Long, D.; Cheng, L.; Dai, F. Recent Advances in Silk Fibroin-Based Composites for Bone Repair Applications: A Review. Polymers 2025, 17, 772. https://doi.org/10.3390/polym17060772
Zhu S, Zhang Q, Xu X, Liu Z, Cheng G, Long D, Cheng L, Dai F. Recent Advances in Silk Fibroin-Based Composites for Bone Repair Applications: A Review. Polymers. 2025; 17(6):772. https://doi.org/10.3390/polym17060772
Chicago/Turabian StyleZhu, Siyu, Qian Zhang, Xiang Xu, Zulan Liu, Guotao Cheng, Dingpei Long, Lan Cheng, and Fangyin Dai. 2025. "Recent Advances in Silk Fibroin-Based Composites for Bone Repair Applications: A Review" Polymers 17, no. 6: 772. https://doi.org/10.3390/polym17060772
APA StyleZhu, S., Zhang, Q., Xu, X., Liu, Z., Cheng, G., Long, D., Cheng, L., & Dai, F. (2025). Recent Advances in Silk Fibroin-Based Composites for Bone Repair Applications: A Review. Polymers, 17(6), 772. https://doi.org/10.3390/polym17060772






