Nanofibrous Membrane-Based Stretchable Electrochemical Sweat Sensor for pH Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TPU Electrospun Membrane
2.3. Preparation of Silver Nanowires
2.4. Preparation of MWCNT Dispersion
2.5. Preparation of TPU Membrane Loaded with AgNWs
2.6. Preparation of TPU Membrane Loaded with AgNWs and MWCNT
2.7. Preparation of pH-Sensing Electrode
2.8. Characterizations
3. Results and Discussion
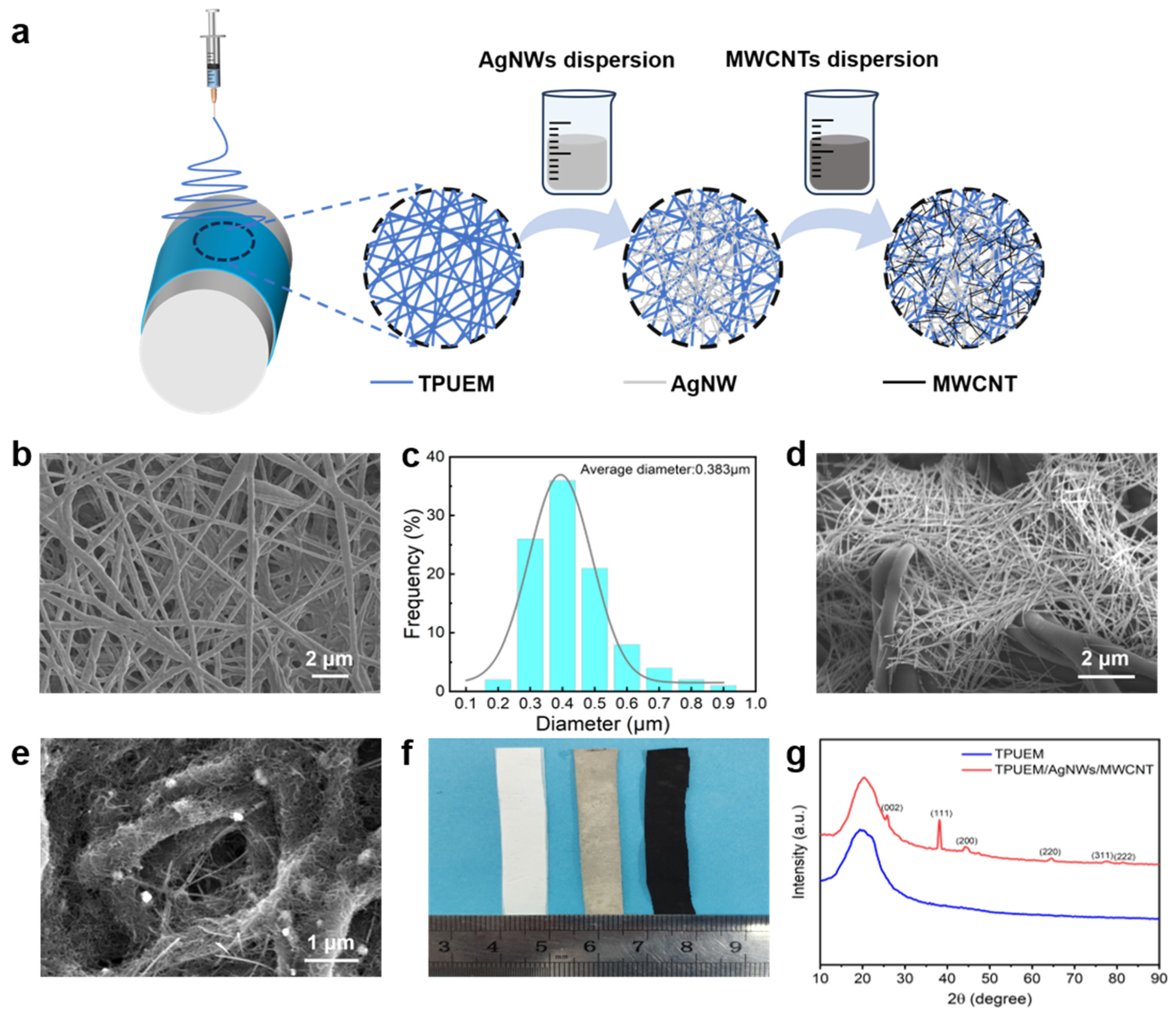
3.1. Fabrication of the Stretchable Electrode
3.2. Electrical Conductivity
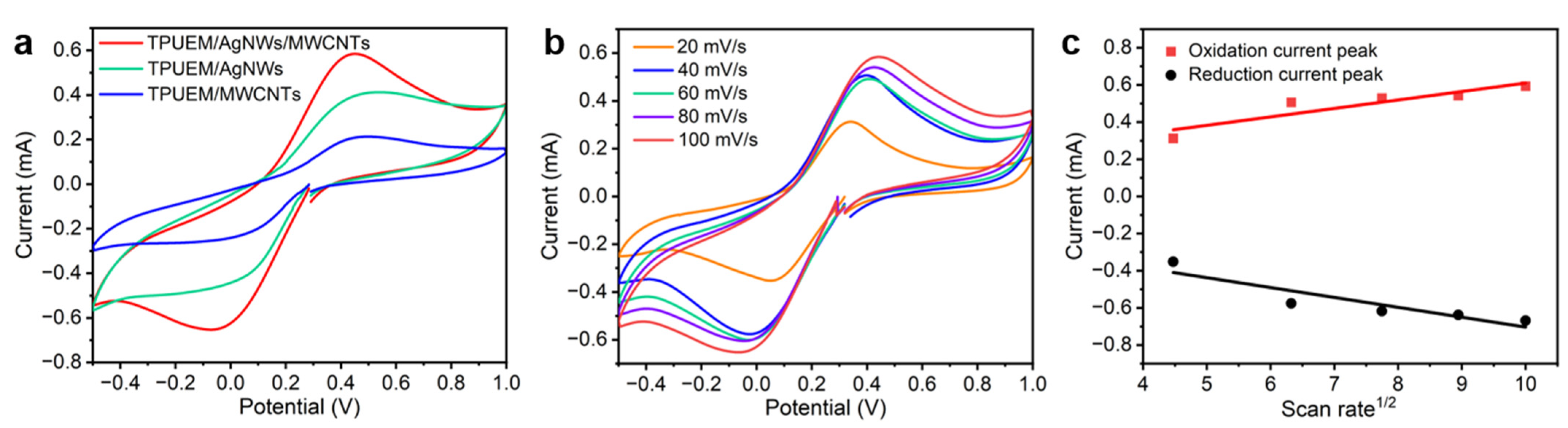
3.3. Electrochemical Performance
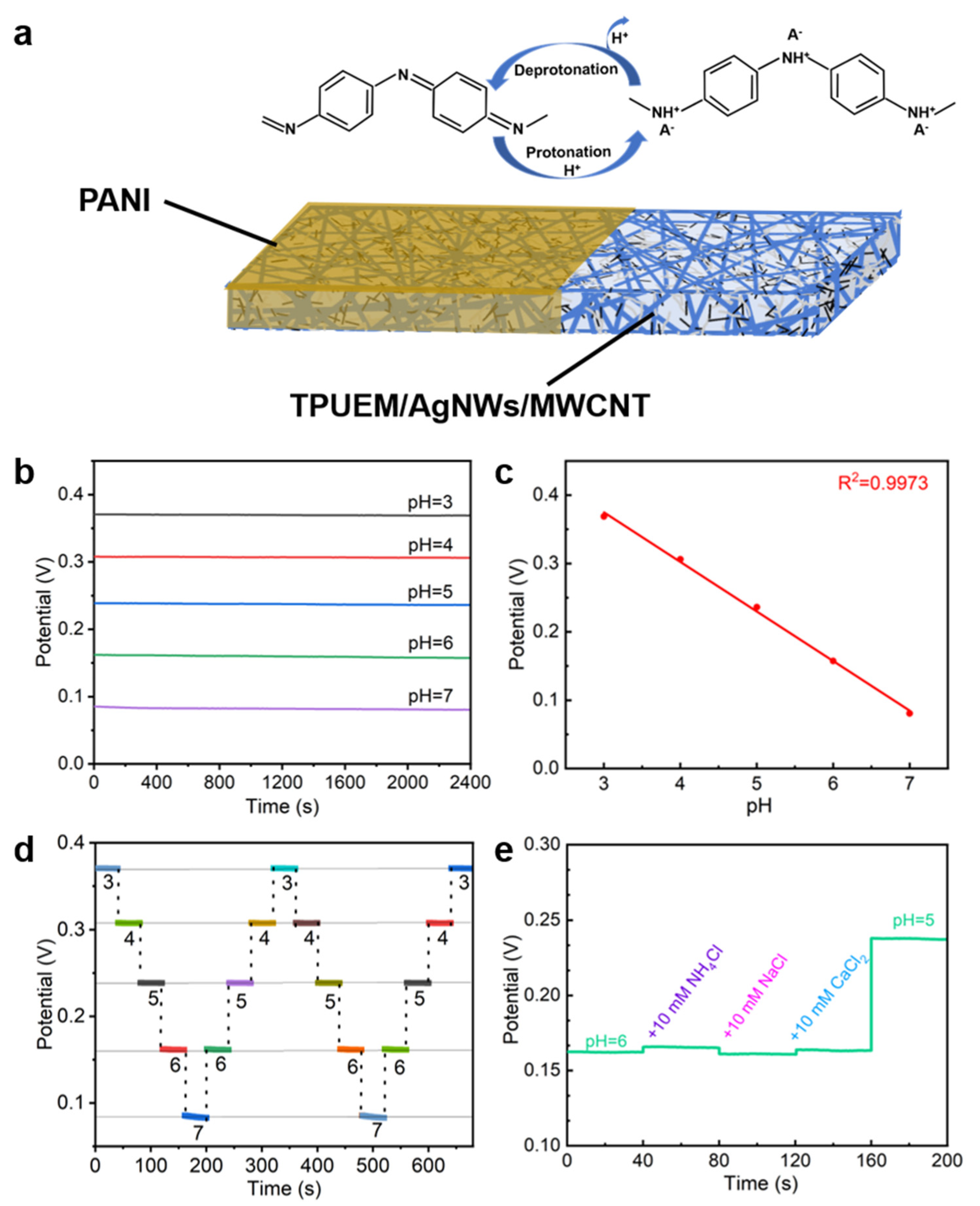
3.4. Sensing Performance
3.5. Sensing Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.L.; Qin, Y.; Jin, Z.H.; Hu, X.B.; Chen, M.M.; Liu, R.; Amatore, C.; Huang, W.H. A Stretchable Electrochemical Sensor for Inducing and Monitoring Cell Mechanotransduction in Real Time. Angew. Chem. Int. Ed 2017, 56, 9454–9458. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.H.; Liu, Y.L.; Chen, J.J.; Cai, S.L.; Xu, J.Q.; Huang, W.H. Conductive Polymer-Coated Carbon Nanotubes to Construct Stretchable and Transparent Electrochemical Sensors. Anal. Chem. 2017, 89, 2032–2038. [Google Scholar] [CrossRef]
- Kim, J.; Kumar, R.; Bandodkar, A.J.; Wang, J. Advanced Materials for Printed Wearable Electrochemical Devices: A Review. Adv. Electron. Mater. 2016, 3, 1600260. [Google Scholar] [CrossRef]
- Xu, K.C.; Ko, S.H.; Chen, J. Advances in Wearable and Implantable Bioelectronics for Precision Medicine. Bio-Des. Manuf. 2024, 7, 383–387. [Google Scholar] [CrossRef]
- Choi, S.; Lee, H.; Ghaffari, R.; Hyeon, T.; Kim, D.H. Recent Advances in Flexible and Stretchable Bio-Electronic Devices Integrated with Nanomaterials. Adv. Mater. 2016, 28, 4203–4218. [Google Scholar] [CrossRef] [PubMed]
- Sannicolo, T.; Lagrange, M.; Cabos, A.; Celle, C.; Simonato, J.P.; Bellet, D. Metallic Nanowire-Based Transparent Electrodes for Next Generation Flexible Devices: A Review. Small 2016, 12, 6052–6075. [Google Scholar] [CrossRef]
- Cho, Y.; Park, S.; Lee, J.; Yu, K.J. Emerging Materials and Technologies with Applications in Flexible Neural Implants: A Comprehensive Review of Current Issues with Neural Devices. Adv. Mater. 2021, 33, 2005786. [Google Scholar] [CrossRef]
- Choi, J.; Ghaffari, R.; Baker, L.B.; Rogers, J.A. Skin-Interfaced Systems for Sweat Collection and Analytics. Sci. Adv. 2018, 4, eaar3921. [Google Scholar] [CrossRef]
- Sonner, Z.; Wilder, E.; Heikenfeld, J.; Kasting, G.; Beyette, F.; Swaile, D.; Sherman, F.; Joyce, J.; Hagen, J.; Kelley-Loughnane, N.; et al. The Microfluidics of the Eccrine Sweat Gland, Including Biomarker Partitioning, Transport, and Biosensing Implications. Biomicrofluidics 2015, 9, 031301. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Avila, B.E.F.; Wang, J. Wearable Biosensors for Healthcare Monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Gao, F.P.; Liu, C.X.; Zhang, L.C.; Liu, T.Z.; Wang, Z.; Song, Z.X.; Cai, H.Y.; Fang, Z.; Chen, J.M.; Wang, J.B.; et al. Wearable and Flexible Electrochemical Sensors for Sweat Analysis: A Review. Microsyst. Nanoeng. 2023, 9, 1. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jeerapan, I.; Wang, J. Wearable Chemical Sensors: Present Challenges and Future Prospects. ACS Sens. 2016, 1, 464–482. [Google Scholar] [CrossRef]
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable Sweat Sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar] [CrossRef]
- Yang, M.; Sun, N.; Lai, X.; Wu, J.; Wu, L.; Zhao, X.; Feng, L. Paper-Based Sandwich-Structured Wearable Sensor with Sebum Filtering for Continuous Detection of Sweat pH. ACS Sens. 2023, 8, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Escobedo, P.; Ramos-Lorente, C.E.; Martínez-Olmos, A.; Carvajal, M.A.; Ortega-Muñoz, M.; de Orbe-Payá, I.; Hernández-Mateo, F.; Santoyo-González, F.; Capitán-Vallvey, L.F.; Palma, A.J.; et al. Wireless Wearable Wristband for Continuous Sweat pH Monitoring. Sens. Actuators B Chem. 2021, 327, 128948. [Google Scholar] [CrossRef]
- Rahimi, R.; Ochoa, M.; Parupudi, T.; Zhao, X.; Yazdi, I.K.; Dokmeci, M.R.; Tamayol, A.; Khademhosseini, A.; Ziaie, B. A Low-Cost Flexible pH Sensor Array for Wound Assessment. Sens. Actuators B Chem. 2016, 229, 609–617. [Google Scholar] [CrossRef]
- Mazzara, F.; Patella, B.; D’Agostino, C.; Bruno, M.G.; Carbone, S.; Lopresti, F.; Aiello, G.; Torino, C.; Vilasi, A.; O’Riordan, A.; et al. PANI-Based Wearable Electrochemical Sensor for pH Sweat Monitoring. Chemosensors 2021, 9, 169. [Google Scholar] [CrossRef]
- O’Neill, S.J.K.; Huang, Z.H.; Chen, X.Y.; Sala, R.L.; McCune, J.A.; Malliaras, G.G.; Scherman, O.A. Highly Stretchable Dynamic Hydrogels for Soft Multilayer Electronics. Sci. Adv. 2024, 10, eadn5142. [Google Scholar] [CrossRef]
- Zhao, Y.; Jin, K.Q.; Li, J.D.; Sheng, K.K.; Huang, W.H.; Liu, Y.L. Flexible and Stretchable Electrochemical Sensors for Biological Monitoring. Adv. Mater. 2023, 2305917. [Google Scholar] [CrossRef]
- Wang, R.; Zhai, Q.; Zhao, Y.; An, T.; Gong, S.; Guo, Z.; Shi, Q.; Yong, Z.; Cheng, W. Stretchable Gold Fiber-Based Wearable Electrochemical Sensor toward pH Monitoring. J. Mater. Chem. B 2020, 8, 3655–3660. [Google Scholar] [CrossRef]
- Kil, M.S.; Kim, S.J.; Park, H.J.; Yoon, J.H.; Jeong, J.M.; Choi, B.G. Highly Stretchable Sensor Based on Fluid Dynamics-Assisted Graphene Inks for Real-Time Monitoring of Sweat. ACS Appl. Mater. Interfaces 2022, 14, 48072–48080. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.Z.; Guan, M.X.; Xia, L.Z.; Chen, Y.; Mai, J.H.; Zhao, C.; Liao, C.R. Highly Stretchable and Robust Electrochemical Sensor Based on 3D Graphene Oxide-CNT Composite for Detecting Ammonium in Sweat. Biosensors 2023, 13, 409. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Gong, S.; Wang, Y.; Lyu, Q.; Liu, Y.; Ling, Y.; Wang, J.; Simon, G.P.; Cheng, W. Enokitake Mushroom-Like Standing Gold Nanowires toward Wearable Noninvasive Bimodal Glucose and Strain Sensing. ACS Appl. Mater. Interfaces 2019, 11, 9724–9729. [Google Scholar] [CrossRef]
- Fang, C.Q.; Lei, W.Q.; Zhou, X.; Yu, Q.; Cheng, Y.L. Preparation and Characterization of Waterborne Polyurethane Containing PET Waste/PPG as Soft Segment. J. Appl. Polym. Sci. 2015, 132, 42757. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, B.; Zheng, G.; Liu, X.; Li, T.; Yan, C.; Cheng, C.; Dai, K.; Liu, C.; Shen, C.; et al. Continuously Prepared Highly Conductive and Stretchable SWNT/MWNT Synergistically Composited Electrospun Thermoplastic Polyurethane Yarns for Wearable Sensing. J. Mater. Chem. C 2018, 6, 2258–2269. [Google Scholar] [CrossRef]
- Huang, J.; Li, D.; Zhao, M.; Mensah, A.; Lv, P.; Tian, X.; Huang, F.; Ke, H.; Wei, Q. Highly Sensitive and Stretchable CNT-Bridged AgNP Strain Sensor Based on TPU Electrospun Membrane for Human Motion Detection. Adv. Electron. Mater. 2019, 5, 1900241. [Google Scholar] [CrossRef]
- Zeng, J.; Zhu, H.X.; Jiang, S.S.; Wang, P.J.; Zhou, S.S.; Li, J.L.; Song, B.; Huang, Y.W.; Lin, S.D.; Wang, B. A Cost-Effective Purification Strategy of Ultra-Long AgNWs for Enhancing Stability and Durability of AgNWs/PU Sensor. Colloid Surf. A 2024, 696, 134329. [Google Scholar] [CrossRef]
- Lee, P.; Ham, J.; Lee, J.; Hong, S.; Han, S.; Suh, Y.D.; Lee, S.E.; Yeo, J.; Lee, S.S.; Lee, D.; et al. Highly Stretchable or Transparent Conductor Fabrication by a Hierarchical Multiscale Hybrid Nanocomposite. Adv. Funct. Mater. 2014, 24, 5671–5678. [Google Scholar] [CrossRef]
- Ma, X.; Wu, X.; Luo, W.; Liu, Z.; Wang, F.; Yu, H. Large-Scale Wearable Textile-Based Sweat Sensor with High Sensitivity, Rapid Response, and Stable Electrochemical Performance. ACS Appl. Mater. Interfaces 2024, 16, 18202–18212. [Google Scholar] [CrossRef]
- Li, Y.; Mao, Y.; Xiao, C.; Xu, X.; Li, X. Flexible pH Sensor Based on a Conductive PANI Membrane for pH Monitoring. RSC Adv. 2019, 10, 21–28. [Google Scholar] [CrossRef]
- Zahed, M.A.; Barman, S.C.; Das, P.S.; Sharifuzzaman, M.; Yoon, H.S.; Yoon, S.H.; Park, J.Y. Highly Flexible and Conductive Poly (3, 4-Ethylene Dioxythiophene)-Poly (Styrene Sulfonate) Anchored 3-Dimensional Porous Graphene Network-Based Electrochemical Biosensor for Glucose and pH Detection in Human Perspiration. Biosens. Bioelectron. 2020, 160, 112220. [Google Scholar] [CrossRef]
- Choudhury, S.; Deepak, D.; Bhattacharya, G.; McLaughlign, J.; Roy, S.S. MoS2-Polyaniline Based Flexible Electrochemical Biosensor: Toward pH Monitoring in Human Sweat. Macromol. Mater. Eng. 2023, 308, 2300007777. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, Y.; Zhao, S.; Zhu, R.; Zhao, J.; Cui, G. Highly Sensitive pH Sensor Based on Flexible Polyaniline Matrix for Synchronal Sweat Monitoring. Microchem. J. 2023, 185, 108092. [Google Scholar] [CrossRef]
- Zhao, H.; Dai, Z.; He, T.; Zhu, S.; Yan, X.; Yang, J. Fabrication of Pani-Modified Pvdf Nanofibrous Yarn for pH Sensor. e-Polymers 2021, 22, 69–74. [Google Scholar] [CrossRef]
- Bignall, L.; Magnenet, C.; Ramsamy, C.; Lakard, S.; Vassal, S.; Lakard, B. Polyaniline-Based Flexible Sensor for pH Monitoring in Oxidizing Environments. Chemosensors 2024, 12, 97. [Google Scholar] [CrossRef]
- Lu, H.; Lei, T.; Zhang, L.; Chen, S.; Chen, R.; Li, X.; Wang, Y.; Zhu, J.; Chen, M.; Zhang, K.; et al. Flexible Polyaniline@Carbon Nanofiber Membrane pH Electrode for Health Care. Microchem. J. 2024, 200, 110436. [Google Scholar] [CrossRef]
- Lee, J.; Llerena Zambrano, B.; Woo, J.; Yoon, K.; Lee, T. Recent Advances in 1d Stretchable Electrodes and Devices for Textile and Wearable Electronics: Materials, Fabrications, and Applications. Adv. Mater. 2020, 32, 1902532. [Google Scholar] [CrossRef] [PubMed]


| Materials | Bendable | Stretchable | Sensitivity (mV/pH) | pH Range | Reference |
|---|---|---|---|---|---|
| PANI/gold fiber | × | √ | 60.6 | 4–8 | [20] |
| PANI/rGO/ITO-PET | × | × | 62.3 | 2–8 | [17] |
| PANI/phytic/interdigital gold electrode | √ | × | 69.3 | 4–9 | [33] |
| MoS2-PANI/SPCE | √ | × | 70.4 | 4–8 | [32] |
| PANI/PVDF yarn | × | × | 48.5 | 4–8 | [34] |
| PANI/H2SO4/gold layer | × | × | 73.4 | 3–8 | [35] |
| PANI/PP/LIG | × | × | 75.1 | 4–7 | [31] |
| PANI/interdigital electrodes/PI | √ | × | 58.6 | 5.5–8.6 | [30] |
| PANI/carbon nanofiber membrane | √ | × | 76.2 | 4.0–8.3 | [36] |
| PANI/TPUEM/AgNWs/MWCNT | √ | √ | 82.5 | 3–7 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Ma, B.; Xu, Z.; Zhao, Y. Nanofibrous Membrane-Based Stretchable Electrochemical Sweat Sensor for pH Detection. Polymers 2025, 17, 663. https://doi.org/10.3390/polym17050663
Zhang L, Ma B, Xu Z, Zhao Y. Nanofibrous Membrane-Based Stretchable Electrochemical Sweat Sensor for pH Detection. Polymers. 2025; 17(5):663. https://doi.org/10.3390/polym17050663
Chicago/Turabian StyleZhang, Longzhou, Baoyuan Ma, Zhiguang Xu, and Yan Zhao. 2025. "Nanofibrous Membrane-Based Stretchable Electrochemical Sweat Sensor for pH Detection" Polymers 17, no. 5: 663. https://doi.org/10.3390/polym17050663
APA StyleZhang, L., Ma, B., Xu, Z., & Zhao, Y. (2025). Nanofibrous Membrane-Based Stretchable Electrochemical Sweat Sensor for pH Detection. Polymers, 17(5), 663. https://doi.org/10.3390/polym17050663







