4.1. Discussion of Characterization Extract of Grape Seed Condensed Tannins
The physicochemical characterization of P indicates that the TPC, approximately 230 GAEmg/g, is a high value since according to the portal antioxidants from the Institute of Nutrition and Food Technology (INTA), Chardonnay, Black seedless, and Crimson grape samples have a TPC of 200, 94, and 53 GAEmg/g in dry weight, respectively [
34]. These results are representative of a high antioxidant activity close to 90% inhibition of ABTS●+ and DPPH● radicals, which is due to a high content of equivalent antioxidants of Trolox 8.8 ± 0.3, 9.2 ± 0.7, 9.4 ± 0.6 (mmol/L) for P, P-Dep, and AG, respectively, in agreement with Xu and collaborators, who analyzed the pomace of different grape varieties, finding equivalent antioxidant activities of Trolox of para varieties Viognier 3.5 and Vidal Blanc 7.7 µmol/g [
35].
The MPS polymeric dispersions is in a relatively dispersed range with an IPD of 3.5, indicating the presence of P from monomers to at least octamers, consistent with 16.6 µm particle size, an average mDP of 7. 5.
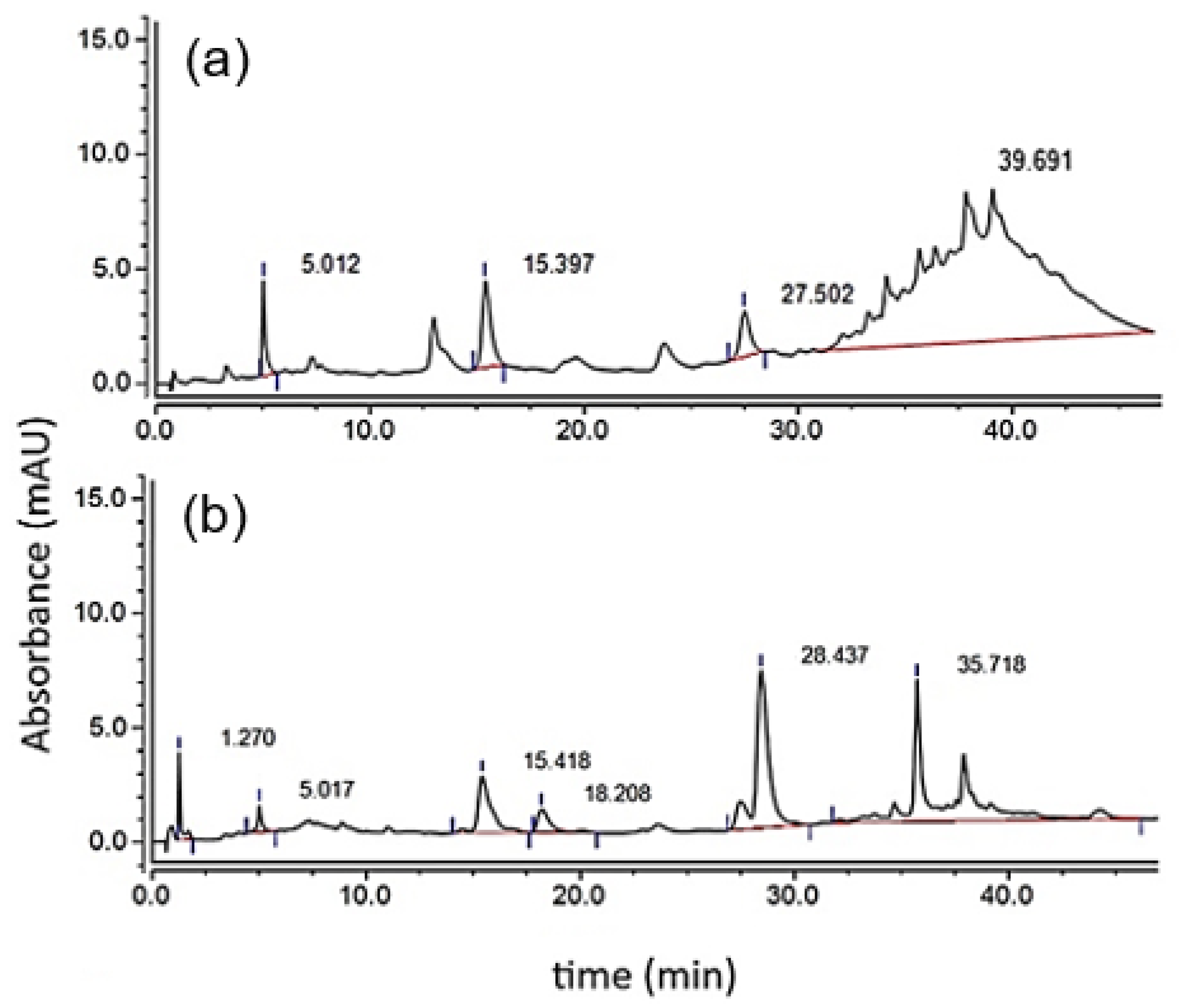
Calibration curves were carried out with an external standard of the main components present in condensed grape seed tannins (P) as reported in the literature to identify the species corresponding to each peak in the chromatograms corresponding to both P and P-Dep: GA (5.0 min), Ca (15.4 min), and Ep (27.5 min) [
36]. Additionally, Cys (1.3 min) and thiolated adducts of catechin (18.2 min) and epicatechin (28.4 min) were identified [
21] (
Table 4). These results are consistent with what was reported by Bianchi and collaborators in their Bioprotocol to determine the
of condensed tannins in which it is specified that, although there are no external standards for thiolated compounds, these appear immediately after the monomer with which they formed the adduct [
21]. The final peak is seen with a retention time between approximately 35 and 45 min (
Figure 1a); these multiple peaks correspond to the condensed tannins fractions—that is, to flavan-3-ol polymers [
37,
38]. Furthermore, the 28.4 min area increase can be seen (
Figure 1b), which corresponds to the thiolated adducts of extensional Ep, confirming that Ep is the main monomer of the P under study. This peak overlapped with terminal Ep, so they had to deconvulse [
39].
Table 4 identifies the percentage of added P (
) contained in the tannin samples, which corresponds to more than 90% of the total mass. The main degree of polymerization (
) of 8, which is indicative that this fraction of the sample is highly polymeric [
13,
40]. Furthermore, the depolymerization reaction yield with cysteamine under these conditions was 57.5%.
4.2. Studies of the Effect of Temperature on the TPC in P Comparing Two Heating Methods: Microwave (M) and Thermostatic Bath (TB)
In
Table 5, it is possible to see that there is a decrease in the measured TPC values when the samples of P are subjected to heating both in a microwave synthesis platform and in a thermostatic bath. It is clear that TPC decay is present in all the TB tests and only in M at 80 °C. In contrast, there is no decay for TPC in M at 40 °C and M at 60 °C, providing a stable environment for our experiments.
Studies that have investigated the impact of temperature on polyphenol yield are mainly based on drying fruits and vegetables and extracting polyphenols from them. These studies have provided information on the thermal lability of plant polyphenols from different plant materials, which have indicated that external heat induces the loss of polyphenol content [
41,
42].
Thermal degradation is the most common mechanism explaining the drop in polyphenol yield during high-temperature extractions, as conventional extraction studies have shown that thermal degradation generally occurs at 80 °C [
43]. However, it is important to consider that the duration of heating, storage conditions before heating, and exposure to oxygen play important roles in determining the effect of temperature on polyphenols [
44].
It is also important to note that antioxidants can not only oxidize in the face of temperature increases; they can also degrade. In research carried out by Palma et al., the results of the extraction of polyphenols with boiling methanol (65 °C) showed that catechin experienced a 40% reduction in its concentration [
45], while Loncaric and collaborators demonstrated that increasing the temperature to 100 °C causes shorter half-lives of catechin and epicatechin [
46].
It should be noted that the studies are not carried out directly on a specific type of antioxidant as has been carried out in this study, and they involve all the components of the fruits and/or vegetables studied. In this sense, it has been shown that extracts with a higher carbohydrate content and lower tannin purity tend to have decreased thermal stability [
47]. Therefore, the effect of heating under the proposed conditions is carried out directly on the antioxidants, without room for other types of reactions and, consequently, other degradation products.
To understand the behavior of polyphenols during exposure to high temperatures, it is necessary to understand all the factors that affect this parameter. Thermal degradation of different types of polyphenols occurs at different temperatures but depends on the pretreatment, type of solvent, pH, treatment time, environment, and source of the material. Regarding the antioxidant capacity, it is possible to observe that it follows the same thermal degradation pattern as phenolic compounds [
48].
In this study, it is observed that there is a decline in the TPC; however, when comparing these values with what is described in the literature, it is possible to see that the TPC value continues to be within the averages for antioxidant extracts. The lowest TPC value recorded in this experiment was for TB 80 °C at 60 min and corresponds to 87.5 ± 2.1 (
Table 5) (EAGmg/g), while Chardonnay, Black seeds, and Crimson grape samples have a CFT of 200, 94, and 53 EAGmg/g in dry weight, respectively [
34].
Regarding the ANOVA analysis of the results, three effects are significant (
Table 6) since they have a
p-value < 0.05, indicating that they are significantly different from zero with a confidence level of 95.0%. Furthermore, the model fit describes that the experimental design factors predict 70% of the TPC.
The literature consistently shows that increasing temperature and time significantly affects TPC. However, this study also shows that the method by which these compounds are heated has a significant effect.
Microwaves are electromagnetic fields ranging from 300 MHz to 300 GHz with two perpendicular oscillating fields: electric and magnetic [
49]. It is considered a non-thermal and “green” technology since it conforms to the criteria established by the Environmental Protection Agency (EPA) due to reduction in reaction times, energy consumption, use of solvents, and CO2 emissions in comparison with conventional heating methods [
43].
Therefore, microwave equipment is a non-contact heat source that can provide more effective heating, accelerating energy transfer and reducing the thermal gradient [
50]. Grigoras et al. (2012) [
51] conducted a comparative study of conventional methods, maceration, and extraction using pressurized liquid, ultrasound, and microwaves. The microwave-assisted methodology provided the highest concentration of bioactive compounds in the apple extract. Boukroufa et al. (2015) [
52] presented a study from laboratory to pilot scale for the extraction and separation of volatile and non-volatile compounds from boldo leaves using microwaves, concluding that this provided lower energy and time consumption because hydrodistillation required 30 min to initiate azeotropic distillation, while microwave-assisted extraction only required 5 min. This research indicates that microwaves quickly raise the volumetric temperature without overheating the surface, provide better process control, and achieve greater energy efficiency than conventional heating methods [
53]. Some authors suggest that electric fields can alter the conformation of molecules, which are susceptible to structural changes that affect their functionality [
54,
55], as microwaves do not possess the energy necessary to break covalent bonds [
35,
56].
When comparing traditional and novel thermal processes, the discussion should focus on matching the temperature profiles since the results do not depend on any given mathematical model but on thermodynamic and molecular parameters [
57]. Finally, it should be noted that microwave heating is an isothermal process where the temperature of the samples is uniform. In addition, the heating occurs due to the stretching and contraction of the bonds of the molecules with a high dielectric constant (water). In contrast, in conventional heating, the thermal gradient causes a higher temperature on the surface of the reactor and, therefore, the stretching of the bonds of all the molecules (in this case, the phenolic groups), giving them energy to activate oxidation reactions [
58].
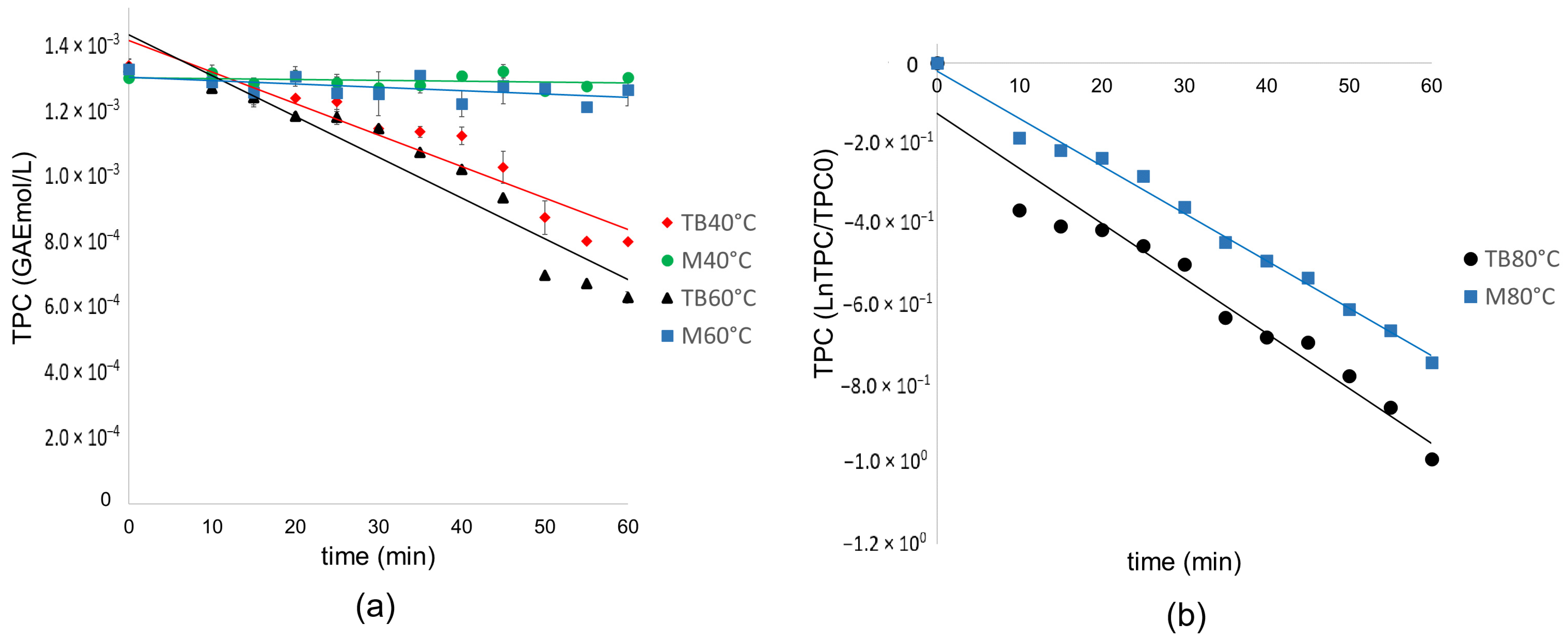
In
Figure 2a, for the M 40 °C and M 60 °C runs, there is no significant degradation of the TPC, which is why the data do not fit any reaction model and have a dispersion within the standard deviation.
The linear regression plots (
Table 7) were fitted with a higher coefficient of determination to the equation TPC = k t + TPC0 in which TPC corresponds to the molar concentration of equivalent polyphenols of gallic acid, therefore following a zero-order reaction model for TB 40 °C and TB 60 °C; however, a change is seen in the degradation mechanism for TB 80 °C, adjusting to a first order linear regression, which indicates that at this temperature the concentration of one species is affecting the degradation mechanism oxidation. This same behavior is observed for M80 °C, which fits a first-order linear regression.
It has been shown that flavonoids’ antioxidant activity is due to aromatic OH groups (phenols). Polyphenols’ chemical properties, in terms of the availability of phenolic hydrogens as hydrogen donor radical scavengers, suggest that they will have antioxidant activity [
59].
Although the degradation rate depends on temperature, the number of soluble solids, and pH, several authors report that these kinetics follow first-order kinetics [
60,
61,
62]. Zapata and collaborators (2001) showed that the thermal degradation of phenolic compounds follows first-order kinetics in the range of typical food processes (70–90 °C). The antioxidant activity of the extracts is directly related, following the same pattern [
48], and Loncaric and collaborators (2018) demonstrate that the thermal degradation of catechin and epicatechin follows first-order kinetics [
46].
The reducing capacity of flavan-3-ol involves a complex oxidation process that varies depending on the pH; it is produced by a consecutive proton donation mechanism by which phenol is converted into a phenoxyl radical (semiquinone), which is reversible, followed by electron transfer by which this radical is converted into quinone, which is irreversible [
63]. These structures are almost identical to the original flavan-3-ol and cannot be detected by conventional methods such as thiolysis [
41].
It is inferred that in TB at 40 °C and 60 °C, the oxidation mechanism occurs by deprotonating the benzene catechol ring and following zero-order kinetics. Therefore, it is inferred that this oxidation occurs without depending on the concentration of the species.
For TB80 °C and M80 °C, the oxidation mechanism follows two steps: deprotonation and electron transfer. These results agree with previous research in which it is shown that thermal degradation in general for polyphenols occurs at 80 °C [
43]. In comparison, the degradation of condensed tannins remains low at temperatures that do not exceed 50 °C [
41]. Let us consider that water contains approximately 9 mg/L of oxygen in a saturated state. Ethanol can contain up to 40 mg/L (under standard pressure and temperature conditions) [
64]; it is important to highlight that the reactors are closed in this study. The reaction occurs under reflux, which indicates that there may be a redissolution of oxygen. Low temperatures have been considered to inhibit the degradation of active ingredients. However, low temperatures significantly increase operation times [
41]. Therefore, this study seeks to use the highest possible temperature with a non-significant loss of TPC in microwaves, which is 60 °C, demonstrating that this method is optimal for heating polyphenols in contrast to the thermostatic bath.
4.3. Feasibility of Depolymerization of P Whit GA, Reaction Optimization and Kinetics Analysis
Table 8 describes the experimental design and results obtained for optimizing the P depolymerization reaction. In general, the reaction is feasible for all experimental runs, with an RY over 70% and, under optimized conditions, around 100%.
In the case of RY, there are four significant effects: the percentage of ethanol (S); the P/S ratio; and, in turn, the interaction between them; and finally, the interaction between P/S and P/GA (
Table 9).
The chemical methods investigated for the depolymerization of polymeric CT with the addition of (+)-catechin, (−)-epicatechin, and (−)-epigallocatechin gallate as a chain-disrupting nucleophile pose a significant limitation in their use due to their high costs. In addition, they include the use of catalysts or alcoholic solutions of mineral acids that raise problems in terms of their toxicity, flammability and environmental impact [
23,
65]. Due to this, in this work, it is proposed to use a highly accessible and low-cost nucleophile, such as GA, and a GRAS solvent such as ethanol.
However, chemical reactivity can change dramatically in different solvents, and it is not always clear which solvent properties will affect reaction rates [
66]. In solution, all participants in a chemical reaction are solvated, both reactants and products, including intermediates and transition states. However, the system under study does not represent a solution but rather a polymeric dispersion, in which the dispersed particles have molecular sizes of the order of microns, while a molecule of water or ethanol represents less than 1 nm. However, as described in the literature, CT is soluble in water and ethanol; this is due to the large number of phenols, which can form hydrogen bonds with this type of polar protic solvents, which does not happen with their monomers, which have a very low solubility in water [
67].
Then, the ethanol/water ratio is influential in three respects: in the solubility of the reactants, where, on the one hand, the GA dissolves in both solvents; in the solubility of the products, where the catechin and epicatechin monomers are better solubilized as the proportion of ethanol increases; and finally, in the dielectric constant (ε), which increases as the water content increases. The ε represents the ability of the solvent to dissociate a solute into ions through interatomic and intermolecular interactions [
68]. This last variable becomes very important in this context in which microwaves assist the reaction. Some researchers have reported that the degradation of CT under microwaves occurs at a temperature above 60 °C, and the mDP of the products decreases gradually [
27,
69].
Water, methanol, and ethanol have ε of 79.99, 33.30, and 24.55 (in standard pressure and temperature conditions), respectively [
70], and theoretically, 80% ethanol in 20% water has an ε of 35.64, similar to methanol—that is, the solvent generally used in the depolymerization of condensed tannins [
21].
So, although, in a microwave-assisted reaction, the greater the ε of the solvent, the greater the reaction rate is expected to be; on the other hand, we have the opposite effect on the solvation of the products. This premise can be inferred based on the MPD results. For the MPD, it is observed that only the percentage of ethanol has a significant effect, this is because the solvent has an effect on the solubility of the products, which is reflected in their agglomeration; so, the more aggregated the products are, it means that their affinity with the solvent decreases; therefore, they will look for a way to reduce their energy again by condensing and reversing the reaction, impacting a decrease in the RY. Stepwise polymer growth occurs slowly in condensation reactions, but eventually, long chain polymers are formed [
71].
In water, as the reaction proceeds, the particle size decreases, so their solvation should increase; however, the opposite effect occurs since, although CT is soluble in water, the monomers do not have aqueous solubilities at acidic pH (1.7) GA 86.76 mg/mL catechin and epicatechin 5.59 mg/mL [
72]; this effect can be seen by increasing the percentage of ethanol from 0 to 50%
v/
v, in which the MDP decreases drastically. Although most reactions occur in aqueous media, much remains to be understood regarding their interactions, especially with other more complex particles, considering their sensitivity to factors such as temperature or the presence of an electric field [
73]. This last factor becomes important in this reaction since this external “stress” factor is associated with ionic migration processes, the change in the orientation of the molecular dipole moments, the length of bonds in a molecule, and the distribution of charges in an atom. More information about how depolymerization occurs can be obtained by analyzing the optimized reaction over time.
The reaction’s efficiency is approximately 100%, likely due to the suitable nucleophile and the electromagnetic wave inducing a polarization reaction in the macromolecular structure. The polar bonds quickly absorbed the microwave energy, leading to a decrease in activation energy, an increase in reaction activity within the polar bonds, and an enhancement of the depolymerization effect [
27,
69].
These results are mainly because depolymerization occurs by the formation of a flavan-3-ol carbocation followed by the formation of an adduct with the GA nucleophile, which forms an ester between catechin/epicatechin, a GA homologous to catechin/epicatechin gallate. However, under the reaction conditions, this ester is hydrolyzed, giving rise to epicatechin monomer and the recovery of GA [
74].
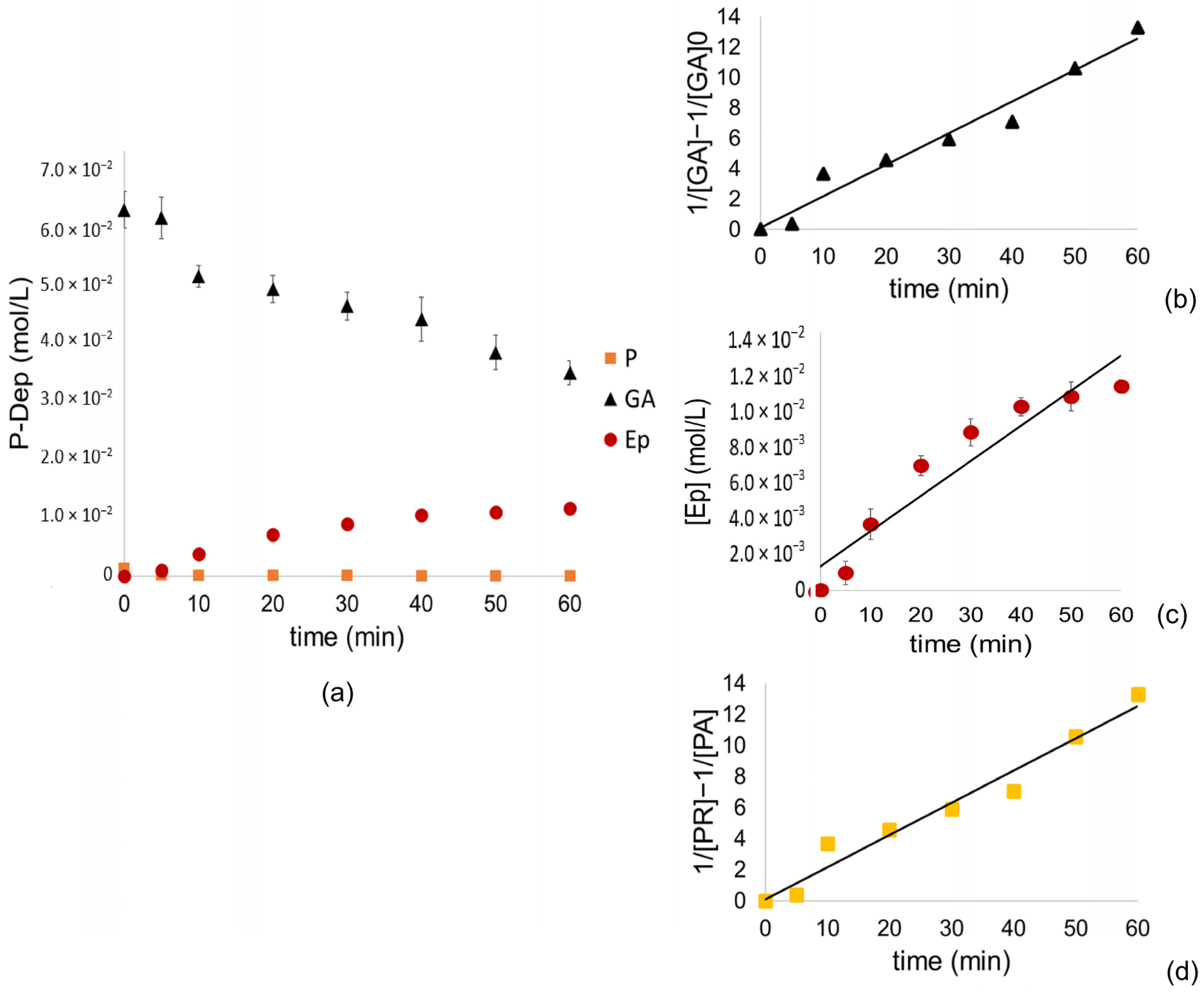
Therefore, the formation of epicatechin does not depend on the concentration of AG or P in this case, following a constant rate formation with zero-order kinetics (
Figure 3c) since it relies on the formation of the carbocation, the nucleophilic attack and the hydrolysis of the ester consecutively, processes that are spontaneous in these reaction conditions. However, it is complex to understand all the processes that may be involved in the formation of the final products; it has been described in the literature that the main reaction products can follow several fragmentation routes, including quinone cleavage, retro-cleavage, Diels–Alder, heterocyclic ring fission, and gallate loss [
24,
40].
On the other hand, it is possible to observe an abrupt drop in the concentration of PR during the first 5 min of the reaction, which results in a linear adjustment of second-order kinetics. The same adjustment is observed for GA, which could be consumed by forming adducts with cyanidins formed by the oxidation of catechin and epicatechins [
24,
40,
65].
When analyzing the reaction products by UHPLC in the absorption range of 545 nm, seven chromatographic peaks at 36.8 can be seen: 39.6; 41.6; 43.6; 44.2; 45.0; and 49.1 min (
Figure S1). These peaks do not appear in the suspension at 0 time and increase over time with zero-order kinetics (
Table S1). This absorption range is typical for cyanidings, one of the main formation products of the cleavage of procyanidins in acid alcohol [
40].
Ethanol recovery is crucial for enhancing the sustainability and cost-effectiveness of our process. This solvent can be efficiently recovered via vacuum evaporation using a rotary evaporator, a well-established technique for solvent recovery in polyphenol extraction [
75,
76]. The solvent can be further purified through nitrogen bubbling and ultrasonication, which ensures minimal solvent loss and prevents oxidation of bioactive compounds [
77]. Studies indicate that over 90% of ethanol can be recovered under optimized vacuum conditions, significantly reducing solvent consumption [
75,
78].
Implementing solvent recovery systems reduces operational costs and minimizes environmental impact, aligning with sustainability goals in green chemistry applications [
79,
80].
4.6. Economic Analysis and Future Perspectives
To evaluate the feasibility of the microwave-assisted depolymerization process of grape seed condensed tannins using gallic acid as a nucleophile, we assessed the market value of the products and the cost of consumables and reagents.
The primary product of the depolymerization process is epicatechin, a flavanol with significant antioxidant properties used in pharmaceuticals, nutraceuticals, and cosmetics. The market price of high-purity epicatechin ranges between USD 200 to USD500 per gram, depending on the supplier and purity grade [
79,
85].
The major consumables in our process include ethanol (solvent) and gallic acid (nucleophile). Industrial-grade ethanol costs approximately USD 1 to USD 3 per liter, while gallic acid is priced at around USD 50 to USD 100 per kilogram [
80,
86]. Based on our optimized reaction conditions (80%
v/
v ethanol, 10 mg/mL polymeric tannins, and 5.88 mg/mL gallic acid), the cost per liter of reaction mixture is relatively low compared to the commercial value of epicatechin.
Transitioning from laboratory-scale experiments to pilot-scale production requires designing microwave reactors capable of handling larger volumes while maintaining reaction uniformity [
76,
78,
79]. Developing a continuous flow microwave-assisted extraction system could improve production rates and process efficiency. Continuous microwave processing has been successfully applied in polyphenol extraction and enhances reaction reproducibility [
76,
79,
87]. After depolymerization, purification steps such as membrane filtration and chromatography will be employed to isolate high-purity epicatechin. Advanced spectroscopic and chromatographic techniques will confirm the structural integrity and antioxidant activity of the final product [
79,
80]. Given the food-grade nature of our approach, regulatory approval pathways must be explored. This will facilitate the incorporation of epicatechin into food, nutraceutical, and pharmaceutical formulations [
79,
85].