Green Synthesis of Biocompatible Chiral Gold Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
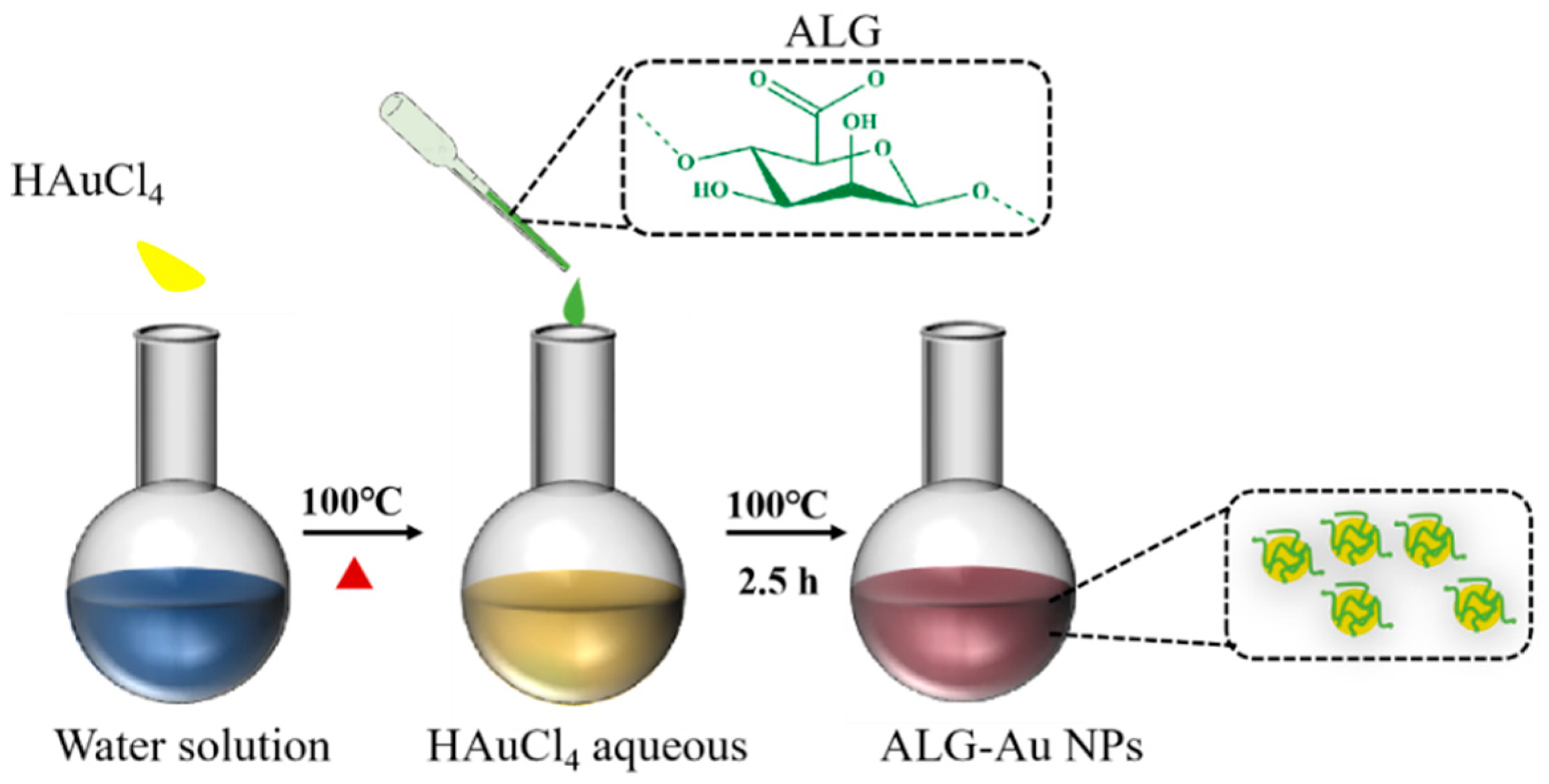
2.2. Preparation of ALG-Au NPs
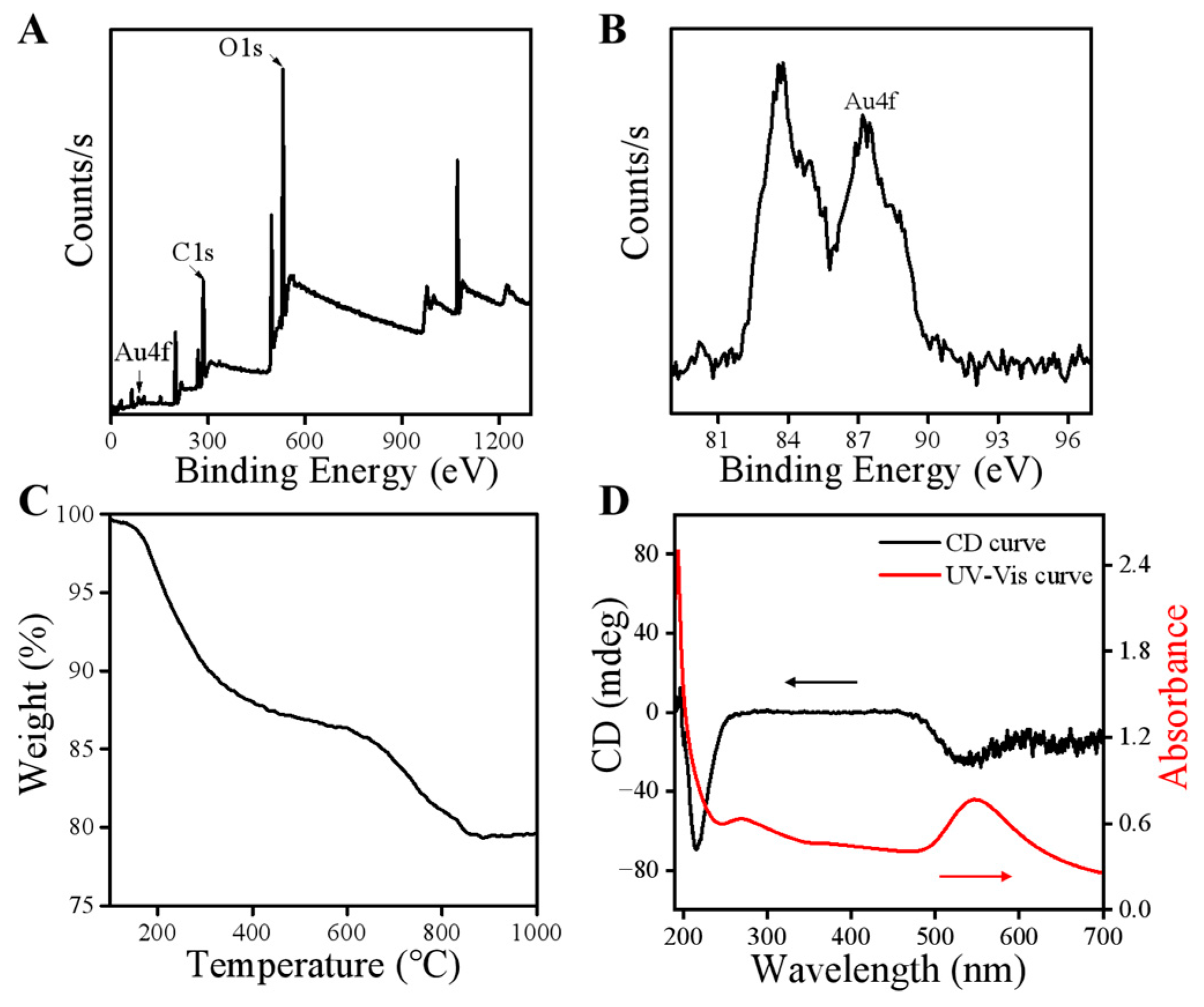
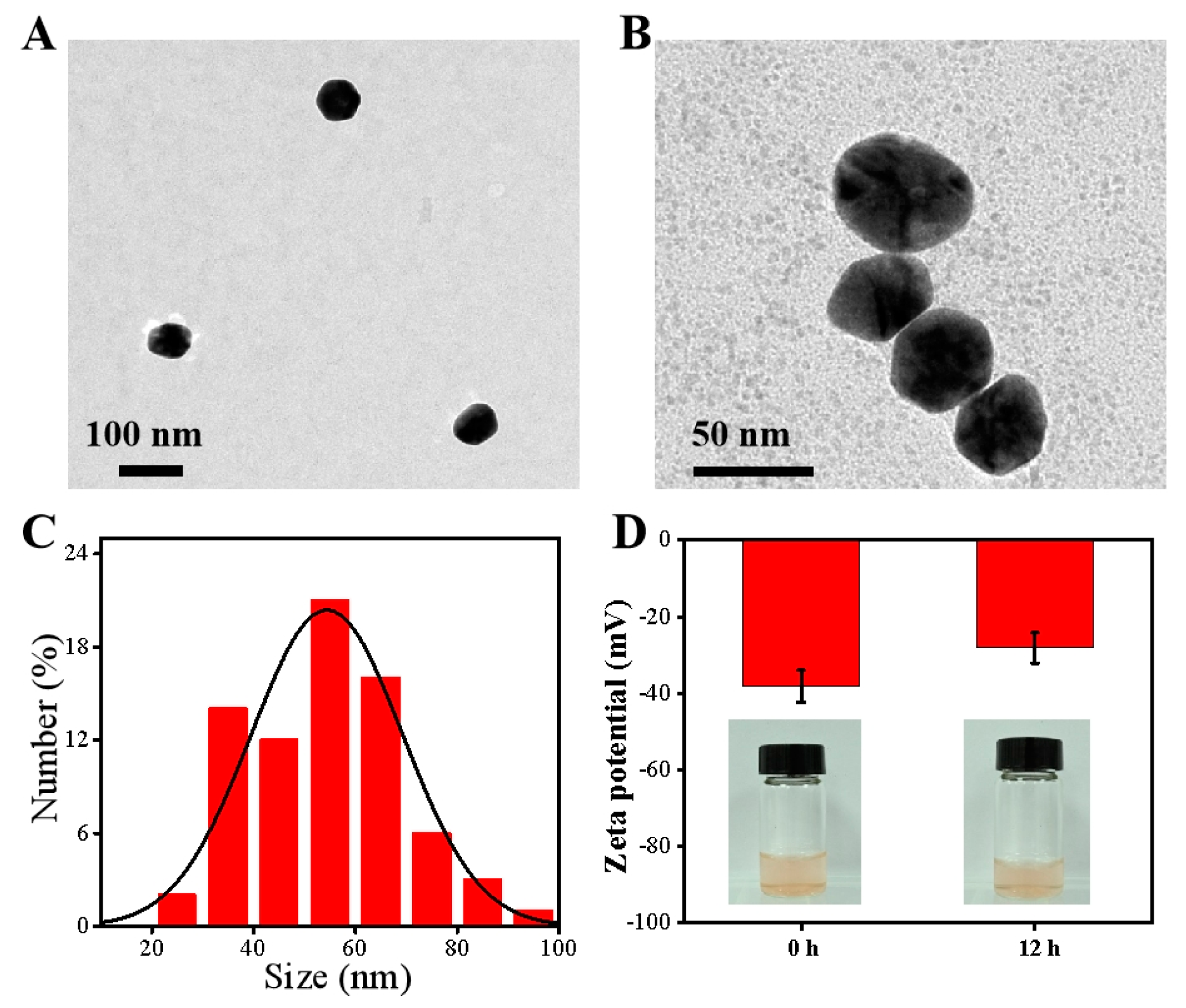
2.3. Characterization of ALG-Au NPs
2.4. Optical Properties of ALG-Au NPs
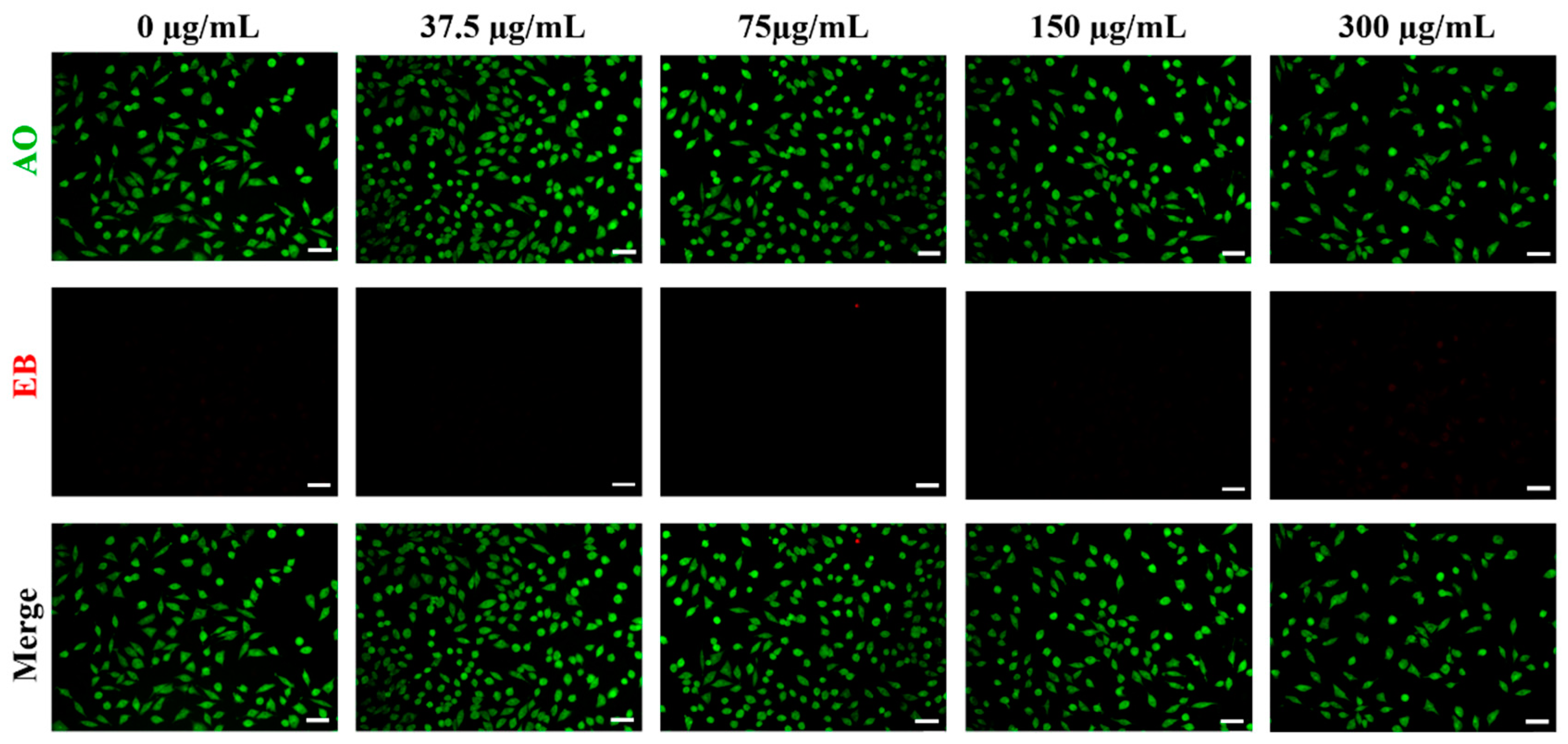
2.5. Biocompatibility Assessment
2.6. Optical Response of ALG-Au NPs to Metal Ions
3. Results and Discussion
3.1. Characterization
3.2. Biocompatibility of ALG-Au NPs
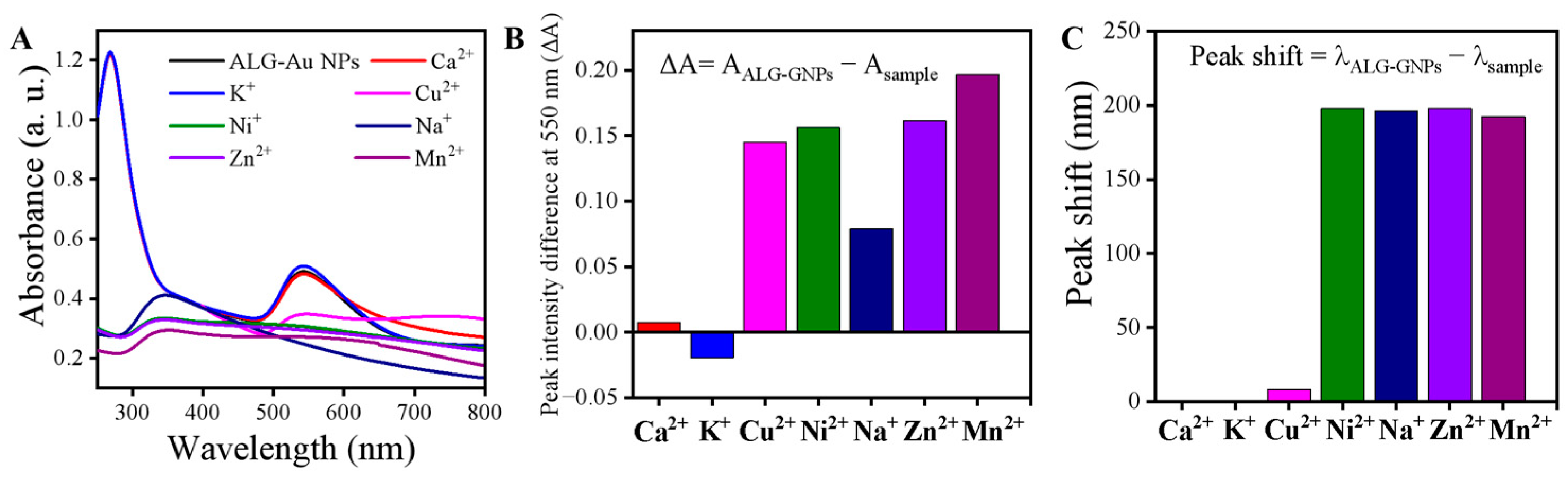
3.3. Optical Response of the ALG-Au NPs to Various Metal Ions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Xu, X.; Xu, L.; Lin, H.; Kuang, H.; Xu, C. Emerging trends in chiral inorganic nanomaterials for enantioselective catalysis. Nat. Commun. 2024, 15, 3506. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, H.; Kuang, H.; Xu, C.; Xu, L. Chiral inorganic nanomaterials for bioapplications. Matter 2023, 6, 1752–1781. [Google Scholar] [CrossRef]
- Hong, T.; Zhou, Q.; Liu, Y.; Guan, J.; Zhou, W.; Tan, S.; Cai, Z. From individuals to families: Design and application of self-similar chiral nanomaterials. Mater. Horiz. 2024, 11, 3975–3995. [Google Scholar] [CrossRef]
- Cao, H.; Yang, E.; Kim, Y.; Zhao, Y.; Ma, W. Biomimetic Chiral Nanomaterials with Selective Catalysis Activity. Adv. Sci. 2024, 11, 2306979. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liang, H.; Yang, Y.; Yu, H. Stability and Reactivity: Positive and Negative Aspects for Nanoparticle Processing. Chem. Rev. 2018, 118, 3209–3250. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yue, X.; Ding, Q.; Lin, H.; Xu, C.; Li, S. Chiral inorganic nanomaterials for biological applications. Nanoscale 2023, 15, 2541–2552. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, L.; Cheng, D.; Wu, T.; Fan, Y.; Wang, Z. Potential Application Prospects of Biomolecule-Modified Two-Dimensional Chiral Nanomaterials in Biomedicine. ACS Biomater. Sci. Eng. 2024, 10, 2022–2040. [Google Scholar] [CrossRef]
- Jiang, S.; Kotov, N.A. Circular Polarized Light Emission in Chiral Inorganic Nanomaterials. Adv. Mater. 2023, 35, 2108431. [Google Scholar] [CrossRef]
- Gautier, G.; Thomas, B. Chiral Gold Nanoparticles. Chem. Rev. 2009, 10, 483–492. [Google Scholar] [CrossRef]
- Li, H.; Gao, X.; Zhang, C.; Ji, Y.; Hu, Z.; Wu, X. Gold-Nanoparticle-Based Chiral Plasmonic Nanostructures and Their Biomedical Applications. Biosensors 2022, 12, 957. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Li, J.; Lu, J.; Liu, H.; Kim, J.; Kim, A.; Yao, L.; Liu, C.; Qian, C.; Hood, Z.; et al. Chiral assemblies of pinwheel superlattices on substrates. Nature 2022, 612, 259–265. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, W.; Xu, L.; Hao, C.; Ma, W.; Sun, M.; Wu, X.; Qin, X.; Colombari, F.; de Moura, A.; et al. Polarization-sensitive optoionic membranes from chiral plasmonic nanoparticles. Nat. Nanotechnol. 2022, 17, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Pedrazo-Tardajos, A.; Claes, N.; Wang, D.; Sánchez-Iglesias, A.; Nandi, P.; Jenkinson, K.; De Meyer, R.; Liz-Marzán, L.M.; Bals, S. Direct visualization of ligands on gold nanoparticles in a liquid environment. Nat. Chem. 2024, 16, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Fukui, T.; Kimura, K. Chiroptical Responses of d-/l-Penicillamine-Capped Gold Clusters under Perturbations of Temperature Change and Phase Transfer. J. Phys. Chem. C 2007, 111, 14968–14976. [Google Scholar] [CrossRef]
- Kariuki, R.; Penman, R.; Bryant, S.; Orrell-Trigg, R.; Meftahi, N.; Crawford, R.; McConville, C.; Bryant, G.; Voitchovsky, K.; Conn, C.; et al. Behavior of Citrate-Capped Ultrasmall Gold Nanoparticles on a Supported Lipid Bilayer Interface at Atomic Resolution. ACS Nano 2022, 16, 17179–17196. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, X.; Vila-Liarte, D.; Wang, S.; de Aberasturi, D.; Liz-Marzán, L.M. Coated Chiral Plasmonic Nanorods with Enhanced Structural Stability. Chem. Mater. 2023, 35, 5689–5698. [Google Scholar] [CrossRef]
- Iravani, S. Green Synthesis of Metal Nanoparticles Using Plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Niznik, L.; Noga, M.; Kobylarz, D.; Frydrych, A.; Krosniak, A.; Kapkae. Gold Nanoparticles (AuNPs)-Toxicity, Safety and Green Synthesis: A Critical Review. Int. J. Mol. Sci. 2024, 25, 4057. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Luo, Y.; Guo, Y.; Zhou, Y.; Liao, X.; Li, D.; Lai, X.; Liu, Y. Development of Alginate-Based Hydrogels: Crosslinking Strategies and Biomedical Applications. Int. J. Biol. Macromol. 2023, 239, 124275. [Google Scholar] [CrossRef]
- Jayeoye, T.J.; Sirimahachai, U.; Rujiralai, T. Sensitive colorimetric detection of ascorbic acid based on seed mediated growth of sodium alginate reduced/stabilized gold nanoparticles. Carbohydr. Polym. 2021, 255, 117376. [Google Scholar] [CrossRef]
- Liu, H.; Nguyen, M.; Thanh Nguyen, M.; Shirato, H.; Yonezawa, T. Green and effective synthesis of gold nanoparticles as an injectable fiducial marker for real-time image gated proton therapy. Mater. Adv. 2022, 3, 5430–5441. [Google Scholar] [CrossRef]
- Shao, Y.; Wu, C.; Wu, T.; Yuan, C.; Chen, S.; Ding, T.; Ye, X.; Hu, Y. Green synthesis of sodium alginate-silver nanoparticles and their antibacterial activity. Int. J. Biol. Macromol. 2018, 111, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Valdez, J.; Gómez, I. One-Step Green Synthesis of Metallic Nanoparticles Using Sodium Alginate. J. Nanomater. 2016, 2016, 9790345. [Google Scholar] [CrossRef]
- Liu, H.; Ikeda, K.; Nguyen, M.; Sato, S.; Matsuda, N.; Tsukamoto, H.; Tokunaga, T.; Yonezawa, T. Alginate-Stabilized Gold Nanoparticles Prepared Using the Microwave-Induced Plasma-in-Liquid Process with Long-Term Storage Stability for Potential Biomedical Applications. ACS Omega 2022, 7, 6238–6247. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Lee, S.; Tark, H.; Nang, M.; Oh, J.; Choi, I. Optical Detection of Copper Ions via Structural Dissociation of Plasmonic Sugar Nanoprobes. Anal. Chem. 2022, 94, 5521–5529. [Google Scholar] [CrossRef] [PubMed]
- Bennacef, C.; Desobry-Banon, S.; Probst, L.; Desobry, S. Advances on alginate use for spherification to encapsulate biomolecules. Food Hydrocoll. 2021, 118, 106782. [Google Scholar] [CrossRef]
- Begum, S.; Pratibha, S.; Rawat, J.; Venugopal, D.; Sahu, P.; Gowda, A.; Qureshi, K.A.; Jaremko, M. Recent Advances in Green Synthesis, Characterization, and Applications of Bioactive Metallic Nanoparticles. Pharmaceutics 2022, 15, 455. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Li, N.; Wang, J.; Liao, L.; Wei, J. Green Synthesis of Biocompatible Chiral Gold Nanoparticles. Polymers 2024, 16, 3333. https://doi.org/10.3390/polym16233333
Fan Y, Li N, Wang J, Liao L, Wei J. Green Synthesis of Biocompatible Chiral Gold Nanoparticles. Polymers. 2024; 16(23):3333. https://doi.org/10.3390/polym16233333
Chicago/Turabian StyleFan, Yuan, Na Li, Jiaolong Wang, Lan Liao, and Junchao Wei. 2024. "Green Synthesis of Biocompatible Chiral Gold Nanoparticles" Polymers 16, no. 23: 3333. https://doi.org/10.3390/polym16233333
APA StyleFan, Y., Li, N., Wang, J., Liao, L., & Wei, J. (2024). Green Synthesis of Biocompatible Chiral Gold Nanoparticles. Polymers, 16(23), 3333. https://doi.org/10.3390/polym16233333









