Green Sulfation of Arabinogalactan in the Melt of a Sulfamic Acid–Urea Mixture
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation of Arabinogalactan
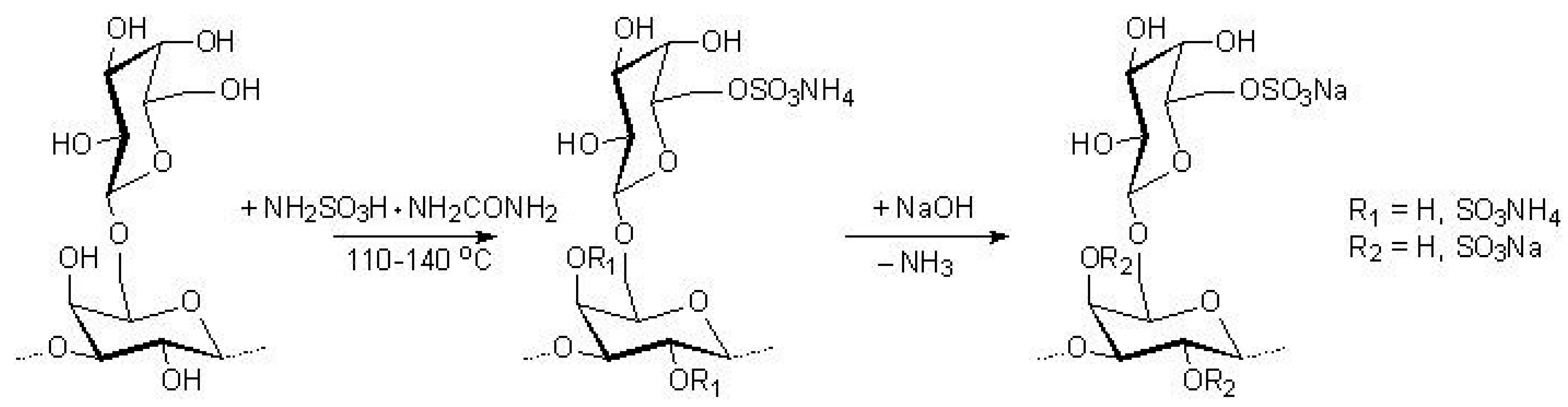
2.3. Sulfation of Arabinogalactan
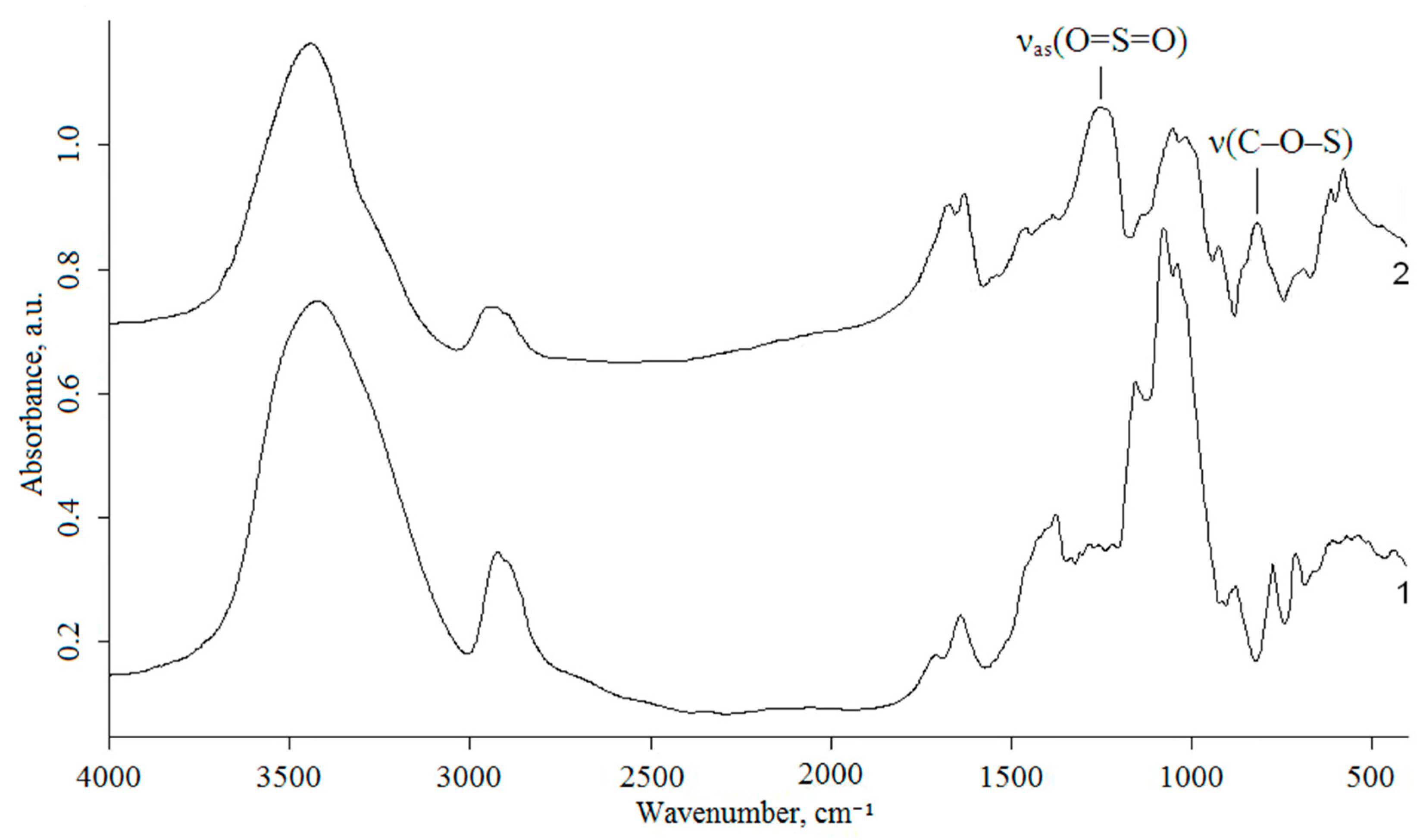
2.4. Characterization Techniques
3. Results and Discussion
3.1. AG Sulfation in the Melt of a Sulfamic Acid–Urea Mixture
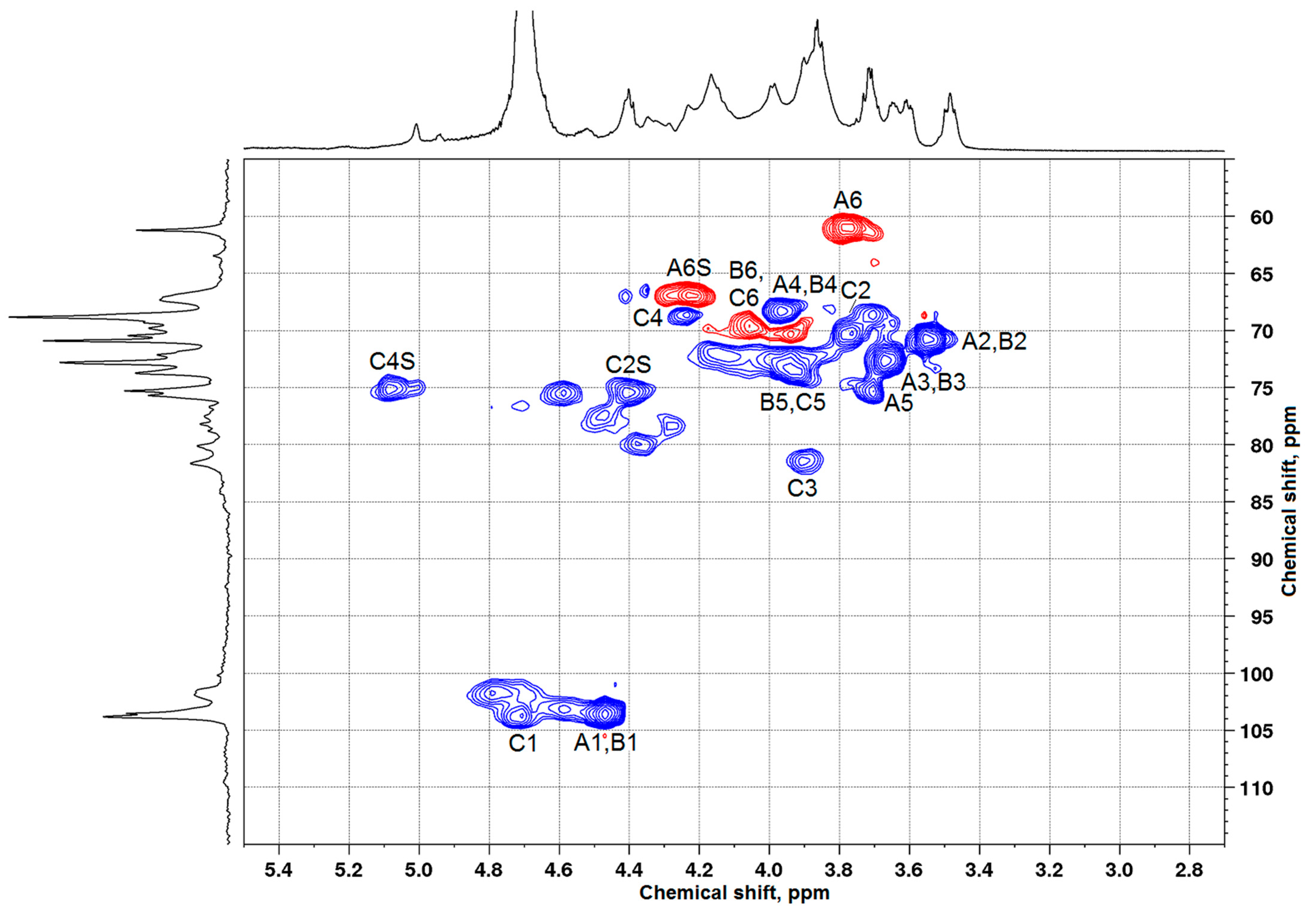
3.2. Characterization of Arabinogalactan and Sulfated Arabinogalactan
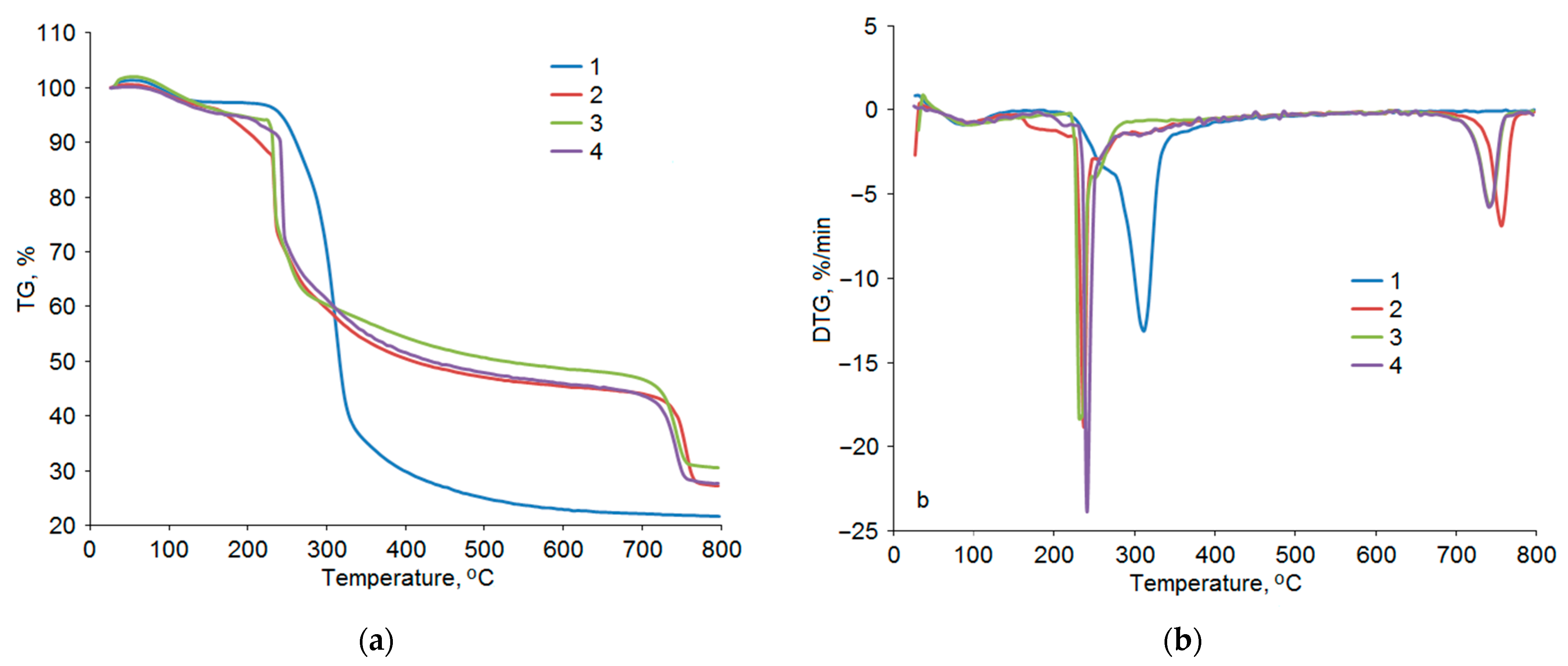
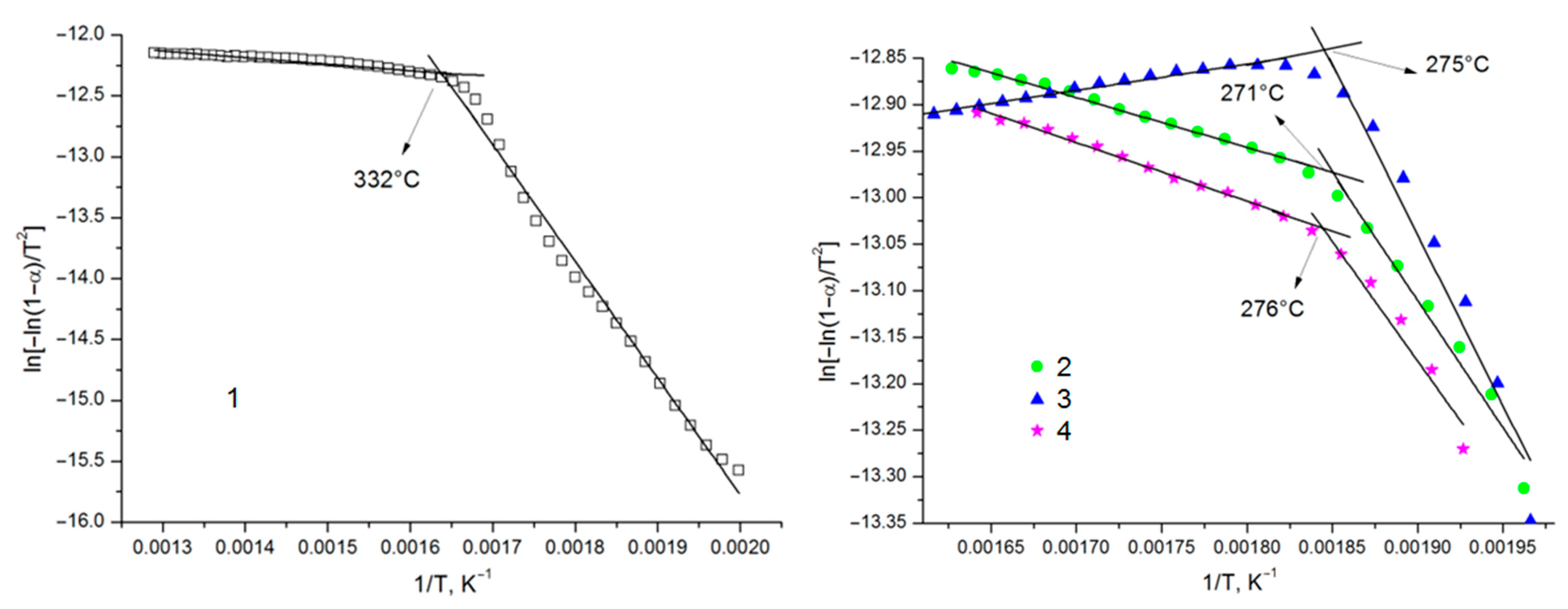
3.3. Thermochemical Properties of Arabinogalactan and Arabinogalactan Sulfates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Govindarajan, S.; Noor, A. Biological activities of plant polysaccharides, mechanism of action and biomedical applications. Res. J. Biotechnol. 2021, 16, 255–272. [Google Scholar] [CrossRef]
- Khvostov, M.V.; Tolstikova, T.G.; Borisov, S.A.; Dushkin, A.V. Application of natural polysaccharides in pharmaceutics. Russ. J. Bioorg. Chem. 2019, 45, 438–450. [Google Scholar] [CrossRef]
- Wang, X.-F.; Chen, X.; Tang, Y.; Wu, J.-M.; Qin, D.-L.; Yu, L.; Yu, C.-L.; Zhou, X.-G.; Wu, A.-G. The therapeutic potential of plant polysaccharides in metabolic diseases. Pharmaceuticals 2022, 15, 1329. [Google Scholar] [CrossRef]
- Dong, X.; Deng, P.; Wang, X.; Peng, C.; Peng, L. Structural characteristics and immunomodulatory effects of polysaccharides extracted from plant seeds: A review. Trends Food Sci. Technol. 2024, 153, 104747. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Zhang, S.; Zhao, Y.; Gao, D.; Xing, J.; Cao, Y.Y.; Xu, G. Purslane (Portulaca oleracea L.) polysaccharide attenuates carbon tetrachloride-induced acute liver injury by modulating the gut microbiota in mice. Genomics 2025, 117, 110983. [Google Scholar] [CrossRef]
- Liu, T.; Ren, Q.; Wang, S.; Gao, J.; Shen, C.; Zhang, S.; Wang, Y.; Guan, F. Chemical modification of polysaccharides: A review of synthetic approaches, biological activity and the structure–activity relationship. Molecules 2023, 28, 6073. [Google Scholar] [CrossRef]
- Goellner, E.M.; Utermoehlen, J.; Kramer, R.; Classen, B. Structure of arabinogalactan from Larix laricina and its reactivity with antibodies direct against type-II-arabinogalactans. Carbohydr. Polym. 2011, 86, 1739–1744. [Google Scholar] [CrossRef]
- Medvedeva, E.N.; Babkin, V.A.; Ostroukhova, L.A. Arabinogalactan from larch—Properties and usage perspectives (review). Chem. Plant Raw Mater. 2003, 1, 27–37. [Google Scholar]
- Ghosh, K.; Takahashi, D.; Kotake, T. Plant type II arabinogalactan: Structural features and modification to increase functionality. Carbohydr. Res. 2023, 529, 108828. [Google Scholar] [CrossRef]
- Kostyro, Y.A.; Stankevich, V.K. New approach to the synthesis of an active substance of Agsular® pharmaceutical for the prevention and treatment of atherosclerosis. Rus. Chem. Bull. 2015, 64, 1576–1580. [Google Scholar] [CrossRef]
- Kostyro, Y.A.; Ganenko, T.V.; Medvedeva, S.A.; Sukhov, B.G.; Trofimov, B.A. Method for Preparing Sulfated Derivatives of Arabinogalactan Possessing Anticoagulating and Hypolipidemic Activity. Russian Federation Patent No. RU2319707, 20 March 2008. [Google Scholar]
- Melo, F.R.; Pereira, M.S.; Foguel, D.; Mourão, P.A.S. Antithrombin-mediated anticoagulant activity of sulfated polysaccharides. Different mechanisms for heparin and sulfated galactans. J. Biol. Chem. 2004, 279, 20824–20835. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Wang, T.; Huang, C.; Lai, C.; Fan, Y.; Yong, Q. Sulfated modification of arabinogalactans from Larix principis-rupprechtii and their antitumor activities. Carbohydr. Polym. 2019, 215, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Vasilyeva, N.Y.; Levdansky, A.V.; Kazachenko, A.S.; Djakovitch, L.; Pinel, C.; Kuznetsov, B.N. Sulfation of mechanically activated arabinogalactan by complex sulfuric anhydride—Pyridine in pyridine medium. J. Sib. Fed. Univ. Chem. 2013, 6, 158–169. [Google Scholar]
- Kazachenko, A.S.; Vasilieva, N.Y.; Malyar, Y.N.; Karacharov, A.A.; Kondrasenko, A.A.; Levdanskiy, A.V.; Borovkova, V.S.; Miroshnikova, A.V.; Issaoui, N.; Kazachenko, A.S.; et al. Sulfation of arabinogalactan with ammonium sulfamate. Biomass Conv. Bioref. 2024, 14, 719–731. [Google Scholar] [CrossRef]
- Vasilyeva, N.Y.; Levdansky, A.V.; Kuznetsov, B.N.; Skvortsova, G.P.; Kazachenko, A.S.; Djakovitch, L.; Pinel, C. Sulfation of arabinogalactan by sulfamic acid in dioxane. Russ. J. Bioorg. Chem. 2015, 41, 725–731. [Google Scholar] [CrossRef]
- Levdansky, A.V.; Vasilyeva, N.Y.; Kondrasenko, A.A.; Levdansky, V.A.; Malyar, Y.N.; Kazachenko, A.S.; Kuznetsov, B.N. Sulfation of arabinogalactan with sulfamic acid under homogeneous conditions in dimethylsulfoxide medium. Wood Sci. Technol. 2021, 55, 1725–1744. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Ukkola, J.; Liimatainen, H. Direct sulfation of cellulose fibers using a reactive deep eutectic solvent to produce highly charged cellulose nanofibers. Cellulose 2019, 26, 2303–2316. [Google Scholar] [CrossRef]
- Ji, X.; Guo, J.; Tian, J.; Ma, K.; Liu, Y. Research progress on degradation methods and product properties of plant polysaccharides. J. Light Industr. 2023, 38, 55–63. [Google Scholar] [CrossRef]
- Babkin, V.A.; Neverova, N.A.; Medvedeva, E.N.; Fedorova, T.E.; Levchuk, A.A. Investigation of physicochemical properties of arabinogalactan of different larch species. Russ. J. Bioorg. Chem. 2016, 42, 707–711. [Google Scholar] [CrossRef]
- Kačuráková, M.; Capek, P.; Sasinková, V.; Wellner, N.; Ebringerová, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Wu, J.; Xu, Y.; Zhu, B.; Liu, K.; Wang, S.; Sheng, Y.; Wang, H.; Shi, S.; Zhang, Q.; Wang, S.; et al. Characterization of an arabinogalactan from the fruit hulls of Ficus pumila Linn. and its immunomodulatory effect. J. Funct. Foods 2020, 73, 104091. [Google Scholar] [CrossRef]
- Pereira, L.; Sousa, A.; Coelho, H.; Amado, A.M.; Ribeiro-Claro, P.J.A. Use of FTIR, FT-Raman and 13C-NMR spectroscopy for identification of some seaweed phycocolloids. Biomol. Eng. 2003, 20, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Ponder, G.R.; Richards, G.N. Arabinogalactan from western larch, part II; a reversible order-disorder transition. J. Carbohydr. Chem. 1997, 16, 195–211. [Google Scholar] [CrossRef]
- Wibowo, E.S.; Park, B.-D. Effect of hemicellulose molecular structure on wettability and surface adhesion to urea–formaldehyde resin adhesives. Wood Sci. Technol. 2022, 56, 1047–1070. [Google Scholar] [CrossRef]
- Tang, S.; Jiang, M.; Huang, C.; Lai, C.; Fan, Y.; Yong, Q. Characterization of arabinogalactans from Larix principis-rupprechtii and their effects on NO production by macrophages. Carbohydr. Polym. 2018, 200, 408–415. [Google Scholar] [CrossRef]
- Cheng, J.; Wei, C.; Li, W.; Wang, Y.; Wang, S.; Huang, Q.; Liu, Y.; He, L. Structural characteristics and enhanced biological activities of partially degraded arabinogalactan from larch sawdust. Int. J. Biol. Macromol. 2021, 171, 550–559. [Google Scholar] [CrossRef]
- Karácsonyi, Š.; Kováčik, V.; Alföldi, J.; Kubačková, M. Chemical and 13C-N.M.R. studies of an arabinogalactan from Larix Sibirica L. Carbohydr. Res. 1984, 134, 265–274. [Google Scholar] [CrossRef]
- Ganenko, T.V.; Tantsyrev, A.P.; Sapozhnikov, A.N.; Khutsishvili, S.S.; Vakulskaya, T.I.; Fadeeva, T.V.; Sukhov, B.G.; Trofimov, B.A. Nanocomposites of silver with arabinogalactan sulfate: Preparation, structure, and antimicrobial activity. Russ. J. Gen. Chem. 2015, 85, 477–484. [Google Scholar] [CrossRef]
- Gane, A.M.; Craik, D.; Munro, S.L.A.; Howlett, G.J.; Clarke, A.E.; Bacic, A. Structural analysis of the carbohydrate moiety of arabinogalactan-proteins from stigmas and styles of Nicotiana alata. Carbohydr. Res. 1995, 277, 67–85. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, G.; Zhao, X.; Wang, Y.; Sun, X.; Jiao, G.; Zhao, X.; Chai, W. Structural characterization of natural ideal 6-O-sulfated agarose from red alga Gloiopeltis furcata. Carbohydr. Polym. 2012, 89, 883–889. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Tomilin, F.N.; Pozdnyakova, A.A.; Vasilyeva, N.Y.; Malyar, Y.N.; Kuznetsova, S.A.; Avramov, P.V. Theoretical DFT interpretation of infrared spectra of biologically active arabinogalactan sulphated derivatives. Chem. Pap. 2020, 74, 4103–4113. [Google Scholar] [CrossRef]
- Malyar, Y.N.; Vasilyeva, N.Y.; Kazachenko, A.S.; Borovkova, V.S.; Skripnikov, A.M.; Miroshnikova, A.V.; Zimonin, D.V.; Ionin, V.A.; Kazachenko, A.S.; Issaoui, N. Modification of arabinogalactan isolated from Larix sibirica Ledeb. into sulfated derivatives with the controlled molecular weights. Molecules 2021, 26, 5364. [Google Scholar] [CrossRef] [PubMed]
- Šesták, J. Thermophysical Properties of Solids: Their Measurements and Theoretical Thermal Analysis; Elsevier: Amsterdam, The Netherlands; New York, NY, USA, 1984. [Google Scholar]
- Gu, J.; Liu, B.; Zhang, Q.S.; Chen, D.Y.; Zhou, J.B. Pyrolysis characteristics study of camellia shell and its three main components based on TG-FTIR. China For. Prod. Ind. 2015, 42, 9–13. [Google Scholar]
- Lobo, R.E.; Gómez, M.I.; Font de Valdez, G.; Torino, M.I. Physicochemical and antioxidant properties of a gastroprotective exopolysaccharide produced by Streptococcus thermophilus CRL1190. Food Hydrocoll. 2019, 96, 625–633. [Google Scholar] [CrossRef]
- Peng, Y.Y.; Wu, S.B. The structural and thermal characteristics of wheat straw hemicelluloses. J. Anal. Appl. Pyrolysis 2010, 88, 134–139. [Google Scholar] [CrossRef]
- Roman, M.; Winter, W.T. Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose. Biomacromolecules 2004, 5, 1671–1677. [Google Scholar] [CrossRef]
- Coats, A.W.; Redfern, J.P. Kinetic parameters from thermogravimetric data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Varhegyi, G.; Antal, M.J., Jr.; Szekely, T.; Szabo, P. Kinetics of the thermal decomposition of cellulose, hemicellulose, and sugarcane bagasse. Energy Fuels 1989, 3, 329–335. [Google Scholar] [CrossRef]
- Willner, T.; Brunner, G. Pyrolysis kinetics of wood and wood components. Chem. Eng. Technol. 2005, 28, 1212–1225. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, M.; Sun, R.; Sun, L. Study on biomass pyrolysis kinetics. J. Eng. Gas Turbines Power 2006, 128, 493–497. [Google Scholar] [CrossRef]
- Du, L.; Wang, J.; Zhang, Y.; Qi, C.; Wolcott, M.P.; Yu, Z. Preparation and characterization of cellulose nanocrystals from the bio-ethanol residuals. Nanomaterials 2017, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, N.; Shaler, S. A study on thermal and nanomechanical performance of cellulose nanomaterials (CNs). Materials 2017, 10, 718. [Google Scholar] [CrossRef] [PubMed]
- Sofla, M.R.K.; Brown, R.J.; Tsuzuki, T.; Rainey, T.J. A comparison of cellulose nanocrystals and cellulose nanofibres extracted from bagasse using acid and ball milling methods. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035004. [Google Scholar] [CrossRef]
- Henrique, M.A.; Neto, W.P.F.; Silvério, H.A.; Martins, D.F.; Gurgel, L.V.A.; da Silva Barud, H.; de Morais, L.C.; Pasquini, D. Kinetic study of the thermal decomposition of cellulose nanocrystals with different polymorphs, cellulose I and II, extracted from different sources and using different types of acids. Ind. Crops Prod. 2015, 76, 128–140. [Google Scholar] [CrossRef]
- Julien, S.; Chornet, E.; Overend, R.P. Influence of acid pretreatment (H2SO4, HCl, HNO3) on reaction selectivity in the vacuum pyrolysis of cellulose. J. Anal. Appl. Pyrol. 1993, 27, 25–43. [Google Scholar] [CrossRef]




| N | Temperature (°C) | Time (h) | Content of Sulfur (wt %) | DS | Yield * (g) |
|---|---|---|---|---|---|
| 1 | 110 | 0.5 | 7.9 | 0.52 | 1.87 |
| 2 | 115 | 0.5 | 11.6 | 0.91 | 2.11 |
| 3 | 120 | 0.5 | 11.3 | 0.89 | 2.03 |
| 4 | 125 | 0.5 | 10.2 | 0.75 | 1.95 |
| 5 | 130 | 0.5 | 8.5 | 0.58 | 1.87 |
| 6 | 140 | 0.5 | 7.7 | 0.50 | 1.79 |
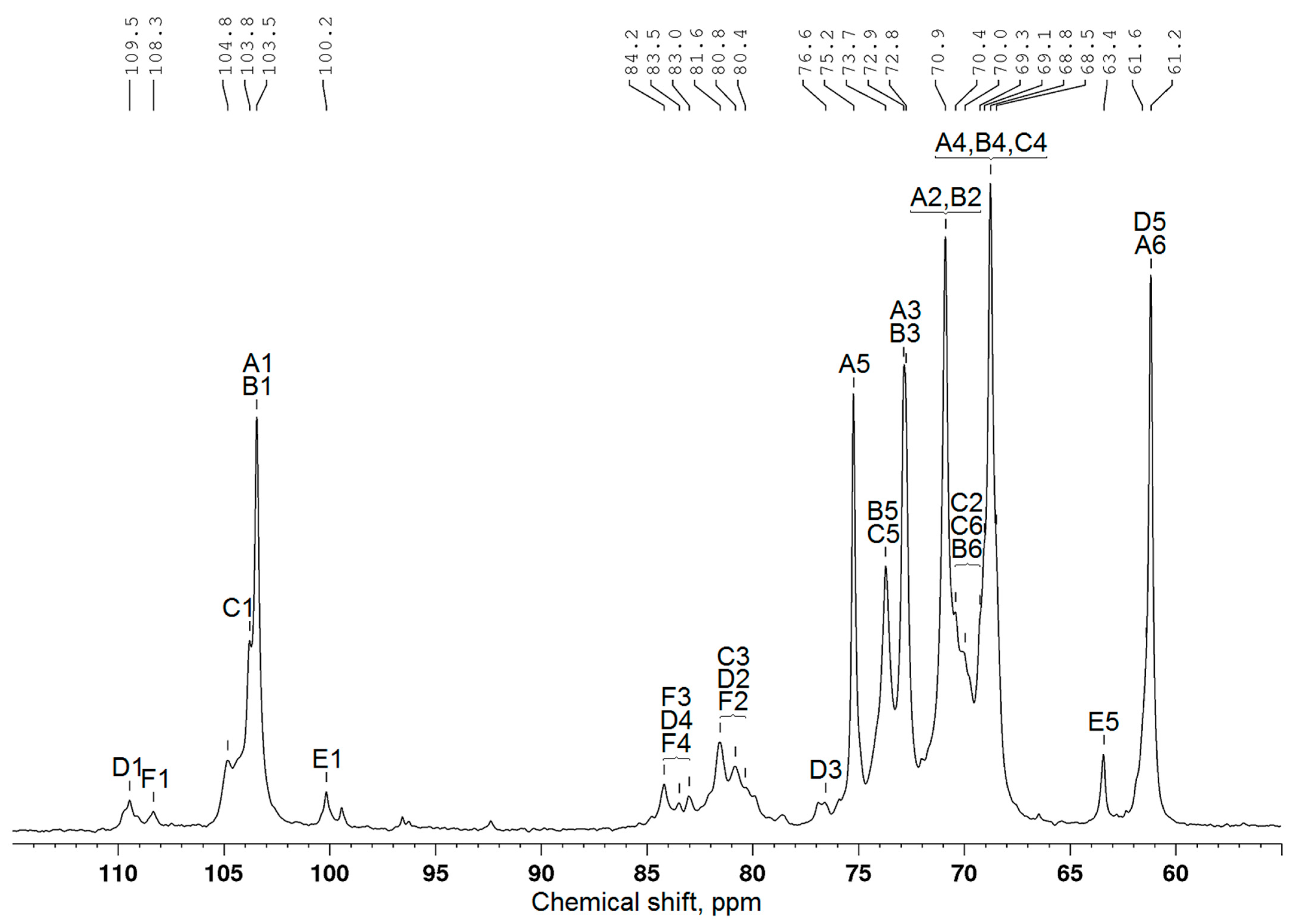
| Structural Unit | Chemical Shift (ppm) | |||||
|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | C6 | |
| β-D-Galp-(1→(A) | 103.5 | 70.9 | 72.9 | 68.8 | 75.2 | 61.2 |
| →6)-β-D-Galp-(1→(B) | 103.5 | 70.9 | 72.8 | 68.8 | 73.7 | 69.3 |
| →3,6)-β-D-Galp-(1→(C) | 103.8 | 70.4 | 81.6 | 68.8 | 73.7 | 70.0 |
| α-L-Araf-(1→(D) | 109.5 | 80.8 | 76.6 | 83.5 | 61.6 | – |
| β-L-Arap-(1→(E) | 100.2 | 68.5 | 68.9 * | 69.1 | 63.4 | – |
| →3)-α-L-Araf-(1→(F) | 108.3 | 80.4 | 84.2 | 83.0 | 61.6 | – |
| Structural Unit | Chemical Shift (ppm) | |||||
|---|---|---|---|---|---|---|
| C1/H1 | C2/H2 | C3/H3 | C4/H4 | C5/H5 | C6/H6 | |
| β-D-Galp-(1→(A) | 103.4/4.42 | 70.8/3.51 | 72.6/3.62 | 68.5/3.90 | 75.4/3.68 | 61.0/3.74 |
| →6)-β-D-Galp-(1→(B) | 103.4/4.42 | 70.8/3.51 | 72.6/3.62 | 68.5/3.90 | 73.6/3.88 | 69.1/4.03 |
| →3,6)-β-D-Galp-(1→(C) | 103.7/4.68 | 70.0/3.74 | 81.3/3.86 | 68.5/4.19 | 73.6/3.88 | 70.8/3.91 |
| α-L-Araf-(1→(D) | 109.3/5.24 | 81.2/4.15 | 76.6/3.86 | 83.9/4.09 | 61.0/3.74 | –/– |
| β-L-Arap-(1→(E) | 99.5/4.97 | n.d. * | n.d. | n.d. | 63.7/3.68 | –/– |
| →3)-α-L-Araf-(1→(F) | 108.2/5.40 | 80.0/4.33 | 84.0/3.92 | 82.9/4.24 | n.d. | –/– |
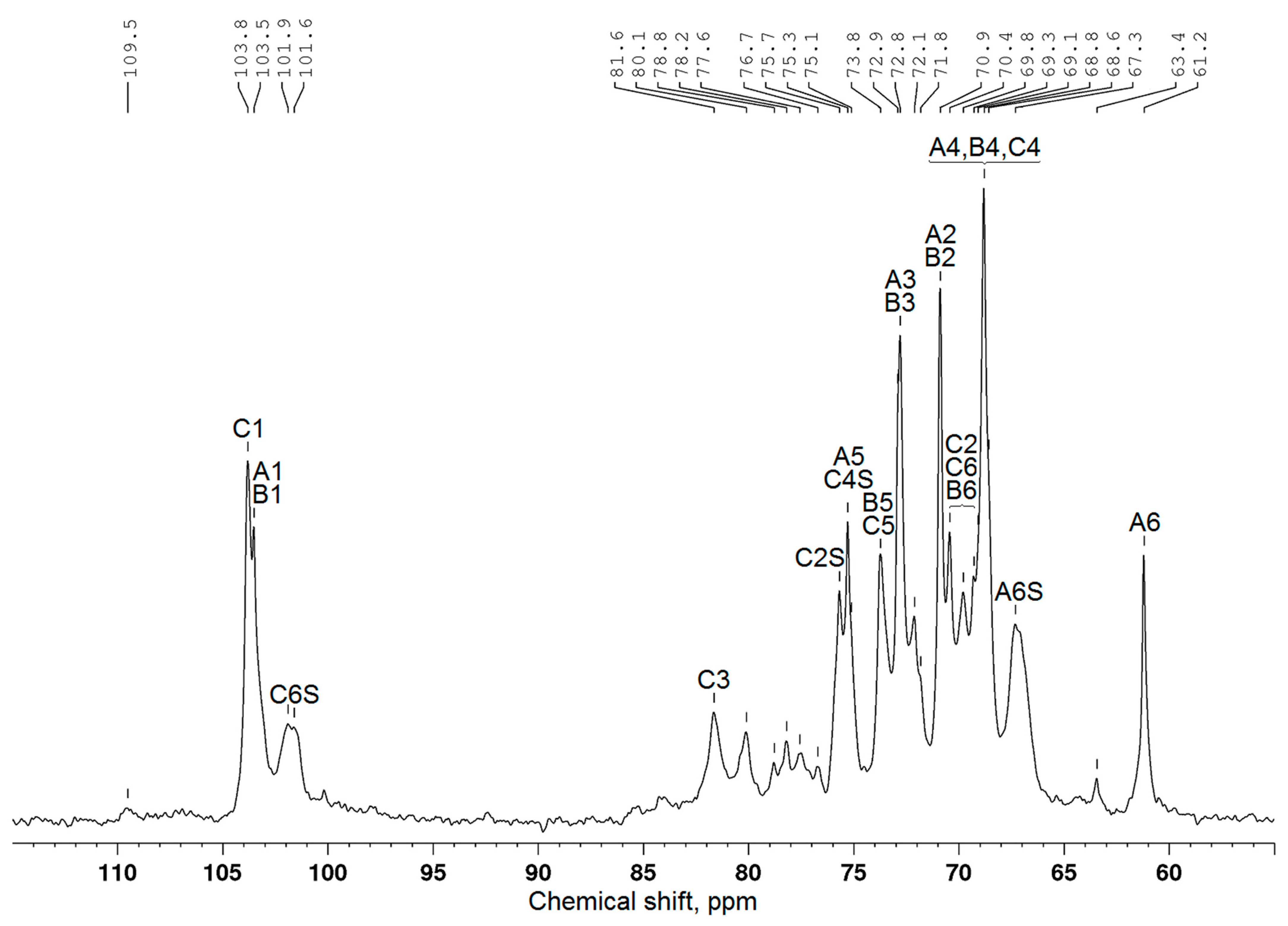
| Structural Unit | Chemical Shift (ppm) | |||||
|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | C6 | |
| β-D-Galp-(1→(A) | 103.5 | 70.9 | 72.9 | 68.8 | 75.3 | 61.2 |
| Sulfated A (AS) | n.d. * | n.d. | n.d. | n.d. | 72.1 | 67.3 |
| →6)-β-D-Galp-(1→(B) | 103.5 | 70.9 | 72.8 | 68.8 | 73.8 | 69.3 |
| Sulfated B (BS) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| →3,6)-β-D-Galp-(1→(C) | 103.8 | 70.4 | 81.6 | 68.8 | 73.8 | 69.8 |
| Sulfated C (CS) | 101.6 | 75.7 | n.d. | 75.1 | n.d. | n.d. |
| Structural Unit | Chemical Shift (ppm) | |||||
|---|---|---|---|---|---|---|
| C1/H1 | C2/H2 | C3/H3 | C4/H4 | C5/H5 | C6/H6 | |
| β-D-Galp-(1→(A) | 103.6/4.47 | 70.8/3.56 | 72.7/3.68 | 68.3/3.97 | 75.4/3.71 | 61.1/3.77 |
| Sulfated A (AS) | 103.2/4.59 | n.d. * | n.d. | n.d. | 72.1/4.15 | 67.0/4.23 |
| →6)-β-D-Galp-(1→(B) | 103.6/4.47 | 70.8/3.56 | 72.7/3.68 | 68.3/3.97 | 73.5/3.94 | 69.7/4.06 |
| Sulfated B (BS) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| →3,6)-β-D-Galp-(1→(C) | 103.7/4.71 | 70.3/3.77 | 81.4/3.91 | 68.6/4.19 | 73.5/3.94 | 70.3/3.94 |
| Sulfated C (CS) | 101.8/4.80 | 75.5/4.41 | n.d. | 75.2/5.08 | n.d. | n.d. |
| Sample | Mn, Da | Mw, Da | PDI |
|---|---|---|---|
| AG | 7505 | 9527 | 1.27 |
| sulfated AG obtained at 110 °C | 9229 | 13,516 | 1.46 |
| sulfated AG obtained at 115 °C | 8922 | 13,198 | 1.48 |
| sulfated AG obtained at 120 °C | 8786 | 12,890 | 1.47 |
| sulfated AG obtained at 125 °C | 9315 | 12,983 | 1.39 |
| sulfated AG obtained at 130 °C | 6148 | 11,766 | 1.91 |
| sulfated AG obtained at 140 °C | 7666 | 12,909 | 1.68 |
| Sample | Temperature Range, °C | Equation of the Kinetic Curve | Activation Energy E (kJ/mol) | Correlation Factor R2 |
|---|---|---|---|---|
| AG | 227–327 332–502 | y = −9580.2x + 3.3848 y = −536.92x − 11.435 | 79.6 4.5 | 0.9894 0.9737 |
| sulfated AG obtained at 110 °C | 236–266 271–341 | y = −2732.3x − 7.9194 y = −535.5x − 11.982 | 22.7 4.5 | 0.9725 0.9880 |
| sulfated AG obtained at 125 °C | 235–270 275–350 | y = −3672.4x − 6.0628 y = −282.7x − 13.365 | 30.5 2.4 | 0.9492 0.9775 |
| sulfated AG obtained at 140 °C | 246–271 276–336 | y = −2567x − 8.2984 y = −630.7x − 11.869 | 21.3 5.2 | 0.9541 0.9940 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levdansky, V.A.; Levdansky, A.V.; Malyar, Y.N.; Ivanenko, T.Y.; Fetisova, O.Y.; Kazachenko, A.S.; Kuznetsov, B.N. Green Sulfation of Arabinogalactan in the Melt of a Sulfamic Acid–Urea Mixture. Polymers 2025, 17, 642. https://doi.org/10.3390/polym17050642
Levdansky VA, Levdansky AV, Malyar YN, Ivanenko TY, Fetisova OY, Kazachenko AS, Kuznetsov BN. Green Sulfation of Arabinogalactan in the Melt of a Sulfamic Acid–Urea Mixture. Polymers. 2025; 17(5):642. https://doi.org/10.3390/polym17050642
Chicago/Turabian StyleLevdansky, Vladimir A., Alexander V. Levdansky, Yuriy N. Malyar, Timur Yu. Ivanenko, Olga Yu. Fetisova, Aleksandr S. Kazachenko, and Boris N. Kuznetsov. 2025. "Green Sulfation of Arabinogalactan in the Melt of a Sulfamic Acid–Urea Mixture" Polymers 17, no. 5: 642. https://doi.org/10.3390/polym17050642
APA StyleLevdansky, V. A., Levdansky, A. V., Malyar, Y. N., Ivanenko, T. Y., Fetisova, O. Y., Kazachenko, A. S., & Kuznetsov, B. N. (2025). Green Sulfation of Arabinogalactan in the Melt of a Sulfamic Acid–Urea Mixture. Polymers, 17(5), 642. https://doi.org/10.3390/polym17050642








