Recent Advances in Polymer Recycling: A Review of Chemical and Biological Processes for Sustainable Solutions
Abstract
1. Introduction
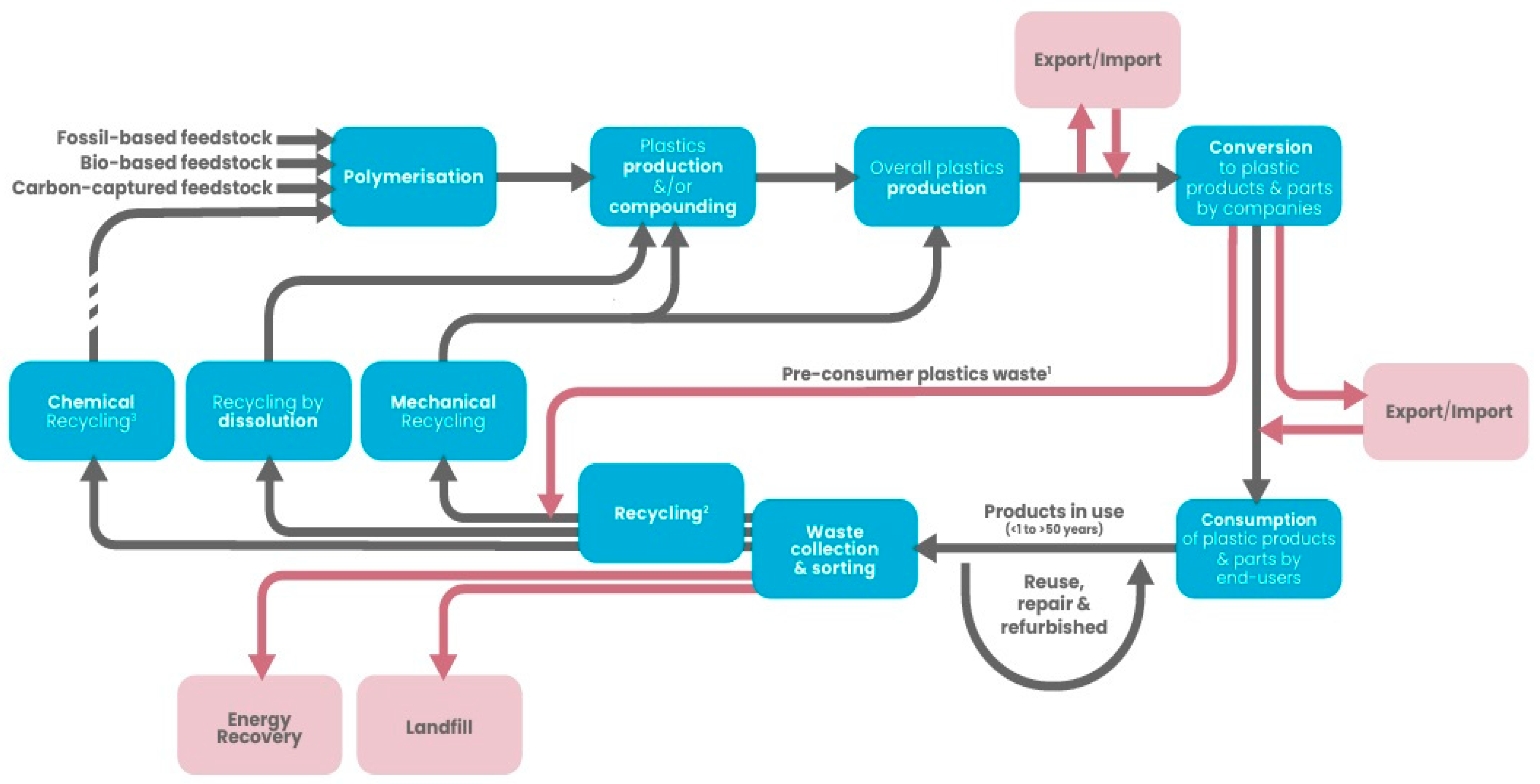
2. Advancing Towards a Circular Plastics Economy
3. Plastic Polymers: Types and Industrial Applications
- LDPE—Low-Density Polyethylene (Thermoplastic)
- Thermosetting: Vinyl ester
- HDPE—High-Density Polyethylene (Thermoplastic)
- Thermosetting: Phenol formaldehyde resin
- PP—Polypropylene (Thermoplastic)
- Thermosetting: Silicon
- PS—Polystyrene (Thermoplastic)
- Thermosetting: Polyester
- PET—Polyethylene Terephthalate (Thermoplastic)
- Thermosetting: Urea—Formaldehyde
- PVC—Polyvinyl chloride (Thermoplastic)
- Thermosetting: Bakelite
4. Current Advances in Plastic Recycling: Methods and Trends
4.1. Primary Mechanical Recycling: Fundamentals and Techniques
- Recovered materials are reintroduced rapidly into the manufacturing process.
- Contaminants can either be effectively removed or are inconsequential to the final product, particularly when incorporated into specific layers of the recycled material.
- The polymer retains sufficient thermal stability to withstand subsequent high-temperature processing stages.
4.2. Secondary Mechanical Recycling: Techniques and Material Integrity
- Availability and logistics related to waste materials, including collection, storage, and transport costs.
- The physical form and size of the waste, whether it be flakes, fibers, or other shapes.
- The complexity of the material composition, particularly if the waste contains multiple components with varying melting points.
- The price differential between virgin and recycled polymers can make secondary recycling of high-value technical polymers financially attractive.
- The presence of additives that influence the recyclate’s characteristics, such as odor or color, may restrict its use in end products unless purification, deodorizing, or decolorizing processes are applied.
- The availability and costs of advanced separation, detection, and purification technologies.
- Environmental considerations include dust generation, noise from mechanical processing, energy consumption, and the potential toxicity of solvents used in purification processes.
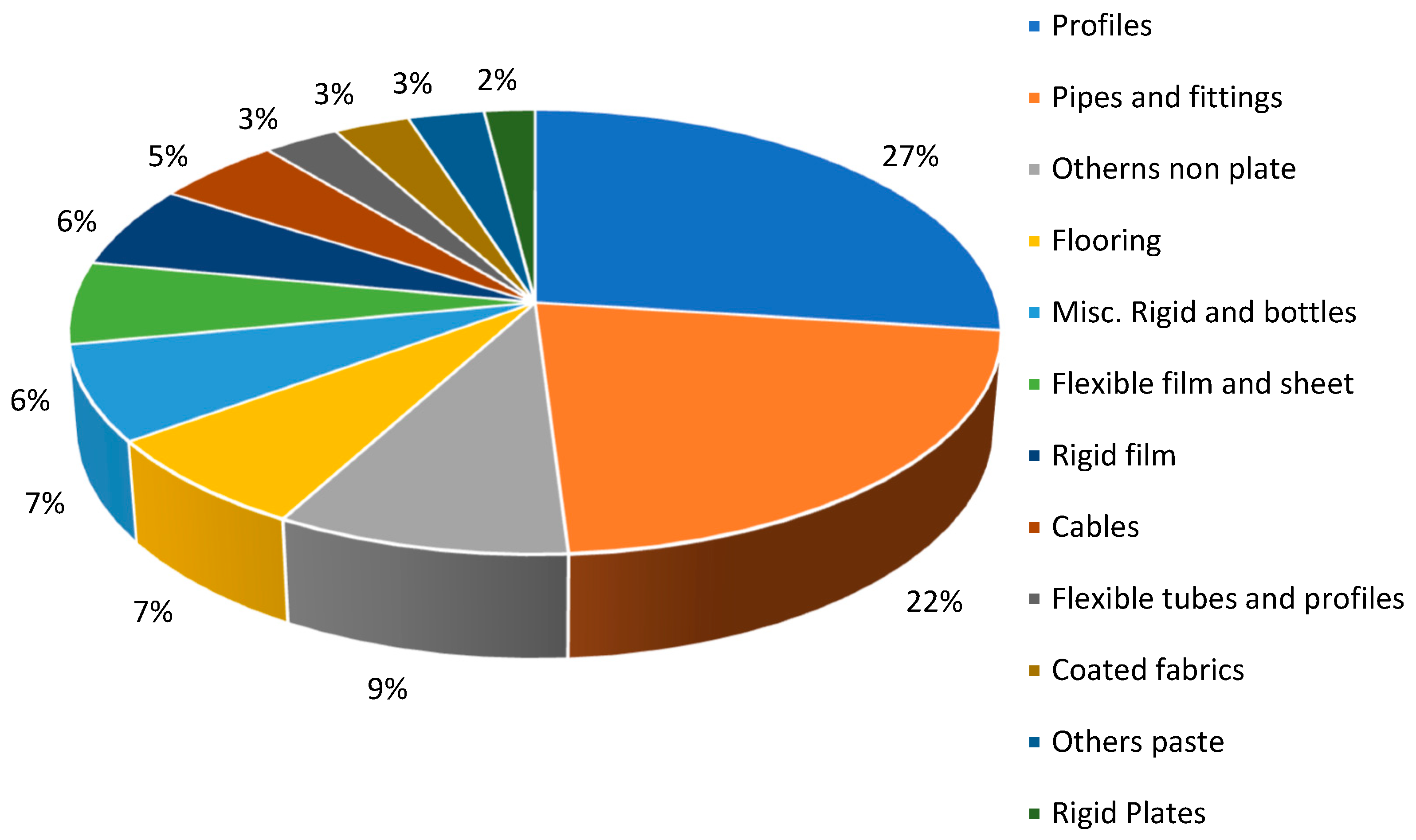
Utilization of Recycled Polymers as Fillers in Composite Materials
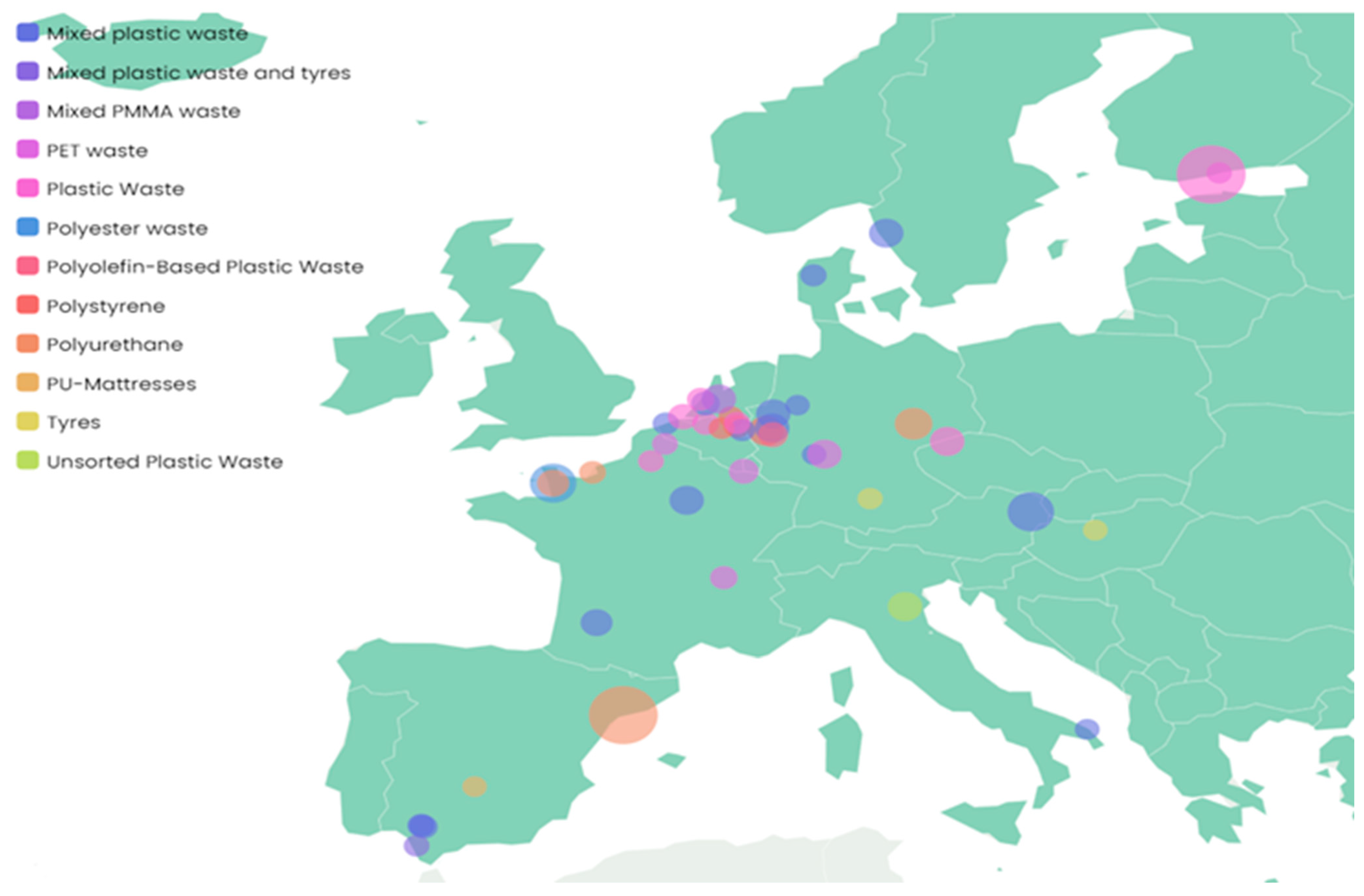
4.3. Chemical Recycling Solutions for End-of-Life Polymers
4.3.1. Purification
4.3.2. Depolymerization
Depolymerization of PET in SPI Code 1
Depolymerization of HDPE, LPDE and PP for SPI Code 2, 4, and 5
Depolymerization of PVC for SPI Code 3
Depolymerization of PS for SPI Code 6
4.3.3. Feedstock Recycling—Pyrolysis
4.3.4. Benefits of Chemical Recycling
4.4. Biodegradation of Polymers: Pathways to Sustainable Polymer Recycling
4.4.1. Hydrolytic Degradation Mechanisms in Synthetic Polymers
4.4.2. Thermolysis and Thermal Decomposition of Polymers
4.4.3. Chemical Decomposition: Oxidation and Thermo-Oxidative Processes
4.4.4. Mechanisms of Polymer Degradation: Chemical and Microbial Interactions
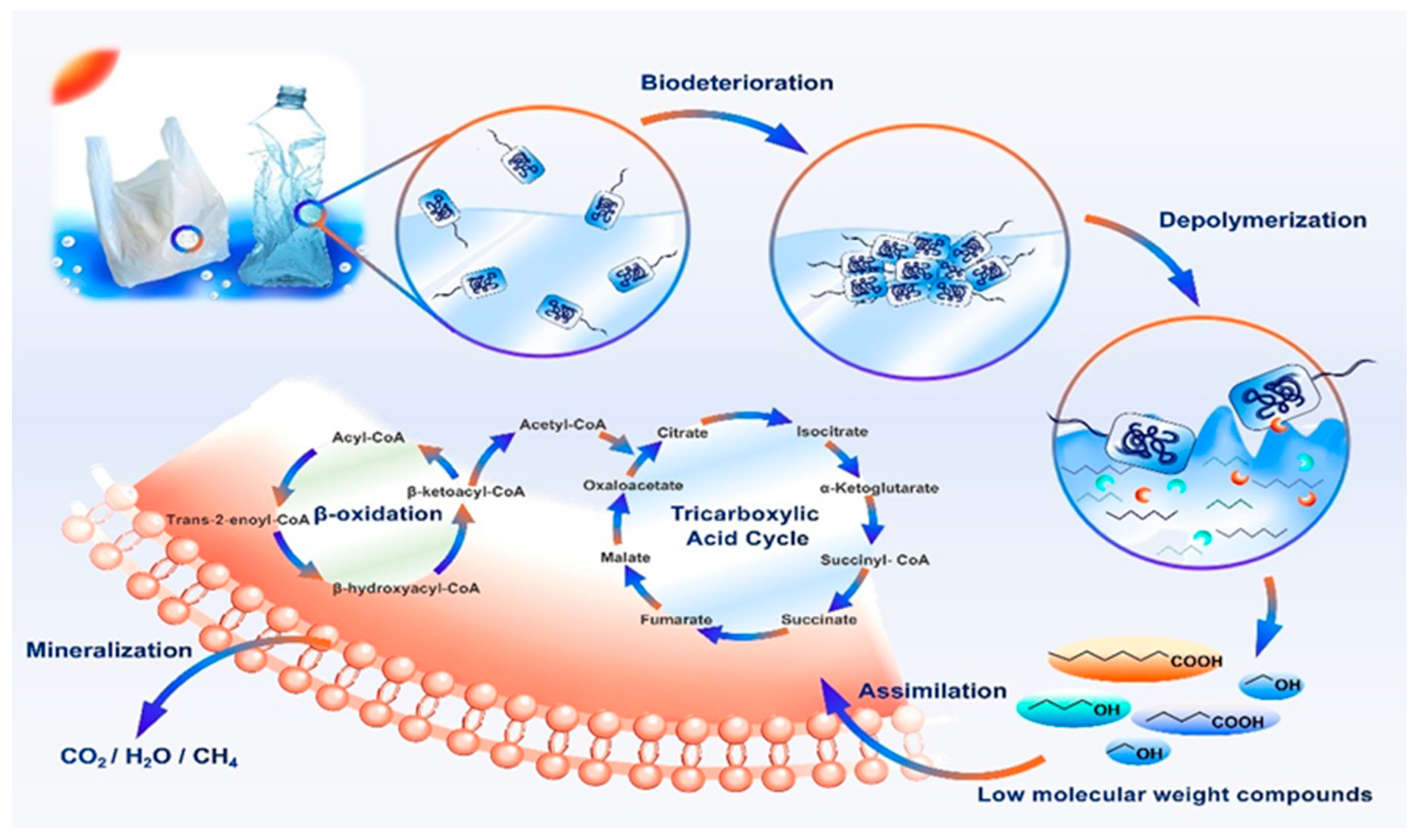
4.4.5. Phases of Polymer Biodegradation
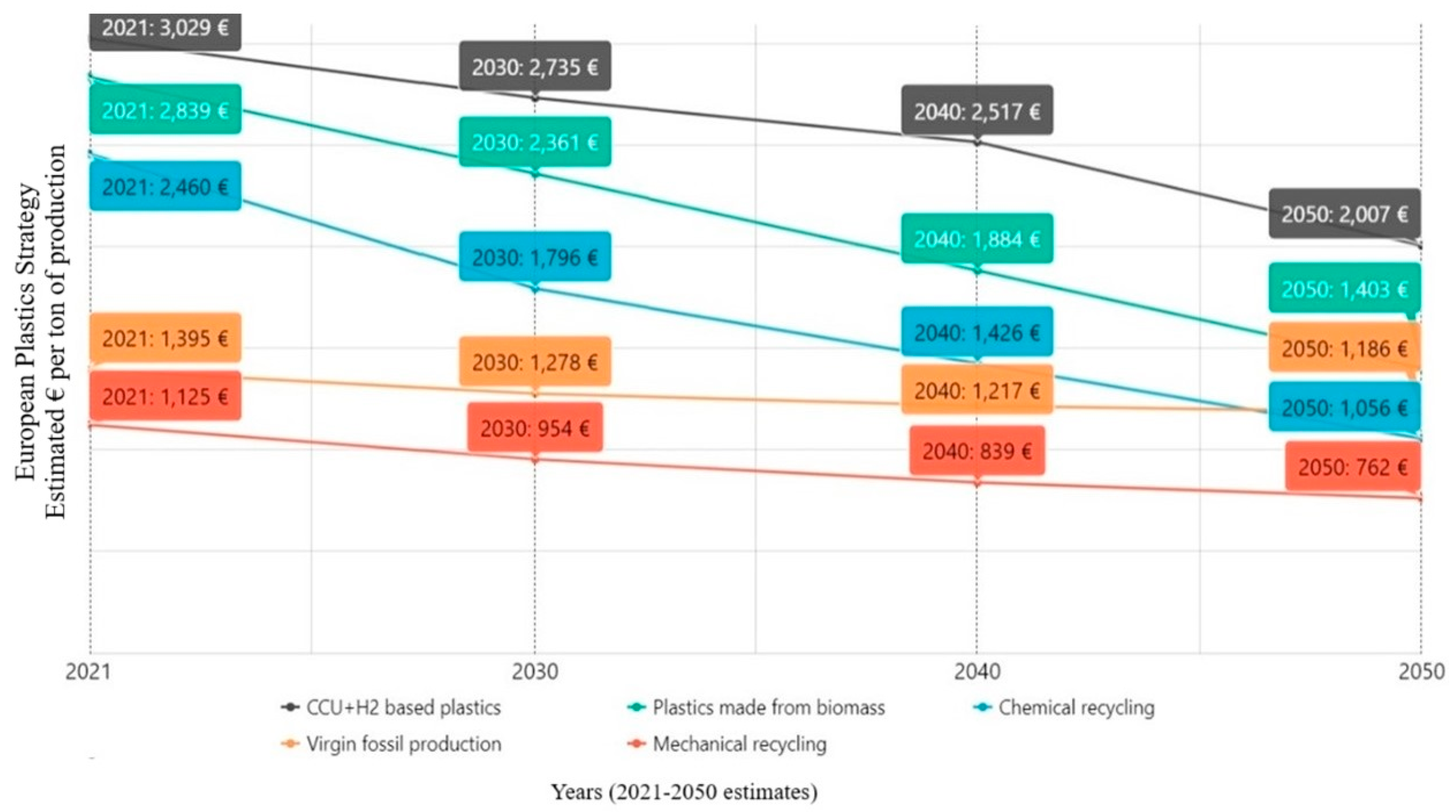
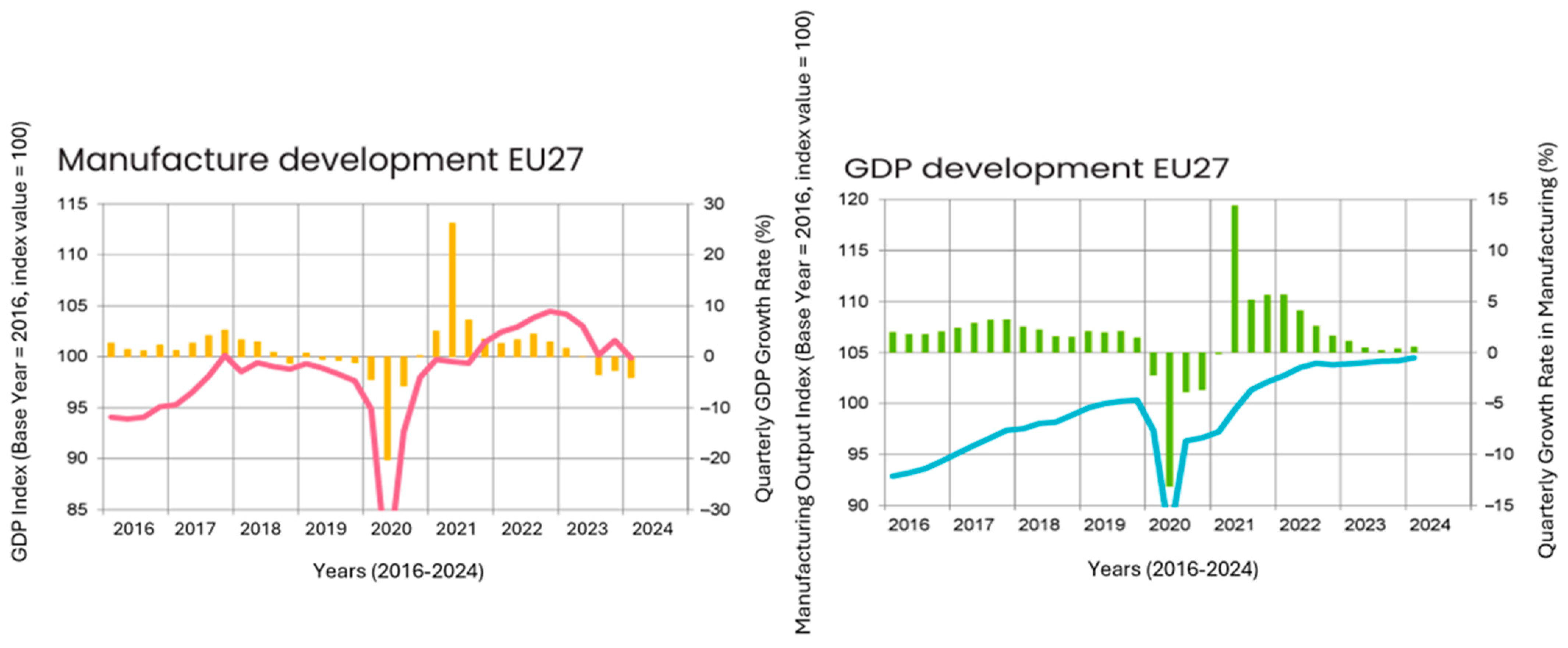
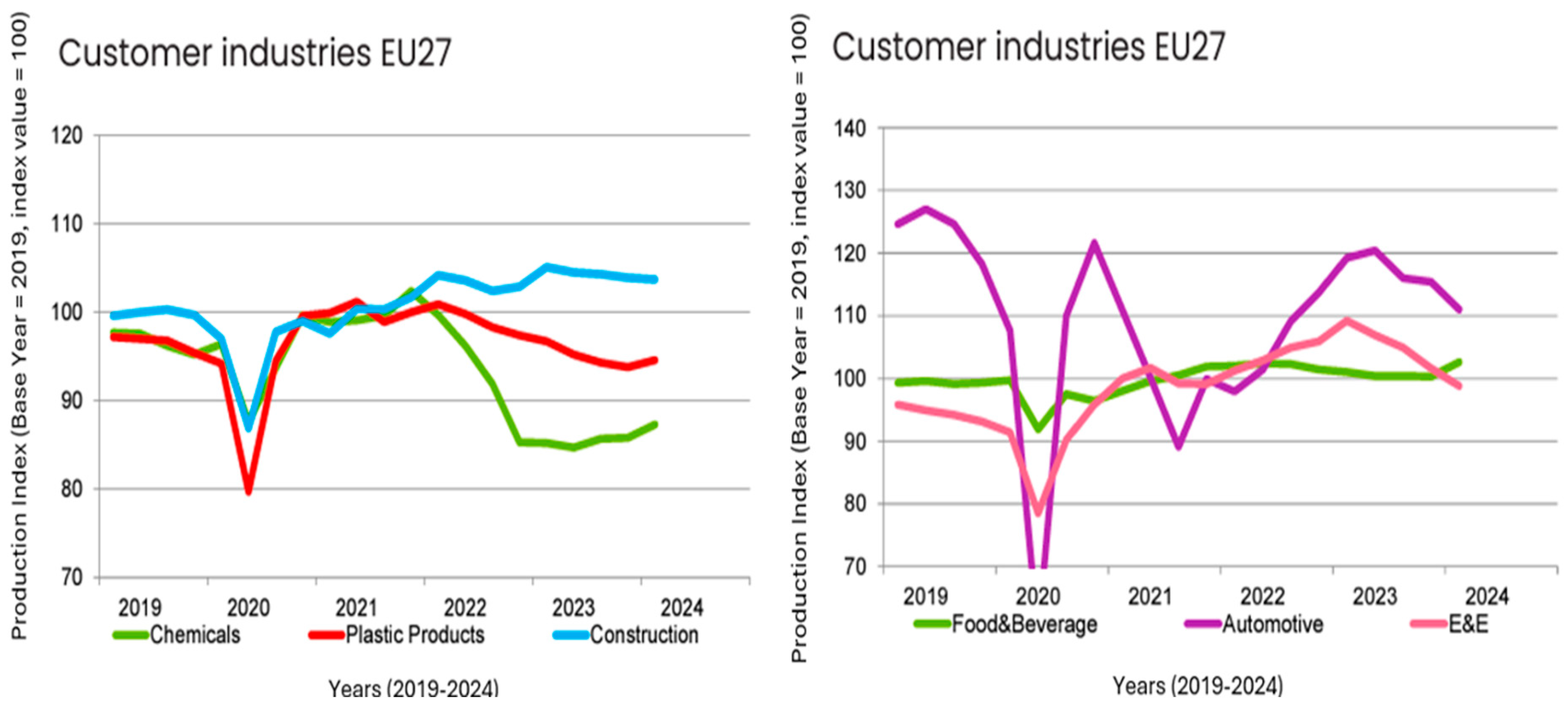
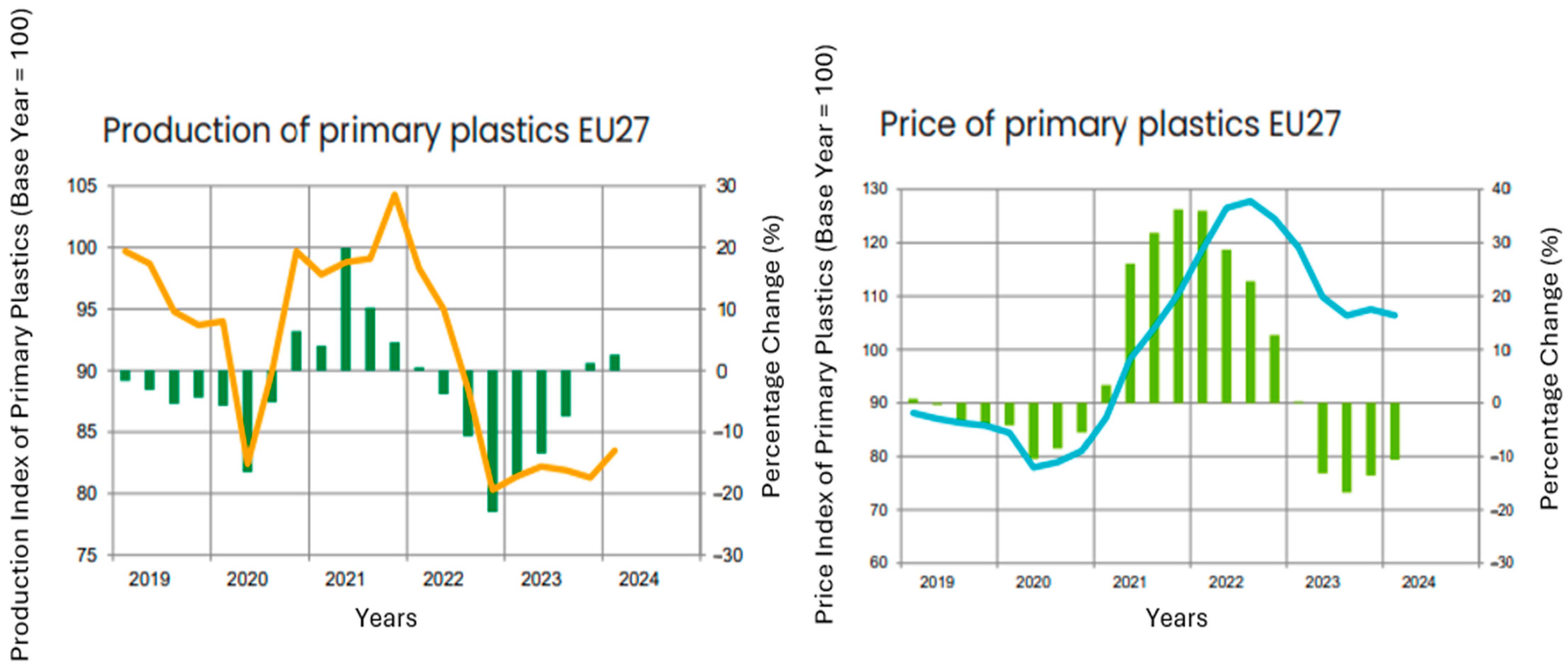
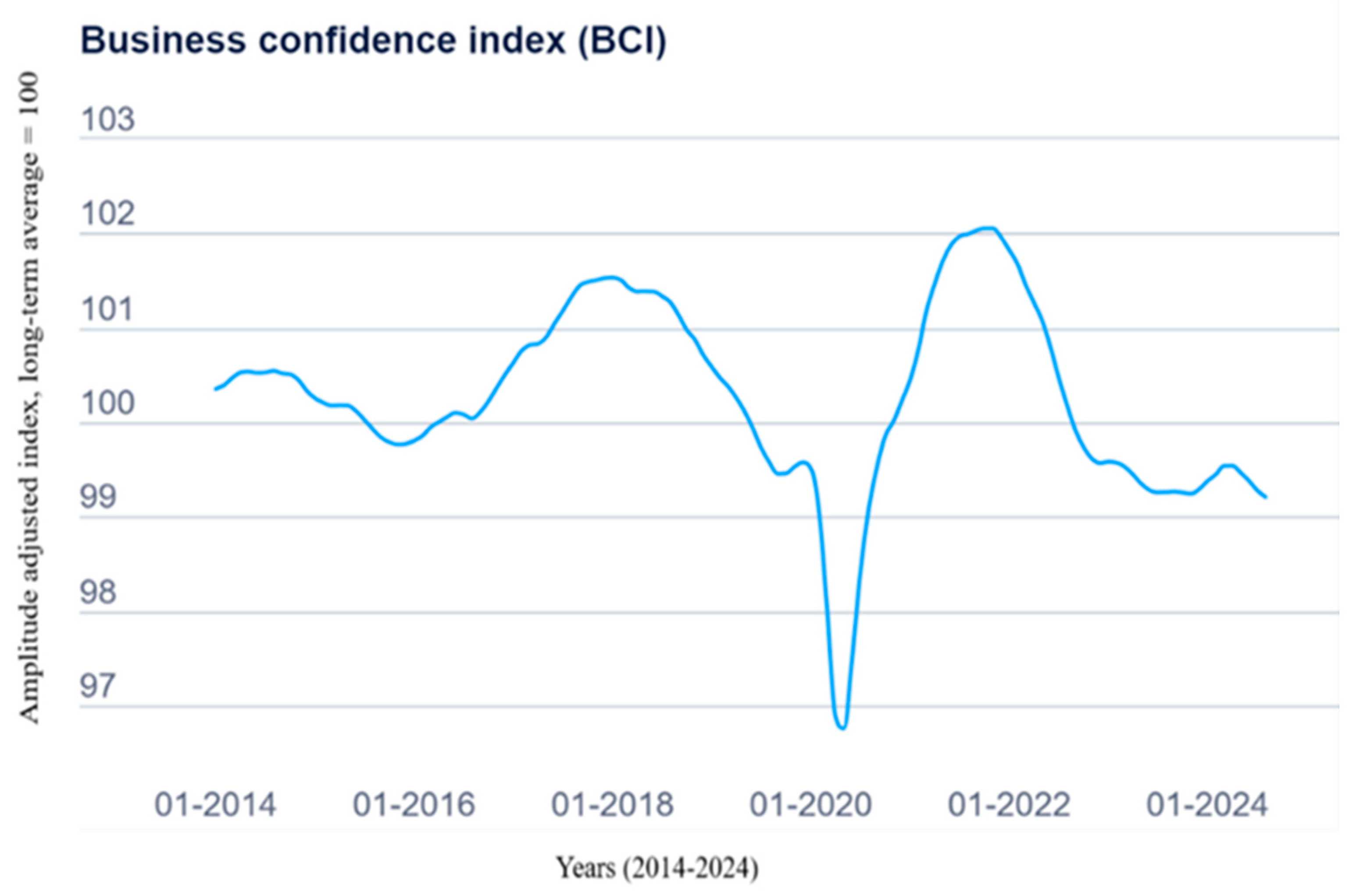
5. Quarterly Report—Q1/2024
5.1. Automotive Sector
5.2. Production of Plastics
5.3. Foreign Trade
5.4. European Plastics Manufacturers
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastic ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- World Steel Association (WSA). Steel Statistical Yearbooks 1978 to 2016. Available online: www.worldsteel.org/steel-by-topic/statistics/steel-statistical-yearbook-.html (accessed on 28 October 2024).
- U.S. Geological Survey (USGS). Cement Statistics and Information. Available online: https://minerals.usgs.gov/minerals/pubs/commodity/cement/ (accessed on 28 October 2024).
- Khalid, M.Y.; Arif, Z.U.; Ahmed, W.; Arshad, H. Recent trends in recycling and reusing techniques of different plastic and polymers and their composite materials. Sustain. Mater. Technol. 2022, 31, e00382. [Google Scholar] [CrossRef]
- Wu, J.H.; Zhang, C.; Meng, Q.N.; Liu, B.C.; Sun, Y.H.; Wen, M.; Ma, S.M.; He, L.K. Study on tensile properties of carbon fiber reinforced AA7075 composites at high temperatures. Mater. Sci. Eng. A 2021, 825, 141931. [Google Scholar] [CrossRef]
- Cardona-Vivas, N.; Correa, M.A.; Colorado, H.A. Multifunctional composites obtained from combination of conductive polymer with different contents of primary battery waste powders. Sustain. Mater. Technol. 2021, 28, e00281. [Google Scholar] [CrossRef]
- Olivera, S.; Muralidhara, H.B.; Venkatesh, K.; Guna, V.K.; Gopalakrishna, K.; Kumar, K.Y. Potential applications of cellulose and chitosan nanoparticles/composites in wastewater treatment: A review. Carbohyd Polym. 2016, 153, 600–618. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.; Miranda, D.; Almeida, A.M.; Silva, M.M.; Meseguer-Duenas, J.M.; Ribelles, J.L.G.; Lanceros-Méndez, S.; Costa, C.M. Solid polymer electrolytes based on lithium bis(trifuoromethanesulfonyl)imide/poly(vinylidene fluoride-co-hexafluoropropylene) for safer rechargeable lithium-ion batteries. Sustain. Mater. Technol. 2019, 21, e00104. [Google Scholar]
- Selvan, R.T.; Raja, P.V.; Mangal, P.; Mohan, N.; Bhowmik, S. Recycling technology of epoxy glass fiber and epoxy carbon fiber composites used in aerospace vehicles. J. Compos. Mater. 2021, 55, 3281–3292. [Google Scholar] [CrossRef]
- Stergiou, V.; Konstantopoulos, G.; Charitidis, C.A. Carbon Fiber Reinforces Plastics in Space: Life Cycle Assessment towards Improved Sustainability of Space Vehicles. J. Compos. Sci. 2022, 6, 144. [Google Scholar] [CrossRef]
- Sommer, V.; Walther, G. Recycling and recovery infrastructures for glass and carbon fibre reinforced plastic waste from wind energy industry: A European case study. Waste Manag. 2021, 121, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef] [PubMed]
- Ignatyev, I.A.; Thielemans, W.; Vander Beke, B. Recycling of Polymers: A review. ChemSusChem 2014, 7, 1579–1593. [Google Scholar] [CrossRef] [PubMed]
- Dobransky, J.; Pollak, M.; Kocisko, M.; Kascak, J.; Torokova, M. Effect of Technological Waste on Rheological Properties of the Polymer Composite. Manuf. Technol. 2021, 21, 51–55. [Google Scholar] [CrossRef]
- Jung, H.Y.N.; Shin, G.; Kwak, H.; Hao, L.T.; Jegal, J.; Kim, H.J.; Jeon, H.; Park, J.; Oh, D.X. Review of polymer technologies for improving the recycling and upcycling efficiency of plastic waste. Chemosphere 2023, 320, 138089. [Google Scholar] [CrossRef] [PubMed]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef] [PubMed]
- Hubo, S.; Delva, L.; Van Damme, N.; Ragaert, K. Blending of recycled mixed polyolefins with recycled polypropylene: Effect on physical and mechanical properties. AIP Conf. Proc. 2016, 1779, 140006. [Google Scholar]
- Hubo, S.; Leite, L.; Martins, C.; Ragaert, K. Evaluation of post-industrial and post-consumer polyolefin-based polymer waste streams for injection moulding. In Proceedings of the 6th Polymer and Moulds Innovation Conference, Guimaraes, Portugal, 10–12 September 2014. [Google Scholar]
- Correira, J.R.; Almeida, N.M.; Figueira, J.R. Recycling of FRP composites: Reusing fine GFRP waste in concrete mixtures. J. Clean. Prod. 2011, 19, 1745–1753. [Google Scholar] [CrossRef]
- Gharde, S.; Kandasubramanian, B. Mechanothermal and chemical recycling methodologies for the Fibre Reinforced Plastic (FRP). Environ. Technol. Innov. 2019, 14, 100311. [Google Scholar] [CrossRef]
- Berger, F.; Gauvin, F.; Brouwers, H.J.H. The recycling potential of wood waste into wood-wool/cement composite. Constr. Build. Mater. 2020, 260, 119786. [Google Scholar] [CrossRef]
- Yang, L.; Sáez, E.R.; Nagel, U.; Thomason, J.L. Can thermally degraded glass fibre be regenerated for closed-loop recycling of thermosetting composites? Compos. Part A Appl. Sci. Manuf. 2015, 72, 167–174. [Google Scholar] [CrossRef]
- Ferdous, W.; Manalo, A.; Siddique, R.; Mendis, P.; Yan, Z.G.; Wong, H.S.; Lokuge, W.; Aravinthan, T.; Schubel, P. Recycling of landfill wastes (tyres, plastics and glass) in construction—A review on global waste generation, performance, application and future opportunities. Resour. Conserv. Recycl. 2021, 173, 105745. [Google Scholar] [CrossRef]
- Demets, R.; Van Kets, K.; Huysveld, S.; Dewulf, J.; De Meester, S.; Ragaert, K. Addressing the complex challenge of understanding and quantifying substitutability for recycled plastics. Resour. Conserv. Recycl. 2021, 174, 105826. [Google Scholar] [CrossRef]
- Ellis, L.D.; Rorrer, N.A.; Sullivan, K.P.; Otto, M.; McGeehan, J.E.; Román-Leshkov, Y.; Wierckx, N.; Beckham, G.T. Chemical and biological catalysis for plastics recycling and upcycling. Nat. Catal. 2021, 4, 539–556. [Google Scholar] [CrossRef]
- Thuy, V.T.T.; Hao, L.T.; Jeon, H.; Koo, J.M.; Park, J.; Lee, E.S.; Hwang, S.Y.; Choi, S.; Park, J.; Dongyeop, X.O. Sustainable, self-cleaning, transparent, and moisture/oxygen-barrier coating films for food packaging. Green. Chem. 2021, 23, 2658–2667. [Google Scholar] [CrossRef]
- The Circular Economy for Plastics—A European Analysis 2024: Plastics Europe. Retrieved from Plastics Europe. Available online: https://plasticseurope.org/knowledge-hub/the-circular-economy-for-plastics-a-european-analysis-2024/ (accessed on 30 October 2024).
- SYSTEMIQ. ReShaping Plastics: Pathways to a Circular, Climate Neutral Plastics System in Europe. 2022. Available online: https://plasticseurope.org/wp-content/uploads/2022/04/SYSTEMIQ-ReShapingPlastics-April2022.pdf (accessed on 30 October 2024).
- Schirmeister, C.G.; Mulhaupt, R. Closing the Carbon Loop in the Circular Plastics Economy. Macromol. Rapid Commun. 2022, 43, 2200247. [Google Scholar] [CrossRef] [PubMed]
- European Commission. An EU Action Plan for the Circular Economy. COM (2015) 614. 2015. Available online: https://www.eea.europa.eu/policy-documents/com-2015-0614-final (accessed on 2 November 2024).
- Piontek, W. The Circular Plastics Economy and the Instruments to Implement it. Econ. Environ. 2019, 3, 18–33. [Google Scholar]
- Foschi, E.; Bonoli, A. The Commitment of Packaging Industry in the Framework of the European Strategy for Plastics in a Circular Economy. Adm. Sci. 2019, 9, 18. [Google Scholar] [CrossRef]
- The Plastics Transition: Plastics Europe. Retrieved from Plastics Europe. 2024. Available online: https://plasticseurope.org/changingplasticsforgood/the-plastics-transition/ (accessed on 2 November 2024).
- Von Vacano, B.; Mangold, H.; Vandermeulen, G.W.M.; Battagliarin, G.; Hofmann, M.; Bean, J.; Kuenkel, A. Sustainable Design of Structural and Functional Polymers for a Circular Economy. Angew. Chem. Int. Ed. 2022, 62, e202210823. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, C.; Mayers, K.; Lee, J.; Murphy, R. Recycling plastics from e-waste: Implications for effective eco-design. J. Ind. Ecol. 2023, 27, 1370–1388. [Google Scholar] [CrossRef]
- Jagadeesh, P.; Rangappa, S.M.; Siengchin, S.; Puttegowda, M.; Thiagamani, S.M.K.; Rajeshkumar, G.; Kumar, M.H.; Oladijo, O.P.; Fiore, V.; Cuadrado, M. Sustainable recycling technologies for thermoplastic polymers and their composites: A review of the state of the art. Polym. Compos. 2022, 43, 5831–5862. [Google Scholar] [CrossRef]
- Daborn, G.R.; Derry, R. Crogenic communition in scrap recycling. Resour. Conserv. Recycl. 1998, 1, 49–63. [Google Scholar] [CrossRef]
- Singh, N.; Hui, D.; Singh, R.; Ahuja, I.P.S.; Feo, L.; Fraternali, F. Recycling of plastic solid waste: A state of art review and future applications. Compos. Part B Eng. 2017, 115, 409–422. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.S.; Ma, C.; Qiao, Y.; Yao, H. Thermal degradation of PVC: A review. Waste Manag. 2016, 48, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Rezakalla, A.I.; Petrovna, S.T. Study of Mechanical Properties of Recycled Polyethylene of High and Low Density. Mater. Plast. 2021, 58, 210–215. [Google Scholar] [CrossRef]
- Li, X.M.; Mahadas, N.A.; Zhang, M.X.; Depodesta, J.; Stefik, M.; Tang, C.B. Sustainable high-density polyethylene via chemical recycling: From modification to polymerization methods. Polymer 2024, 295, 126698. [Google Scholar] [CrossRef]
- Wang, J.C.; Li, Y.C.; Qiu, Z.M.; Zhang, Y.Q. Experimental research on compressive properties of recycling polypropylene (PP) fiber recycled coarse aggregate concrete. J. Build. Eng. 2023, 76, 107403. [Google Scholar] [CrossRef]
- Germane, L.; Berzina, A.; Eglitis, R.; Iesalnieks, M.; Lungevics, J.; Linarts, A.; Sutka, A.; Lapcinskis, L. Physical and Chemical Surface Modification of Recycled Polystyrene Films for Improved Triboelectric Properties. Energy Technol. 2024, 12, 2400762. [Google Scholar] [CrossRef]
- Nistico, R. Polyethylene terephthalate (PET) in the packaging industry. Polym. Test. 2020, 90, 106707. [Google Scholar] [CrossRef]
- Babaei, M.; Jalilian, M.; Shahbaz, K. Chemical recycling of Polyethylene terephthalate: A mini-review. J. Environ. Chem. Eng. 2024, 12, 112507. [Google Scholar] [CrossRef]
- Ait-Touchente, Z.; Khellaf, M.; Raffin, G.; Lebaz, N.; Elaissari, A. Recent advances in polyvinyl chloride (PVC) recycling. Polym. Adv. Technol. 2023, 35, e6228. [Google Scholar] [CrossRef]
- The European Council of Vinyl Manufacturers. PVC Applications 2024, Retrieved from ECMV. Available online: https://pvc.org/pvc-applications/ (accessed on 2 November 2024).
- Fleischhaker, F.; Haehnel, A.P.; Misske, A.M.; Blanchot, M.; Haremza, S.; Barner-Kowollik, C. Glass-Transition-, Melting-, and Decomposition Temperatures of Tailored Polyacrylates and Polymethacrylates: General Trends and Structure-Property Relationships. Macromol. Chem. Phys. 2014, 215, 1192–1200. [Google Scholar] [CrossRef]
- Li, H.Q.; Aguirre-Villegas, H.A.; Allen, R.D.; Bai, X.L.; Benson, C.H.; Beckham, G.T.; Bradshaw, S.L.; Brown, J.L.; Brown, R.C.; Cecon, V.S.; et al. Expanding plastics recycling technologies: Chemical aspects, technology status and challenges. Green. Chem. 2022, 25, 791–792. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.; Ahuja, I.P.S.; Hashmi, M.S.J. Processing techniques of polymeric materials and their reinforced composites. Adv. Mater. Res. 2020, 6, 591–607. [Google Scholar] [CrossRef]
- Kolluru, S.; Thakur, A.; Tamakuwala, D.; Kumar, V.V.; Ramakrishna, S.; Chandran, S. Sustainable recycling of polymers: A comprehensive review. Polym. Bull. 2024, 81, 9569–9610. [Google Scholar] [CrossRef]
- Goodship, V. Plastic Recycling. Sci. Prog. 2007, 90, 245–268. [Google Scholar] [CrossRef] [PubMed]
- Zinser, J. Top 4 Benefits of Customized, Closed-Loop and End-of-Life Recycling Programs for Automakers; Novelis Inc. 2023. Available online: https://investors.novelis.com/news-events/press-releases/detail/25/top-4-benefits-of-customized-closed-loop-and-end-of-life-recycling-programs-for-automakers (accessed on 2 November 2024).
- Garcia, J.C.; Marcilla, A.; Beltran, M. The effect of adding processed PVC on the rheology of PVC plastisols. Polymer 1998, 39, 2261–2267. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. The valorization of plastic solid waste (PSW) by primary to quaternary routes: From re-use to energy and chemicals. Prog. Energ. Combust. 2010, 36, 103–129. [Google Scholar] [CrossRef]
- Baillie, C.; Matovic, D.; Thamae, T.; Vaja, S. Waste-based composites-Poverty reducing solutions to environmental problems. Resour. Conserv. Recycl. 2011, 55, 973–978. [Google Scholar] [CrossRef]
- Francis, R. Recycling of Polymers: Methods, Characterization and Applications; Wiley-VCH: Hoboken, NJ, USA, 2017. [Google Scholar]
- Achilias, D.S. Material Recycling—Trends and Perspectives; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Dobry, A.; Boyerkawenoki, F. Phase separation in polymer solution. J. Polym. Sci. 1947, 2, 90–100. [Google Scholar] [CrossRef]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastic recycling: Challenges and opportunities. Philos. Trans. R. Soc. B 2009, 364, 2115–2126. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Bosnea, L.A. Recycling of polymeric materials used for food packaging: Current status and perspectives. Food Rev. Int. 2001, 17, 291–346. [Google Scholar] [CrossRef]
- Brems, A.; Baeyens, J.; Dewil, R. Recycling and recovery of post-consumer plastic solid waste in a European context. Therm. Sci. 2012, 16, 669–685. [Google Scholar] [CrossRef]
- Gaur, A.; Shukla, A.; Saxena, D.; Maiti, P. Polymer Composites for Structural, Device, and Biomedical Applications. Mater. Sci. Technol. 2019, 1–34. [Google Scholar] [CrossRef]
- Oladele, I.O.; Otomosho, T.F.; Adediran, A.A. Polymer-based composites: An indispensable material for present and future applications. Int. J. Polym. Sci. 2020, 2020, 8834518. [Google Scholar] [CrossRef]
- Dubey, S.C.H.; Mishra, V.; Sharma, A. A review on polymer composites with waste material as reinforcement. Mater. Today Proc. 2021, 47, 2846–2851. [Google Scholar] [CrossRef]
- Kovačevič, T.; Brzic, S.; Kalagasidis, M. Reuse potential of functionalized thermoplastic waste as reinforcement for thermoset polymers: Mechanical properties and erosion resistance. J. Compos. Mater. 2021, 55, 4207–4220. [Google Scholar] [CrossRef]
- Stielmann, M. Closing the loop on chemical recycling in Europe. In European Plastics Manufacturers Plan 7.2 Billion Euros of Investment in Chemical Recycling; Press Release: Brussels, Belgium, 2021. [Google Scholar]
- Chemical Recycling—British Plastics Federation. 2024. Available online: https://www.bpf.co.uk/plastipedia/chemical-recycling-101.aspx (accessed on 4 November 2024).
- Qin, B.; Zhang, X. On Depolymerization. CCS Chem. 2024, 6, 297–312. [Google Scholar] [CrossRef]
- Chanda, M. Chemical aspects of polymer recycling. Adv. Ind. Eng. Polym. Res. 2021, 4, 133–150. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, L.; Gu, J.; Yuan, H.; Chen, Y. Chemical recycling of plastic wastes via homogenous catalysis: A review. Chem. Eng. J. 2024, 479, 147853. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Lv, X.D.; Ni, H.G. Solvent-based separation and recycling of waste plastics: A review. Chemosphere 2018, 209, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Chemical Recycling—Plastics Europe. 2024. Available online: https://plasticseurope.org/sustainability/circularity/recycling/chemical-recycling/ (accessed on 4 November 2024).
- Punkkinen, H.; Oasmaa, A.; Laatikainen-Luntama, J.; Nieminen, M.; Laine-Ylijoki, J. Thermal conversion of plastic-containing waste: A review. VTT Res. Rep. 2017, D4, 1–22. [Google Scholar]
- Kamber, N.E.; Tsujii, Y.; Keets, K.; Waymouth, R.M. The depolymerization of poly (ethylene terephthalate) (PET) using N-heterocyclic carbenes from ionic liquids. J. Chem. Educ. 2010, 87, 519–521. [Google Scholar] [CrossRef]
- Shi, L.; Zhu, L. Recent Advances and Challenges in Enzymatic Depolymerization and Recycling of PET wastes. ChemBioChem 2024, 25, e202300578. [Google Scholar] [CrossRef]
- Avadanei, M.; Drobota, M.; Stoica, I.; Rusu, E.; Barboiu, V. Surface morphology and amide concentration depth profile of aminolyzed poly (ethylene terephthalate) films. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 5456–5467. [Google Scholar] [CrossRef]
- Miao, Y.; Jouanne, A.; Yokochi, A. Current Technologies in Depolymerization Process and the Road Ahead. J. Polym. 2021, 13, 449. [Google Scholar] [CrossRef] [PubMed]
- Scheirs, J.; Kaminsky, W. Feedstock Recycling and Pyrolysis of Waste Plastics; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2006; pp. 709–728. [Google Scholar]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, M.J., Jr.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub-and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Moriya, T.; Enomoto, H. Characteristics of polyethylene cracking in supercritical water compared to thermal cracking. Polym. Degrad. Stab. 1999, 65, 373–386. [Google Scholar] [CrossRef]
- Mio, H.; Saeki, S.; Kano, J.; Saito, F. Estimation of mechanochemical dechlorination rate of poly (vinyl chloride). Environ. Sci. Technol. 2002, 36, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Dement’ev, K.I.; Palankoev, T.A.; Alekseeva, O.A.; Babkin, I.A.; Maksimov, A.L. Thermal depolymerization of polystyrene in highly aromatic hydrocarbon medium. J. Anal. Appl. Pyrolysis 2019, 142, 104612. [Google Scholar] [CrossRef]
- Miskolczi, N.; Bartha, I.; Deák, G. Thermal degradation of polyethylene and polystyrene from the packaging industry over different catalysts into fuel-like feed stocks. Polym. Degrad. Stab. 2006, 91, 517–526. [Google Scholar] [CrossRef]
- Garforth, A.A.; Ali, S.; Hernández-Martínez, J.; Akah, A. Feedstock recycling of polymer wastes. Curr. Opin. Solid State Mater. Sci. 2004, 8, 419–425. [Google Scholar] [CrossRef]
- Lord, H.L.; Pfannkoch, E.A. Sample Preparation Automation for GC Injection. Comp. Samp Prep. 2012, 2, 597–612. [Google Scholar]
- Basu, P. Pyrolisis. Biomass Gasification, Pyrolisis and Torrefaction (Third Edition)—Practical Desing and Theory; Elsevier: Halifax, NS, Canada, 2018. [Google Scholar]
- Canevarolo, S.V., Jr. Ciência dos Polímeros; Artliber: São Paulo, Brazil, 2006; p. 277. [Google Scholar]
- Silva, R.R.A.; Marques, C.S.; Arruda, T.R.; Teixeira, S.C.; Oliveira, T.V. Biodegradation of Polymers: Stages, Measurement, Standards and Prospects. Macromol 2023, 3, 371–399. [Google Scholar] [CrossRef]
- Lade, V.G.; Chavhan, S.K.; Shirsat, S.P.; Bhanvase, B.A. Processes for the treatment of biomedical wastes: Challenges and issues. 360-Degree Waste Manag. 2023, 2, 123–138. [Google Scholar]
- Zeenat; Elahi, A.; Bukhari, D.A.; Shamim, S.; Rehman, A. Plastics degradation by microbes: A sustainable approach. J. King Saud. Univ.-Sci. 2021, 33, 101538. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Shi, W.; Valencak, T.G.; Zhang, Y.A.; Liu, G.X.; Ren, D.X. Biodegradation of conventional plastics: Candidate organisms and potential mechanisms. Sci. Total Environ. 2023, 885, 163908. [Google Scholar] [CrossRef] [PubMed]
- Pathak, V.M.; Navneet. Review on the current status of polymer degradation: A microbial approach. Bioresour. Bioproces 2017, 4, 15. [Google Scholar] [CrossRef]
- Kliem, S.; Kreutzbruck, M.; Bonten, C. Review on the Biological Degradation of Polymers in Various Environments. Materials 2020, 13, 4586. [Google Scholar] [CrossRef] [PubMed]
- Magnin, A.; Pollet, E.; Phalip, V.; Avérous, L. Evaluation of biological degradation of polyurethanes. Biotechnol. Adv. 2020, 39, 107457. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarzadeh, B.; Almasi, H. Biodegradable Polymers. Biodegrad.-Life Sci. 2013, 23, 141–185. [Google Scholar]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent advances in biodegradable polymers for sustainable applications. Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Kumagai, S.; Yoshioka, T. Feedstock Recycling via Waste Plastic Pyrolysis. J. Jpn. Pet. Inst. 2016, 59, 243–253. [Google Scholar] [CrossRef]
- Rai, P.; Mehrotra, S.; Priya, S.; Gnansounou, E.; Sharma, S.K. Recent advances in the sustainable design and applications of biodegradable polymers. Bioresour. Technol. 2021, 325, 124739. [Google Scholar] [CrossRef]
- Lyu, S.; Untereker, D. Degradability of Polymers for Implantable Biomedical Devices. Int. J. Mol. Sci. 2009, 10, 4033–4065. [Google Scholar] [CrossRef] [PubMed]
- Kabir, E.; Kaur, R.; Lee, J.; Kim, K.-H.; Kwon, E.E. Prospects of biopolymer technology as an alternative option for non-degradable plastics and sustainable management of plastic wastes. J. Clean. Prod. 2020, 258, 120536. [Google Scholar] [CrossRef]
- Grynova, G.; Hodgson, J.L.; Coote, M.L. Revising the mechanism of polymer autooxidation. Org. Biomol. Chem. 2011, 9, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Quarterly Report Q1/2024—European Plastics Manufacturers (EU 27). Available online: https://plasticseurope.org/knowledge-hub/quarterly-report-q1-2024/ (accessed on 6 November 2024).
- OECD (2024)—Business Confidence Index (BCI). Available online: https://www.oecd.org/en/data/indicators/business-confidence-index-bci.html?oecdcontrol-b2a0dbca4d-var3=2010-02&oecdcontrol-b2a0dbca4d-var4=2024-12 (accessed on 6 November 2024).
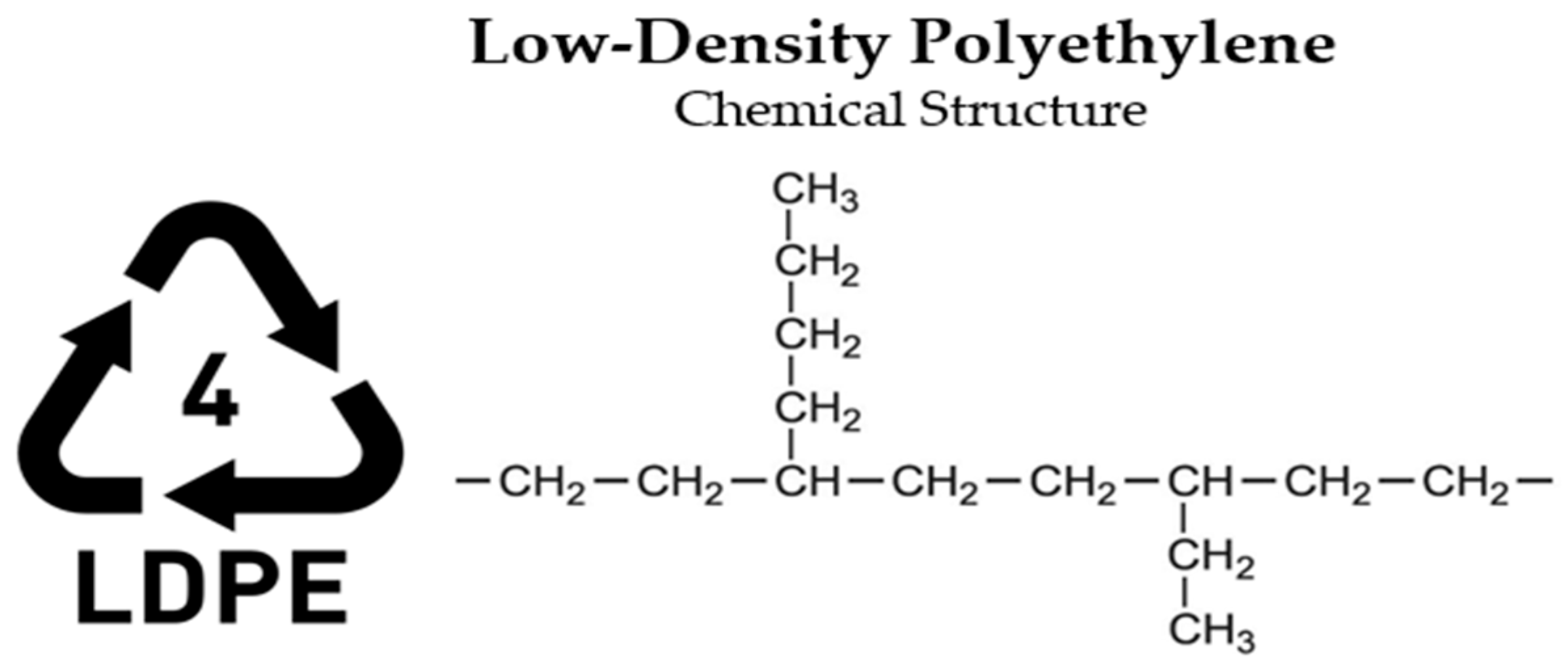



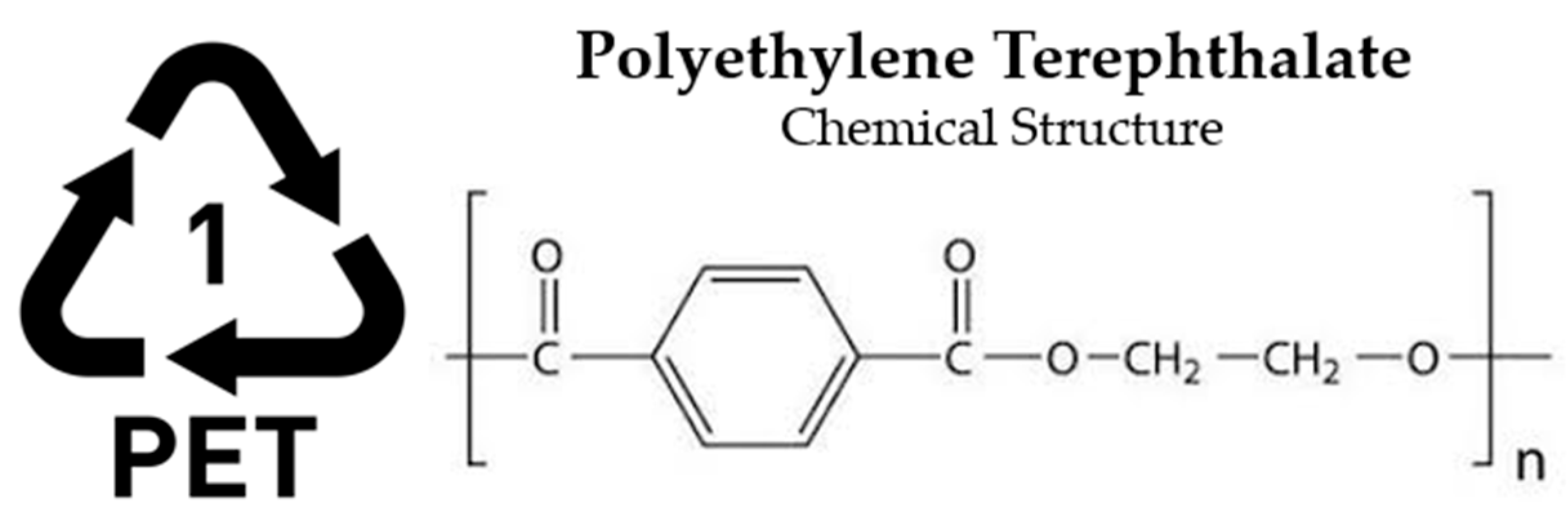








| Material | Morphology | Density (g.cm−3) | Glass Transition Temperature (°C) | Melting Temperature (°C) |
|---|---|---|---|---|
| LPDE | Semi-crystalline (40–55%) | 0.91–0.93 | −130 to −100/−30 to −10 | 110–115 |
| HDPE | Semi-crystalline (60–80%) | 0.94–0.96 | −130 to −100 | 125–135 |
| PP | Semi-crystalline | 0.90–0.91 | −20 to −21 | 160–165 |
| PS | Amorphous or Semicrystalline | 1.05 | 80–105 | - |
| PET | Semi-crystalline | 1.33–1.4 | 70–85 | 245–260 |
| PVC | Amorphous | 1.6–1.35 | −50 to −80 | - |
| Material | Initial Degradation Temp. (°C) | Tensile Strength (MPa) | Tensile Modulus (MPa) | Strain at Break (%) |
|---|---|---|---|---|
| LPDE | 487–498 | 8–23 | 200–500 | 300–1000 |
| HDPE | 480–498 | 18–35 | 700–1400 | 100–1000 |
| PP | 450–470 | 21–37 | 1100–1300 | 20–800 |
| PS | 415–425 | 45–65 | 3200–3250 | 3–4 |
| PET | 425–445 | 47 | 3100 | 50–300 |
| PVC | 290–315 | 10–25 | - | 170–400 |
| 2023 | Q1/24 | Q1/24-Q1/24 | ||
|---|---|---|---|---|
| % to Year | % to Year | % to Quarter | % to Year | |
| GDP world | 2.5 | 2.4 | 0.6 | 2.4 |
| GDP EU27 | 0.5 | 0.6 | 0.3 | 0.6 |
| Man. World | 2.0 | 2.8 | 1.1 | 2.8 |
| Man. EU27 | −1.1 | −4.1 | −1.7 | −4.1 |
| 2023 | Q1/24 | Q1/24-Q1/24 | ||
|---|---|---|---|---|
| Industry | % to Year | % to Year | % to Quarter | % to Year |
| Food | −1.5 | 1.6 | 2.3 | 1.6 |
| Automotive | 11.6 | −6.9 | −3.8 | −6.9 |
| E&E | −8.1 | −9.5 | −2.7 | −9.5 |
| Plastic | −4.1 | −2.2 | 0.9 | −2.2 |
| Chemicals | −8.5 | 2.4 | 1.8 | 2.4 |
| Construction | 1.1 | −1.3 | −0.3 | −1.3 |
| 2023 | Q1/24 | Q1/24-Q1/24 | ||
|---|---|---|---|---|
| Industry | % to Year | % to Year | % to Quarter | % to Year |
| Production | −9.7 | 2.6 | 2.7 | 2.6 |
| Producer prices | −11.0 | −10.6 | −1.0 | −10.6 |
| 2023 | Q1/24 | Q1/24-Q1/24 | ||
|---|---|---|---|---|
| Regions | % to Year | % to Year | % to Quarter | % to Year |
| Extra EU27 | −0.5 | 14.7 | −21.7 | 7.3 |
| Asia | 7.4 | 9.7 | −27.4 | 1.2 |
| Rest Europe | −5.9 | 18.2 | −18.0 | 11.4 |
| N. America | −0.4 | 20.8 | −13.5 | 17.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peti, D.; Dobránsky, J.; Michalík, P. Recent Advances in Polymer Recycling: A Review of Chemical and Biological Processes for Sustainable Solutions. Polymers 2025, 17, 603. https://doi.org/10.3390/polym17050603
Peti D, Dobránsky J, Michalík P. Recent Advances in Polymer Recycling: A Review of Chemical and Biological Processes for Sustainable Solutions. Polymers. 2025; 17(5):603. https://doi.org/10.3390/polym17050603
Chicago/Turabian StylePeti, Damián, Jozef Dobránsky, and Peter Michalík. 2025. "Recent Advances in Polymer Recycling: A Review of Chemical and Biological Processes for Sustainable Solutions" Polymers 17, no. 5: 603. https://doi.org/10.3390/polym17050603
APA StylePeti, D., Dobránsky, J., & Michalík, P. (2025). Recent Advances in Polymer Recycling: A Review of Chemical and Biological Processes for Sustainable Solutions. Polymers, 17(5), 603. https://doi.org/10.3390/polym17050603








