Study of Polyhedral Oligomeric Silsesquioxane-Modified Superhydrophilic Transparent Coating in Antifogging, Antifrost and Self-Cleaning
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation Process
2.2.1. Preparation of POSS-SH4-EG4
2.2.2. Preparation of the Coating
2.3. Characterization
2.3.1. FTIR Spectroscopy
2.3.2. H NMR Spectroscopy
2.3.3. UV-Visible Near-Infrared Spectroscopy
2.3.4. X-Ray Photoelectron Spectroscopy (XPS)
2.3.5. Scanning Electron Microscopy (SEM)
2.3.6. Surface Wettability Assessment
2.3.7. Antifogging Performance Evaluation
2.3.8. Antifrost Performance Evaluation
2.3.9. Self-Cleaning Performance Evaluation
3. Results and Discussion
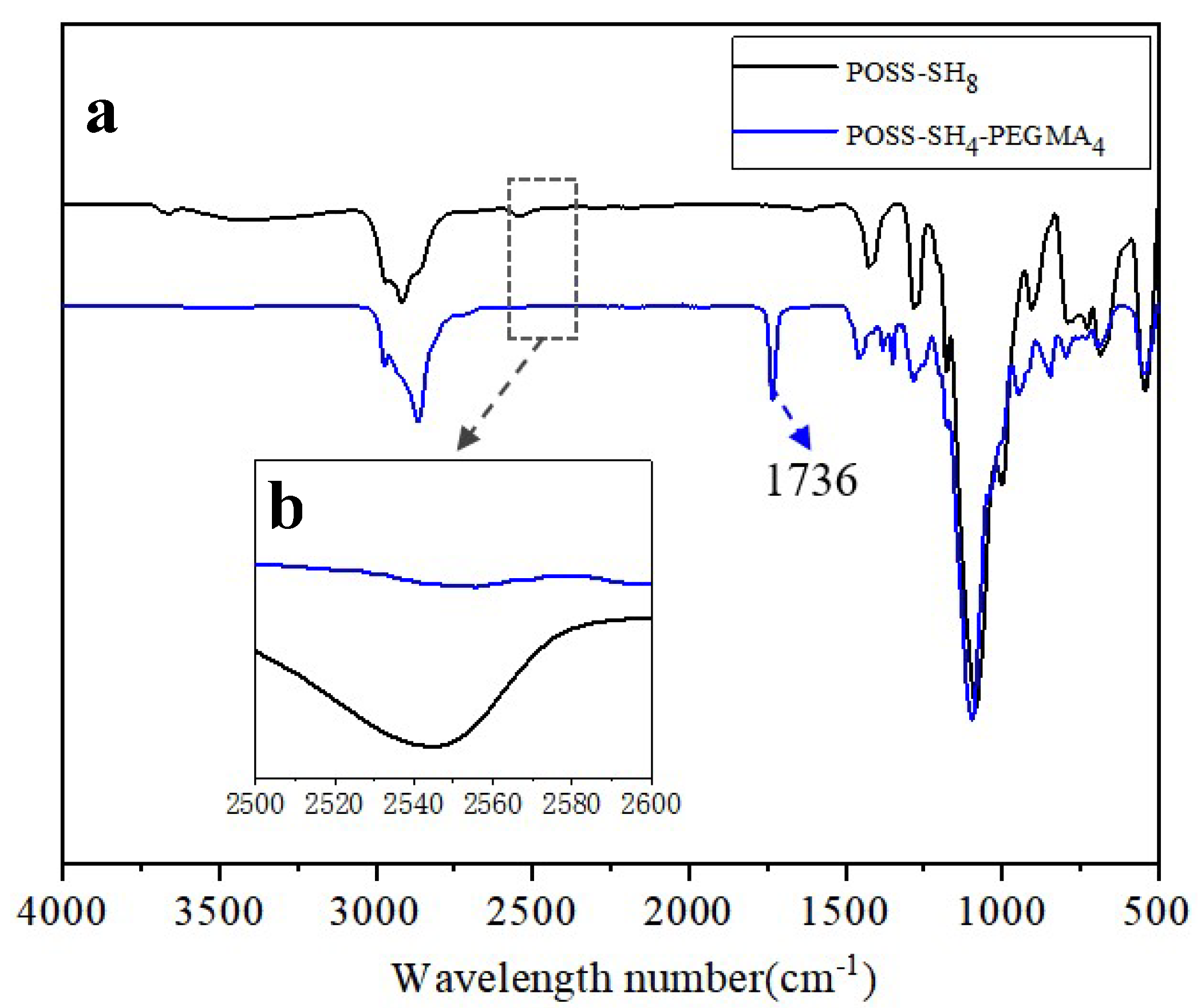
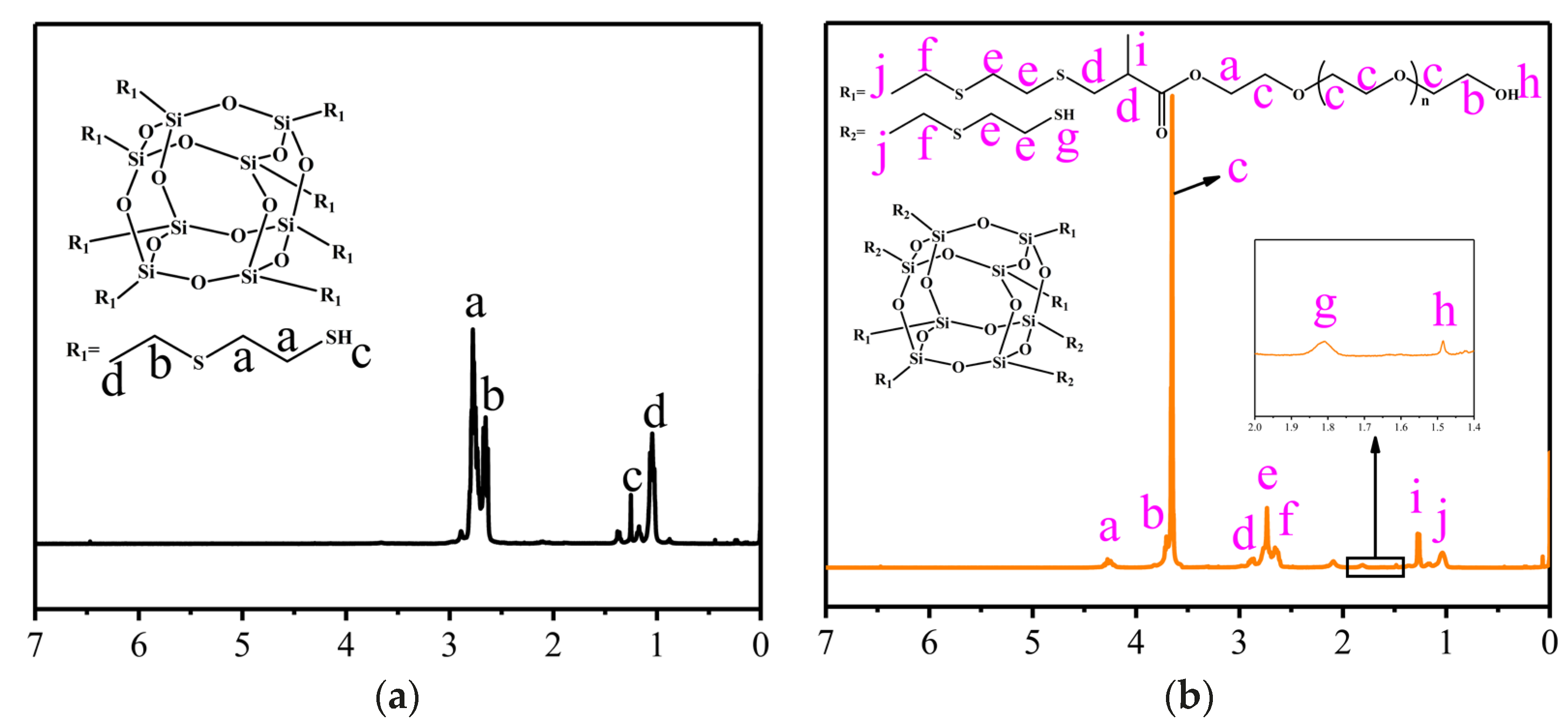
3.1. Characterization of POSS-SH8 and POSS-SH4-EG4
3.2. Hydrophilic Evaluation of Coating
3.3. Hydrophilic Evaluation
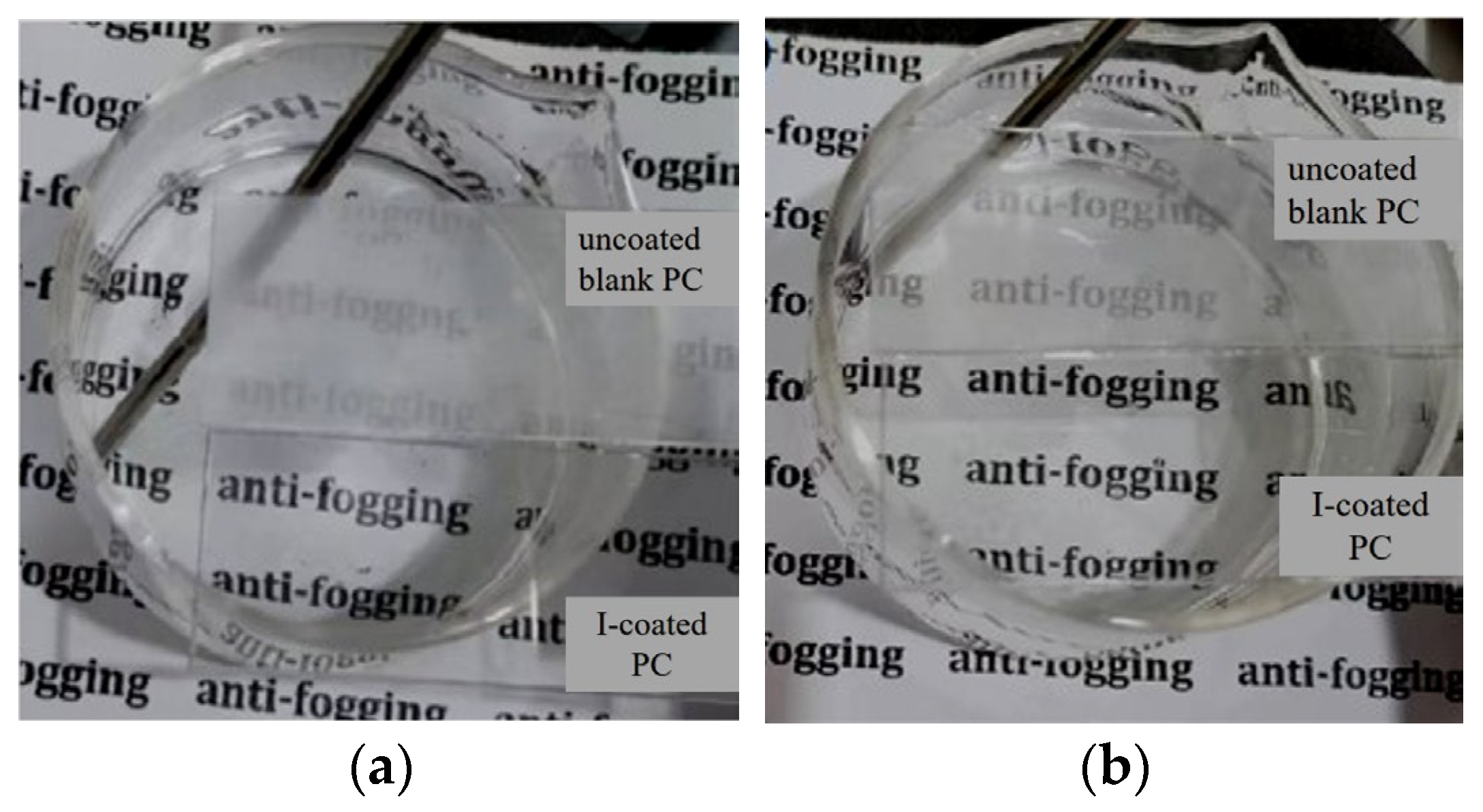
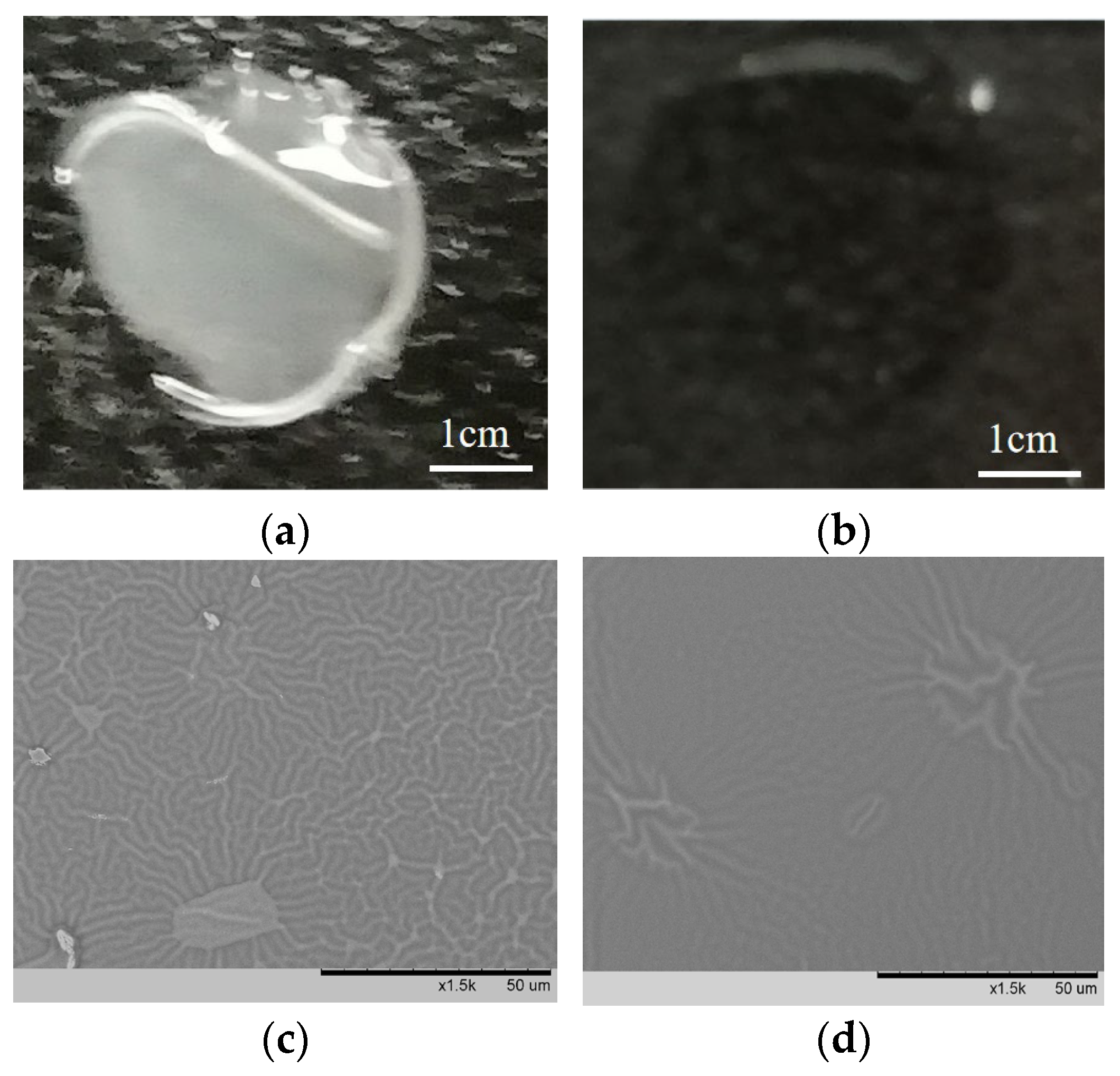
3.4. Antifogging Analysis
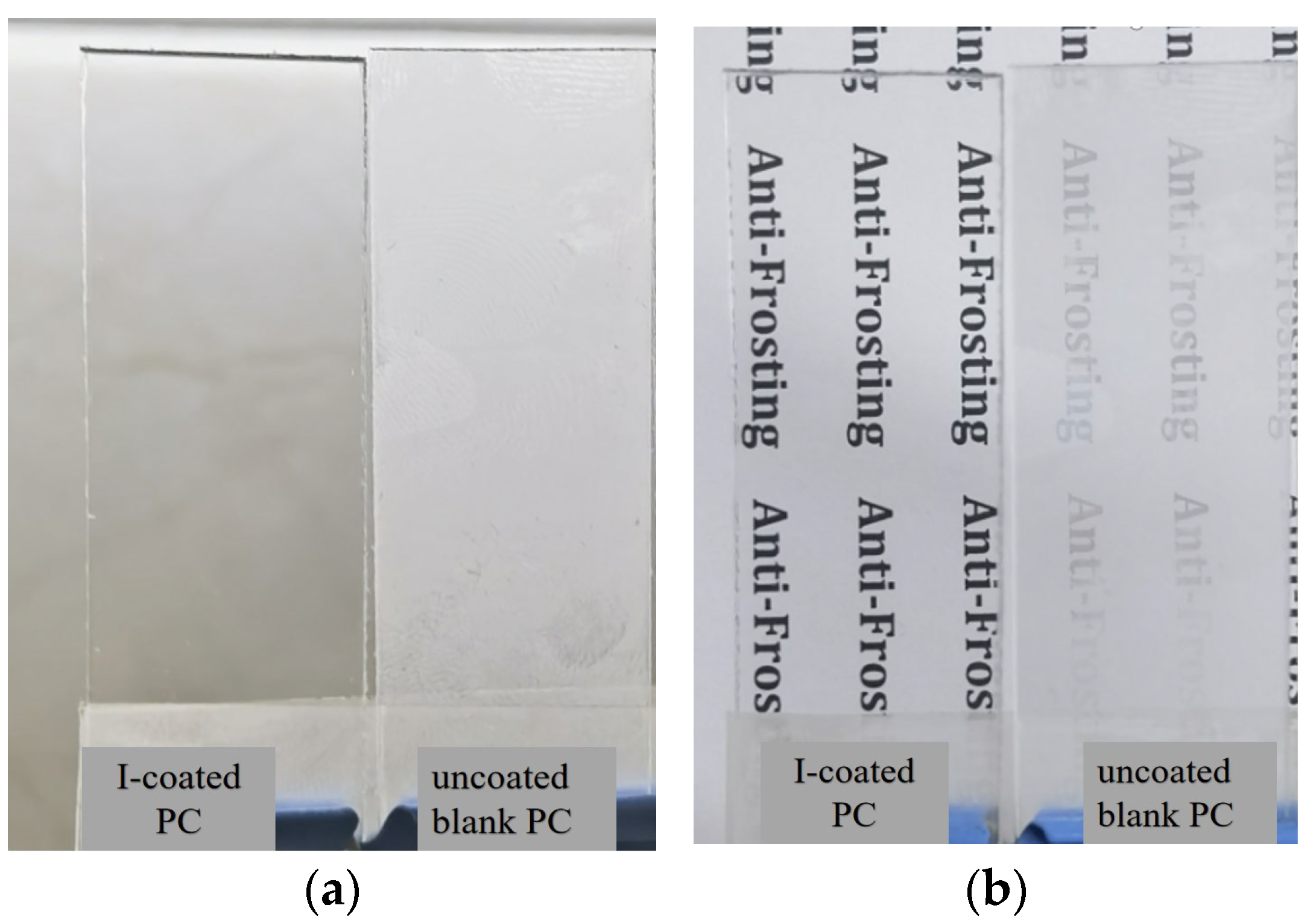
3.5. Antifrost Test
3.6. Self-Cleaning Performance
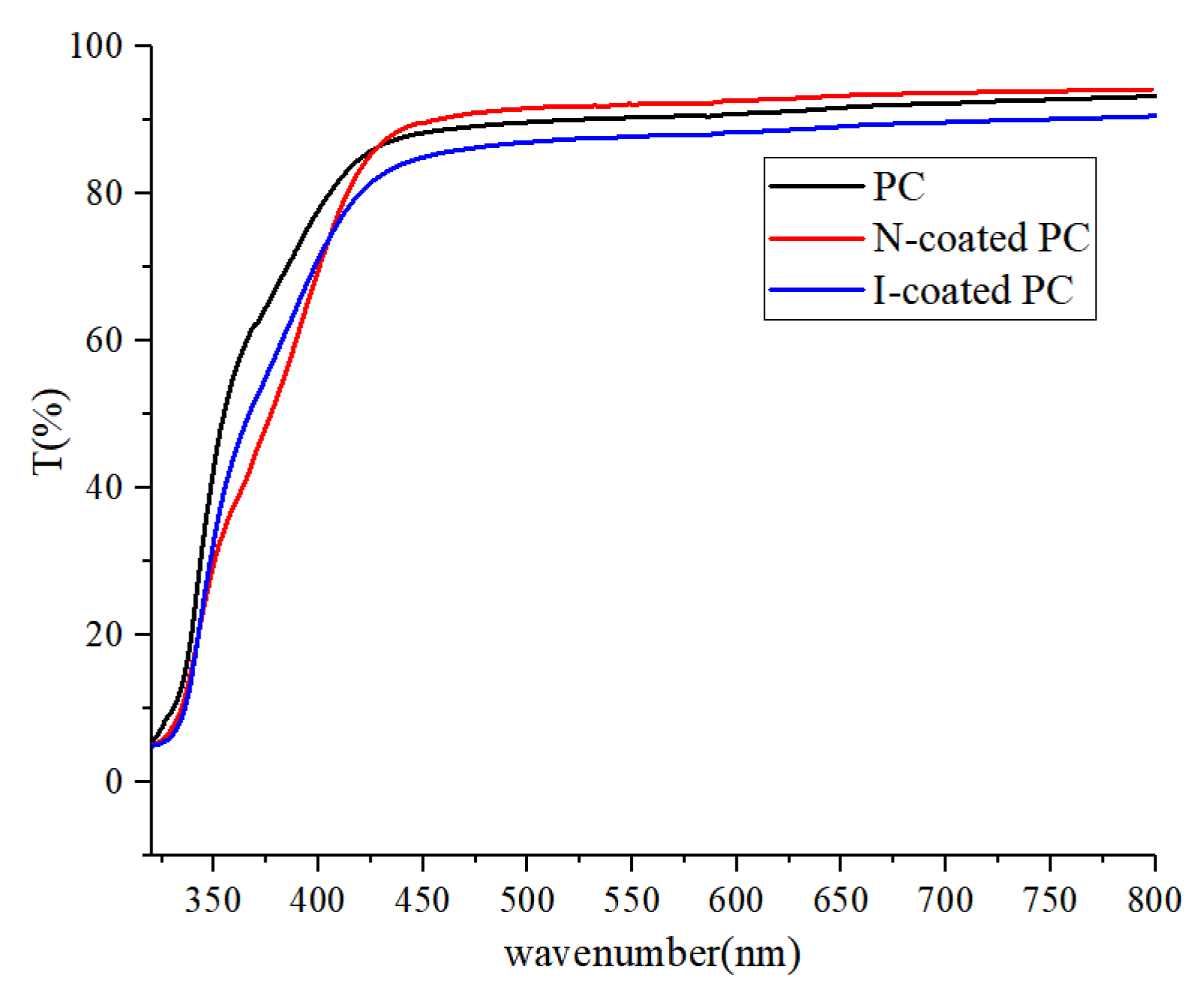
3.7. Water Resistance
3.8. Surface Structure
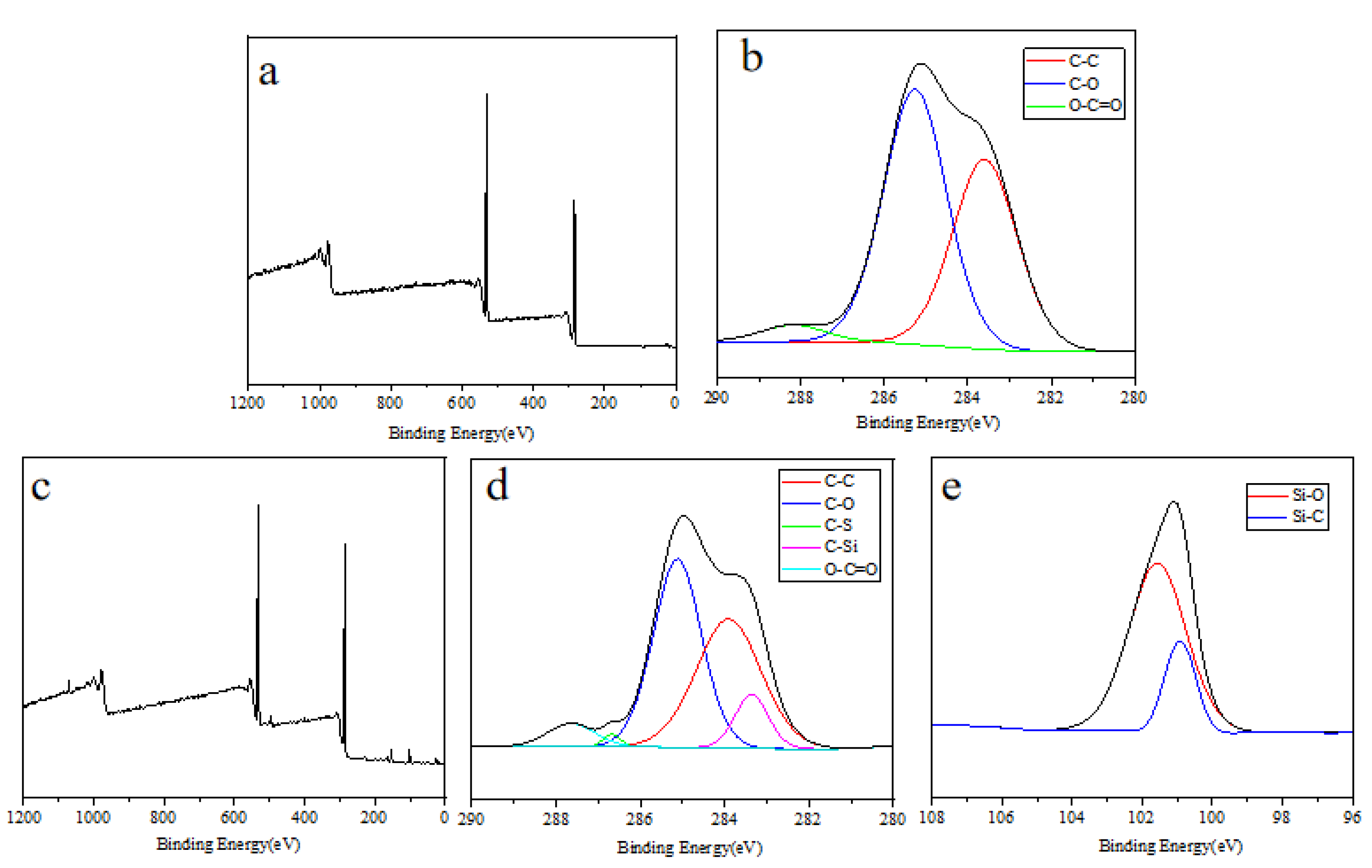
3.9. Surface Element
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, T.A.; Hsu, W.J.; Hung, T.H.; Hu, S.W.; Tsao, H.K.; Zou, C.L.; Lin, L.C.; Kang, Y.H.; Chen, J.J.; Kang, D.Y. Toward Long-Lasting Low-Haze Antifog Coatings through the Deposition of Zeolites. Ind. Eng. Chem. Res. 2020, 59, 13042–13050. [Google Scholar] [CrossRef]
- Chang, C.C.; Lin, Z.M.; Cheng, L.P. Preparation of superhydrophilic nanosilica/polyacrylate hard coatings on plastic substrate for antifogging and frost-resistant applications. J. Appl. Polym. Sci. 2019, 136, 48144. [Google Scholar] [CrossRef]
- Yao, B.L.; Zhao, H.Q.; Wang, L.K.; Liu, Y.; Zheng, C.S.; Li, H.P.; Sun, C.Q. Synthesis of acrylate-based UV/thermal dual-cure coatings for antifogging. J. Coat. Technol. 2018, 15, 149–158. [Google Scholar] [CrossRef]
- Sason, E.; Kolitz-Domb, M.; Chill, J.H.; Margel, S. Engineering of Durable Antifog Thin Coatings on Plastic Films by UV-Curing of Proteinoid Prepolymers with PEG-Diacrylate Monomers. ACS Omega 2019, 4, 9352–9360. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhou, Q.; Guo, Z.Y.; Song, L.N.; Meng, F.D.; Tong, Z.M.; Zhan, X.L.; Liua, Q.; Ren, Y.Y.; Zhang, Q.H. A Facile Strategy to Construct Anti-Swelling, Antibacterial and Antifogging Coatings for Protection of Medical Goggles. Macromol Biosci. 2023, 23, 2300099. [Google Scholar] [CrossRef] [PubMed]
- Resetco, C.; Hendriks, B.; Nezha Badi, N.; Du Prez, F. Thiol-ene chemistry for polymer coatings and surface modification-building in sustainability and performance. Mater. Horiz. 2017, 4, 1041–1053. [Google Scholar] [CrossRef]
- Li, T.; Wang, Y.; Liu, X.J.; Yang, S.B.; Zhang, S.W.; Wang, S.B.; Dong, W.F. Transparent UV-Cured Polyurethane−Surfactant Coatings with Antifogging Properties. ACS Appl. Polym. Mater. 2022, 4, 8147–8155. [Google Scholar] [CrossRef]
- Kanovsky, N.; Margel, S. Fabrication of Transparent Silica/PEG Smooth Thin Coatings on Polymeric Films for Antifogging Applications. ACS Omega 2022, 7, 20505–20514. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, y.; Wang, L.; Yu, L.; Cen, Y.; Zhu, T.; Yu, D.; Chen, C. A novel organic-inorganic zwitterionic acrylate polymer for high-performance anti-fog coating. Prog. Org. Coat. 2020, 142, 105578. [Google Scholar] [CrossRef]
- Manabe, K.; Norikane, Y. Graphene composite self-healing antifog/frost-resist transparent coatings with zwitter-wettability. Surf. Interfaces 2023, 42, 103363. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, L.; Li, X.; Cai, Z. Hydrophilic/hydrophobic poly (AA-co-BA-co-BPA) anti-fog coating with excellent water resistance and self-healing properties. Prog. Org. Coat. 2024, 187, 108071. [Google Scholar] [CrossRef]
- Xu, L.; He, J. Antifogging and Antireflection Coatings Fabricated by Integrating Solid and Mesoporous Silica Nanoparticles without Any Post-Treatments. ACS Appl. Mater. Interfaces 2012, 4, 3293–3299. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.D.; Weng, D.; Chen, L.; Wang, J.D. Effective large-scale deicing based on the interfacial toughness tuning of a UV-curable PDMS coating. Mater. Today Phys. 2023, 35, 101134. [Google Scholar] [CrossRef]
- Calvez, I.; Davoudi, S.; Szczepanski, C.R.; Landry, V. Low-gloss UV-curable coatings: Light mechanisms, formulations and processes-A review. Prog. Org. Coat. 2022, 171, 107039. [Google Scholar] [CrossRef]
- Jafarifard, S.; Ebrahimi, M.; Sharif, F. Antistatic epoxy acrylate/graphene oxide UV-curable coatings with improved shrinkage and adhesion strength. Prog. Org. Coat. 2023, 182, 107595. [Google Scholar] [CrossRef]
- Li, C.; Li, X.H.; Tao, C.; Ren, L.X.; Zhao, Y.H.; Bai, S.; Yuan, X.Y. Amphiphilic Antifogging/Anti-Icing Coatings Containing POSSPDMAEMA-b-PSBMA. ACS Appl. Mater.-Als Interfaces 2017, 9, 22959–22969. [Google Scholar] [CrossRef]
- Chrusciel, J.J.; Lesniak, E. Modification of epoxy resins with functional silanes, polysiloxanes, silsesquioxanes, silica and silicates. Prog. Polym. Sci. 2015, 41, 67–121. [Google Scholar] [CrossRef]
- Zhu, W.B.; Xu, Y.Z.; He, J.X.; Dong, X. Transparent Superhydrophobic Coatings with Mechanical and Chemical Stability Prepared by Modified Polyhedral Oligosilsesquioxanes via UV-Curable Method. Coatings 2023, 13, 498. [Google Scholar] [CrossRef]
- Zhong, H.; Liu, X.; Yu, B.; Zhou, S. Fast UV-Curable Zwitter-Wettable Coatings with Reliable Antifogging/Frost-Resisting Performances. Biomimetics 2022, 7, 162. [Google Scholar] [CrossRef]
- England, M.W.; Urata, C.; Dunderdale, G.J.; Hozumi, A. Anti-Fogging/Self-Healing Properties of Clay-Containing Transparent Nanocomposite Thin Films. ACS Appl. Mater. Interfaces 2016, 8, 4318–4322. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, Y.; Feng, K.; Wei, J.; Liu, J.; Wu, Y.; Pei, X.; Yu, B.; Cai, M.; Zhou, F. “Brush-like” Amphiphilic Polymer for Environmental Adaptive Coating. ACS Appl. Mater. Interfaces 2022, 14, 18901–18909. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.L.; Kong, R.X.; Gao, Y.J.; Zhang, L.B.; Zhu, J.T. Bioinspired adhesive coatings frompolyethylenimine and tannic acid complexes exhibiting antifogging, self-cleaning, and antibacterial capabilities. J. Colloid Interface Sci. 2021, 603, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.F.; Zhu, W.B.; He, J.X.; Dong, X. Water Resistant Hydrophilic Anti-fog Coating Constructed by Polyether Modified Polyhedral Oligosilsesquioxanes. Surf. Technol. 2020, 49, 123–131. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Wu, C.; He, J.; Dong, X. Study of Polyhedral Oligomeric Silsesquioxane-Modified Superhydrophilic Transparent Coating in Antifogging, Antifrost and Self-Cleaning. Polymers 2025, 17, 599. https://doi.org/10.3390/polym17050599
Zhu W, Wu C, He J, Dong X. Study of Polyhedral Oligomeric Silsesquioxane-Modified Superhydrophilic Transparent Coating in Antifogging, Antifrost and Self-Cleaning. Polymers. 2025; 17(5):599. https://doi.org/10.3390/polym17050599
Chicago/Turabian StyleZhu, Weibiao, Chengfeng Wu, Jinxin He, and Xia Dong. 2025. "Study of Polyhedral Oligomeric Silsesquioxane-Modified Superhydrophilic Transparent Coating in Antifogging, Antifrost and Self-Cleaning" Polymers 17, no. 5: 599. https://doi.org/10.3390/polym17050599
APA StyleZhu, W., Wu, C., He, J., & Dong, X. (2025). Study of Polyhedral Oligomeric Silsesquioxane-Modified Superhydrophilic Transparent Coating in Antifogging, Antifrost and Self-Cleaning. Polymers, 17(5), 599. https://doi.org/10.3390/polym17050599




