Influence of Molecular Weight and Temperature on the Pyrolysis Behavior of Polyethylene
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pyrolysis–Gas Chromatography/Mass Spectrometry (Py-GC/MS)
2.3. Calculations
3. Results and Discussion
3.1. Principal Pyrolysis Products Formed from PE
3.2. Influence of Pyrolysis Temperature and PE Molecular Weight on Generation of Pyrolysis Products
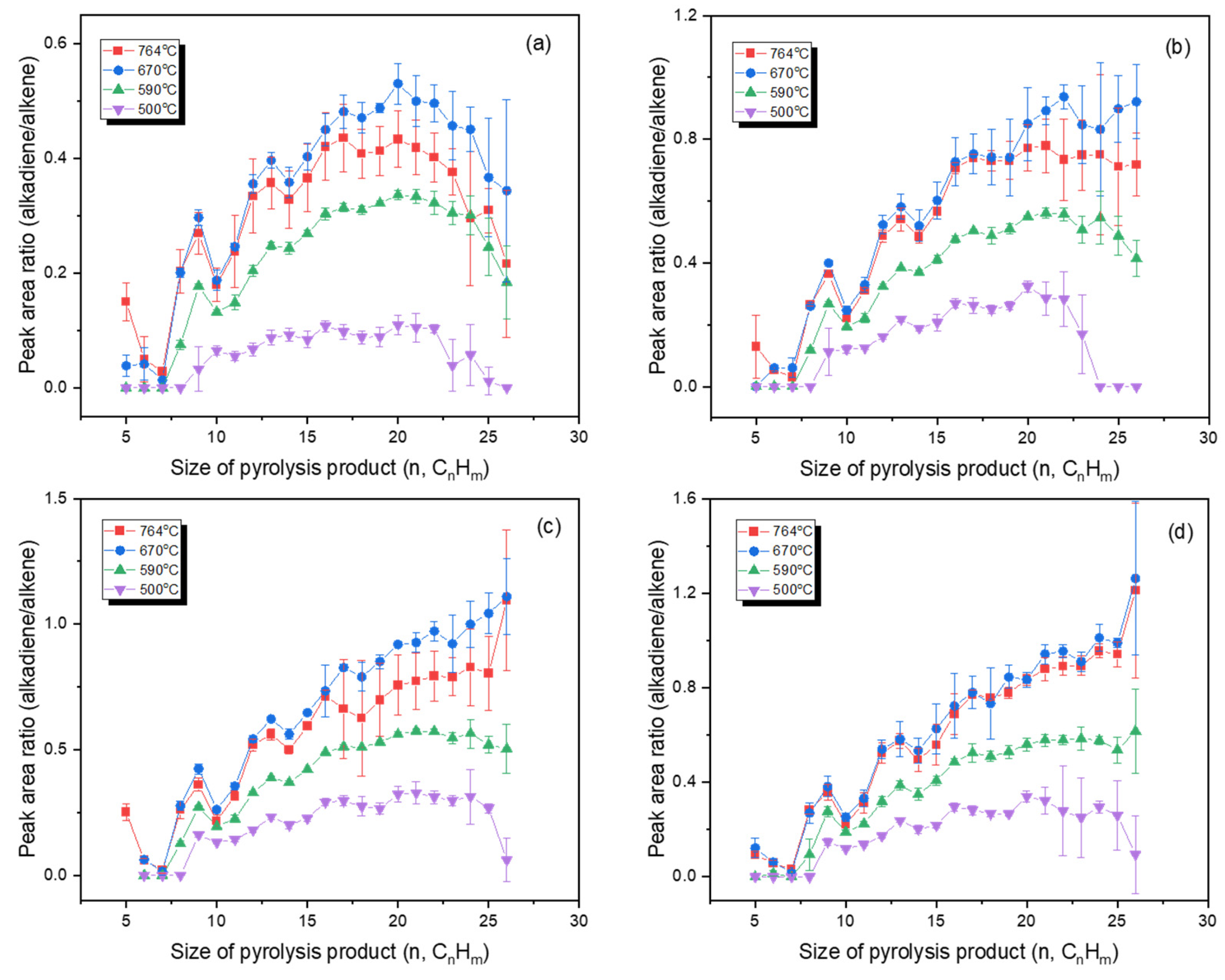
3.3. Comparison of Favorability for Productions of Alkanes, Alkenes, and Alkadienes from PE by Pyrolysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ronca, S. Polyethylene, Brydson’s Plastics Materials; Elsevier: Amsterdam, The Netherlands, 2017; pp. 247–278. [Google Scholar]
- Ahmad, H.; Rodrigue, D. Crosslinked polyethylene: A review on the crosslinking techniques, manufacturing methods, applications, and recycling. Polym. Sci. Eng. 2022, 62, 2376–2401. [Google Scholar] [CrossRef]
- Plastics Europe, Plastics—The Fast Facts 2024. 2024. Available online: https://plasticseurope.org/ (accessed on 30 January 2025).
- Dou, Y.; Rodrigue, D. Morphological, thermal and mechanical properties of recycled HDPE foams via rotational molding. J. Cell. Plast. 2021, 58, 305–323. [Google Scholar] [CrossRef] [PubMed]
- Demirors, M. The History of Polyethylene; American Chemical Society: Washington, DC, USA, 2011; pp. 115–145. [Google Scholar]
- Sharuddin, S.D.A.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef]
- Xu, W.; Chen, W.-Q.; Jiang, D.; Zhang, C.; Ma, Z.; Ren, Y.; Shi, L. Evolution of the global polyethylene waste trade system. Ecosyst. Health Sustain. 2020, 6, 1756925. [Google Scholar] [CrossRef]
- Achilias, D.S.; Giannoulis, A.; Papageorgiou, G.Z. Recycling of polymers from plastic packaging materials using the dissolution–reprecipitation technique. Polym. Bull. 2009, 63, 449–465. [Google Scholar] [CrossRef]
- Thiounn, T.; Smith, R.C. Advances and approaches for chemical recycling of plastic waste. J. Polym. Sci. 2020, 58, 1347–1364. [Google Scholar] [CrossRef]
- Achilias, D.S.; Karayannidis, G.P. The chemical recycling of PET in the framework of sustainable development. Water Air Soil Pollut. Focus 2004, 4, 385–396. [Google Scholar] [CrossRef]
- Karayannidis, G.P.; Achilias, D.S. Chemical recycling of poly(ethylene terephthalate). Macromol. Mater. Eng. 2007, 292, 128–146. [Google Scholar] [CrossRef]
- Achilias, D.S.; Roupakias, C.; Megalokonomos, P.; Lappas, A.A.; Antonakou, E.V. Chemical recycling of plastic wastes made from polyethylene (LDPE and HDPE) and polypropylene (PP). J. Hazard. Mater. 2007, 149, 536–542. [Google Scholar] [CrossRef]
- Papaspyrides, C.D.; Poulakis, J.G.; Varelides, P.C. A model recycling process for low density polyethylene. Res. Conserv. Recycl. 1994, 12, 177–184. [Google Scholar] [CrossRef]
- Poulakis, J.G.; Papaspyrides, C.D. The dissolution/reprecipitation technique applied on high density polyethylene: I. model recycling experiments. Adv. Polym. Technol. 1995, 14, 237–242. [Google Scholar] [CrossRef]
- Hellenic Solid Waste Management Association. 2007. Available online: https://www.eedsa.gr/ (accessed on 30 January 2025).
- Vicente, G.; Aguado, J.; Serrano, D.P.; Sánchez, N. HDPE chemical recycling promoted by phenol solvent. J. Anal. Appl. Pyrolysis 2009, 85, 366–371. [Google Scholar] [CrossRef]
- Aguado, J.; Serrano, D.; Miguel, G.S.; Castro, M.C.; Madrid, S. Feedstock recycling of polyethylene in a two-step thermo-catalytic reaction system. J. Anal. Appl. Pyrolysis 2007, 79, 415–423. [Google Scholar] [CrossRef]
- Aguado, J.; Serrano, D.P. Feedstock Recycling of Plastic Wastes; The Royal Society of Chemistry: Cambridge, UK, 1999; pp. 73–84. [Google Scholar]
- Achilias, D.S.; Megalokonomos, P.; Karayannidis, G.P. Current trends in chemical recycling of polyolefins. J. Environ. Prot. Ecol. 2006, 7, 407–413. [Google Scholar]
- Kaminski, W.; Schlesselmann, B.; Simon, C. Olefins from polyolefins and mixed plastics by pyrolysis. J. Anal. Appl. Pyrolysis 1995, 32, 19–27. [Google Scholar] [CrossRef]
- Plastics Europe, Circular Economy for Plastics Data for 2022. 2024. Available online: https://plasticseurope.org/ (accessed on 30 January 2025).
- Williams, P.T.; Williams, E.A. Fluidised bed pyrolysis of low density polyethylene to produce petrochemical feedstock. J. Anal. Appl. Pyrolysis 1999, 51, 107–126. [Google Scholar] [CrossRef]
- Kaminsky, W.; Sim, J.-S. Pyrolysis of mixed plastics into aromatics. J. Anal. Appl. Pyrolysis 1999, 51, 127–134. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Sumi, K. Thermal decomposition products of polyethylene. J. Polym. Sci. Polym. Chem. 1968, 6, 415–424. [Google Scholar] [CrossRef]
- Kiran, E.; Gillham, J.K. Pyrolysis-molecular weight chromatography: A new on-line system for analysis of polymers. II. Thermal decomposition of polyolefins: Polyethylene, polypropylene, polyisobutylene. J. Appl. Polym. Sci. 1976, 20, 2045–2068. [Google Scholar] [CrossRef]
- Lattimer, R.P. Pyrolysis field ionization mass spectrometry of polyolefins. J. Anal. Appl. Pyrolysis 1995, 31, 203–225. [Google Scholar] [CrossRef]
- Spartan’10; Wave function Inc.: Irvine, CA, USA, 2011.
- Halgren, T.A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Halgren, T.A. Merck molecular force field. II. MMFF94 van der Waals and electrostatic parameters for intermolecular interactions. J. Comput. Chem. 1996, 17, 520–552. [Google Scholar] [CrossRef]
- Gaussian 09, Revision, C.01; Gaussian Inc.: Wallingford, CT, USA, 2010.








| Chemical | Energy (kJ/mol) |
|---|---|
| C30H62 | −3,100,554 |
| C10H22 (n-decane) | −1,035,633 |
| C10H20 (1-decene) | −1,032,389 |
| C10H18 (α,ω-decadiene) | −1,029,144 |
| C30H61• | −3,098,790 |
| CH3(CH2)9• | −1,033,868 |
| •(CH2)10• | −1,031,931 |
| H | −1319 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chae, E.; Choi, S.-S. Influence of Molecular Weight and Temperature on the Pyrolysis Behavior of Polyethylene. Polymers 2025, 17, 576. https://doi.org/10.3390/polym17050576
Chae E, Choi S-S. Influence of Molecular Weight and Temperature on the Pyrolysis Behavior of Polyethylene. Polymers. 2025; 17(5):576. https://doi.org/10.3390/polym17050576
Chicago/Turabian StyleChae, Eunji, and Sung-Seen Choi. 2025. "Influence of Molecular Weight and Temperature on the Pyrolysis Behavior of Polyethylene" Polymers 17, no. 5: 576. https://doi.org/10.3390/polym17050576
APA StyleChae, E., & Choi, S.-S. (2025). Influence of Molecular Weight and Temperature on the Pyrolysis Behavior of Polyethylene. Polymers, 17(5), 576. https://doi.org/10.3390/polym17050576





