Chitosan-Lemongrass Essential Oil on Paperboard for Active Food Packaging Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Paperboard
2.3. Experimental Design
2.4. Analysis
2.4.1. Color Parameters
2.4.2. Contact Angle
2.4.3. Thermal Analysis: TG/DTG and DSC
2.4.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.5. X-Ray Diffraction
2.4.6. Tensile Properties
2.4.7. Taber Stiffness
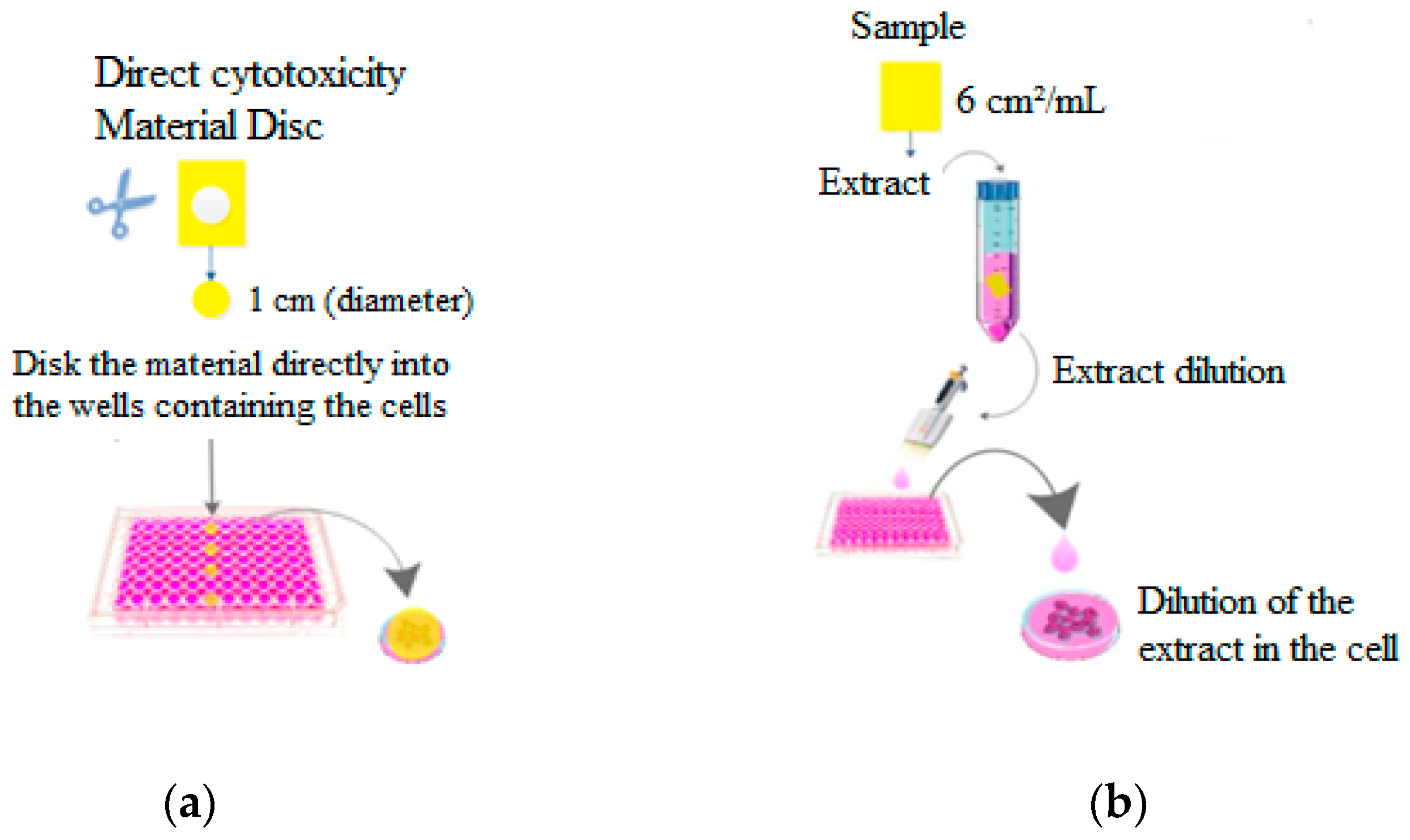
2.4.8. Direct and Indirect Cytotoxicity of the Active Film-Paper System
2.5. Statistical Analysis
3. Results
3.1. Color Parameters
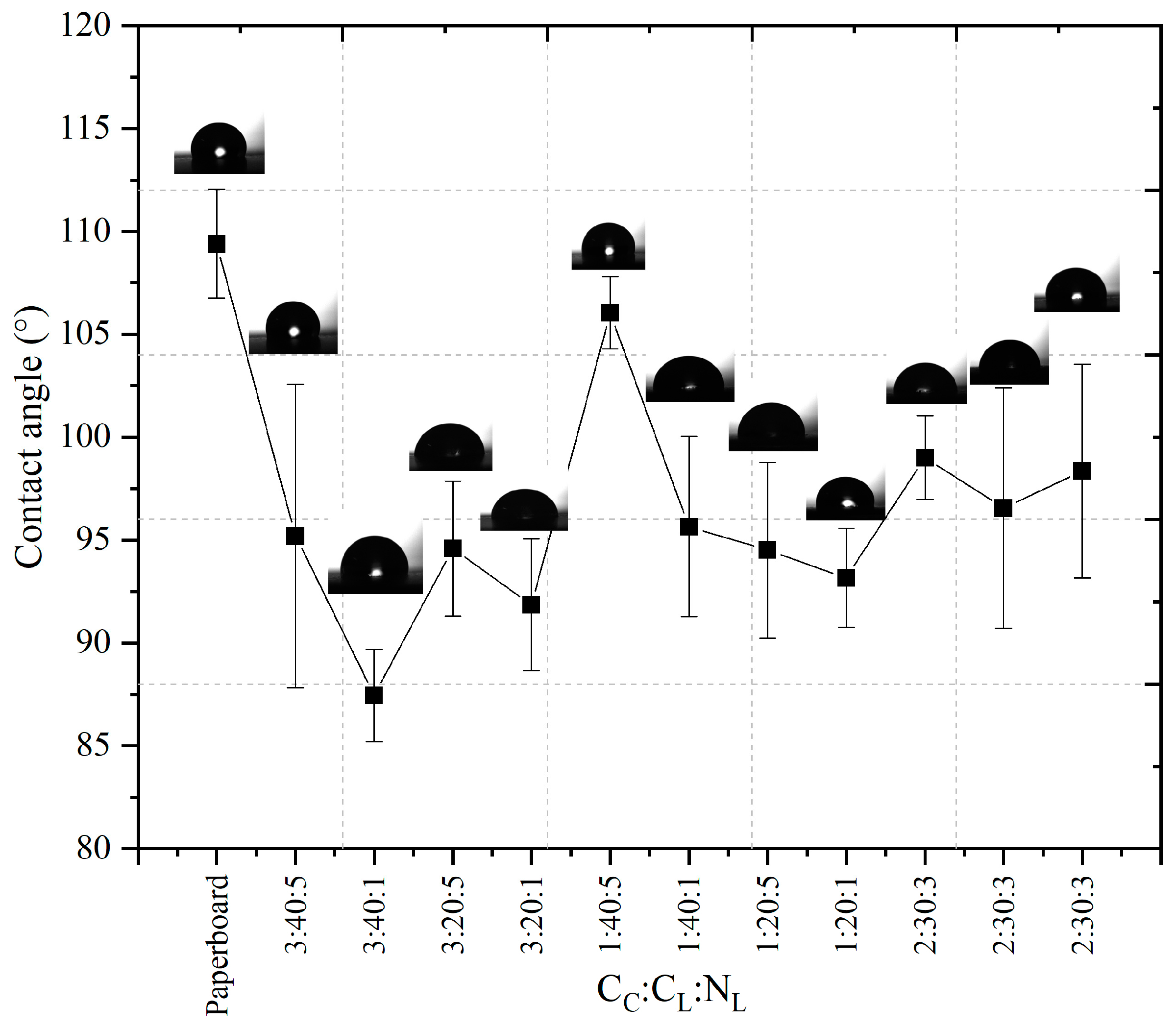
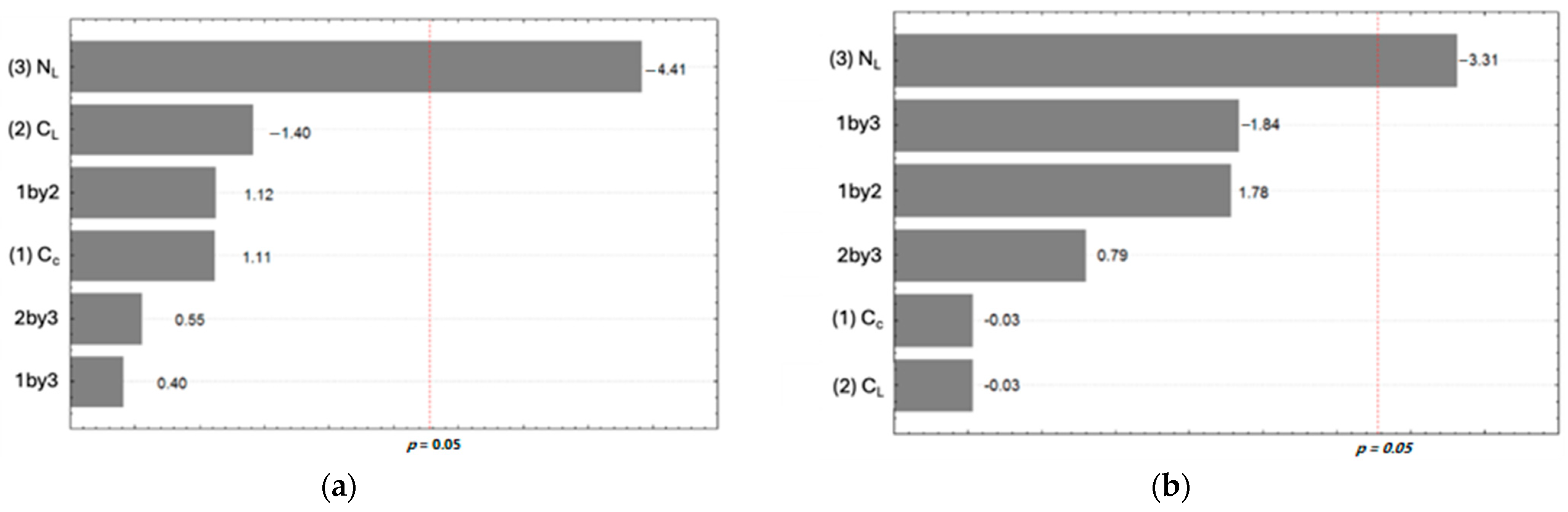
3.2. Contact Angle (CA)
3.3. Tensile Properties
3.4. Taber Stiffness
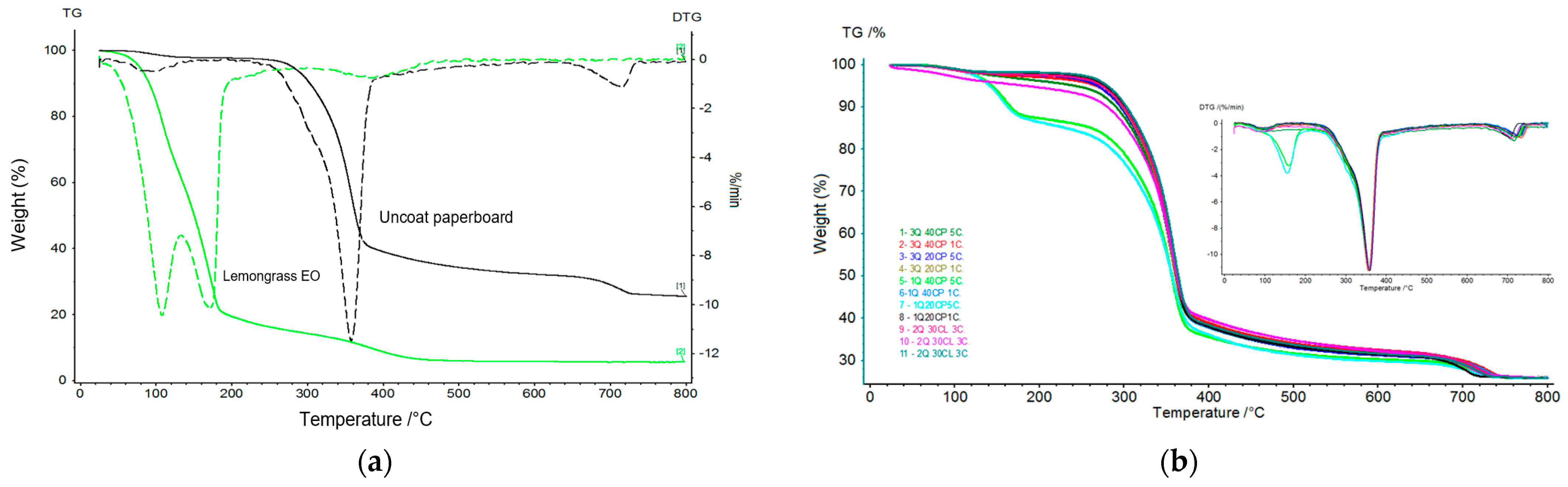
3.5. Thermal Analysis: TG/DTG and DSC
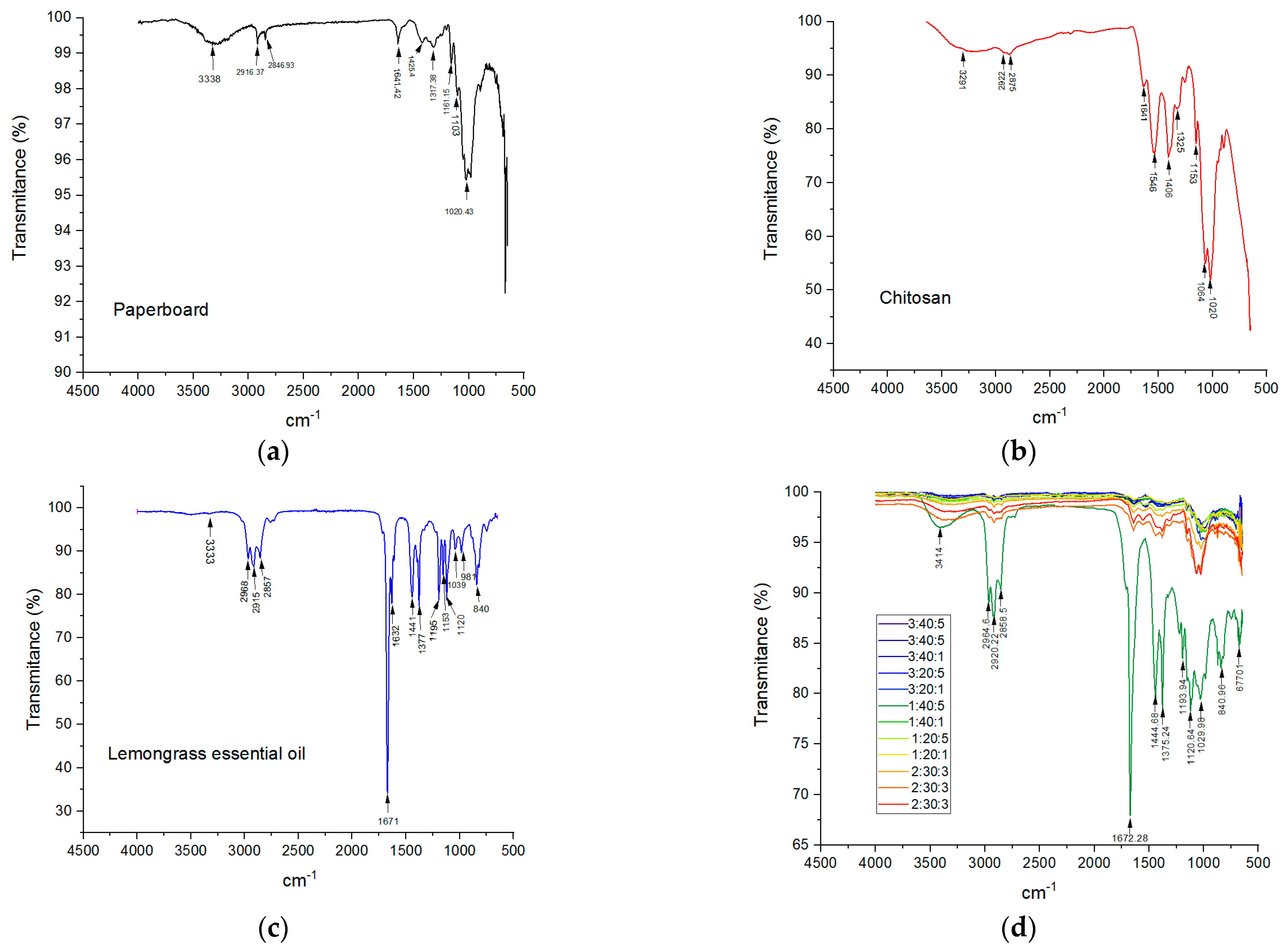
3.6. FTIR-ATR Spectra
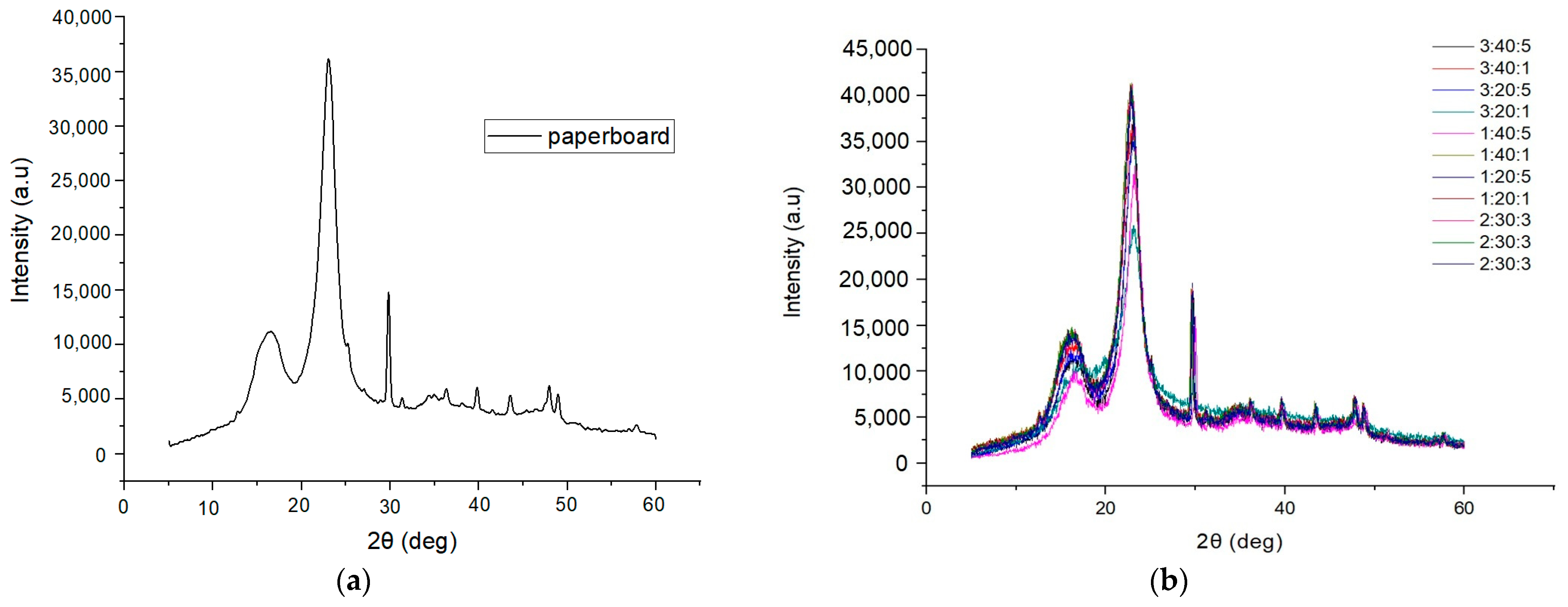
3.7. X-Ray Diffraction (XRD)
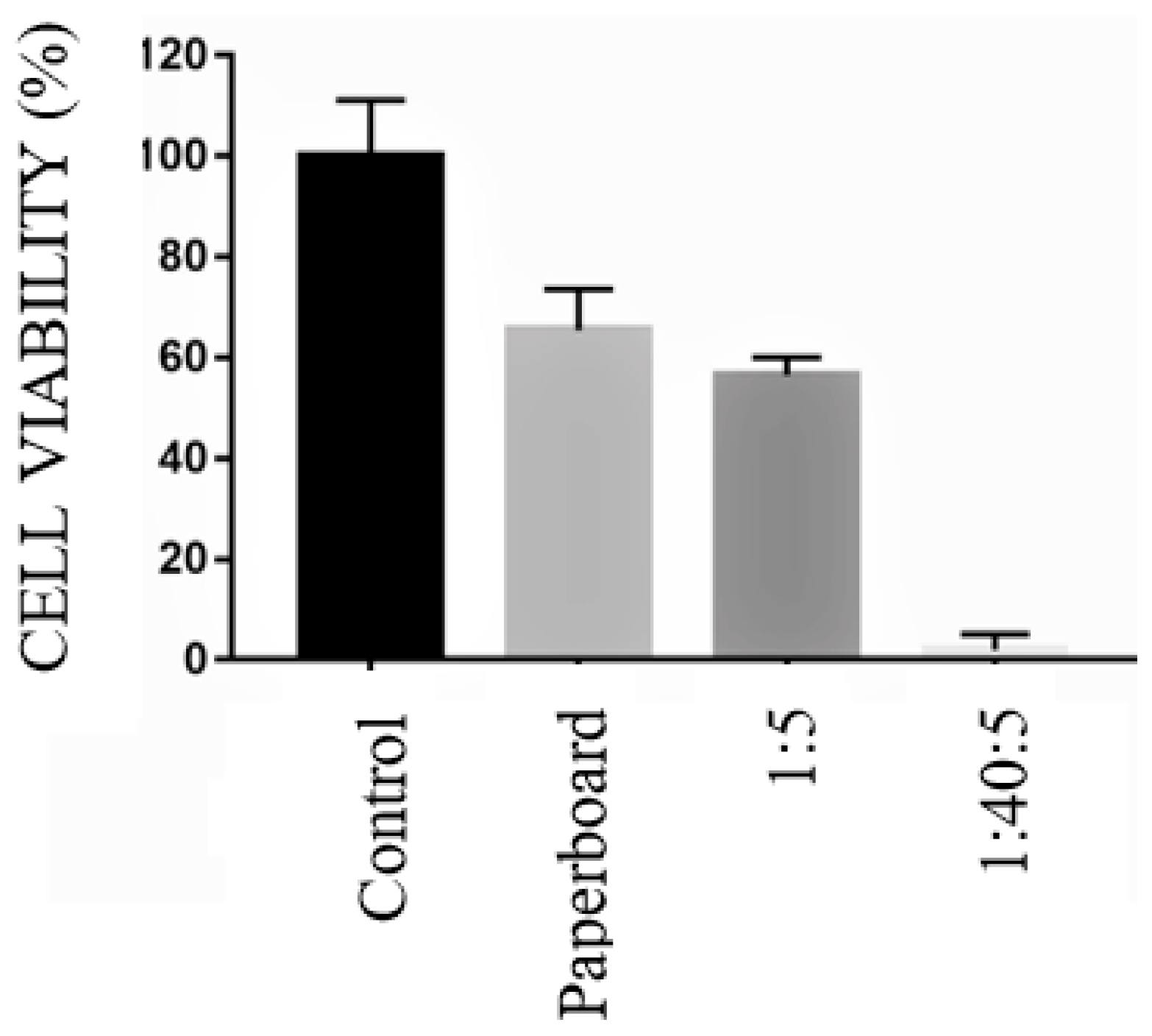
3.8. Cytotoxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tian, B.; Liu, J.; Yang, W.; Wan, J.B. Biopolymer Food Packaging Films Incorporated with Essential Oils. J. Agric. Food Chem. 2023, 71, 1325–1347. [Google Scholar] [CrossRef] [PubMed]
- Koketso Ncube, L.; Ude, A.U.; Nifise Ogunmuyiwa, E.; Zulkifli, R.; Beas, I.N. An Overview of Plastic Waste Generation and Management in Food Packaging Industries. Recycling 2021, 6, 12. [Google Scholar] [CrossRef]
- Din, M.I.; Ghaffar, T.; Najeeb, J.; Hussain, Z.; Khalid, R.; Zahid, H. Potential Perspectives of Biodegradable Plastics for Food Packaging Application-Review of Properties and Recent Developments. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2020, 37, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Farmahini-farahani, M.; Hearn, P.O.; Xiao, H.; Ocampo, H. An Overview of Bio-Based Polymers for Packaging Materials. J. Bioresour. Bioprod. 2016, 1, 106–113. [Google Scholar]
- Song, Z.; Tang, J.; Li, J.; Xiao, H. Plasma-Induced Polymerization for Enhancing Paper Hydrophobicity. Carbohydr. Polym. 2013, 92, 928–933. [Google Scholar] [CrossRef]
- Adibi, A.; Trinh, B.M.; Mekonnen, T.H. Recent Progress in Sustainable Barrier Paper Coating for Food Packaging Applications. Prog. Org. Coat. 2023, 181, 107566. [Google Scholar] [CrossRef]
- Poulose, S.; Toriseva, J.; Lahti, J.; Jönkkäri, I.; Hedenqvist, M.S.; Kuusipalo, J. A Green High Barrier Solution for Paperboard Packaging Based on Potato Fruit Juice, Poly(Lactic Acid), and Poly(Butylene Adipate Terephthalate). ACS Appl. Polym. Mater. 2022, 4, 4179–4188. [Google Scholar] [CrossRef]
- Kansal, D.; Rabnawaz, M. Fabrication of Oil- and Water-Resistant Paper without Creating Microplastics on Disposal. J. Appl. Polym. Sci. 2021, 138, 49692. [Google Scholar] [CrossRef]
- Bordenave, N.; Grelier, S.; Pichavant, F.; Coma, V. Water and Moisture Susceptibility of Chitosan and Paper-Based Materials: Structure-Property Relationships. J. Agric. Food Chem. 2007, 55, 9479–9488. [Google Scholar] [CrossRef]
- Srinivasa, P.C.; Ramesh, M.N.; Kumar, K.R.; Tharanathan, R.N. Properties and Sorption Studies of Chitosan—Polyvinyl Alcohol Blend Films. Carbohydr. Polym. 2003, 53, 431–438. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R. A Review of Chitin and Chitosan Applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Grinfelds, U.; Zoldners, J.; Passas, R.; Vikele, L. Effect of chitosan on properties of paper for packaging. Cellul. Chem. Technol. 2017, 51, 67–73. [Google Scholar]
- Kjellgren, H.; Ga, M. Barrier and Surface Properties of Chitosan-Coated Greaseproof Paper. Carbohydr. Polym. 2006, 65, 453–460. [Google Scholar] [CrossRef]
- Khwaldia, K.; Basta, A.H.; Aloui, H.; El-Saied, H. Chitosan-Caseinate Bilayer Coatings for Paper Packaging Materials. Carbohydr. Polym. 2014, 99, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, C.M.P.; Oliveira, E.N.; Franco, T.T. Chitosan Tailor-Made Films: The Effects of Additives on Barrier and Mechanical Properties. Packag. Technol. Sci. 2009, 22, 161–170. [Google Scholar] [CrossRef]
- Tanpichai, S.; Srimarut, Y.; Woraprayote, W.; Malila, Y. Chitosan Coating for the Preparation of Multilayer Coated Paper for Food-Contact Packaging: Wettability, Mechanical Properties, and Overall Migration. Int. J. Biol. Macromol. 2022, 213, 534–545. [Google Scholar] [CrossRef]
- Jumaa, M.; Mu, B.W. Physicochemical Properties of Chitosan-Lipid Emulsions and Their Stability during the Autoclaving Process. Int. J. Pharm. 1999, 183, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2017, 17, 165–199. [Google Scholar] [CrossRef] [PubMed]
- de Fátima Silva, M.; Maciel, V.B.V.; Noletto, A.P.R.; Venturini, A.C.; de Carvalho, R.A.; Yoshida, C.M.P. Chitosan Active Coating on Paperboard Surface Forming an Anti-Insect Grain-Based Food Packaging. Packag. Technol. Sci. 2022, 35, 361–372. [Google Scholar] [CrossRef]
- Djebbi, T.; Ascrizzi, R.; Bedini, S.; Farina, P.; Sanmartin, C.; Mediouni Ben Jemâa, J.; Bozzini, M.F.; Flamini, G.; Conti, B. Physicochemical and Repellent Properties of Chitosan Films Loaded with Essential Oils for Producing an Active Packaging Effective Against the Food Pest Sitophilus oryzae. J. Stored Prod. Res. 2024, 106, 102297. [Google Scholar] [CrossRef]
- Negrelle, R.R.B.; Gomes, E.C. Cymbopogon citratus (DC.) Stapf: Chemical Composition and Biological Activities. Rev. Bras. De Plantas Med. 2007, 9, 80–92. [Google Scholar]
- Lawal, O.A.; Ogundajo, A.L.; Avoseh, N.O.; Ogunwande, I.A. Cymbopogon citratus. In Medicinal Spices and Vegetables from Africa: Therapeutic Potential Against Metabolic, Inflammatory, Infectious and Systemic Diseases; Academic Press: Cambridge, MA, USA, 2017; pp. 397–423. [Google Scholar] [CrossRef]
- Saddiq, A.A.; Khayyat, S.A. Chemical and Antimicrobial Studies of Monoterpene: Citral. Pestic. Biochem. Physiol. 2010, 98, 89–93. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, H.; Qian, L. Beeswax-Chitosan Emulsion Coated Paper with Enhanced Water Vapor Barrier Efficiency. Appl. Surf. Sci. 2014, 300, 80–85. [Google Scholar] [CrossRef]
- Despond, S.; Espuche, E.; Cartier, N.; Domard, A. Barrier Properties of Paper-Chitosan and Paper-Chitosan-Carnauba Wax Films. J. Appl. Polym. Sci. 2005, 98, 704–710. [Google Scholar] [CrossRef]
- ASTM. Annual Book of ASTM Standards; American Society for Testing & Materials: West Conshohocken, PA, USA, 2010; ISBN 0-8031-2586-0/0-8031-2561-5/0-8031-2565-8. [Google Scholar]
- Yoshida, C.M.P.; Maciel, V.B.V.; Mendonça, M.E.D.; Franco, T.T. Chitosan Biobased and Intelligent Films: Monitoring PH Variations. LWT-Food Sci. Technol. 2014, 55, 83–89. [Google Scholar] [CrossRef]
- ASTM D724-99; Annual Book of ASTM Standards. Standard Test Method for Surface Wettability of Paper (Angle-of-Contact Method). ASTM International: West Conshohocken, PA, USA, 2003.
- Rabe, A.; Aliero, B.; Galadima, A.; Baqi, A. Thermal Decomposition of Camel Grass and Lemon Grass. J. Sci. Res. Rep. 2018, 17, 1–4. [Google Scholar] [CrossRef]
- Kriegner, D.; Matěj, Z.; Kužel, R.; Holý, V. Powder Diffraction in Bragg-Brentano Geometry with Straight Linear Detectors. J. Appl. Crystallogr. 2015, 48, 613–618. [Google Scholar] [CrossRef]
- ASTM D828-16e1; Annual Book of ASTM Standards. Standard Test Method for Tensile Properties of Paper and Paperboard Using Constant-Rate-of-Elongation Apparatus. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM D5342-97; Annual Book of ASTM Standards. Standard Test Method for Resistence to Bending of Paper Paperboard (Taber-Type Tester in Basic Configuration). ASTM International: West Conshohocken, PA, USA, 2007.
- ISO 10993-5; Biological Evaluation of Medical Devices—Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009; Volume 5.
- ISO 10993-12; Biological Evaluation of Medical Devices-Part 12: Sample Preparation and Reference Materials Évaluation Biologique Des Dispositifs Médicaux-Partie 12: Préparation Des Échantillons et Matériaux de Référence Copyright Protected Document. ISO: Geneva, Switzerland, 2021.
- Gällstedt, B.M.; Brottman, A.; Hedenqvist, M.S. Packaging-Related Properties of Protein- and Chitosan-Coated Paper and Science. Packag. Technol. Sci. Int. J. 2005, 18, 161–170. [Google Scholar] [CrossRef]
- Stepien, M.; Saarinen, J.J.; Teisala, H.; Tuominen, M.; Aromaa, M.; Kuusipalo, J.; Mäkelä, J.M.; Toivakka, M. Surface Chemical Characterization of Nanoparticle Coated Paperboard. Appl. Surf. Sci. 2012, 258, 3119–3125. [Google Scholar] [CrossRef]
- Kannangara, D.; Shen, W. Roughness Effects of Cellulose and Paper Substrates on Water Drop Impact and Recoil. Colloids Surf. A Physicochem. Eng. Asp. 2008, 330, 151–160. [Google Scholar] [CrossRef]
- Modaressi, H.; Garnier, G. Mechanism of Wetting and Absorption of Water Droplets on Sized Paper: Effects of Chemical and Physical Heterogeneity. Langmuir 2002, 18, 642–649. [Google Scholar] [CrossRef]
- Guillaume, C.; Pinte, J.; Gontard, N.; Gastaldi, E. Wheat Gluten-Coated Papers for Bio-Based Food Packaging: Structure, Surface and Transfer Properties. Food Res. Int. 2010, 43, 1395–1401. [Google Scholar] [CrossRef]
- Rhim, J.; Lee, J.; Hong, S. Water Resistance and Mechanical Properties of Biopolymer (Alginate and Soy Protein) Coated Paperboards. LWT-Food Sci. Technol. 2006, 39, 806–813. [Google Scholar] [CrossRef]
- Gal, M.R.; Rahmaninia, M.; Hubbe, M.A. A Comprehensive Review of Chitosan Applications in Paper Science and Technologies. Carbohydr. Polym. 2023, 309, 120665. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.B.; Yoshida, C.M.P.; Reis, A.P.C.; Franco, T.T. Application of Chitosan Emulsion as a Coating on Kraft Paper. Polym. Int. 2011, 60, 963–969. [Google Scholar] [CrossRef]
- Abdelrazek, E.M.; Elashmawi, I.S.; Labeeb, S. Chitosan Filler Effects on the Experimental Characterization, Spectroscopic Investigation and Thermal Studies of PVA/PVP Blend Films. Physica B 2010, 405, 2021–2027. [Google Scholar] [CrossRef]
- Fazzio, P.; Martinez, M.; Benites, C.; Regina, M.; Maciel, W. Thermal Characterization of Orange, Lemongrass, and Basil Essential Oils. Chem. Eng. Trans. 2011, 24, 463–468. [Google Scholar] [CrossRef]
- Poletto, M.; Pistor, V.; Zattera, A.J. Structural Characteristics and Thermal Properties of Native Cellulose. Cellul.–Fundam. Asp. 2013, 2, 45–68. [Google Scholar] [CrossRef]
- Habibie, S.; Hamzah, M.; Anggaravidya, M.; Kalembang, E. The Effect of Chitosan on Physical and Mechanical Properties of Paper. J. Chem. Eng. Mater. Sci. 2016, 7, 1–10. [Google Scholar]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Preparation and Physicochemical Evaluation of Chitosan/Poly(Vinyl Alcohol)/Pectin Ternary Film for Food-Packaging Applications. Carbohydr. Polym. 2010, 79, 711–716. [Google Scholar] [CrossRef]
- Dai, D.; Fan, M. Investigation of the Dislocation of Natural Fibres by Fourier-Transform Infrared Spectroscopy. Vib. Spectrosc. 2011, 55, 300–306. [Google Scholar] [CrossRef]
- Mauricio-Sánchez, R.A.; Salazar, R.; Luna-Bárcenas, J.G.; Mendoza-Galván, A. FTIR Spectroscopy Studies on the Spontaneous Neutralization of Chitosan Acetate Films by Moisture Conditioning. Vib. Spectrosc. 2018, 94, 1–6. [Google Scholar] [CrossRef]
- Vazquez-Briones, M.D.C.; Hernandez, L.R.; Guerrero-Beltran, J.A. Physicochemical and Antioxidant Properties of Cymbopogon citratus Essential Oil. J. Food Res. 2015, 4, 36. [Google Scholar] [CrossRef]
- Wany, A.; Kumar, A.; Nallapeta, S.; Jha, S.; Nigam, V.K.; Pandey, D.M. Extraction and Characterization of Essential Oil Components Based on Geraniol and Citronellol from Java Citronella (Cymbopogon Winterianus Jowitt). Plant Growth Regul. 2014, 73, 133–145. [Google Scholar] [CrossRef]
- Cristina Lengowski, E.; Ines Bolzon de Muniz, G.; Nisgoski, S.; Luiz Esteves Magalhães, W. Scientia ForeStaliS Avaliação de Métodos de Obtenção de Celulose Com Diferentes Graus de Cristalinidade (Cellulose Acquirement Evaluation Methods with Different Degrees of Crystallinity). Sci. For. 2013, 41, 185–194. [Google Scholar]
- Chen, J.; Han, X.; Fang, Z.; Cheng, F.; Zhao, B.; Lu, P.; Li, J.; Dai, J.; Lacey, S.; Elspas, R.; et al. Rapid Dissolving-Debonding Strategy for Optically Transparent Paper Production. Sci. Rep. 2015, 5, 17703. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Chen, L.; Huang, L.; Cao, S.; Luo, X.; Liu, K. Novel Antimicrobial Chitosan-Cellulose Composite Films Bioconjugated with Silver Nanoparticles. Ind. Crops Prod. 2015, 70, 395–403. [Google Scholar] [CrossRef]
- Xu, Y.X.; Kim, K.M.; Hanna, M.A.; Nag, D. Chitosan-Starch Composite Film: Preparation and Characterization. Ind. Crops Prod. 2005, 21, 185–192. [Google Scholar] [CrossRef]
- Baker, B.P.; Grant, J.A. Active Ingredients Eligible for Minimum Risk Pesticide Use: Overview of the Profiles; New York State IPM Program: Geneva, NY, USA, 2018; pp. 1–19. [Google Scholar]
- FDA. Code of Federal Regulations. Food and Drugs. Food for Human Consumption. Title 21, 3rd ed.; FDA: Silver Spring, MD, USA, 2018. [Google Scholar]
- Boukhatem, M.N.; Ferhat, M.A.; Kameli, A.; Saidi, F.; Kebir, H.T. Lemon Grass (Cymbopogon citratus) Essential Oil as a Potent Anti-Inflammatory and Antifungal Drugs. Libyan J. Med. 2014, 9, 25431. [Google Scholar] [CrossRef]
- Munhuweyi, K.; Caleb, O.J.; Lennox, C.L.; van Reenen, A.J.; Opara, U.L. In Vitro and in Vivo Antifungal Activity of Chitosan-Essential Oils against Pomegranate Fruit Pathogens. Postharvest Biol. Technol. 2017, 129, 9–22. [Google Scholar] [CrossRef]
- Hadjilouka, A.; Polychronopoulou, M.; Paramithiotis, S.; Tzamalis, P.; Drosinos, E.H. Effect of Lemongrass Essential Oil Vapors on Microbial Dynamics and Listeria Monocytogenes Survival on Rocket and Melon Stored under Different Packaging Conditions and Temperatures. Microorganisms 2015, 3, 535–550. [Google Scholar] [CrossRef] [PubMed]


| Test | Levels Coded (Real Values) | Formulation (CC:CL:NL) | ||
|---|---|---|---|---|
| CC | CL | NL | ||
| 1 | 1 (3%) | 1 (40%) | 1 (5NL) | 3:40:5 |
| 2 | 1 (3%) | 1 (40%) | −1 (1NL) | 3:40:1 |
| 3 | 1 (3%) | −1 (20%) | 1 (5NL) | 3:20:5 |
| 4 | 1 (3%) | −1 (20%) | −1 (1NL) | 3:20:1 |
| 5 | −1 (1%) | 1 (40%) | 1 (5NL) | 1:40:5 |
| 6 | −1 (1%) | 1 (40%) | −1 (1NL) | 1:40:1 |
| 7 | −1 (1%) | −1 (20%) | 1 (5NL) | 1:20:5 |
| 8 | −1 (1%) | −1 (20%) | −1 (1NL) | 1:20:1 |
| 9 | 0 (2%) | 0 (30%) | 0 (3NL) | 2:30:3 |
| 10 | 0 (2%) | 0 (30%) | 0 (3NL) | 2:30:3 |
| 11 | 0 (2%) | 0 (30%) | 0 (3NL) | 2:30:3 |
| Test | Formulation (CC:CL:NL) | L* | a* | b* | Hab (°) |
|---|---|---|---|---|---|
| 1 | 3:40:5 | 86.93 ± 1.03 | 0.68 ± 0.18 | 21.42 ± 1.35 | 88.21 ± 0.41 |
| 2 | 3:40:1 | 83.76 ± 0.48 | 1.95 ± 0.29 | 15.47 ± 1.11 | 82.78 ± 1.14 |
| 3 | 3:20:5 | 83.08 ± 1.62 | 0.30 ± 0.24 | 25.11 ± 3.91 | 89.36 ± 0.48 |
| 4 | 3:20:1 | 80.69 ± 0.86 | 2.75 ± 0.17 | 15.26 ± 0.51 | 79.78 ± 0.33 |
| 5 | 1:40:5 | 81.16 ± 2.19 | 2.16 ± 0.17 | 22.83 ± 1.12 | 84.56 ± 0.48 |
| 6 | 1:40:1 | 82.88 ± 0.55 | 2.91 ± 0.11 | 15.04 ± 0.55 | 79.04 ± 0.09 |
| 7 | 1:20:5 | 82.69 ± 0.84 | 1.72 ± 0.16 | 16.19 ± 0.66 | 83.94 ± 0.38 |
| 8 | 1:20:1 | 81.26 ± 0.26 | 3.16 ± 0.02 | 16.42 ± 0.11 | 79.12 ± 0.05 |
| 9 | 2:30:3 | 81.88 ± 1.30 | 1.90 ± 0.17 | 15.53 ± 0.48 | 83.05 ± 0.47 |
| 10 | 2:30:3 | 81.59 ± 0.70 | 1.83 ± 0.34 | 15.21 ± 0.35 | 83.46 ± 1.34 |
| 11 | 2:30:3 | 82.76 ± 0.46 | 1.95 ± 0.29 | 15.47 ± 1.11 | 82.78 ± 1.14 |
| Test | Formulation (CC:CL:NL) | Average Thickness (µm) | TS (MPa) | Єr (%) | Rt (mN) | |||
|---|---|---|---|---|---|---|---|---|
| MD | CD | MD | CD | MD | CD | |||
| 1 | 3:40:5 | 435 ± 3 | 26.14 ± 1.21 | 14.06 ± 0.61 | 2.16 ± 0.14 | 5.11 ± 0.25 | 15.67 ± 0.58 | 7.48 ± 0.17 |
| 2 | 3:40:1 | 394 ± 4 | 27.61 ± 1.30 | 15.15 ± 0.23 | 2.05 ± 0.16 | 5.60 ± 0.41 | 15.30 ± 0.31 | 6.96 ± 0.47 |
| 3 | 3:20:5 | 487 ± 2 | 25.02 ± 1.46 | 12.93 ± 1.10 | 2.21 ± 0.14 | 4.54 ± 0.33 | 17.16 ± 0.69 | 8.75 ± 0.40 |
| 4 | 3:20:1 | 399 ± 4 | 29.12 ± 2.16 | 15.65 ± 1.18 | 2.16 ± 0.21 | 5.63 ± 0.28 | 16.18 ± 0.58 | 7.60 ± 0.46 |
| 5 | 1:40:5 | 448 ± 2 | 23.38 ± 1.47 | 13.27 ± 0.65 | 1.95 ± 0.09 | 4.94 ± 0.26 | 15.23 ± 0.95 | 6.96 ± 0.40 |
| 6 | 1:40:1 | 399 ± 4 | 27.27 ± 1.26 | 16.40 ± 0.82 | 2.05 ± 0.13 | 5.23 ± 0.40 | 15.05 ± 0.73 | 7.33 ± 0.32 |
| 7 | 1:20:5 | 423 ± 4 | 25.68 ± 1.48 | 15.47 ± 0.78 | 2.18 ± 0.13 | 5.28 ± 0.25 | 15.54 ± 0.75 | 7.60 ± 0.40 |
| 8 | 1:20:1 | 385 ± 2 | 28.48 ± 1.04 | 17.09 ± 0.63 | 2.12 ± 0.12 | 5.44 ± 0.26 | 14.56 ± 0.45 | 7.67 ± 0.33 |
| 9 | 2:30:3 | 426 ± 4 | 26.46 ± 1.06 | 13.93 ± 0.12 | 2.21 ± 0.10 | 5.41 ± 0.24 | 16.28 ± 0.47 | 7.58 ± 0.54 |
| 10 | 2:30:3 | 424 ± 4 | 26.01 ± 1.02 | 14.36 ± 0.76 | 2.22 ± 0.12 | 5.00 ± 0.47 | 16.28 ± 0.80 | 7.60 ± 0.52 |
| 11 | 2:30:3 | 429 ± 3 | 25.10 ± 0.90 | 14.00 ± 1.09 | 2.19 ± 0.14 | 5.03 ± 0.36 | 15.79 ± 0.87 | 8.12 ± 0.76 |
| Component | Wave Number (cm−1) | Interpretation |
|---|---|---|
| Cellulose [48] | 3338 2916, 2846 1161, 1103 1425 1020 | O-H stretching C-H stretching C-O-C stretching HCH and OCH in-plane bending vibration C–C, C–OH, and C–H ring and side group vibrations |
| Chitosan [49] | 3291 2922 2875 1641 1325 1546 1153 1064, 1020 | N-H and O-H stretching and intramolecular hydrogen bond stretching C-H symmetric stretching C-H asymmetric stretching C=O stretching of amide I (presence of residual N-acetyl groups) C-N stretching of amide III (presence of residual N-acetyl groups) N-H bending of amide II asymmetric stretching of the C-O-C bridge C-O stretching |
| Lemongrass essential oil [50,51] | 2968 2915 and 2857 1671 1632 1441 1377 1195–1120 840 | predominant asymmetric stretching of -CH3 corresponding to an alkyl saturated aliphatic group -CH2 symmetric and asymmetric stretching C=C vibrations (cis and trans), confirming the presence of conjugated double bonds (C=C-CHO) in citral stretching of C=O of the aldehyde group -CH2 bending -CH3 bending stretching of -C-O and vibrations of the -CH skeleton CH=CH trans unsaturation and 1, 3 disubstitution or 1, 4 disubstitution were also observed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.d.F.; Ernesto, J.V.; Rinaldi, A.R.; Noletto, A.P.R.; Lopes, P.S.; Carvalho, R.A.d.; Garcia, V.A.d.S.; Yoshida, C.M.P. Chitosan-Lemongrass Essential Oil on Paperboard for Active Food Packaging Applications. Polymers 2025, 17, 473. https://doi.org/10.3390/polym17040473
Silva MdF, Ernesto JV, Rinaldi AR, Noletto APR, Lopes PS, Carvalho RAd, Garcia VAdS, Yoshida CMP. Chitosan-Lemongrass Essential Oil on Paperboard for Active Food Packaging Applications. Polymers. 2025; 17(4):473. https://doi.org/10.3390/polym17040473
Chicago/Turabian StyleSilva, Mariangela de Fátima, Julia Vaz Ernesto, Alessandra Rigo Rinaldi, Ana Paula Reis Noletto, Patricia Santos Lopes, Rosemary Aparecida de Carvalho, Vitor Augusto dos Santos Garcia, and Cristiana Maria Pedroso Yoshida. 2025. "Chitosan-Lemongrass Essential Oil on Paperboard for Active Food Packaging Applications" Polymers 17, no. 4: 473. https://doi.org/10.3390/polym17040473
APA StyleSilva, M. d. F., Ernesto, J. V., Rinaldi, A. R., Noletto, A. P. R., Lopes, P. S., Carvalho, R. A. d., Garcia, V. A. d. S., & Yoshida, C. M. P. (2025). Chitosan-Lemongrass Essential Oil on Paperboard for Active Food Packaging Applications. Polymers, 17(4), 473. https://doi.org/10.3390/polym17040473








