Encapsulation of Essential Oils Using Hemp Protein Isolate–Gallic Acid Conjugates: Characterization and Functional Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Hemp Protein Isolate
2.3. Preparation of HPI–Gallic Acid Conjugates
2.4. Encapsulation of Essential Oil by Using HPI–Gallic Acid Conjugates
2.5. Determination of Encapsulation Efficiency
2.6. Particle Size of Microcapsules in Dispersion
2.7. Fourier Transform Infrared (FTIR) Spectroscopy Analysis of Microcapsules
2.8. Thermal Properties of Microcapsules
2.9. Acquiring Microstructure of Microcapsules
2.10. Controlled Release of Essential Oil from Microcapsules
2.11. Measurement of Antioxidant and Antimicrobial Properties
2.12. Statistical Analysis
3. Results and Discussion
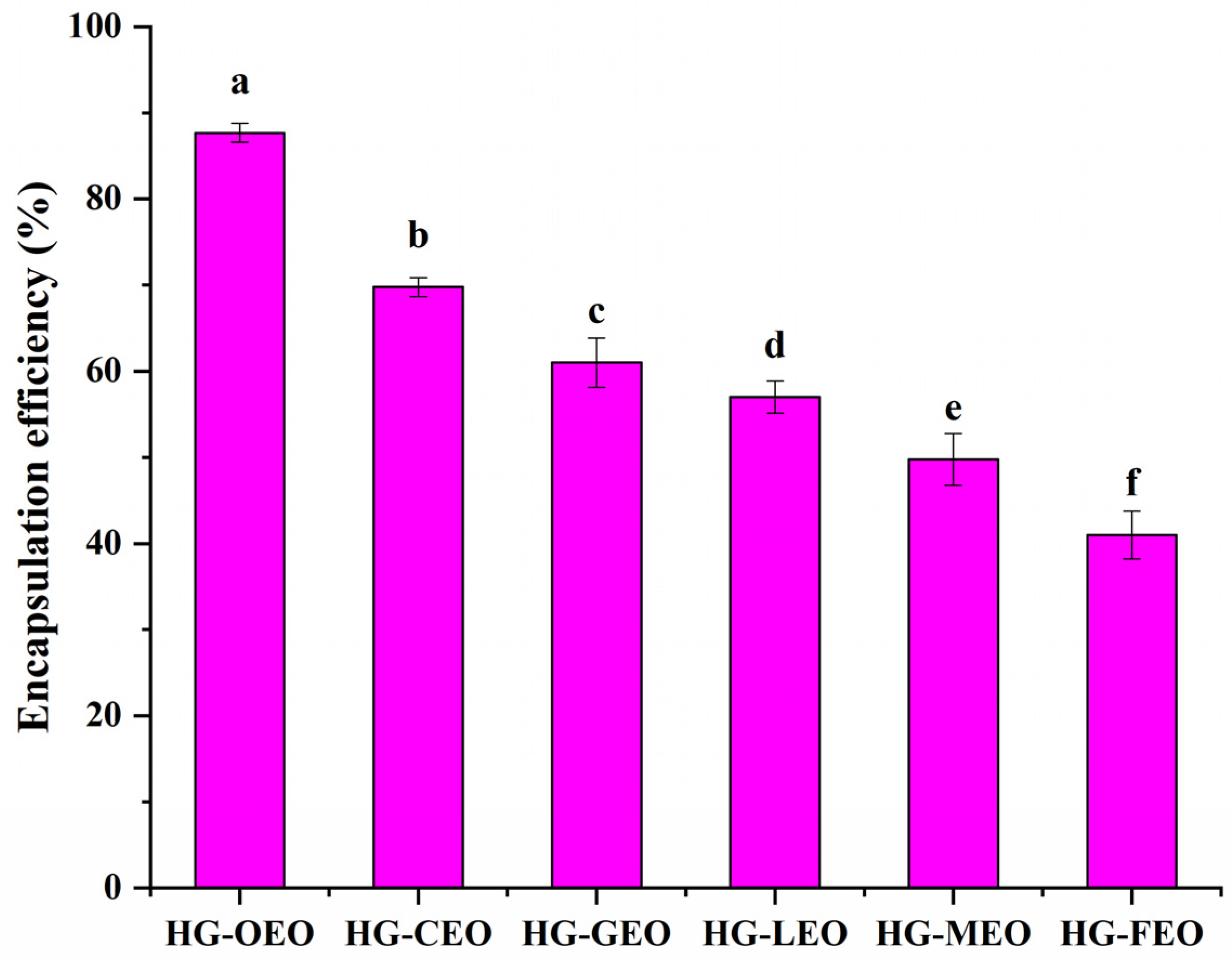
3.1. Effect of Essential Oil Type on the Encapsulation Efficiency
3.2. Effect of Essential Oils on the Microstructure of Capsules in an Aqueous Medium
3.3. Effect of Essential Oils on the Particle Size of Capsules in Aqueous Phase
3.4. Interaction of Essential Oils with the Wall Materials of Capsules
3.5. Effect of Essential Oils on the Thermal Properties of Capsules
3.6. Effect of Essential Oils on the Microstructure of Capsules
3.7. Effect of Essential Oils on Their Controlled Release
3.8. Effect of Essential Oils on the Antioxidant Properties of Microcapsules
3.9. Effect of Essential Oils on the Antimicrobial Properties of Capsules
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HG | Conjugates of hemp protein isolate and gallic acid |
| LEO | Essential oil from lemon |
| GEO | Essential oil from grapefruit |
| CEO | Essential oil from camellia |
| FEO | Essential oil from fragrans |
| OEO | Essential oil from oregano |
| MEO | Essential oil from mustard |
| HPI | Hemp protein isolate |
References
- Ashaq, B.; Rasool, K.; Habib, S.; Bashir, I.; Nisar, N.; Mustafa, S.; Ayaz, Q.; Nayik, G.A.; Uddin, J.; Ramniwas, S.; et al. Insights into chemistry, extraction and industrial application of lemon grass essential oil—A review of recent advances. Food Chem. X 2024, 22, 101521. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, L.P.; Silva, E.K. Pulsed electric field-assisted extraction techniques for obtaining vegetable oils and essential oils: Recent progress and opportunities for the food industry. Sep. Purif. Technol. 2025, 354, 128833. [Google Scholar] [CrossRef]
- Song, C.; Feng, X.; Wang, Z.; Yan, X.; Hao, C. Bioactivities of the essential oils derived from four species of non-host plants against diamondback moth Plutella xylostella. Acta Phytophylacica Sin. 2022, 49, 671–682. [Google Scholar]
- Catani, L.; Grassi, E.; Cocozza di Montanara, A.; Guidi, L.; Sandulli, R.; Manachini, B.; Semprucci, F. Essential oils and their applications in agriculture and agricultural products: A literature analysis through VOSviewer. Biocatal. Agric. Biotechnol. 2022, 45, 102502. [Google Scholar] [CrossRef]
- Hedayati, S.; Tarahi, M.; Iraji, A.; Hashempur, M.H. Recent developments in the encapsulation of lavender essential oil. Adv. Colloid Interface Sci. 2024, 331, 103229. [Google Scholar] [CrossRef]
- Lakshmayya, N.S.V.; Mishra, A.K.; Mohanta, Y.K.; Panda, J.; Naik, B.; Mishra, B.; Varma, R.S. Essential oils-based nano-emulsion system for food safety and preservation: Current status and future prospects. Biocatal. Agric. Biotechnol. 2023, 53, 102897. [Google Scholar] [CrossRef]
- Pandita, G.; de Souza, C.K.; Gonçalves, M.J.; Jasińska, J.M.; Jamróz, E.; Roy, S. Recent progress on Pickering emulsion stabilized essential oil added biopolymer-based film for food packaging applications: A review. Int. J. Biol. Macromol. 2024, 269, 132067. [Google Scholar] [CrossRef]
- Haikal, R.R.; El Salakawy, N.; Ibrahim, A.; Ali, S.L.; Mamdouh, W. Synergistic antioxidant and antibacterial effects of a Zn-ascorbate metal–organic framework loaded with marjoram essential oil. Nanoscale Adv. 2024, 6, 4664–4671. [Google Scholar] [CrossRef]
- Wang, C.; Tu, Q.; Ye, Z.; Shi, Y.; Xiao, M.; Fang, Y.; Lu, Y.; You, R. Active constituents, encapsulation technology, bioactivities and applications in food industry by essential oils of Litsea cubeba (Lour) Pers: A review. Trends Food Sci. Technol. 2024, 153, 104728. [Google Scholar] [CrossRef]
- Pires, J.B.; Santos, F.N.d.; Costa, I.H.d.L.; Kringel, D.H.; Zavareze, E.d.R.; Dias, A.R.G. Essential oil encapsulation by electrospinning and electrospraying using food proteins: A review. Food Res. Int. 2023, 170, 112970. [Google Scholar] [CrossRef]
- Chen, H.; Xu, B.; Wang, Y.; Li, W.; He, D.; Zhang, Y.; Zhang, X.; Xing, X. Emerging natural hemp seed proteins and their functions for nutraceutical applications. Food Sci. Hum. Wellness 2023, 12, 929–941. [Google Scholar] [CrossRef]
- Liu, X.; Xue, F.; Adhikari, B. Recent advances in plant protein modification: Spotlight on hemp protein. Sustain. Food Technol. 2024, 2, 893–907. [Google Scholar] [CrossRef]
- Shen, P.; Gao, Z.; Fang, B.; Rao, J.; Chen, B. Ferreting out the secrets of industrial hemp protein as emerging functional food ingredients. Trends Food Sci. Technol. 2021, 112, 1–15. [Google Scholar] [CrossRef]
- Liu, X.; Xue, F.; Adhikari, B. Production of hemp protein isolate-polyphenol conjugates through ultrasound and alkali treatment methods and their characterization. Future Foods 2023, 7, 100210. [Google Scholar] [CrossRef]
- Liu, X.; Shi, Y.; Wang, M.; Adhikari, B.; Xue, F. Covalent conjugation of hemp protein isolates with curcumin via ultrasound to improve its structural and functional properties. Food Chem. 2025, 482, 144096. [Google Scholar] [CrossRef]
- Xue, F.; Gu, Y.; Wang, Y.; Li, C.; Adhikari, B. Encapsulation of essential oil in emulsion based edible films prepared by soy protein isolate-gum acacia conjugates. Food Hydrocoll. 2019, 96, 178–189. [Google Scholar] [CrossRef]
- Liu, X.; Xue, F.; Adhikari, B. Encapsulation of essential oils using hemp protein isolate–gum Arabic complex coacervates and evaluation of the capsules. Sustain. Food Technol. 2023, 1, 426–436. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Y.; Tang, K.; He, N.; Özcan, M.M. Antioxidant effect and acaricidal potential against camel tick, Hyalomma dromedarii of the essential oil hydrodistilled from Myristica fragrans Houtt. (Nutmeg). Vet. Parasitol. 2024, 332, 110339. [Google Scholar] [CrossRef]
- Lin, J.; Dai, J.; Yang, Q.; Li, J.; Xiao, J.; Zhang, Y.; Huang, Y.; Wang, L.; Chen, P.; Xu, B.; et al. Preparation and characterization of Salecan β-glucan-based edible film loaded with lemon essential oil nanoemulsion: Effects on the preservation of chilled pork. Food Chem. 2025, 478, 143598. [Google Scholar] [CrossRef]
- Li, K.; Han, G.; Lu, S.; Xu, X.; Dong, H.; Wang, H.; Luan, F.; Jiang, X.; Liu, T.; Zhao, Y. Inhibition effect of non-contact biocontrol bacteria and plant essential oil mixture on the generation of N-nitrosamines in deli meat during storage. Food Chem. X 2024, 24, 101897. [Google Scholar] [CrossRef]
- Chen, K.; Jiang, J.; Tian, Y.; Guo, Y.; He, T.; Xie, Y.; Wu, K.; Zhu, F.; Jiang, F. Improved konjac glucomannan/curdlan-based emulsion coating by mung bean protein addition for cherry tomato preservation. Int. J. Biol. Macromol. 2025, 291, 139080. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yan, H.; Zhang, Z.-F.; Zeng, J.-M.; Zhang, Y.; Wang, J.-T.; Ma, W.-J.; Wang, M.-Q.; Peng, Q.-H.; Lv, H.-P.; et al. Assessment of the contribution of chiral odorants to aroma property of baked green teas using an efficient sequential stir bar sorptive extraction approach. Food Chem. 2021, 365, 130615. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, M.; Xue, F.; Adhikari, B. Application of ultrasound treatment to improve the technofunctional properties of hemp protein isolate. Future Foods 2022, 6, 100176. [Google Scholar] [CrossRef]
- Xue, F.; Zhao, X.; Li, C.; Adhikari, B. Modification of plum seed protein isolate via enzymatic hydrolysis, polyphenol conjugation and polysaccharide complexation to enhance emulsification and encapsulation of essential oils. Int. J. Biol. Macromol. 2025, 306, 141812. [Google Scholar] [CrossRef]
- Liu, S.; Low, N.H.; Nickerson, M.T. Entrapment of Flaxseed Oil Within Gelatin-Gum Arabic Capsules. J. Am. Oil Chem. Soc. 2010, 87, 809–815. [Google Scholar] [CrossRef]
- Olaitan, M.O.; Orababa, O.Q.; Shittu, R.B.; Obunukwu, G.M.; Kade, A.E.; Arowolo, M.T.; Oyediran, A.A.; Yusuff, R.A. Prevalence of ESBL-producing Escherichia coli in sub-Saharan Africa: A meta-analysis using a One Health approach. One Health 2025, 20, 101090. [Google Scholar] [CrossRef]
- Prosty, C.; Noutsios, D.; Lee, T.C.; Daneman, N.; Davis, J.S.; Jager, N.G.L.; Ghanem-Zoubi, N.; Goodman, A.L.; Kaasch, A.J.; Kouijzer, I.; et al. Cefazolin vs. antistaphylococcal penicillins for the treatment of methicillin-susceptible Staphylococcus aureus bacteraemia: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2025; in press. [Google Scholar] [CrossRef]
- Heckert Bastos, L.P.; Vicente, J.; Corrêa dos Santos, C.H.; Geraldo de Carvalho, M.; Garcia-Rojas, E.E. Encapsulation of black pepper (Piper nigrum L.) essential oil with gelatin and sodium alginate by complex coacervation. Food Hydrocoll. 2020, 102, 105605. [Google Scholar] [CrossRef]
- Hernández-Nava, R.; López-Malo, A.; Palou, E.; Ramírez-Corona, N.; Jiménez-Munguía, M.T. Encapsulation of oregano essential oil (Origanum vulgare) by complex coacervation between gelatin and chia mucilage and its properties after spray drying. Food Hydrocoll. 2020, 109, 106077. [Google Scholar] [CrossRef]
- Bastos, L.P.H.; dos Santos, C.H.C.; de Carvalho, M.G.; Garcia-Rojas, E.E. Encapsulation of the black pepper (Piper nigrum L.) essential oil by lactoferrin-sodium alginate complex coacervates: Structural characterization and simulated gastrointestinal conditions. Food Chem. 2020, 316, 126345. [Google Scholar] [CrossRef]
- de Matos, E.F.; Scopel, B.S.; Dettmer, A. Citronella essential oil microencapsulation by complex coacervation with leather waste gelatin and sodium alginate. J. Environ. Chem. Eng. 2018, 6, 1989–1994. [Google Scholar] [CrossRef]
- He, L.; Xu, Q.; Xin, W.; Gu, H.; Lin, Y.; Sun, P. Microencapsulation of Angelica sinensis essential oil by complex coacervation using chitosan and gelatin: Optimization, characterization, in vitro digestion, and biological activity. Int. J. Biol. Macromol. 2025, 316, 144365. [Google Scholar] [CrossRef] [PubMed]
- Plati, F.; Paraskevopoulou, A. Hemp protein isolate–gum Arabic complex coacervates as a means for oregano essential oil encapsulation. Comparison with whey protein isolate–gum Arabic system. Food Hydrocoll. 2023, 136, 108284. [Google Scholar] [CrossRef]
- Prata, A.S.; Grosso, C.R.F. Influence of the Oil Phase on the Microencapsulation by Complex Coacervation. J. Am. Oil Chem. Soc. 2015, 92, 1063–1072. [Google Scholar] [CrossRef]
- Tabatabaei, M.; Rajaei, A.; Hosseini, E.; Aghbashlo, M.; Gupta, V.K.; Lam, S.S. Effect of type of fatty acid attached to chitosan on walnut oil-in-water Pickering emulsion properties. Carbohydr. Polym. 2022, 291, 119566. [Google Scholar] [CrossRef]
- Giatropoulos, A.; Karamaouna, F.; Ampatzi, A.; Papachristos, D.; Michaelakis, A. Sublethal effects of oregano essential oil and its major compound carvacrol on biological parameters of Aedes albopictus (Diptera: Culicidae). Exp. Parasitol. 2022, 242, 108392. [Google Scholar] [CrossRef]
- Darío Pierini, G.; Andrés Bortolato, S.; Noel Robledo, S.; Raquel Alcaraz, M.; Fernández, H.; Casimiro Goicoechea, H.; Alicia Zon, M. Second-order electrochemical data generation to quantify carvacrol in oregano essential oils. Food Chem. 2022, 368, 130840. [Google Scholar] [CrossRef]
- Dong, H.; You, Y.; Wang, N.; Wang, M.; Song, T.; He, Y.; Zou, Y.; He, Y.; Peng, T.; Mei, L. Development of amphipathic derivatives of thymol and carvacrol as potent broad-spectrum antibacterial agents. Eur. J. Med. Chem. 2024, 276, 116716. [Google Scholar] [CrossRef]
- Sargolzaee, G.; Salehi, E.A.; Mahdian, E.; Naji-Tabasi, S. Encapsulation of Boswellia essential oil by quinoa protein concentrate-cress seed gum complex coacervates: Enrichment of white cheese. LWT 2024, 212, 116960. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Ju, R.; Bhandari, B.; Liu, K. Antibacterial mechanisms of star anise essential oil microcapsules encapsulated by rice protein-depolymerized pectin electrostatic complexation and its application in crab meatballs. Int. J. Food Microbiol. 2023, 384, 109963. [Google Scholar] [CrossRef]
- Nawaz, S.; Shahzadi, S.; Oneeb, M.; Hassan, J.U.; Ahmad, S.; Ramakarshina, S.; Sundarrajan, S.; Ban, D. Biocontrol Potential of Nano-Encapsulated Essential Oil Crosslinked with Chitosan Nanoparticles Against Tick-Borne Diseases. Colloids Surf. A Physicochem. Eng. Asp. 2025, 720, 137166. [Google Scholar] [CrossRef]
- Bansal, H.; Sharma, A.; Singh, S.; Mehta, S.K. Fabrication and application of buckwheat starch-based sustained-release composite films infused with curry leaf essential oil/β-cyclodextrin micro-capsules for preservation of green grapes. Food Hydrocoll. 2025, 159, 110666. [Google Scholar] [CrossRef]
- Guo, Q.; Li, S.; Du, G.; Chen, H.; Yan, X.; Chang, S.; Yue, T.; Yuan, Y. Formulation and characterization of microcapsules encapsulating carvacrol using complex coacervation crosslinked with tannic acid. LWT 2022, 165, 113683. [Google Scholar] [CrossRef]
- Jamwal, R.; Amit; Kumari, S.; Sharma, S.; Kelly, S.; Cannavan, A.; Singh, D.K. Recent trends in the use of FTIR spectroscopy integrated with chemometrics for the detection of edible oil adulteration. Vib. Spectrosc. 2021, 113, 103222. [Google Scholar] [CrossRef]
- Mei, L.; Ji, Q.; Jin, Z.; Guo, T.; Yu, K.; Ding, W.; Liu, C.; Wu, Y.; Zhang, N. Nano-microencapsulation of tea seed oil via modified complex coacervation with propolis and phosphatidylcholine for improving antioxidant activity. LWT 2022, 163, 113550. [Google Scholar] [CrossRef]
- Ramli, N.A.; Ali, N.; Hamzah, S.; Yatim, N.I. Physicochemical characteristics of liposome encapsulation of stingless bees’ propolis. Heliyon 2021, 7, e06649. [Google Scholar] [CrossRef]
- Ukwatta, R.H.; Yuan, R.; Ma, Y.; Xiong, X.; Hu, Y.; Li, C.; Xue, F. Effect of lipid addition on the physiochemical, structural, and photoactive antibacterial properties of cornstarch-chlorophyllin composite film. Food Res. Int. 2025, 202, 115699. [Google Scholar] [CrossRef]
- Liu, X.; Xue, F.; Li, C.; Adhikari, B. Physicochemical properties of films produced using nanoemulsions stabilized by carboxymethyl chitosan-peptide conjugates and application in blueberry preservation. Int. J. Biol. Macromol. 2022, 202, 26–36. [Google Scholar] [CrossRef]
- Asbahani, A.E.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; Mousadik, A.E.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Bezerra, F.M.; Carmona, O.G.; Carmona, C.G.; Lis, M.J.; de Moraes, F.F. Controlled release of microencapsulated citronella essential oil on cotton and polyester matrices. Cellulose 2016, 23, 1459–1470. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Chen, Y.; Ma, X.; Xia, M. Chitosan and procyanidin composite films with high antioxidant activity and pH responsivity for cheese packaging. Food Chem. 2021, 338, 128013. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Pei, J.; Zhu, S.; Song, Y.; Xiong, X.; Xue, F. Development of Chitosan/Peptide Films: Physical, Antibacterial and Antioxidant Properties. Coatings 2020, 10, 1193. [Google Scholar] [CrossRef]
- Volić, M.; Pećinar, I.; Micić, D.; Đorđević, V.; Pešić, R.; Nedović, V.; Obradović, N. Design and characterization of whey protein nanocarriers for thyme essential oil encapsulation obtained by freeze-drying. Food Chem. 2022, 386, 132749. [Google Scholar] [CrossRef] [PubMed]
- Koupantsis, T.; Pavlidou, E.; Paraskevopoulou, A. Glycerol and tannic acid as applied in the preparation of milk proteins–CMC complex coavervates for flavour encapsulation. Food Hydrocoll. 2016, 57, 62–71. [Google Scholar] [CrossRef]
- Volić, M.; Pajić-Lijaković, I.; Djordjević, V.; Knežević-Jugović, Z.; Pećinar, I.; Stevanović-Dajić, Z.; Veljović, Đ.; Hadnadjev, M.; Bugarski, B. Alginate/soy protein system for essential oil encapsulation with intestinal delivery. Carbohydr. Polym. 2018, 200, 15–24. [Google Scholar] [CrossRef]
- Li, C.; Pei, J.; Xiong, X.; Xue, F. Encapsulation of Grapefruit Essential Oil in Emulsion-Based Edible Film Prepared by Plum (Pruni Domesticae Semen) Seed Protein Isolate and Gum Acacia Conjugates. Coatings 2020, 10, 784. [Google Scholar] [CrossRef]
- Papadopoulou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the use of the Weibull function for the discernment of drug release mechanisms. Int. J. Pharm. 2006, 309, 44–50. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Zhang, M.; Li, H.; Liu, F.; Ou, S.; Liu, P.; Zheng, J. Enhancement of antioxidant, carbonyl scavenging and anti-glycation activities of polysaccharide-based hydrocolloids by covalent grafting with gallic acid. Int. J. Biol. Macromol. 2025, 307, 141855. [Google Scholar] [CrossRef]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; Camp, J.v.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, C.; Yang, W.; Gong, Y.; Zhang, X.; Li, J.; Wu, D. Dual cross-linking with tannic acid and transglutaminase improves microcapsule stability and encapsulates lemon essential oil for food preservation. Food Chem. 2025, 465, 142173. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Al-Harrasi, A.; Shah, Y.A.; Saif Alrasbi, A.N.; Jawad, M.; Koca, E.; Aydemir, L.Y.; Alamoudi, J.A.; Almoshari, Y.; Mohan, S. Structural, mechanical, barrier and antioxidant properties of pectin and xanthan gum edible films loaded with grapefruit essential oil. Heliyon 2024, 10, e25501. [Google Scholar] [CrossRef] [PubMed]
- Shetta, A.; Osama, A.; Hanafi, A.A.; Ali, I.H.; El Salakawy, N.; Mamdouh, W. Box-Behnken Design for optimizing the synthesis of chitosan/PVA/Camellia sinensis essential oil composite: Thermal stability, in vitro release, antioxidant, in silico studies and antibacterial activities. Int. J. Biol. Macromol. 2025, 309, 142724. [Google Scholar] [CrossRef]
- Goli, S.A.H.; Keramat, S.; Soleimanian-Zad, S.; Ghasemi Baghabrishami, R. Antioxidant and antimicrobial efficacy of microencapsulated mustard essential oil against Escherichia coli and Salmonella Enteritidis in mayonnaise. Int. J. Food Microbiol. 2024, 410, 110484. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Salvia-Trujillo, L.; Rojas-Graü, M.A.; Martín-Belloso, O. Edible films from essential-oil-loaded nanoemulsions: Physicochemical characterization and antimicrobial properties. Food Hydrocoll. 2015, 47, 168–177. [Google Scholar] [CrossRef]
- Cao, Y.; Hu, Q.; Xue, F. Comparative Characterization of Oil Body Proteins from Hemp, Plum, and Jujube Seed and Their Application in Curcumin-Loaded Artificial Oleosomes. Polymers 2025, 17, 1346. [Google Scholar] [CrossRef]
- Yuan, R.; Yi, M.; Xue, F.; Xiong, X.; Adhikari, B.; Li, C. Comparative analysis of physicochemical properties and functionalities of artificial oil bodies stabilized by different seed oil body proteins. Int. J. Biol. Macromol. 2025, 307, 141893. [Google Scholar] [CrossRef]
- da Silva, B.D.; do Rosário, D.K.A.; Weitz, D.A.; Conte-Junior, C.A. Essential oil nanoemulsions: Properties, development, and application in meat and meat products. Trends Food Sci. Technol. 2022, 121, 1–13. [Google Scholar] [CrossRef]
- Cai, F.; Duan, Z.; Yu, D.; Song, Z.; Lu, P. Antimicrobial packaging activity enhancement by lemon essential oil pickering emulsion stabilized with nanocellulose microgel particles. Food Packag. Shelf Life 2025, 47, 101439. [Google Scholar] [CrossRef]
- Özogul, Y.; Özogul, F.; Kulawik, P. The antimicrobial effect of grapefruit peel essential oil and its nanoemulsion on fish spoilage bacteria and food-borne pathogens. LWT 2021, 136, 110362. [Google Scholar] [CrossRef]
- Wang, W.; Leng, Z.; Liu, Q.; Zhao, J.; Li, S. Nano-emulsification of Osmanthus essential oil: Characterizations, stability and molecular interactions explaining antibacterial activity. Ind. Crops Prod. 2024, 219, 118987. [Google Scholar] [CrossRef]
- Chevalier, R.C.; Almeida, N.A.; de Oliveira Rocha, L.; Cunha, R.L. Antimicrobial potential of oregano essential oil vehiculated in Pickering cellulose nanofibers-stabilized emulsions. Int. J. Biol. Macromol. 2024, 275, 133457. [Google Scholar] [CrossRef]
- Peng, C.; Zhao, S.-Q.; Zhang, J.; Huang, G.-Y.; Chen, L.-Y.; Zhao, F.-Y. Chemical composition, antimicrobial property and microencapsulation of Mustard (Sinapis alba) seed essential oil by complex coacervation. Food Chem. 2014, 165, 560–568. [Google Scholar] [CrossRef] [PubMed]









| Sample | Peppas Model | Weibull Model | ||||
|---|---|---|---|---|---|---|
| R2 | Model Constants | R2 | Model Constants | |||
| Kp | n | a | b | |||
| HG-LEO | 0.9590 | 0.0160 | 0.6802 | 0.9509 | 0.0083 | 0.8860 |
| HG-GEO | 0.9630 | 0.0146 | 0.6682 | 0.9674 | 0.0094 | 0.8139 |
| HG-CEO | 0.9370 | 0.0013 | 1.0970 | 0.9535 | 0.0008 | 1.2423 |
| HG-FEO | 0.9601 | 0.2249 | 0.2114 | 0.9425 | 0.1804 | 0.3548 |
| HG-OEO | 0.9077 | 0.0002 | 1.4230 | 0.9157 | 0.0001 | 1.5551 |
| HG-MEO | 0.8629 | 0.0695 | 0.4286 | 0.9352 | 0.0482 | 0.5912 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhu, H.; Xue, F. Encapsulation of Essential Oils Using Hemp Protein Isolate–Gallic Acid Conjugates: Characterization and Functional Evaluation. Polymers 2025, 17, 1724. https://doi.org/10.3390/polym17131724
Zhang X, Zhu H, Xue F. Encapsulation of Essential Oils Using Hemp Protein Isolate–Gallic Acid Conjugates: Characterization and Functional Evaluation. Polymers. 2025; 17(13):1724. https://doi.org/10.3390/polym17131724
Chicago/Turabian StyleZhang, Xinyu, Haoran Zhu, and Feng Xue. 2025. "Encapsulation of Essential Oils Using Hemp Protein Isolate–Gallic Acid Conjugates: Characterization and Functional Evaluation" Polymers 17, no. 13: 1724. https://doi.org/10.3390/polym17131724
APA StyleZhang, X., Zhu, H., & Xue, F. (2025). Encapsulation of Essential Oils Using Hemp Protein Isolate–Gallic Acid Conjugates: Characterization and Functional Evaluation. Polymers, 17(13), 1724. https://doi.org/10.3390/polym17131724






